Published online Nov 7, 2016. doi: 10.3748/wjg.v22.i41.9141
Peer-review started: June 29, 2016
First decision: July 29, 2016
Revised: August 12, 2016
Accepted: September 12, 2016
Article in press: September 12, 2016
Published online: November 7, 2016
Processing time: 130 Days and 0 Hours
To investigated the relationships between HER2, c-Jun N-terminal kinase (JNK) and protein kinase B (AKT) with respect to metastatic potential of HER2-positive gastric cancer (GC) cells.
Immunohistochemistry was performed on tissue array slides containing 423 human GC specimens. Using HER2-positve GC cell lines SNU-216 and NCI-N87, HER2 expression was silenced by RNA interference, and the activations of JNK and AKT were suppressed by SP600125 and LY294002, respectively. Transwell assay, Western blot, semi-quantitative reverse transcription-polymerase chain reaction and immunofluorescence staining were used in cell culture experiments.
In GC specimens, HER2, JNK, and AKT activations were positively correlated with each other. In vitro analysis revealed a positive regulatory feedback loop between HER2 and JNK in GC cell lines and the role of JNK as a downstream effector of AKT in the HER2/AKT signaling pathway. JNK inhibition suppressed migratory capacity through reversing EMT and dual inhibition of JNK and AKT induced a more profound effect on cancer cell motility.
HER2, JNK and AKT in human GC specimens are positively associated with each other. JNK and AKT, downstream effectors of HER2, co-operatively contribute to the metastatic potential of HER2-positive GC cells. Thus, targeting of these two molecules in combination with HER2 downregulation may be a good approach to combat HER2-positive GC.
Core tip: We investigated the significance of c-Jun N-terminal kinase (JNK) and its interaction with protein kinase B (AKT) in the HER2 signaling with respect to metastatic potential of HER2-positive gastric cancer (GC). In clinical GC samples, we found positive relationships between HER2, JNK and AKT. Inhibition studies using HER2-positive SNU-216 and NCI-N87 GC cell lines demonstrated that positive crosstalk exists between HER2 and JNK, and that JNK is a downstream signaling molecule of AKT. In addition, JNK and AKT increased EMT and co-operatively contributed to the metastatic potential of HER2-positive GC cell lines. Thus, HER2 signaling contributes to GC metastasis through activation of AKT/JNK/EMT pathway.
- Citation: Choi Y, Ko YS, Park J, Choi Y, Kim Y, Pyo JS, Jang BG, Hwang DH, Kim WH, Lee BL. HER2-induced metastasis is mediated by AKT/JNK/EMT signaling pathway in gastric cancer. World J Gastroenterol 2016; 22(41): 9141-9153
- URL: https://www.wjgnet.com/1007-9327/full/v22/i41/9141.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i41.9141
Gastric cancer (GC) is the fourth most common malignancy and the second leading cause of cancer death worldwide[1]. Although metastasis is the major obstacle in the treatment of malignant cancer, the underlying molecular mechanism responsible for GC metastasis needs to be further elucidated.
Human epidermal growth factor receptor 2 (HER2/ERBB2/neu), a member of the epidermal growth factor receptor family of receptor tyrosine kinases[2], is overexpressed in 7%-34% of GC cases[3]. Since recent studies have reported a high concordance between HER2 protein overexpression in immunohistochemistry and gene amplification by fluorescence in situ hybridization or chromogenic in situ hybridization[4], HER2 overexpression seems to be directly correlated with HER2 amplification in most cases[5]. Although our previous study[6] showed that HER2 downregulation decreased cell migration, invasion and metastasis of GC, the efficacy of anti-HER2 treatment of GC patients was limited due to intrinsic and acquired drug resistance. However, the underlying molecular mechanism of HER2-induced GC metastasis remains largely unknown.
Major downstream signaling pathways of HER2 include the mitogen-activated protein kinase (MAPK) pathway and phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT) pathway[7]. MAPKs are serine (Ser)/threonine (Thr)-specific protein kinases and include extracellular signal-regulated kinases (ERKs), p38 MAPK and c-Jun N-terminal kinases (JNKs). After phosphorylation, MAPKs are activated and can translocate to the nucleus followed by regulation of various transcription factors[8], which control the proliferation, differentiation, survival and migration of specific cell types. The specific role of individual MAPKs is dependent on cell-context and cell-type[9].
Aberrant expression and activation of JNK is found in many cancer cell lines and tissue samples of cancer patients[9]. In general, JNK has been established as a key kinase in cancer cell apoptosis[10]. Recently, the role of JNK in HER2 signaling pathway has gained much attention, because JNK activation plays a critical role in the lapatinib-resistance in HER2-positve breast cancer cells[11,12]. However, regarding GC, the biological significance of JNK in relation to HER2 signaling has not been reported. Thus, the role of JNK and its interaction with other signaling molecules in HER2-positive GC need to be investigated.
It has been shown that AKT promotes cell migration and invasion of GC cells in vitro[13] and that Akt activation significantly correlates with HER2 expression in GC specimens[14]. Although the association between AKT and JNK in various cancer cells has been reported previously[10,15-17], the results have been inconsistent. In breast cancer cells, AKT induced JNK activation[15]. In lung cancer cells, JNK induced AKT activation[16]. In glioblastoma cells, there was crosstalk between JNK and AKT[10]. In addition, in osteosarcoma cells, JNK inhibited AKT activation[17]. Thus, these findings indicate that the association between JNK and AKT could be different according to the cancer cell type investigated. Thus, interaction of these two molecules in terms of cancer cell metastasis of each cancer type needs to be elucidated. However, the relationship between JNK and AKT in GC has not been described previously.
The present study performed a large scale immunohistochemical analysis and examined the associations between the activation of HER2, JNK and AKT in 423 GC specimens. In addition, we evaluated the in vitro effect of these molecules alone or in combination on the metastatic potential of HER2-positive GC cell lines SUN-216 and NCI-N87. Furthermore, the effect of JNK/AKT inhibition on epithelial-mesenchymal transition (EMT) of these cell lines were investigated since previous studies have demonstrated that EMT plays a critical role in not only tumor metastasis but also drug resistance[18].
