Published online Nov 7, 2016. doi: 10.3748/wjg.v22.i41.9057
Peer-review started: August 3, 2016
First decision: August 22, 2016
Revised: September 9, 2016
Accepted: October 19, 2016
Article in press: October 19, 2016
Published online: November 7, 2016
Processing time: 96 Days and 16.7 Hours
The past decade has witnessed an outstanding scientific production focused towards the possible clinical applications of mesenchymal stromal cells (MSCs) in autoimmune and chronic inflammatory diseases. This raised the need of novel standards to adequately address quality, efficacy and safety issues of this advanced therapy. The development of a streamlined regulation is currently hampered by the complexity of analyzing dynamic biological entities rather than chemicals. Although numerous pieces of evidence show efficacy in reducing intestinal inflammation, some inconsistencies between the mechanisms of action of rodent vs human MSCs suggest caution before assigning translational value to preclinical studies. Preliminary evidence from clinical trials showed efficacy of MSCs in the treatment of fistulizing Crohn’s disease (CD), and preparations of heterologous MSCs for CD treatment are currently tested in ongoing clinical trials. However, safety issues, especially in long-term treatment, still require solid clinical data. In this regard, standardized guidelines for appropriate dosing and methods of infusion could enhance the likelihood to predict more accurately the number of responders and the duration of remission periods. In addition, elucidating MSC mechanisms of action could lead to novel and more reliable formulations such as those derived from the MSCs themselves (e.g., supernatants).
Core tip: Mesenchymal stromal cells (MSCs) release immunomodulatory mediators upon inflammatory stimuli. This behavior is attractive for the development of advanced therapeutic strategies applied to several intestinal disorders where inflammation is a key pathophysiological feature. In order to assess quality, efficacy and safety of MSC-based therapy, a novel approach to pharmacokinetics/pharmacodynamics (PK/PD) is mandatory. This must rely on careful assessment of cell phenotype, signaling and homing mechanisms. In this regard, experimental models must take advantage of the most updated knowledge in order to reflect the PK/PD mechanisms in humans. Finally, an alternative approach to the “whole-cell treatment” applies MSC-derived mediators alone in order to avoid the hypothesized serious adverse events deriving from a biological entity mostly acting systemically.
- Citation: Dothel G, Raschi E, Rimondini R, De Ponti F. Mesenchymal stromal cell-based therapy: Regulatory and translational aspects in gastroenterology. World J Gastroenterol 2016; 22(41): 9057-9068
- URL: https://www.wjgnet.com/1007-9327/full/v22/i41/9057.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i41.9057
Mesenchymal stromal cells (MSCs) were first isolated from bone marrow as a population of adherent cells, characterized by a non-phagocytic fibroblast-like morphology[1]. Later, MSCs were described in several different tissues, (bone marrow-derived MSCs): cartilage, adipose tissue (adipose-derived stem cells - ASC), tendon and muscle.
The International Society for Cellular Therapy established 3 minimum criteria that MSCs must fulfill in vitro: (1) adherence to plastic; (2) expression of specific surface antigens (CD73+, CD90+, CD105+, CD34-, CD45-, CD11b-, CD14-, CD19-, CD79a, HLA-DR-); and (3) differentiation potential (osteogenic, chondrogenic and adipogenic lineages)[1]. Considering this multi-lineage differentiation capacity, MSCs were first considered as a therapeutic tool in bone and cartilage diseases, aiming at tissue regeneration. Later, hematopoietic stem cells (HSCs) transplantation was tested in facilitating engraftment and treating steroid-resistant acute-graft-versus-host disease.
More recently, the ability of MSCs to home and promote tissue repair and counteract inflammatory status prompted their investigation in a variety of inflammatory immune-mediated disorders, with about 250 clinical studies already registered[2].
