Published online Nov 7, 2016. doi: 10.3748/wjg.v22.i41.9205
Peer-review started: June 28, 2016
First decision: August 8, 2016
Revised: August 20, 2016
Accepted: September 14, 2016
Article in press: September 14, 2016
Published online: November 7, 2016
Processing time: 135 Days and 2.1 Hours
To assess the performance of proposed scores specific for acute-on-chronic liver failure in predicting short-term mortality among patients with alcoholic hepatitis.
We retrospectively collected data from 264 patients with clinically diagnosed alcoholic hepatitis from January to December 2013 at 21 academic hospitals in Korea. The performance for predicting short-term mortality was calculated for Chronic Liver Failure-Sequential Organ Failure Assessment (CLIF-SOFA), CLIF Consortium Organ Failure score (CLIF-C OFs), Maddrey’s discriminant function (DF), age, bilirubin, international normalized ratio and creatinine score (ABIC), Glasgow Alcoholic Hepatitis Score (GAHS), model for end-stage liver disease (MELD), and MELD-Na.
Of 264 patients, 32 (12%) patients died within 28 d. The area under receiver operating characteristic curve of CLIF-SOFA, CLIF-C OFs, DF, ABIC, GAHS, MELD, and MELD-Na was 0.86 (0.81-0.90), 0.89 (0.84-0.92), 0.79 (0.74-0.84), 0.78 (0.72-0.83), 0.81 (0.76-0.86), 0.83 (0.78-0.88), and 0.83 (0.78-0.88), respectively, for 28-d mortality. The performance of CLIF-SOFA had no statistically significant differences for 28-d mortality. The performance of CLIF-C OFs was superior to that of DF, ABIC, and GAHS, while comparable to that of MELD and MELD-Na in predicting 28-d mortality. A CLIF-SOFA score of 8 had 78.1% sensitivity and 79.7% specificity, and CLIF-C OFs of 10 had 68.8% sensitivity and 91.4% specificity for predicting 28-d mortality.
CLIF-SOFA and CLIF-C OF scores performed well, with comparable predictive ability for short-term mortality compared to the commonly used scoring systems in patients with alcoholic hepatitis.
Core tip: Alcoholic hepatitis (AH) often leads to acute-on-chronic liver failure (ACLF), which is characterized by acute hepatic decompensation of chronic liver disease, organ failure, and high short-term mortality. We investigated the prognostic utilities of proposed scores specific for ACLF in predicting short-term mortality among patients with AH. Chronic Liver Failure (CLIF)-Sequential Organ Failure Assessment and CLIF Consortium Organ Failure score performed well, and showed comparable predictive ability for short-term mortality compared to commonly used scoring systems proposed for AH. The present study suggests that scores proposed for ACLF could be useful in predicting short-term morality in patients with AH.
- Citation: Kim HY, Kim CW, Kim TY, Song DS, Sinn DH, Yoon EL, Jung YK, Suk KT, Lee SS, Lee CH, Kim TH, Kim JH, Yim HJ, Kim SE, Baik SK, Lee BS, Jang JY, Kim YS, Kim SG, Yang JM, Sohn JH, Lee HJ, Park SH, Choi EH, Kim DJ, Korean Acute-on-Chronic Liver Failure Study Group. Assessment of scoring systems for acute-on-chronic liver failure at predicting short-term mortality in patients with alcoholic hepatitis. World J Gastroenterol 2016; 22(41): 9205-9213
- URL: https://www.wjgnet.com/1007-9327/full/v22/i41/9205.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i41.9205
Alcoholic hepatitis (AH) is an acute inflammatory syndrome that occurs in patients after long-term alcohol misuse. The clinical spectrum of AH is diverse, ranging from mild to severe[1]. AH may deteriorate rapidly in its severe form, which has a high 30-d mortality of up to 50%[2,3]. AH often presents with acute deterioration superimposed on chronic liver disease[4], comprising acute-on-chronic liver failure (ACLF). ACLF is a distinct entity that was recently defined on the basis of acute decompensation, organ failure, and high short-term mortality. One of the leading causes of ACLF is active alcoholism, presenting in about 25% of patients with ACLF[5].
Given that short-term mortality is high in severe AH, it is crucial to assess disease severity and identify patients at greater risk of death in the management of patients with AH. Various scoring systems have been proposed to assess the severity of AH and to predict prognosis in these patients. Maddrey’s discriminant function (DF) has proven helpful in scoring disease severity and guiding specific treatment in AH[6]. DF ≥ 32 is associated with a high short-term mortality, therefore it is used as the threshold for corticosteroid or pentoxifylline therapy[7]. The age, bilirubin, international normalized ratio (INR), and creatinine score (ABIC) was proposed to risk stratify patients with AH into low, intermediate, and high risk of death using cut-off values of 6.71 and 9.0[8]. The Glasgow Alcoholic Hepatitis Score (GAHS) is based on age, serum bilirubin, blood urea, prothrombin time (PT), and peripheral blood white blood cell (WBC) count[9]. A GAHS ≥ 9 identifies patients with a high risk of death[10]. In addition to these disease-specific models, the model for end-stage liver disease (MELD) and modified MELD including sodium (MELD-Na) have been found to predict prognosis in AH with good accuracy[11-14].