A total of 423 surgically resected gastric carcinoma cases were obtained from the Department of Pathology, Seoul National University College of Medicine from 2 January to 29 December, 2006. Eight paraffin tissue array blocks were prepared as previously described[19]. Briefly, core tissue biopsies (2 mm in diameter) were taken from individual paraffin-embedded gastric tumors (donor blocks) and arranged in a new recipient paraffin block (tissue array block) using a trephine apparatus. Each tissue block was able to contain up to 60 cases, allowing eight array blocks to contain 423 cases. The staining results of the different intratumoral areas of gastric carcinomas in these tissue array blocks showed an excellent agreement[20]. A core was chosen from each case for analysis. We defined an adequate case as a tumor occupying more than 10% of the core area. Sections of 4 μm thicknesses were cut from each tissue array block, deparaffinized, and rehydrated. This protocol was reviewed and approved by the Institutional Review Board of Seoul National University.
Immunohistochemistry was performed after antigen retrieval using a Bond-max automated immunostainer (Leica Microsystems, Newcastle, United Kingdom). The primary antibodies used were against HER2 (1:100, DAKO, Glostrup, Denmark), active form of JNK phosphorylated at Thr183 and Tyr185 (pJNK) (1:50, Cell Signaling Technology, Beverly, MA, United States) and active form of AKT phosphorylated at Ser473 (pAKT) (1:100, New England Biolabs, Beverly, MA, United States). Antibody binding was detected with the Bond Polymer Refine Detection kit (Leica Microsystems). All immunostained sections were then lightly counterstained with Mayer’s haematoxylin. Throughout the above analysis, negative controls were prepared by omitting the primary antibody.
For statistical analysis, the results of immunostaining were considered positive if immunoreactivity (nuclear pJNK, and nuclear and cytoplasmic pAKT) was seen in ≥ 10% of the tumor cells, as described in previous studies[20,21]. Regarding HER2 immunostaining, immunoreactivity was scored in accordance with the HER2 scoring system for GC as described in a previous study[22]. Briefly, cases showing weak to strong staining of the entire or basolateral membrane in ≥ 10% of the tumour cells were considered HER2 immuno-positive.
Human GC cell lines SNU-216 and NCI-N87 were purchased from the Korean Cell Line Bank (Seoul, Korea). Cells were cultured in RPMI1640 (Life Technologies, Grand Island, NY, United States) supplemented with 10% fetal bovine serum (FBS), 2 mg/mL sodium bicarbonate, 100 U/mL penicillin, and 100 μg/mL streptomycin (Life Technoloiges) at 37 °C in a humidified 95% air and 5% CO2 atmosphere.
Lentiviral particles containing non-targeting shRNA or HER2 shRNA were purchased (Sigma, St. Louis, MO, United States). The sequence of HER2 shRNA was 5’-CCGGTGTCAGTATCCAGGCTTTGTACTCGAGTACAAAGCCTGGATACTGACATTTTTG-3’. The control shRNA particles contain four base pair mismatches within the short hairpin sequence to any known human or mouse gene. Viral infection was performed by incubating GC cells in the culture medium containing lentiviral particles for 12 h in the presence of 5 μg/mL Polybrene (Santa Cruz Biotechnology, Santa Cruz, CA, United States). Pooled puromycin (2 μg/mL)-resistant cells were used for further analysis.
Cell lysates were prepared in 100-200 μL of 1 × sodium dodecyl sulfate (SDS) lysis buffer [125 mM Tris-HCl (pH 6.8), 4% SDS, 0.004% bromophenol blue, and 20% glycerol]. Protein contents were measured using BCA Protein Assay Reagent (Pierce, Rockford, IL, United States). Equal amounts of proteins were separated on an 8% discontinuous SDS-polyacrylamide gel and electrophoretically transferred to PVDF membranes (Millipore Corporation, Billerica, MA, United States) blocked with 5% nonfat dry milk in phosphate-buffered saline-Tween 20 (0.1%, v/v) for 1 h. The membranes were then incubated at 4 °C overnight. The primary antibodies used were against phospho-HER2Tyr1221/1222 (1:1000, Cell Signaling Technology), HER2 (1:1000, Cell Signaling Technology), pJNK (1:1000, Cell Signaling Technology), JNK (1:1000, Cell Signaling Technology), E-cadherin (1:1000, BD Biosciences, San Jose, United States), Snail (1:1000, Santa Cruz Biotechnology), Vimentin (1:1000, Neomarkers), pAKT (1:1000, Cell signaling Technology), matrix metalloproteinase (MMP9) (1:1000; Neomarkers, Fremont, CA, United States) and β-actin (1:1000, Santa Cruz Biotechnology). Horse-radish peroxidase-conjugated anti-rabbit IgG (1:4000, Santa Cruz Biotechnology) or anti-mouse IgG (1:4000, Santa Cruz Biotechnology) was used as a secondary antibody. Enhanced chemiluminescence (Pierce) was used to detect the immunoreactive proteins. Equal protein loading was confirmed by β-actin.
SNU-216 cells (1 × 104 cells/well) were cultured on 4-well chamber slide (Thermo Scientific, Rockford, IL, United States). After 24 h, cells were fixed with 4% paraformaldehyde for 10 min, and blocked with 5% normal donkey serum containing 0.5% Triton X-100 for 5 min. Cells were incubated overnight at 4 °C with mixture of the following primary antibodies: rabbit anti-HER2 (1:200; Cell Signaling Technology) and mouse anti-pJNK (1:200, Santa Cruz Biotechnology). Alexa fluor-555-conjugated anti-rabbit IgG (1:200, Life Technologies) and Alexa fluo-488-conjugated anti-mouse IgG (1:200, Life Technologies) were used as secondary antibodies. To examine whether JNK inhibition reorganizes cytoskeleton, filamentous actin (F-actin) was visualized. Cells were incubated with 165 nmol/L Alexa Fluor-633-conjugated phalloidin (Invitrogen, Carlsbad, CA, United States) for 10 min, followed by 4’6’-diamidio-2-phenoylindole (DAPI) staining. Immunofluorescence was observed under a fluorescence microscope.
Cells were seeded and allowed to attach for 24 h. To inhibit endogenous JNK activity, cancer cells were treated with a specific JNK inhibitor SP600125 (20 μmol/L for SNU-216 and 30 μmol/L for NCI-N87) (Cell Signaling Technology) dissolved in dimethylsulfoxide (DMSO). For AKT inhibition, cells were treated with 20 μmol/L of a PI3K/AKT inhibitor LY294002 (Cell Signaling Technology) dissolved in DMSO, as described in previous study[23].