Therefore, it is not surprising that MSCs are being extensively assessed as a possible therapy of intestinal disorders where inflammation represents a key pathophysiological feature, especially considering the epidemiologic burden of inflammatory bowel diseases (IBDs). There are three intriguing aspects that attract interest and warrant further investigation to fully exploit the potential therapeutic properties of MSCs.
First, it is widely accepted that MSCs do not per se inhibit inflammation, but require activation by an inflammatory environment in the host to produce their immunoregulatory effect. The presence of an inflammatory environment activates MSCs, which in turn shift into an immune-suppressive phenotype, whereas, conversely, the lack of inflammatory stimuli prompts MSCs to adopt a pro-inflammatory phenotype[3]. In fact, Duijvestein et al[4] found that administration of IFN-γ in MSC culture medium increases their therapeutic potential in a model of trinitrobenzene sulfonate (TNBS)-induced colitis.
Second, healing properties of MSCs appear largely dependent on the release of soluble factors and chemokines produced by the cells themselves and/or by local microenvironment, while their survival does not seem necessary for clinical benefit[5]: the detection of biologically active compounds derived from MSCs implies that a “cell-free” therapy could be an alternative[6-8].
Third, homing and migration are still incompletely characterized, but are likely to be influenced by multiple factors such as age and number of passages, culture conditions and the delivery method[9]. Notably, different studies indicated that intravenous-injected MSCs are trapped in the lungs upon first passage[10].
From a regulatory standpoint, stem cells are regulated both in Europe and United States under specific legislation. In Europe, stem cells can only be used under two regulatory frameworks: approved clinical trial or compassionate use, according to the Regulation 1394/2007 of the European Parliament and of the Council on advanced therapy medicinal products and amending Directive 2001/83/EC.
Recently, the European Commission launched a public stakeholder consultation on the draft “Guidelines on Good Manufacturing Practice for Advanced Therapy Medicinal Products (ATMPs)” (ending on 26 September 2016). The main purpose is to provide all the requirements to assure identity/consistency, quality, safety and efficacy in a way that takes advantage of what has been learnt in the development of quality standards of medicinal products of chemical origin, taking into account the specific requirements of the ATMP. Importantly, internal audit personnel, formally designated as “quality personnel”, must certify several steps of quality control and batch release. Special provisions regard investigational ATMPs which may be developed in academic or hospitals and cannot ensure all the information required in conventional procedures (e.g., potency). However, risk-based approach and the application of good manufacturing processes (GMP) are mandatory regardless the site of production. Among other indications, blinding of the cell characterization procedure is suggested in case of investigational ATMPs, and special provisions are in place for automated productions[11].
The multifaceted immuno-modulating properties of MSCs, capable of interacting with both the adaptive and innate immune system, make them an attractive source to restore immune homeostasis in the gut and even coordinate tissue remodeling during the healing process.
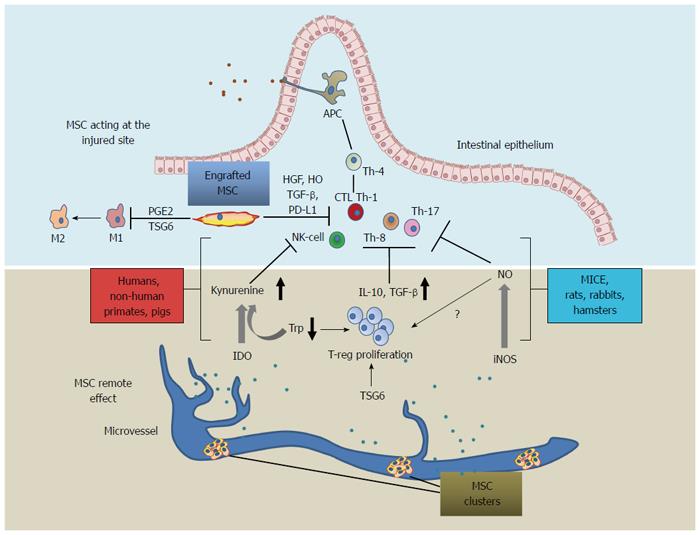
MSCs were demonstrated to inhibit in vitro differentiation of T lymphocytes into Th1 and Th17 cells, suppress cytotoxic T cells proliferation through secretion of anti-proliferative soluble factors such as hepatocyte growth factor (HGF), TGF-β, prostaglandin E2, indoleamine 2,3-dioxygenase (IDO), nitric oxide (NO) and heme-oxigenase-1 (HO-1). In addition, MSCs possess the ability to polarize T cells towards a regulatory phenotype, interfere with differentiation and maturation of monocytes towards dendritic cells, induce dendritic cells to acquire a tolerogenic phenotype, and down-regulate NK activation (Table 1).