Meanwhile, several models have been proposed to predict mortality in patients with ACLF. The European Association for Study of Liver/Chronic Liver Failure Consortium (EASL-CLIF Consortium) has modified the sequential organ failure assessment (SOFA) score to include factors specific to liver disease. This modified SOFA score adapted for patients with cirrhosis (CLIF-SOFA score) was shown to predict mortality in acute deterioration of chronic liver disease[5]. A simplified CLIF-SOFA score (CLIF Consortium Organ Failure score, CLIF-C OFs) is easy to calculate, and has similar prognostic accuracy compared to the CLIF-SOFA score[15].
The ability of scores proposed for ACLF to predict survival in patients with AH is largely unknown. The aim of this study was to validate the utility of ACLF scoring systems and compare the predictive ability of these scores with that of other commonly used prognostic models in predicting outcomes for patients with AH.
Consecutive patients with acutely decompensated alcoholic liver disease and active alcoholism were retrospectively enrolled from 21 Korean academic hospitals from January to December 2013. The inclusion criteria were history of recent excess alcohol consumption within the last 2 mo (> 50 g/d for males and > 40 g/d for females) and a clinical diagnosis of alcoholic hepatitis. Alcoholic hepatitis was clinically diagnosed as the combination of serum bilirubin more than 3 mg/dL, elevated aspartate aminotransferase (AST) but < 400 U/L, and an AST to alanine aminotransferase (ALT) ratio of > 1.5[16]. Key exclusion criteria were the presence of other causes of liver disease, infection, gastrointestinal bleeding, drug-induced hepatitis, and hepatocellular carcinoma. Medical treatment for severe AH was left to the physician’s discretion at each institute, although it usually included corticosteroids and/or pentoxifylline. Baseline clinical characteristics, laboratory findings, and survival 28 and 90 d following hospitalization were retrospectively identified by chart review. This study was approved by the Institutional Ethics Committee of all participating institutions.
For each patient, DF[6], ABIC[8], GAHS[9], MELD[14], MELD-Na[17], CLIF-SOFA[5], and CLIF-C OF[15] were calculated using laboratory data from the day of hospitalization. The formulas used to calculate prognostic models are listed in Table 1.
| Scores | Formulas | ||||
| DF[6] | 4.6 × [patient’s prothrombin time-control prothrombin time (s)] + total bilirubin (mg/dL) | ||||
| ABIC score[8] | (age × 0.1) + [serum bilirubin (mg/dL) × 0.08] + [serum creatinine (mg/dL) × 0.3] + (INR × 0.8) | ||||
| GAHS[9] | Age, blood urea nitrogen, white blood cell count, serum bilirubin, and INR; each scored 1-3 | ||||
| MELD score[14] | MELD = 3.78 ln[bilirubin (mg/dL)] + 11.20ln(INR)+ 9.57ln[creatinine (mg/dL)] + 6.43 | ||||
| MELD-Na score[17] | MELD + 1.59 (135-Na), with maximum and minimum Na of 135 and 120 mEq/L, respectively | ||||