Semiquantitative reverse transcription-polymerase chain reaction (SQ RT-PCR) was performed to determine the transcript level of HER2 in human gastric cancer cells, and the amplification of β-actin transcripts was used as the control to normalize the transcript levels of HER2. Total RNAs were isolated using TRIZOL reagent (Invitrogen), and reverse transcription was performed to synthesize cDNAs in a 20 μg reaction mixture containing each gene-specific primer, 1 μg RNA, 2 × reaction buffer, 0.4 μg Taq polymerase, and 1.2 mM MgCl2. The cDNAs of HER2 transcripts were all amplified for 20 cycles (denaturation at 94 °C for 30 s, annealing at 52 °C for 30 s, and extension at 70 °C for 30 s), and the cDNAs of b-actin transcripts were amplified for 18 cycles (94 °C for 30 s, 52 °C for 30 s, and 70 °C for 30 s). The PCR cycling numbers had been optimized to avoid the amplification saturation. Then, 5 μL of RT-PCR product was separated on 1% agarose gels, which were subsequently stained with ethidium bromide. Primer sequences were 5’-GGGAGAGAGTTCTGAGGATT-3’ and 5’-CGTCCGTAGAAAGGTAGTTG-3’ for HER2, and 5’-ACACCTTCTACAATGAGCTG-3’ and 5’-CATGATGGAGTTGAAGG TAG-3’ for β-actin.
A 24-well Insert System with an 8 μm pore size polyethylene terephthalate membrane was purchased from BD Biosciences. Transwell inserts were coated with Matrigel, followed by rehydration with medium for 2 h. Ten percent FBS-containing medium was placed in the lower chambers to be used as a chemoattractant. SNU-216 cells (1 × 104 cells/insert) or NCI-N87 cells (5 × 104 cells/insert) in 300 μL volume of 1% FBS-containing medium. After incubation for 48 h at 37 °C, non-invasive cells were removed with a cotton swab. Invasive cells on the bottom surface of the insert were stained with 0.2% crystal violet in 20% methanol for 30 min and were photographed with an inverted microscope. Stained cells were lysed with 10% SDS for 30 min, and absorbance was measured at 570 nm using an ELISA reader (Bio-Rad) as described previously[6]. Migration assays were performed the same way as the invasion assays, using Transwell compartment except that Matrigel was not included[6].
SNU-216 (2 × 104 cells/each well) and NCI-N87 cells (5 × 104 cells/each well) were seeded into 24-well plates and were allowed to grow for 3 d. Cell numbers were measured indirectly by using the crystal violet assay as reported by Kim et al[24]. Briefly, cells were stained with 0.2% crystal violet aqueous solution in 20% methanol for 10 min, dissolved in 10% SDS, transferred into 96-well plates, and the absorbance was measured at 570 nm using an ELISA reader (Bio-Rad, Hercules, CA, United States).
For tissue array analysis, statistical analyses were conducted using SPSS version 11.0 statistical software program (SPSS, Chicago, IL, United States), and the χ2 test was used to determine the correlations between the expressions of HER2, pJNK and pAKT. For cell culture experiments, data were analyzed using GraphPad Prism software for Windows 7 (version 4; GraphPad Software, San Diego, CA, United States), and the significances of the results were determined by the two-tailed Student’s t-test. P values of < 0.05 were considered statistically significant for all statistical analyses.
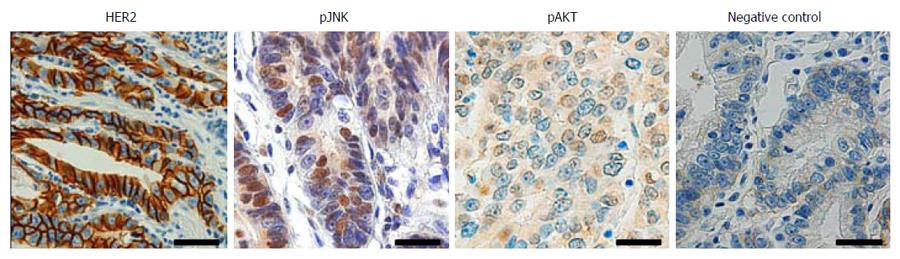
To investigate the association between HER2, JNK and AKT in human GC, immunohistochemical tissue array analysis of 423 human GC specimens was performed. Figure 1 shows the representative findings of the immunohistochemical stainings. Cancer cells with membranous HER2 expression were considered to exhibit HER2 activation, and those with nuclear staining of pJNK, regardless of cytoplasmic staining, were considered to exhibit JNK activation. For pAKT staining, immunoreactivity in both nucleus and cytoplasm was interpreted to show AKT activation. We found positive immunoreactivity for membranous HER2 in 56 (14%), pJNK in 130 (31%) and pAKT in 36 (8%) of 423 GC cases, respectively. Data concerning the correlations between the expressions of membranous HER2, nuclear pJNK and pAKT are summarized in Table 1. HER2 activation was found to be positively correlated with JNK activation (P = 0.035) and AKT activation (P = 0.029). In addition, JNK activation was also correlated with AKT activation (P = 0.025).