| Bioactive molecule | Mouse | Rat | G.pig | Pig | Human | Clinical biomarker | Major mechanisms | Ref. |
| TSG-6 | X | X | X | NA | X | X | Reduction of IL-6, IFN-γ, and TNF-α, induction of T-reg lymphocytes | [5,18,19,27,72-76] |
| IDO | NA | X | X | X | Apoptosis of cytolytic lymphocytes, IL-10 induction T-reg proliferation | [13,15,16,52,77] | ||
| iNOS | X | X | X | Inhibition of effector lymphocytes through IL-10 | [13,17] | |||
| PGE2 | X | X | X | Macrophage conversion to M2 phenotype, NK cell inhibition, IDO induction | [12,78-80] | |||
| IL-10 | X | X | X | X | X | X | T-reg lymphocyte Induction | [12,13,15,17,19,54,78] |
| TGF-β | X | X | X | X | T-reg induction | [81-83] | ||
| PD-L1 | X | X | X | X | CD8+ lymphocyte inhibition | [12] |
Taken together, these findings document the in vitro properties of MSCs and strongly support their anti-inflammatory mechanisms involved in IBDs[12]. Importantly, MSC production of IDO depends on IFNγ or TNFα in combination with IL-1β. The latter in turn catabolizes tryptophan producing kynurenins, a potent T-reg lymphocyte inducer[13,14]. MSC-dependent induction of IDO correlates with disease rating in humans[15,16] and, to a lesser extent, in mice where MSC anti-inflammatory action is mostly mediated by iNOS[13,17] (Table 1). Notably, IDO or iNOS-mediated MSC activity was recently shown to correlate with a specific phylogenetic tree (Figure 1)[13].
Tumor necrosis factor-inducible gene 6 protein (TSG-6) mediates MSC action in both mice and humans[18,19]. Originally, this molecule was extensively studied for its anti-inflammatory properties associated with extracellular matrix rearrangement[20] and, more recently, as an effector of MSCs[18]. MSC-secreted TSG-6 decreases NF-κB in macrophages, which in turn secretes PGE-2[21] (Figure 1). Moreover, MSCs knocked down for TSG-6 do not exert any therapeutic action.
Unfortunately, TSG-6 production in vitro is scarcely achieved because of its tendency to form complexes with hyaluronans. This finding is in line with the observations of Sala and colleagues, who detected the formation of intra-peritoneal TSG-6 mediated aggregates of MSCs in a mouse model secreting IL-10. As a consequence, the authors question the relevance of MSC engraftment as a therapeutic mechanism[19].
Whether the anti-inflammatory properties of MSCs are local or depend on the release of soluble mediators from a distance is a matter of debate[22,23].
Intravenous injection of MSCs has been thoroughly investigated to test the claim that it can restore tissue function after myocardial infarction[24-26]. During the expansion phase in vitro, the size of cell bodies tends to increase and this favors their entrapment in microvessels and capillaries once they are infused[23,27,28]. This phenomenon could explain the extremely high percentage of MSCs localized in the lung after intravenous injection[18,26,29]. Recently, mouse models have confirmed the pulmonary localization of MSCs[30-33]. Furthermore, infusion of MSCs led to pulmonary parenchymal edema and hemorrhage at the highest dose tested[34]. Wang and colleagues proposed an in silico approach to predict the time course of the localization of MSCs after intravenous injection in vivo[33]. Notably, this analysis allowed to calculate changes in MSC distribution occurring in disease states entailing loss of function of specific organs (e.g., heart failure). In line with other studies, supply through the hepatic artery was then suggested as an alternative route of administration to avoid lung overload[23,33].