| CLIF-SOFA score[5] | 0 | 1 | 2 | 3 | 4 |
| Liver (bilirubin, mg/dL) | < 1.2 | ≥ 1.2 to < 2.0 | ≥ 2.0 to < 6.0 | ≥ 6.0 to < 12.0 | ≥ 12.0 |
| Renal (creatinine, mg/dL) | < 1.2 | ≥ 1.2 to < 2.0 | ≥ 2.0 to < 3.5 | ≥ 3.5 to < 5.0 or use of RRT | ≥ 5.0 |
| CNS (HE grade) | No HE | I | II | III | IV |
| Coagulation (INR) | < 1.1 | ≥ 1.1 to < 1.25 | ≥ 1.25 to < 1.5 | ≥ 1.5 to < 2.5 | ≥ 2.5 or platelet ≤ 20 × 109/L |
| Cardiovascular (hypotension) | MAP ≥ 70 | MAP < 70 | Dopamine ≤ 5 or dobutamine (any dose)1 or terlipressin | Dopamine > 5 or epi ≤ 0.1 or norepi ≤ 0.11 | Dopamine > 15 or epi > 0.1 or norepi > 0.11 |
| Respiration | |||||
| PaO2/FiO2 | > 400 | > 300 to ≤ 400 | > 200 to ≤ 300 | > 100 to ≤ 200 | ≤ 100 |
| Or SpO2/FiO2 | > 512 | > 357 to ≤ 512 | > 214 to ≤ 357 | > 89 to ≤ 214 | ≤ 89 |
| CLIF-C OF score[15] | 1 | 2 | 3 | ||
| Liver (bilirubin, mg/dL) | < 6 | ≥ 6.0 to < 12.0 | ≥ 12 | ||
| Renal (creatinine, mg/dL) | < 2 | ≥ 2.0 to < 3.5 | ≥ 3.5 or RRT | ||
| CNS (HE grade) | 0 | 1-2 | 3-4 | ||
| Coagulation (INR) | < 2.0 | ≥ 2.0 to < 2.5 | ≥ 2.5 | ||
| Cardiovascular (hypotension) | MAP ≥ 70 | MAP < 70 | Vasopressors | ||
| Respiration | |||||
| PaO2/FiO2 | > 300 | ≤ 300 and > 200 | ≤ 200 | ||
| Or SpO2/FiO2 | > 357 | > 214 and ≤ 357 | ≤ 214 | ||
Data are expressed as mean ± SD for continuous variables. Categorical variables are expressed as frequencies (percentage). Differences between two groups were compared using a t-test for continuous variables and χ2-test for categorical variables. Cumulative survival curves were estimated by the Kaplan-Meier method. The log-rank statistic was used to test for significant differences between the curves. The prognostic utility of various scoring systems for predicting mortality at 28 or 90 d was assessed using the area under receiver operating characteristics curves (AUROCs). Comparison between AUROCs was performed by DeLong’s test. For each model, the sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) were calculated using originally proposed cut-off values: 32 for DF, 9.0 for ABIC, 9 for GAHS, 21 for MELD, and 28 for MELD-Na[6,8,9,13,18]. For CLIF-SOFA and CLIF-C OFs, predictive performance was calculated using an optimal cut-off point with the best sensitivity and specificity from the cohort[19]. Analyses were performed using SPSS version 18 (SPSS, IBM, Chicago, IL, USA). The comparisons between AUROCs were performed using MedCalc version 16.4.3 (Medisoftware, Mariakerke, Belgium).
The study population comprised 264 consecutive patients with a clinical diagnosis of AH who met the inclusion criteria. Table 2 describes baseline characteristics and prognostic scores of enrolled patients. The mean age was 48.8 ± 9.1 years, and males were predominant (77.3%). Overall, 28-d mortality was 12.0% and 90-d mortality was 19.0%. The differences between 28-d survivors and non-survivors are presented in Table 2. Patients who died within 28 d had higher baseline WBC count, bilirubin, INR, creatinine, and lower albumin and gammaglutamyl transferase (GGT) compared to patients who survived. Prognostic scores including DF, ABIC, GAHS, MELD, MELD-Na, CLIF-SOFA, and CLIF-C OFs, were significantly higher in non-survivors compared to 28-d survivors.