| HER2 (n) | Total | ||
| Positive | Negative | ||
| Total | 56 | 367 | 423 |
| pJNK | |||
| Positive | 24 | 106 | 130 |
| Negative | 32 | 261 | 293 |
| pAKT | |||
| Positive | 9 | 27 | 36 |
| Negative | 7 | 340 | 347 |
| pJNK (n) | Total | ||
| Positive | Negative | ||
| Total | 130 | 293 | 423 |
| pAKT | |||
| Positive | 17 | 19 | 36 |
| Negative | 113 | 274 | 387 |
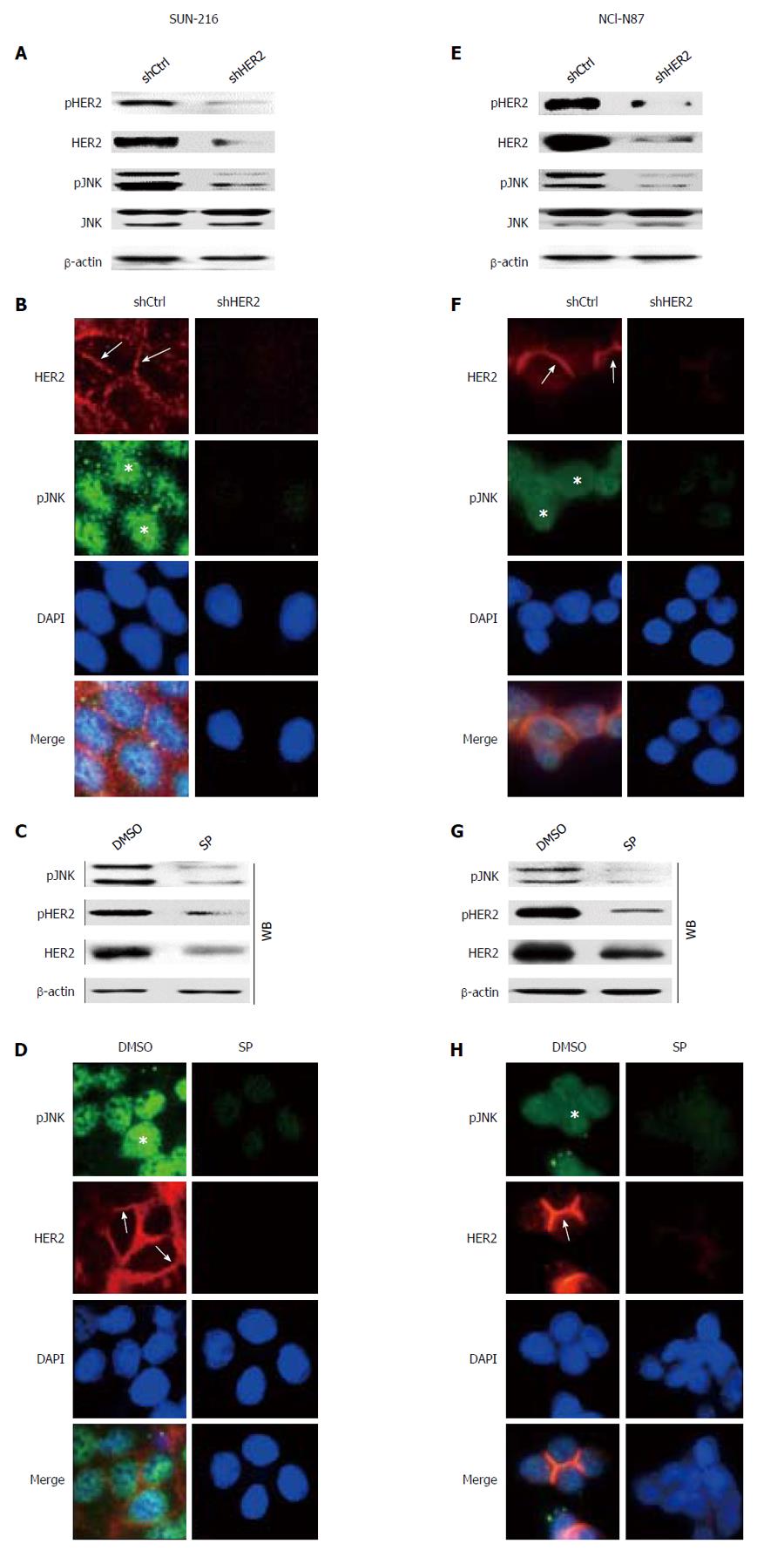
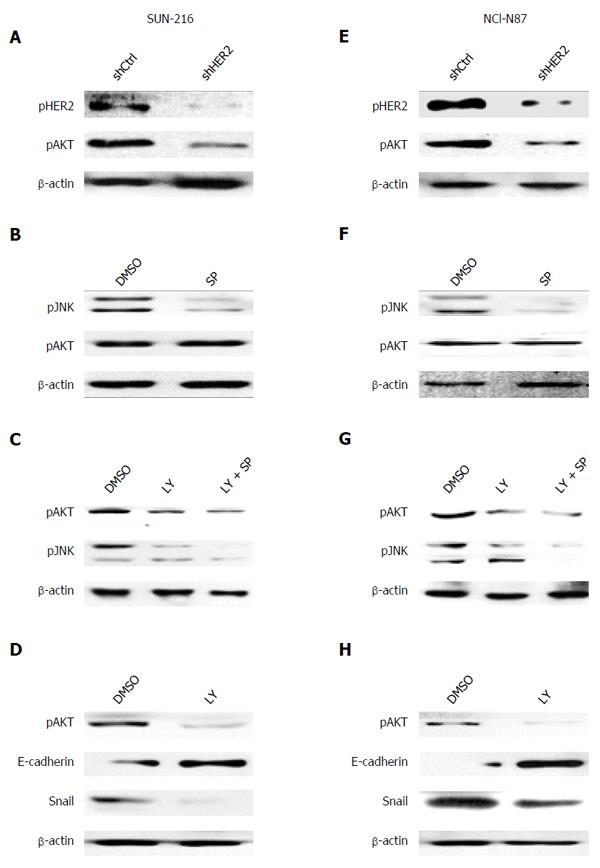
Although a current study[12] reported that HER2 inhibition suppressed JNK activation in HER2-positive breast cancer cells, the relationship between these molecules could be different according to cellular context and cell type. To investigate the direct effect of HER2 on JNK activation, we performed in vitro experiments. Since HER2 protein expression in GC cell lines varied, we selected GC cell lines SNU-216 (Figure 2A-D) and NCI-N87 (Figure 2E-H) showing a high level of HER2 expression[6]. To investigate the relationship between HER2 and JNK in GC cells, we first produced stable cell lines infected with lentiviral particles containing non-targeting (control) or HER2-targeting shRNA. Western blot (Figure 2A and E) confirmed that HER2 shRNA overexpression downregulated HER2 activation (manifested by pHER2 expression) and expression in both cell lines. HER2 silencing also decreased JNK activation (manifested by pJNK expression), but not expression. Additionally, double immunofluorescence staining for HER2 and pJNK were performed (Figure 2B and F). In both cell lines, control shRNA cells showed immunofluorescence for HER2 at the plasma membrane (red) and pJNK staining in the nucleus (green). In contrast, cells with HER2 silencing showed reduced immunofluorescence for both HER2 and pJNK compared to the control cells.