Intraperitoneal injection of autologous and xenogenic (human) MSCs provoked the formation of clusters, which remained outside the site of injury without impeding the antiinflammatory potential[19,30]. Migration and homing are particularly elusive events, either because their detection relies on in situ analysis of functional chemokines and because they depend on a phenotype which can be modified by the initial phases of cell expansion[23]. Importantly, compared to normal tissue inflamed/injured tissues showed an increased tendency to attract circulating MSCs[7,35]. Cell engraftment was reported to be mediated by VCAM-1 and p-selectin[36], whereas diapedesis and extravasion are still unclear, but certainly they are far from resembling lymphocyte’s homing capacity[36].
In light of these findings, the real contribution of MSC homing capacity to the overall immunomodulatory effect is questionable since also pulmonary localization could achieve similar effect. However, ongoing experimental strategies aim at increasing the percentage of MSCs which successfully evade pulmonary and kidney capillary entrapment. As an example, homing receptor CXC-R4 was induced in MSCs by specific culture conditions[37] or viral transfection[38].
Certainly, in situ action of MSCs in large numbers may represent an advantage in those therapies aimed at tissue restitution after inflammation-induced injury. In particular, local MSC administration was particularly efficacious in the treatment of perianal fistula, a complication of Crohn’s disease (CD)[39]. In addition, inhibition of pro-inflammatory cytokines and patients activated T-cell apoptosis was described as a cell-to-cell mediated mechanism of MSCs[16].
Differently from murine MSCs, which undergo rapid senescence, human MSCs can be maintained and expanded in vitro after multiple passages[40]. Number of cells plated, serum and number of passages can markedly influence MSC phenotype and immunomodulatory activity, the latter causing cellular senescence by different mechanisms, the most important of which is shortening of telomeres. A careful control of MSC phenotype during the expansion phase can avoid development of transformed and oncogenic MSC populations[41].
Cryogenic preservation represents an important aspect to be taken into account to obtain positive outcomes in clinical trials and reproducibility of preclinical studies. Enhanced immunomodulation was reported to be linked to “fresh” MSC preparations compared to cryo-conserved MSC batches[42]. In contrast, Luetzkendorf and colleagues extensively demonstrated an intact phenotype after MSC thawing under GMP conditions and a similar activity in the leukocyte proliferation assay[43,44]. Higher rate of MSC proliferation can be achieved with hypoxic conditions in culture, so as to reproduce the niche environment, whereas IFNγ/IFNγ + IL1-β pretreatment enhance IDO-mediated T-reg expansion[16,43,44].
Recent efforts were focused on innovative methods for MSC expansions, which apply microcarriers to increase the surface for cell adhesion in bioreactors. This apparatus allows the large-scale production needed for the formulation of commercial products[45-48]. The application of this novel technology highlighted the need for further standard in-culture conditions aimed at stabilizing MSC phenotype. These include medium flow rate, sheer tension[49] and cell-microcarrier interactions[50].
Finally, since MSC cultures are generally prone to be contaminated by leukocytes and because of the markedly unstable phenotype of MSCs under different lab methodologies, a number of recommendations has been released on the use of MSCs for therapeutic purposes[44,51].
Due to the multiplicity of ligand-receptors systems and indirect mechanisms of action entailed in cell-based therapies, classical pharmacological standards are hardly applicable.