| Variable | Total cohort | 28-d survivors | 28-d nonsurvivors | P value |
| (n = 264) | (n = 232) | (n = 32) | ||
| Age (yr) | 48.8 ± 9.1 | 48.6 ± 9.2 | 48.3 ± 8.1 | 0.865 |
| Men, n (%) | 204 (77.3%) | 175 (75.4%) | 29 (90.6%) | 0.070 |
| Presence of cirrhosis, n (%) | 240 (90.9%) | 208 (89.7%) | 32 (100%) | 0.092 |
| SIRS, n (%) | 76 (28.8%) | 63 (27.2%) | 13 (40.6%) | 0.144 |
| Mean blood pressure (mmHg) | 89.3 ± 15.9 | 90.1 ± 15.1 | 83.5 ± 20.0 | 0.082 |
| WBC count (× 109/L) | 9.6 ± 6.0 | 9.2 ± 5.9 | 12.0 ± 6.7 | 0.013 |
| Platelet count (× 109/L) | 109.2 ± 71.5 | 110.6 ± 72.0 | 98.7 ± 68.0 | 0.377 |
| Albumin (g/dL) | 2.7 ± 0.6 | 2.7 ± 0.5 | 2.3 ± 0.6 | < 0.0001 |
| Bilirubin (mg/dL) | 11.3 ± 8.6 | 10.3 ± 8.0 | 18.7 ± 9.7 | < 0.0001 |
| AST (U/L) | 157.7 ± 86.6 | 160.5 ± 88.4 | 137.3 ± 70.7 | 0.098 |
| ALT (U/L) | 49.9 ± 32.4 | 50.4 ± 33.3 | 46.4 ± 24.6 | 0.514 |
| GGT (U/L) | 428.4 ± 437.5 | 455.4 ± 448.4 | 236.9 ± 290.5 | 0.001 |
| Prothrombin time (s) | 18.0 ± 5.2 | 17.3 ± 3.9 | 22.8 ± 9.6 | 0.004 |
| INR | 1.7 ± 0.6 | 1.6 ± 0.4 | 2.3 ± 0.9 | 0.001 |
| Creatinine (mg/dL) | 1.2 ± 1.5 | 0.9 ± 0.7 | 2.9 ± 3.6 | 0.005 |
| Sodium (mEq/L) | 133.5 ± 6.6 | 133.8 ± 6.3 | 131.1 ± 8.2 | 0.078 |
| DF score | 38.8 ± 28.2 | 34.9 ± 21.6 | 68.4 ± 48.0 | 0.001 |
| ABIC score | 7.5 ± 1.4 | 7.3 ± 1.1 | 9.0 ± 1.9 | < 0.0001 |
| GAHS | 7.4 ± 1.5 | 7.2 ± 1.3 | 9.1 ± 1.6 | < 0.0001 |
| MELD score | 21.6 ± 6.9 | 20.3 ± 5.5 | 30.6 ± 9.1 | < 0.0001 |
| MELD-Na score | 24.3 ± 6.8 | 23.1 ± 5.9 | 32.3 ± 7.2 | < 0.0001 |
| CLIF-SOFA score | 6.8 ± 3.1 | 6.1 ± 2.1 | 11.5 ± 4.6 | < 0.0001 |
| CLIF-C OF score | 7.9 ± 2.0 | 7.4 ± 1.4 | 11.2 ± 2.9 | < 0.0001 |
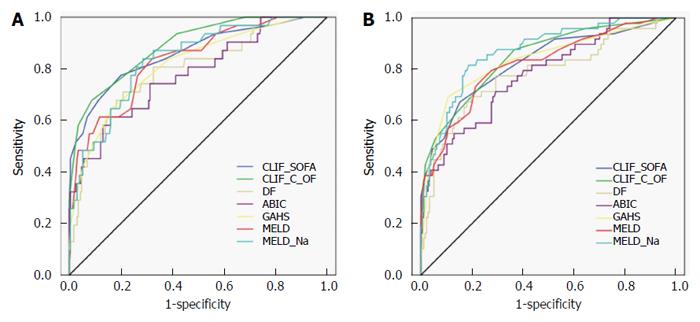
The ROC curves for various scores are shown in Figure 1. Table 3 shows the predictive accuracy of DF, ABIC, GAHS, MELD, MELD-Na, CLIF-SOFA, and CLIF-C OFs. The AUROCs of CLIF-SOFA and CLIF-C OF for 28-d mortality were 0.86 (0.81-0.90) and 0.89 (0.84-0.92) respectively. The AUROC of CLIF-SOFA was comparable to those of other scoring systems for alcoholic hepatitis in predicting 28-d mortality, such as DF, ABIC, GAHS, MELD, and MELD-Na score [AUROC (95%CI): 0.79 (0.74-0.84) for DF, 0.78 (0.72-0.83) for ABIC, 0.81 (0.76-0.86) for GAHS, 0.83 (0.78-0.88) for MELD, and 0.83 (0.78-0.88) for MELD-Na]. The AUROC of CLIF-C OFs was superior to those of DF, ABIC, and GAHS (P = 0.005 for DF, P = 0.006 for ABIC, P = 0.046 for GAHS), but was comparable to those of MELD and MELD-Na scores in predicting 28-d mortality. There were no significant differences between predictive abilities of the DF, ABIC, GAHS, MELD, MELD-Na, CLIF-SOFA, and CLIF-C OFs for 90-d mortality (except between CLIF-C OFs and DF; P = 0.02).