Next, we examined whether JNK has a role in HER2 activation and expression. Western blot and RT-PCR (Figure 2C and G) showed that protein expressions of pHER2 and HER2 as well as HER2 mRNA expression were substantially decreased by SP600125 treatment. Consistently, immunofluorescence staining (Figure 2D and H) revealed that SP600125-treated cells showed faint stainability for both pJNK and HER2 compared to DMSO control cells. Taken together, these results indicate that JNK controls and is controlled by HER2 with a positive relationship.
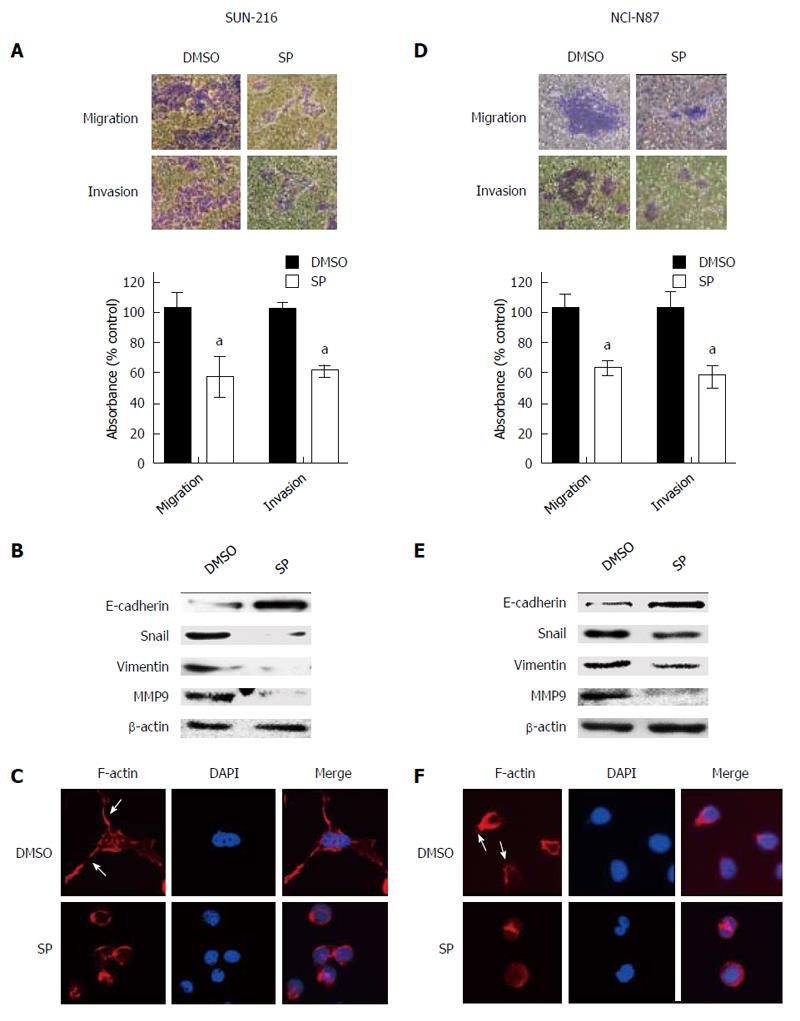
To evaluate the effect of JNK on the metastatic potential of HER2-positive GC cells, Transwell assay was performed. Figure 3A shows that SP600125 treatment for 48 h significantly suppressed the cancer cell migration (by 43%, P = 0.0288) and invasion (by 39%, P = 0.005) of SNU-216 cells compared to DMSO control cells. Consistent results were shown in NCI-N87 cells (Figure 3D).
In the initial steps of metastasis of carcinoma cells, epithelial cancer cells change their phenotype to mesenchymal phenotype and become motile and invasive by a process called EMT[25]. To examine whether JNK activation is related to EMT phenotype of cancer cells, Western blot (Figure 3B and E) was performed. After SP600125 treatment for 24 h, the expression of the representative epithelial marker E-cadherin enhanced, whereas the expressions of mesenchymal markers Snail, Vimentin and MMP9 reduced. To further confirm these results, immunofluorescence staining was performed. Since actin-dependent membrane protrusions are regarded as a critical determinant of EMT[26], we examined actin organization. Staining of F-actin with fluorescein isothiocynate-conjugated phalloidin revealed that SP600125 treatment induced apparent changes in actin organization, leading to the loss of many filopodia-like cellular projections shown in DMSO control cells (Figure 3C and F).
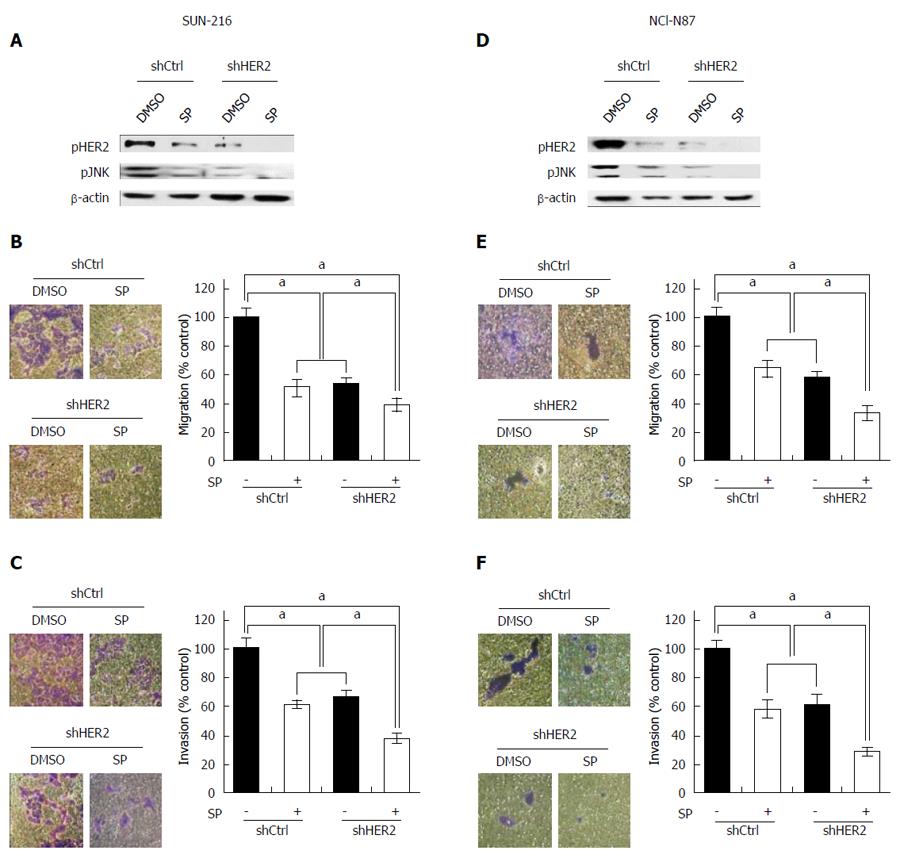
The above results indicate that JNK enhances metastatic potential and that positive crosstalk exists between HER2 and JNK in GC cells. To investigate whether the combination of JNK and HER2 has an additive effect on GC cell metastasis, we performed dual inhibition of these molecules. Western blot showed that combination of HER2 downregulation and SP600125 treatment induced lower protein expressions of pHER2 and pJNK than individual inhibitions (Figure 4A and D). Consistently, cell migration assay using SNU-216 cells (Figure 4B) showed that SP600125 treatment decreased cell motility by 49%, and HER2 downregulation by 47% compared to control cells. Dual inhibition of HER2 and JNK further decreased cell motility by 61% compared to control cells. Similar results were shown in the invasion assay (by 63% in cells with dual inhibition compared to control cells) (Figure 4C). Consistent results were shown in the experiments using NCI-N87 cells (Figure 4E and F).