Considering systemic treatments, IDO-mediated serological shift of T-cells toward a regulatory phenotype has been indicated as a reliable mechanism in clinical studies; therefore the rate of tryptophan catabolism by increased concentration of kynurenin might be of value as a biomarker, or in alternative, serum concentration of IDO (Table 1).
On the contrary, clinical studies on TSG-6 serum concentrations after infusion of MSCs are still lacking. Concomitant observations by different research groups showed inconsistent levels of MSC-secreted TSG-6, which could depend on different culture conditions[18]. Therefore, this could lead to consider TSG-6 as a biomarker of efficacy of MSC preparation rather than a clinical biomarker, together with IDO or even a novel bioactive compound per se[16,52].
Animal models of colitis transferred with human MSCs or autologous MSCs show marked improvement of bowel wall architecture and the overall immunological parameters[7,19,31,32,40,53-56]. However, given the aforementioned species-specific differences with rodents, shared biomarkers such as TSG-6 should be evaluated for further proof of concept studies. Moreover, the dinitrobenzene (DNBS) or TNBS model should be preferred over the dextran sodium sulphate (DSS) model for investigational studies on acquired immunity, since the former mimics more closely features of human chronic inflammation[57].
Inflammatory bowel diseases (IBD) are characterized by chronic recurrent intestinal inflammatory episodes and an exaggerated immune response to luminal antigens. IBD include ulcerative colitis, where inflammation is localized to the colonic mucosa, and CD, where inflammation extends to the entire intestinal tract with focal mucosal inflammation[58]. Complications of CD include transmural inflammation, fistula, bowel wall thickening/strictures and extra-intestinal inflammatory manifestations. As IBD entails aberrant cell-mediated immune response, MSC-based therapy is increasingly considered as a potentially valuable therapeutic strategy.
At present, HSCs and MSCs have been tested in several clinical trials, although with unpredictable and partially conflicting results[59,60]. Clinical experience on the use of HSCs is limited, with transient benefit in severe refractory CD, and hampered by toxicities, thus suggesting that this procedure has to be performed in highly experienced centers[61]. A recent first-in-human safety trial (single infusion of autologous bone marrow-derived mesenchymal stromal cells in 12 subjects with CD using three doses in the range of 2-10 millions of cells/kg BW) was partly reassuring on feasibility and safety aspects. Only two patients experienced serious events that were possibly related to MSC infusion (appendicitis and C. difficile colitis). Five patients required hospitalizations likely due to the moderate to severe nature of their underlying CD and not the MSC infusions[52].
Two major instances of MSC-based therapies must be faced in future clinical trials: safety and efficacy of allogeneic MSC preparations, which would avoid the time-consuming phase of cell expansion before injection, and the possible unwanted interactions with the patients’ ongoing therapies, i.e., biological agents and other immunomodulators. Indeed, previous trials on perianal fistulas in CD enrolled patients refractory to standard therapies. Initial trials required discontinuation of immunomodulators, whereas the latest studies allowed continuation of therapy if the dose was maintained stable for several months. Some authors have envisioned an adjuvant role of MSCs to control residual fistulas[62]. In this regard, encouraging results come from the positive outcomes of a recent phase III trial on efficacy and safety of allogeneic ASC treatment of patients with refractory CD and complex perianal fistula. Local injection of allogeneic ASC shortened time to remission over placebo. Importantly, the study also addressed efficacy and safety parameters with concomitant anti-TNFα therapy, immunomodulators (i.e., azathioprine, 6-mercaptopurine, methotrexate) or antibiotics (i.e. ciprofloxacin or metronidazole)[63] (Table 2). Another ongoing trial generating much expectation is the phase III placebo-controlled double-blind study of Prochymal® (intra-venous injection of allogeneic BM-MSCs) in CD, which plans to enroll 330 patients receiving four infusions over 2 wk of 600-1.2 million cells: results are expected by 2018[64].