| Scores | AUROC (95%CI) | Cutoff | Sensitivity | Specificity | PPV | NPV |
| 28-d mortality | ||||||
| CLIF-SOFA | 0.86 (0.81-0.90) | 8 | 78.1% | 79.7% | 34.7% | 96.4% |
| CLIF-C OF | 0.89 (0.84-0.92) | 10 | 68.8% | 91.4% | 52.4% | 95.5% |
| DF | 0.79 (0.74-0.84) | 32 | 81.3% | 50.9% | 18.6% | 95.2% |
| ABIC | 0.78 (0.72-0.83) | 9 | 43.7% | 93.5% | 48.3% | 92.3% |
| GAHS | 0.81 (0.76-0.86) | 9 | 65.6% | 83.2% | 35.0% | 94.6% |
| MELD | 0.83 (0.78-0.88) | 21 | 87.5% | 57.8% | 22.2% | 97.1% |
| MELD-Na | 0.83 (0.78-0.88) | 28 | 68.8% | 80.2% | 32.4% | 94.9% |
| 90-d mortality | ||||||
| CLIF-SOFA | 0.81 (0.76-0.86) | 8 | 66.7% | 84.3% | 54.8% | 89.8% |
| CLIF-C OF | 0.83 (0.78-0.88) | 10 | 52.9% | 94.4% | 73.0% | 87.5% |
| DF | 0.77 (0.71-0.82) | 32 | 78.4% | 53.4% | 32.5% | 89.6% |
| ABIC | 0.78 (0.72-0.83) | 9 | 39.2% | 96.1% | 74.1% | 84.7% |
| GAHS | 0.82 (0.77-0.87) | 9 | 68.6% | 88.8% | 63.6% | 90.8% |
| MELD | 0.81 (0.77-0.87) | 21 | 82.4% | 61.8% | 38.2% | 92.4% |
| MELD-Na | 0.87 (0.82-0.91) | 28 | 66.7% | 87.6% | 60.7% | 90.2% |
Using a DF cut-off score of ≥ 32, the sensitivity and specificity were 81.3% and 50.9%, respectively, at predicting 28-d mortality. The ABIC score of 9 had 43.7% sensitivity and 93.5% specificity at predicting 28-d mortality. The sensitivity and specificity of MELD ≥ 21 for predicting 28-d mortality were 87.5% and 68.8%, respectively. A MELD-Na of 28 had 68.8% sensitivity and 80.2% specificity. The optimal cut-off points were chosen for CLIF-SOFA and CLIF-C OFs based on receiver operating characteristics curves. Using a CLIF-SOFA cut-off of 8, the sensitivity and specificity of CLIF-SOFA for predicting 28-d mortality were 78.1% and 79.7%, respectively. The sensitivity and specificity of CLIF-C OFs ≥ 10 were 68.8% and 91.4%, respectively, for predicting 28-d mortality.
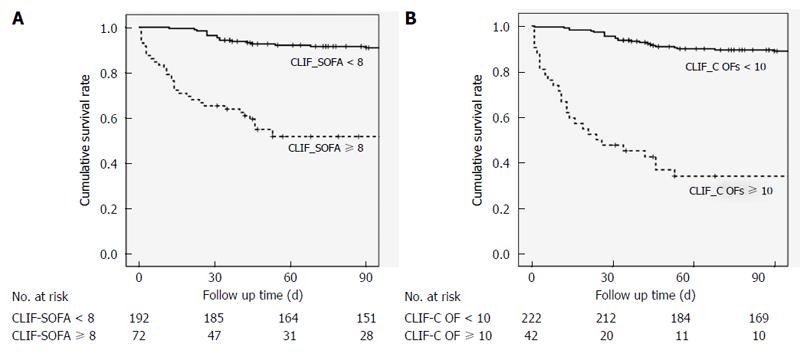
Figure 2 illustrates a survival curve comparing mortality based on CLIF-SOFA score ≥ 8 and < 8 (P < 0.05) with CLIF-C OFs ≥ 10 and < 10 (P < 0.05). Cumulative survival rates differed significantly for patients with a CLIF-SOFA score ≥ 8 and < 8. In addition, patients with CLIF-C OFs < 10 had a significantly better survival rate than those with CLIF-C OFs ≥ 10.
ACLF is a recently established syndrome characterized by acute deterioration of chronic liver disease resulting in organ failure and high short-term mortality. ACLF usually develops following a precipitating insult on cirrhosis, and AH is a common triggering event[5]. The CLIF-SOFA score and CLIF-C OFs are newly proposed scoring systems for cirrhotic patients with acute decompensation[5,15]. However, the value of these scores in predicting outcome for patients with AH remains unclear.
We assessed the prognostic utility of CLIF-SOFA score and CLIF-C OFs for predicting short-term mortality in patients with AH. We also compared the predictive ability of CLIF-SOFA score and CLIF-C OFs with that of DF, ABIC, GAHS, MELD, and MELD-Na in predicting short-term mortality in a multicenter cohort of patients with AH. Our study showed that the CLIF-SOFA score and CLIF-C OFs were excellent for predicting 28- or 90-d mortality with comparable discriminatory power as DF, ABIC, GAHS, MELD, and MELD-Na scores. In particular, the predictive ability of CLIF-C OFs was superior to that of DF, ABIC, and GAHS in predicting 28-d mortality.
In a considerable proportion of cases, the mortality rate of patients with AH is high due to hepatic inflammation and progression to organ failure[2,3]. Early identification of patients with poor outcomes could be used to risk stratify hospitalized patients with AH, ultimately guiding intensive treatment for such cases[20]. Several scoring systems have been introduced to assess severity and predict prognosis in patients with AH.
DF, initially described in 1978, has undergone modifications to replace the absolute value of PT with the prolongation of PT over control[6,7]. DF is easy to calculate and has been validated in many clinical studies. However, the poor standardization of PT and inter-laboratory variation are limitations. Other prognostic models have been proposed for patients with AH, such as the ABIC, GAHS, MELD, and MELD-Na score. Initial research demonstrated that these scores have excellent diagnostic accuracy in predicting short-term outcomes[8,11-14].