In GC, AKT has also been previously described as a downstream effector of HER2 and becomes upregulated in response to HER2 oncogene activation[27,28]. Therefore, we investigated whether AKT is involved in HER2/JNK pathway in GC cell lines SNU-216 and NCI-N87. Our data showed that AKT activation decreased in HER2 shRNA transfectants compared to control shRNA transfectants (Figure 5A and E).
Next, we observed the correlation between JNK and AKT in these cells. Western blot showed that SP600125 treatment did not change AKT activation manifested by pAKT expression (Figure 5B and F). In contrast, AKT inhibition by treatment with a PI3K/AKT inhibitor LY294002 decreased JNK activation in both cell lines (Figure 5C and G). These results were confirmed by dual inhibition of AKT and JNK (Figure 5C and G). These data indicate that AKT is an upstream effector of JNK in HER2/JNK pathway in relation to metastatic potential of HER2-positive GC cells. Additionally, we examined the effect of AKT inhibition on the EMT marker expressions (Figure 5D and H). Western blot showed that LY294002 treatment increased E-cadherin expression but decreased Snail expression, which demonstrated that AKT increases mesenchymal phenotype.
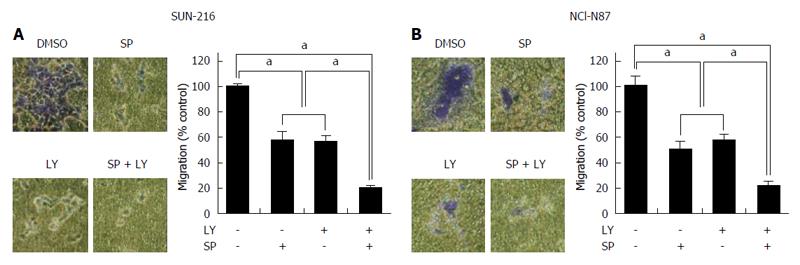
To determine the combined effect of JNK and AKT on the metastatic potential of HER2-positive GC cells, Transwell migration assay was performed (Figure 6A and B). We found that cell migration capacity was significantly suppressed by treatment with either LY294002 (by 46% in SNU-216 cells and by 50% in NCI-N87 cells) or SP600125 (by 45% in SNU-216 and NCI-N87 cells). Dual inhibition of both JNK and AKT led to greater inhibition of migration (by 80% in SNU-216 cells and by 79% in NCI-N87 cells) than single molecule inhibition.
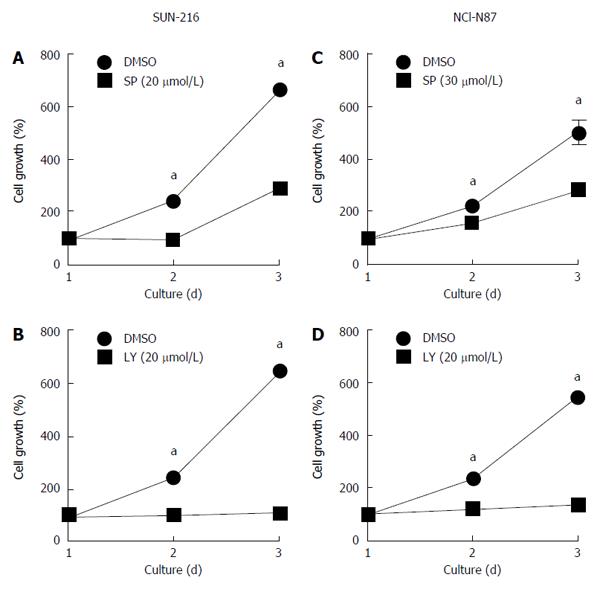
Since cell proliferation and survival affects the results of cell migration and invasion, we examined the effect of treatment with either SP600125 (Figure 7A and C) or LY294002 (Figure 7B and D) on the growth of SNU-216 and NCI-N87 cells. We found that both inhibitors suppressed cell growth of these cell lines compared to DMSO (vehicle) control.
Understanding the oncogenic signaling pathways may lead to the development of therapeutic strategies for cancer treatment. In GC, the pivotal role of HER2 metastasis has been shown[6,29], but not much is known about the downstream effectors in this process. Although most studies have implicated AKT and ERK as promising therapeutic targets for HER2-positive tumors[27,28,30], JNK, especially in breast cancer, is now emerging as an important molecule in HER2 signaling pathways[11,12]. In the present study, we found positive associations between HER2, JNK and AKT in terms of GC metastasis. This is the first study, to the best of our knowledge, to show the associations between JNK and HER2/AKT pathway in GC.