| Ref. | Study design | Patients | Disease duration and characteristics | Assessment and follow-up | Source of cells | Dose | Safety outcomes (terminology) | Key safety results |
| García-Olmo et al[84] (2005) | Phase 1 | 9 patients aged 32-46 | Diagnosis of CD at least 5 years before, unresponsive to medical treatment and unsuccessfully treated by classic surgery at least twice | Weekly follow-up for the first 8 wk, then monthly follow-up up max 30 mo | Autologous ASC | 3-30 × 106 | Not specified | No immediate adverse reactions (e.g., anaphylaxis, allergic reactions) were observed in any of the cases studied |
| Garcia-Olmo et al[85] (2009) | Open-label, multicenter, phase 2 | 24 patients with mean age = 52 received ASC+fibrin glue | Complex perianal fistula (either of cryptoglandular origin or associated with CD). In patients with CD, immunomodulators were used continuously for at least six months and stable for more than eight weeks | Week 8, 16 and then at 3-mo interval up to 12 months | Autologous ASC | 2-4 × 107 | Incidence of adverse events and serious adverse events | 11 adverse events (at week 8), of which two were SAEs, but unrelated to ASC administration. In the following phase, 9 adverse event (perianal sepsis), unrelated to ASC administration |
| Ciccocioppo et al[86] (2011) | Prospective study | 12 patients aged 16-44 (two drop outs) | Patients with CD unresponsive to or unsuitable for all previous medical treatment including biological agents or unsuccessfully treated by surgery | 3, 6 and 12 mo | Autologous BM-MSC | Median 20 × 106 | Changes in vital signs and adverse reactions during the first 6 h after each cellular treatment, and during the following 12-month follow-up | No changes in vital signs and no adverse events were recorded during the procedure and up to the end of the 6-h observation time, or during the 12-mo follow-up period |
| Guadalajara et al[87] (2012) | Retrospective follow up of phase 2 | 24 patients with mean age = 42 | Patients enrolled in previous phase 2 study receiving at least one ASC administration | 8 wk, 1 yr | Autologous ASC | Not specified | Number of adverse events since the final visit of the phase II study (serious or not, severity, causality) | Ischiorectal abscess (patient treated with fibrin glue alone) and a perianal abscess (patient treated with ASCs plus fibrin glue), both toxicity grade I and unrelated to the study treatment. These events occurred at 13 and 21 mo after the original treatment, respectively |
| Herreros et al[88] (2012) | Multicenter randomized single-blind Phase 3 + observational | 200 patients with mean age = 50 | Cryptoglandular complex fistula-in-ano (without CD) | 1, 4 and 12, 14 24, 26, 48 wk | Autologous ASC | 2 × 107 (± fibrin glue) | Incidence of adverse events and SAEs | Approximately 85%-90% of patients experienced an adverse event, but most of these were nonserious. There were 17 different AEs reported in more than 5% of the cases. The most frequent AEs were proctalgia (43.7%), abscess drainage (22.4%), pain (13.7%), perianal abscess (13.1%), pyrexia (0.3%), swelling (6.6%), and pruritus (6.6%). There were no statistically significant differences within groups. There were 37 SAEs in 30 patients. All but 4 SAEs were unrelated to study treatment |
| Lee et al[89] (2013) | Open-label phase 2 | 43 patients with mean age = 26 | Perianal fistulae with CD in patients not treated with infliximab within 3 mo prior to ASC | 4, 6 and 8 wk, 10 mo | Autologous ASC | Depending on the fistula (mean from 15 to 19 × 107) | Systemic tolerance, adverse events and SAEs | Post-operative pain (60%), anal pain (19%) and anal bleeding (7%), unrelated to ASC administration. One hospitalization for vitamin B12 deficit; two grade 3/4 events (exacerbation of disease and peritonitis), unrelated to ASC administration |