CLIF-SOFA score is a newly proposed modified version of the SOFA score applicable to patients with acute decompensation of underlying chronic liver disease[15]. Like the original SOFA score, the CLIF-SOFA score is based on assessment of six organ systems. However, the CLIF-SOFA score also accounts for some special situations in end-stage liver disease by substituting the INR of PT for platelet count and substituting hepatic encephalopathy for Glasgow coma scale. In addition, the use of terlipressin and renal replacement therapy is included. CLIF-C OFs is a simplified version of the CLIF-SOFA score[15]. Respiratory, cardiac, and central nervous (hepatic encephalopathy) systems are components of the CLIF-SOFA score and CLIF-C OFs, but not the DF, ABIC, GAHS, MELD, or MELD-Na scores. Indeed, organ failure is highly associated with increased mortality in ACLF including AH. Therefore, prognostic models incorporating organ failure are promising for use in patients with AH. Many studies have analyzed available prognostic scores that assess AH severity[13,21-26]. Our results are in line with previous observations on the utility of DF, ABIC, GAHS, MELD, and MELD-Na in predicting short-term mortality[21,24-26]. To the best of our knowledge, we are the first to assess scoring systems proposed for ACLF in predicting short-term mortality in AH patients. In our cohort, CLIF-SOFA and CLIF-C OFs perform well for predicting short-term mortality in AH. The AUROCs of CLIF-SOFA in predicting 28- or 90-d mortality were comparable to those of commonly used prognostic scores such as DF, ABIC, GAHS, MELD, and MELD-Na. In addition, the performance characteristic of CLIF-C OFs in predicting 28-d mortality was comparable to those of MELD and MELD-Na scores, and superior to those of DF, ABIC, and GAHS.
In our cohort, the optimal CLIF-SOFA cut-off was 8, which predicted 28-d mortality with 78.1% sensitivity and 79.7% specificity. Moreover, the CLIF-C OFs cutoff at 10 had sensitivity of 68.8% and specificity of 91.4%. The originally proposed cut-offs for DF and MELD lacked specificity (50.9% for DF ≥ 32, 57.8% for MELD ≥ 21). The unoptimized threshold for DF and MELD partially account for the low specificity of DF and MELD score. The NPV of all prognostic models were excellent, in most instances > 90%. In most cases, PPV was lower than 50% (Table 3). This implies that these prognostic models are useful for excluding low-risk patients, rather than identifying those at high risk of death.
Our study has limitations. First, AH was diagnosed based on clinical presentation. Clinical diagnosis poses a 10%-50% risk of erroneous classification as AH[27,28]. Second, medical treatment was determined by physician judgment. Corticosteroid effects may interfere with the predictive ability of prognostic scores, and there is some controversy in the survival benefit provided by corticosteroid treatment[29,30]. Third, we did not evaluate the alcohol relapse rate which might influence mortality and the predictive value of scoring systems. Finally, sequential values of scoring systems were not obtained. Therefore, the dynamic phase of clinical disease may not be reflected. Nevertheless, the strength of this study lies in its multi-center retrospective analysis of consecutive cases. This structure reduces selection bias and improves generalizability. Moreover, the present study is the first investigation of scoring systems proposed for ACLF at predicting the mortality of patients with AH.
In conclusion, the prognostic scores proposed for ACLF, such as CLIF-SOFA or CLIF-C OFs, proved excellent for predicting 28- and 90-d mortality. CLIF-SOFA and CLIF-C OFs had comparable discriminatory power for predicting short-term mortality compared to DF, ABIC, GAHS, MELD, and MELD-Na scores in patients with AH. CLIF-SOFA scores ≥ 8 or CLIF-C OFs ≥ 10 on the day of hospitalization should be regarded as negative short-term prognostic factors. Prospective studies validating the prognostic utility of CLIF-SOFA and CLIF-C OFs in AH are warranted.
Alcoholic hepatitis (AH) occurs in patients after long-term alcohol misuse. AH often presents with acute deterioration superimposed on chronic liver disease, comprising acute-on-chronic liver failure (ACLF), which is characterized by acute decompensation, organ failure, and high short-term mortality. Given that short-term mortality is high in severe AH, various scoring systems have been proposed to assess the severity of AH and to predict prognosis in these patients. Meanwhile, several models have been proposed to predict mortality in patients with ACLF. The ability of scores proposed for ACLF to predict mortality in patients with AH is largely unknown. The aim of this study was to evaluate the utility of ACLF scoring systems and compare the predictive ability of these scores with that of other commonly used prognostic models in predicting outcomes for patients with AH.
ACLF is a recently recognized syndrome, frequently related to active alcoholism. Few studies have analyzed scoring systems proposed for ACLF in patients with AH. The results of this study contribute to evaluating the potential of ACLF scoring systems for AH prognostication.