In the present study, immunohistochemical tissue array analysis of 423 GC specimens demonstrated constitutive activation of HER2 (14%), JNK (31%) and AKT (8%), which were positively related with each other. In addition, cell culture experiments using HER2-positive GC cell lines SNU-216 and NCI-N87 showed that HER2 silencing by RNA interference reduced JNK activation (manifested by pJNK expression), but not JNK expression. On the other hand, pharmacological inhibition of JNK reduced not only HER2 activation (manifested by pHER2 expression) but also HER2 protein and mRNA expressions. These results indicate that there is a positive reciprocal regulatory loop between HER2 and JNK, and that JNK increases HER2 expression at the transcriptional level, possibly through the regulation of the transcription factor. To the best of our knowledge, this is the first report on crosstalk between HER2 and JNK in the regulation of human cancer cells, including GC cells.
The inter-relationship between the PI3K and MAPK pathways is complex and incompletely understood[30]. Although we previously found that both JNK and AKT are overexpressed in GC tissue specimens[20], the relationship between these two molecules in GC has not been reported. In the present study, we found a positive association between the activations of JNK and AKT in human GC tissue specimens. In addition, cell culture experiments showed that treatment of HER2-positive GC cells with a PI3K/AKT inhibitor LY294002 decreased JNK activation, whereas JNK did not modulate AKT activation. Since HER2 downregulation suppressed AKT activation, it seems that HER2 inhibited JNK activation in GC cells through PI3K/AKT signaling.
In the present study, we found that pharmacological inhibition of JNK decreased the expressions of Snail, Vimentin and MMP9, but increased the expression of an epithelial marker E-cadherin. Moreover, SP600125 treatment decreased cancer cell migration and invasion. Since similar effects on EMT were induced by both HER2[6] and AKT, our results indicate that both JNK and AKT might contribute to malignant progression, including metastasis and drug resistance, of HER2-positive GC cells.
Although targeted therapies may increase patient selectivity and treatment efficacy, mostly their effects are not durable when they are used alone. For this reason, combination therapies are often needed for effective treatment of malignant tumors. In the present study, we found that treatment with either SP600125 or LY294002 significantly reduced metastatic potential of HER2-positive GC cells to the similar level, and that co-treatment with both of these inhibitors induced a further decrease compared to treatment with either alone. Thus, our findings suggest that combined targeting of JNK and AKT significantly impairs GC cell migration in concert with HER2 downregulation. However, additional animal experiments to evaluate these inhibitions are needed.
In the present study, we found that either inhibition of JNK/AKT suppresses cell growth in both HER2-positive GC cell lines. Thus, JNK/AKT-induced cell growth of these cell lines might affect the results of cell migration and invasion assays observed in the present study. However, our results also indicate that inhibition of JNK/AKT decreases mesenchymal phenotype in individual GC cells based on the EMT marker expressions. Thus, we speculate that JNK/AKT contributes to metastatic potential as well as cell growth of HER2-positive gastric cancer cells.
In conclusion, our results showed that JNK and AKT are co-expressed in a subset of HER2-positive GC cases, and that HER2, JNK and AKT are positively associated with each other. In addition, inhibition of either JNK or AKT decreased cancer cell motility of HER2-positive GC cells through reversing EMT. Since dual inhibition of JNK and AKT induced more profound effect on cancer cell motility, combined targeting of these molecules might be used to regulate the GC metastasis in a subgroup of GC patients.
Human epidermal growth factor receptor 2 (HER2/ERBB2/neu), a member of the epidermal growth factor receptor family of receptor tyrosine kinases, is overexpressed in 7%-34% of gastric cancer (GC) cases.
The present study performed a large scale immunohistochemical analysis and examined the associations between the activation of HER2, JNK and AKT in 423 GC specimens. In addition, the authors evaluated the in vitro effect of these molecules alone or in combination on the metastatic potential of HER2-positive GC cell lines SUN-216 and NCI-N87.
The effect of JNK/AKT inhibition on epithelial-mesenchymal transition (EMT) of these cell lines were investigated since previous studies have demonstrated that EMT plays a critical role in not only tumor metastasis but also drug resistance.
This manuscript has clearly shown that HER2, JNK and AKT play important roles on invasion of gastric cancer cells. It provided important contribution to the role of anti-HER2 treatment of gastric cancer patients. They performed a large scale analysis and the manuscript was well written. However, certain revisions are required.
| 1. | Oh DY, Doi T, Shirao K, Lee KW, Park SR, Chen Y, Yang L, Valota O, Bang YJ. Phase I Study of Axitinib in Combination with Cisplatin and Capecitabine in Patients with Previously Untreated Advanced Gastric Cancer. Cancer Res Treat. 2015;47:687-696. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 2. | Matsui Y, Inomata M, Tojigamori M, Sonoda K, Shiraishi N, Kitano S. Suppression of tumor growth in human gastric cancer with HER2 overexpression by an anti-HER2 antibody in a murine model. Int J Oncol. 2005;27:681-685. [PubMed] |
| 3. | Peng Z, Zou J, Zhang X, Yang Y, Gao J, Li Y, Li Y, Shen L. HER2 discordance between paired primary gastric cancer and metastasis: a meta-analysis. Chin J Cancer Res. 2015;27:163-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 4. | Gravalos C, Jimeno A. HER2 in gastric cancer: a new prognostic factor and a novel therapeutic target. Ann Oncol. 2008;19:1523-1529. [PubMed] |
| 5. | Kim MA, Jung JE, Lee HE, Yang HK, Kim WH. In situ analysis of HER2 mRNA in gastric carcinoma: comparison with fluorescence in situ hybridization, dual-color silver in situ hybridization, and immunohistochemistry. Hum Pathol. 2013;44:487-494. [PubMed] |
| 6. | Ko YS, Cho SJ, Park J, Kim Y, Choi YJ, Pyo JS, Jang BG, Park JW, Kim WH, Lee BL. Loss of FOXO1 promotes gastric tumour growth and metastasis through upregulation of human epidermal growth factor receptor 2/neu expression. Br J Cancer. 2015;113:1186-1196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 7. | Han JS, Crowe DL. Jun amino-terminal kinase 1 activation promotes cell survival in ErbB2-positive breast cancer. Anticancer Res. 2010;30:3407-3412. [PubMed] |