In this study, Chronic Liver Failure-Sequential Organ Failure Assessment (CLIF-SOFA) and CLIF Consortium Organ Failure score (CLIF-C OFs) performed well in predicting short-term mortality in AH patients. In addition CLIF-SOFA and CLIF-C OFs showed comparable predictive ability for short-term mortality compared to commonly used scoring systems proposed for AH.
This study suggests that scores proposed for ACLF could be useful in predicting short-term morality in patients with AH. In patients with AH, CLIF-SOFA scores ≥ 8 or CLIF-C OFs ≥ 10 could be used as one of the negative short-term prognostic factors.
ACLF: a distinct syndrome with high short-term mortality which is used to characterize patients hospitalized for acute decompensation of cirrhosis who have organ failure. CLIF-SOFA: a modified SOFA score adapted for patients with cirrhosis which is based on the number and type of organ failure to define ACLF.
The author of this paper explored the ability of new scores proposed for patients with cirrhosis and acute decompensation in predicting mortality in alcoholic hepatitis. A promising performance of scores proposed for ACLF in predicting short-term mortality in patients with AH was found, and further prospective studies are needed.
| 1. | Lucey MR, Mathurin P, Morgan TR. Alcoholic hepatitis. N Engl J Med. 2009;360:2758-2769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 659] [Cited by in RCA: 698] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 2. | Porter HP, Simon FR, Pope CE, Volwiler W, Fenster LF. Corticosteroid therapy in severe alcoholic hepatitis. A double-blind drug trial. N Engl J Med. 1971;284:1350-1355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 99] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 3. | Sandahl TD, Jepsen P, Thomsen KL, Vilstrup H. Incidence and mortality of alcoholic hepatitis in Denmark 1999-2008: a nationwide population based cohort study. J Hepatol. 2011;54:760-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 106] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 4. | O’Shea RS, Dasarathy S, McCullough AJ, Practice Guideline Committee of the American Association for the Study of Liver D, Practice Parameters Committee of the American College of G. Alcoholic liver disease. Hepatology. 2010;51:307-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 837] [Cited by in RCA: 862] [Article Influence: 53.9] [Reference Citation Analysis (2)] |
| 5. | Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, Durand F, Gustot T, Saliba F, Domenicali M. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144:1426-137, 1426-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1720] [Cited by in RCA: 2269] [Article Influence: 174.5] [Reference Citation Analysis (6)] |
| 6. | Maddrey WC, Boitnott JK, Bedine MS, Weber FL, Mezey E, White RI. Corticosteroid therapy of alcoholic hepatitis. Gastroenterology. 1978;75:193-199. [PubMed] |
| 7. | Carithers RL, Herlong HF, Diehl AM, Shaw EW, Combes B, Fallon HJ, Maddrey WC. Methylprednisolone therapy in patients with severe alcoholic hepatitis. A randomized multicenter trial. Ann Intern Med. 1989;110:685-690. [PubMed] |
| 8. | Dominguez M, Rincón D, Abraldes JG, Miquel R, Colmenero J, Bellot P, García-Pagán JC, Fernández R, Moreno M, Bañares R. A new scoring system for prognostic stratification of patients with alcoholic hepatitis. Am J Gastroenterol. 2008;103:2747-2756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 235] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 9. | Forrest EH, Evans CD, Stewart S, Phillips M, Oo YH, McAvoy NC, Fisher NC, Singhal S, Brind A, Haydon G. Analysis of factors predictive of mortality in alcoholic hepatitis and derivation and validation of the Glasgow alcoholic hepatitis score. Gut. 2005;54:1174-1179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 267] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 10. | Chayanupatkul M, Liangpunsakul S. Alcoholic hepatitis: a comprehensive review of pathogenesis and treatment. World J Gastroenterol. 2014;20:6279-6286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 34] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 11. | Sheth M, Riggs M, Patel T. Utility of the Mayo End-Stage Liver Disease (MELD) score in assessing prognosis of patients with alcoholic hepatitis. BMC Gastroenterol. 2002;2:2. [PubMed] |
| 12. | Srikureja W, Kyulo NL, Runyon BA, Hu KQ. MELD score is a better prognostic model than Child-Turcotte-Pugh score or Discriminant Function score in patients with alcoholic hepatitis. J Hepatol. 2005;42:700-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 165] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 13. | Vaa BE, Asrani SK, Dunn W, Kamath PS, Shah VH. Influence of serum sodium on MELD-based survival prediction in alcoholic hepatitis. Mayo Clin Proc. 2011;86:37-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, D’Amico G, Dickson ER, Kim WR. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3462] [Cited by in RCA: 3776] [Article Influence: 151.0] [Reference Citation Analysis (2)] |
| 15. | Jalan R, Saliba F, Pavesi M, Amoros A, Moreau R, Ginès P, Levesque E, Durand F, Angeli P, Caraceni P. Development and validation of a prognostic score to predict mortality in patients with acute-on-chronic liver failure. J Hepatol. 2014;61:1038-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 557] [Cited by in RCA: 777] [Article Influence: 64.8] [Reference Citation Analysis (2)] |
| 16. | Crabb DW, Bataller R, Chalasani NP, Kamath PS, Lucey M, Mathurin P, McClain C, McCullough A, Mitchell MC, Morgan TR. Standard Definitions and Common Data Elements for Clinical Trials in Patients With Alcoholic Hepatitis: Recommendation From the NIAAA Alcoholic Hepatitis Consortia. Gastroenterology. 2016;150:785-790. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 464] [Cited by in RCA: 436] [Article Influence: 43.6] [Reference Citation Analysis (0)] |
| 17. | Kim WR, Biggins SW, Kremers WK, Wiesner RH, Kamath PS, Benson JT, Edwards E, Therneau TM. Hyponatremia and mortality among patients on the liver-transplant waiting list. N Engl J Med. 2008;359:1018-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 935] [Cited by in RCA: 1102] [Article Influence: 61.2] [Reference Citation Analysis (0)] |
| 18. | Dunn W, Jamil LH, Brown LS, Wiesner RH, Kim WR, Menon KV, Malinchoc M, Kamath PS, Shah V. MELD accurately predicts mortality in patients with alcoholic hepatitis. Hepatology. 2005;41:353-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 381] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 20. | Mathurin P, Mendenhall CL, Carithers RL, Ramond MJ, Maddrey WC, Garstide P, Rueff B, Naveau S, Chaput JC, Poynard T. Corticosteroids improve short-term survival in patients with severe alcoholic hepatitis (AH): individual data analysis of the last three randomized placebo controlled double blind trials of corticosteroids in severe AH. J Hepatol. 2002;36:480-487. [PubMed] |
| 21. | Papastergiou V, Tsochatzis EA, Pieri G, Thalassinos E, Dhar A, Bruno S, Karatapanis S, Luong TV, O’Beirne J, Patch D. Nine scoring models for short-term mortality in alcoholic hepatitis: cross-validation in a biopsy-proven cohort. Aliment Pharmacol Ther. 2014;39:721-732. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 22. | Goyal SK, Dixit VK, Jain AK, Mohapatra PK, Ghosh JK. Assessment of the Model for End-stage Liver Disease (MELD) Score in Predicting Prognosis of Patients with Alcoholic Hepatitis. J Clin Exp Hepatol. 2014;4:19-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 23. | Mallaiyappan M, Sawalakhe NR, Sasidharan M, Shah DK, Rathi PM, Bhatia SJ. Retrospective and prospective validation of model for end-stage liver disease (MELD) score in predicting mortality in patients of alcoholic liver disease. Trop Gastroenterol. 2013;34:252-258. [PubMed] |
| 24. | Lafferty H, Stanley AJ, Forrest EH. The management of alcoholic hepatitis: a prospective comparison of scoring systems. Aliment Pharmacol Ther. 2013;38:603-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | Palaniyappan N, Subramanian V, Ramappa V, Ryder SD, Kaye P, Aithal GP. The utility of scoring systems in predicting early and late mortality in alcoholic hepatitis: whose score is it anyway? Int J Hepatol. 2012;2012:624675. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 26. | Sandahl TD, Jepsen P, Ott P, Vilstrup H. Validation of prognostic scores for clinical use in patients with alcoholic hepatitis. Scand J Gastroenterol. 2011;46:1127-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 27. | Kryger P, Schlichting P, Dietrichson O, Juhl E. The accuracy of the clinical diagnosis in acute hepatitis and alcoholic liver disease. Clinical versus morphological diagnosis. Scand J Gastroenterol. 1983;18:691-696. [PubMed] |
| 28. | Mookerjee RP, Lackner C, Stauber R, Stadlbauer V, Deheragoda M, Aigelsreiter A, Jalan R. The role of liver biopsy in the diagnosis and prognosis of patients with acute deterioration of alcoholic cirrhosis. J Hepatol. 2011;55:1103-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 112] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 29. | Christensen E, Gluud C. Glucocorticoids are ineffective in alcoholic hepatitis: a meta-analysis adjusting for confounding variables. Gut. 1995;37:113-118. [PubMed] |
| 30. | Rambaldi A, Saconato HH, Christensen E, Thorlund K, Wetterslev J, Gluud C. Systematic review: glucocorticosteroids for alcoholic hepatitis--a Cochrane Hepato-Biliary Group systematic review with meta-analyses and trial sequential analyses of randomized clinical trials. Aliment Pharmacol Ther. 2008;27:1167-1178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 132] [Article Influence: 7.3] [Reference Citation Analysis (1)] |
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Garcia-Martinez R, Roller J S- Editor: Yu J L- Editor: A E- Editor: Wang CH