| 8. | Kyriakis JM, Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev. 2001;81:807-869. [PubMed] |
| 9. | Wagner EF, Nebreda AR. Signal integration by JNK and p38 MAPK pathways in cancer development. Nat Rev Cancer. 2009;9:537-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | Vo VA, Lee JW, Lee HJ, Chun W, Lim SY, Kim SS. Inhibition of JNK potentiates temozolomide-induced cytotoxicity in U87MG glioblastoma cells via suppression of Akt phosphorylation. Anticancer Res. 2014;34:5509-5515. [PubMed] |
| 11. | Phelps-Polirer K, Abt MA, Smith D, Yeh ES. Co-Targeting of JNK and HUNK in Resistant HER2-Positive Breast Cancer. PLoS One. 2016;11:e0153025. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 12. | Gschwantler-Kaulich D, Grunt TW, Muhr D, Wagner R, Kölbl H, Singer CF. HER Specific TKIs Exert Their Antineoplastic Effects on Breast Cancer Cell Lines through the Involvement of STAT5 and JNK. PLoS One. 2016;11:e0146311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Kong D, Li Y, Wang Z, Sarkar FH. Cancer Stem Cells and Epithelial-to-Mesenchymal Transition (EMT)-Phenotypic Cells: Are They Cousins or Twins? Cancers (Basel). 2011;3:716-729. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 252] [Cited by in RCA: 265] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 14. | Yoo YA, Kang MH, Lee HJ, Kim BH, Park JK, Kim HK, Kim JS, Oh SC. Sonic hedgehog pathway promotes metastasis and lymphangiogenesis via activation of Akt, EMT, and MMP-9 pathway in gastric cancer. Cancer Res. 2011;71:7061-7070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 284] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 15. | Sukawa Y, Yamamoto H, Nosho K, Kunimoto H, Suzuki H, Adachi Y, Nakazawa M, Nobuoka T, Kawayama M, Mikami M. Alterations in the human epidermal growth factor receptor 2-phosphatidylinositol 3-kinase-v-Akt pathway in gastric cancer. World J Gastroenterol. 2012;18:6577-6586. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 62] [Cited by in RCA: 72] [Article Influence: 5.1] [Reference Citation Analysis (1)] |
| 16. | Byun HJ, Hong IK, Kim E, Jin YJ, Jeoung DI, Hahn JH, Kim YM, Park SH, Lee H. A splice variant of CD99 increases motility and MMP-9 expression of human breast cancer cells through the AKT-, ERK-, and JNK-dependent AP-1 activation signaling pathways. J Biol Chem. 2006;281:34833-34847. [PubMed] |
| 17. | Ibuki Y, Toyooka T, Zhao X, Yoshida I. Cigarette sidestream smoke induces histone H3 phosphorylation via JNK and PI3K/Akt pathways, leading to the expression of proto-oncogenes. Carcinogenesis. 2014;35:1228-1237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 18. | Yen CC, Hsiao CD, Chen WM, Wen YS, Lin YC, Chang TW, Yao FY, Hung SC, Wang JY, Chiu JH. Cytotoxic effects of 15d-PGJ2 against osteosarcoma through ROS-mediated AKT and cell cycle inhibition. Oncotarget. 2014;5:716-725. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 19. | Lee HS, Lee HK, Kim HS, Yang HK, Kim WH. Tumour suppressor gene expression correlates with gastric cancer prognosis. J Pathol. 2003;200:39-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 143] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 20. | Nam SY, Lee HS, Jung GA, Choi J, Cho SJ, Kim MK, Kim WH, Lee BL. Akt/PKB activation in gastric carcinomas correlates with clinicopathologic variables and prognosis. APMIS. 2003;111:1105-1113. [PubMed] |
| 21. | Choi Y, Park J, Choi Y, Ko YS, Yu DA, Kim Y, Pyo JS, Jang BG, Kim MA, Kim WH. c-Jun N-terminal kinase activation has a prognostic implication and is negatively associated with FOXO1 activation in gastric cancer. BMC Gastroenterol. 2016;16:59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Kim MA, Lee HJ, Yang HK, Bang YJ, Kim WH. Heterogeneous amplification of ERBB2 in primary lesions is responsible for the discordant ERBB2 status of primary and metastatic lesions in gastric carcinoma. Histopathology. 2011;59:822-831. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 115] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 23. | Park J, Ko YS, Yoon J, Kim MA, Park JW, Kim WH, Choi Y, Kim JH, Cheon Y, Lee BL. The forkhead transcription factor FOXO1 mediates cisplatin resistance in gastric cancer cells by activating phosphoinositide 3-kinase/Akt pathway. Gastric Cancer. 2014;17:423-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 24. | Kim WH, Schnaper HW, Nomizu M, Yamada Y, Kleinman HK. Apoptosis in human fibrosarcoma cells is induced by a multimeric synthetic Tyr-Ile-Gly-Ser-Arg (YIGSR)-containing polypeptide from laminin. Cancer Res. 1994;54:5005-5010. [PubMed] |
| 25. | Guarino M, Rubino B, Ballabio G. The role of epithelial-mesenchymal transition in cancer pathology. Pathology. 2007;39:305-318. [PubMed] |
| 26. | Shankar J, Messenberg A, Chan J, Underhill TM, Foster LJ, Nabi IR. Pseudopodial actin dynamics control epithelial-mesenchymal transition in metastatic cancer cells. Cancer Res. 2010;70:3780-3790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 229] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 27. | Nam HJ, Ching KA, Kan J, Kim HP, Han SW, Im SA, Kim TY, Christensen JG, Oh DY, Bang YJ. Evaluation of the antitumor effects and mechanisms of PF00299804, a pan-HER inhibitor, alone or in combination with chemotherapy or targeted agents in gastric cancer. Mol Cancer Ther. 2012;11:439-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 28. | Kim HP, Han SW, Song SH, Jeong EG, Lee MY, Hwang D, Im SA, Bang YJ, Kim TY. Testican-1-mediated epithelial-mesenchymal transition signaling confers acquired resistance to lapatinib in HER2-positive gastric cancer. Oncogene. 2014;33:3334-3341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 95] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 29. | Janjigian YY. Lapatinib in Gastric Cancer: What Is the LOGiCal Next Step? J Clin Oncol. 2016;34:401-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 30. | Saini KS, Loi S, de Azambuja E, Metzger-Filho O, Saini ML, Ignatiadis M, Dancey JE, Piccart-Gebhart MJ. Targeting the PI3K/AKT/mTOR and Raf/MEK/ERK pathways in the treatment of breast cancer. Cancer Treat Rev. 2013;39:935-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 296] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Maehata Y, Matsuda Y, Shimada Y S- Editor: Qi Y L- Editor: A E- Editor: Wang CH