| de la Portilla et al[90] (2013) | Open-label phase 1/2a | 24 patients with mean age = 36 | Diagnosis of CD at least 12 mo before, presence of persistent and active complex perianal fistula with less than three fistulous tracts and/or external opening, non-active luminal CD; no treatment with infliximab or any other anti-TNF agent in the previous 8 wk or tacrolimus or cyclosporine in the previous 4 wk | 10, 12, 22, 24 wk | Allogeneic ASC | 2 × 107 (up to 4 × 107 if no effect) | Incidence of treatment emergent adverse events | 32 treatment-emergent adverse events during the study, the majority of which were of mild to moderate intensity. Five treatment-related AEs were reported: “anal abscess” (3 patients), “pyrexia” (1), and “uterine leiomyoma” (1). Only two SAEs: “pyrexia” and “perianal abscess”, considered to be possibly related to the study treatment and both patients were withdrawn from the study |
| Ciccocioppo et al[91] (2015) | 5-year follow up of an open-label phase 2 | 8 patients with median age = 37 | Refractory CD or inability to undergo standard therapies | 12 mo until 5 yr | Autologous BM-MSC | Not specified | Systemic tolerance, adverse events and SAEs, as specified in the Medical Dictionary for Regulatory Activities terminology | 23 adverse events, mainly consisting of abdominal pain, headache, anal inflammation, diarrhea, erythema, nausea, and fever. All AEs were consistent with exacerbation of the primary disease, but cholecystectomy due to the presence of gallstones. None was attributed to MSC therapy, and no evidence of tumor development or opportunistic infection |
| Cho et al[92] (2013) | Open-label, multicenter, dose-escalation phase 1 | 10 patients with mean age = 26 | Perianal fistula associated with CD | Weeks 8, months 4,6 and 8 | Autologous ASC | 1, 2 and 4 × 107 to 40 × 107 | Adverse events reported after injection with ASCs, serious adverse events during study period, and laboratory toxicity observed after injection with ASCs. (CTCAE version 3.0) | 13 adverse events were reported in seven patients (70%). The adverse events, which were mild or moderate in severity, were not related to study drug. There were no grade 3 or 4 adverse events and no laboratory toxicity greater than grade 3 in this study. Adverse events reported in two or more patients included pain (n = 3) and diarrhea (n = 2). During the study period, two patients reported three SAEs of grade 2 (enterocolitis, seton application, and infliximab administration for new fistulas unrelated to the target fistula) requiring hospitalization |
| Cho et al[93] (2015) | Retrospective analysis of 1-year follow up phase 2 | 42 patients with mean age = 26 | Average duration of CD of 58 mo | 2 yr | Autologous ASC | Average 16.4 × 107 | Systemic tolerance, adverse events, SAEs | 53 adverse events were reported in 30 patients (73.2%), the most common being abdominal pain (17.1%); eczema and exacerbation of disease (9.8%) and anal inflammation, diarrhea, and fever (7.3%). None was related to MSC administration |
| Garcia-Olmo et al[62] (2015) | Observational | 28-76 | Recurrent perianal fistulae who previously undergone at least three surgical interventions | Week 8 and year 1 | Autologous ASC | Not specified | Not specified | No adverse reactions or complications related to MSC administration |
| Molendijk et al[66] (2015) | Phase 2, double-blind, placebo-controlled, randomized study | > 18 | Actively draining perianal fistulizing CD (diagnosis at least 3 months before enrollment) refractory to conventional therapies | 6, 12, and 24 wk | Allogeneic BM-MSC | 1, 3 and 9 × 107 | Primary endpoint: incidence of serious adverse events at week 12. (CTCAE, version 3.0). Secondary end point: incidence of surgical intervention and infections at week 12 and 24 | No infusion reactions; one patient 2 developed fever 6 h after surgery. One patient in each group developed a perianal abscess that required surgical drainage. Reported adverse events: n = 17 (group 1, n = 5), 9 (group 2, n = 5), 10 (group 3, n = 5), 14 (placebo, n = 5). One patient (1 × 107) developed an adenocarcinoma of the cecum with peritoneal carcinomatosis > 15 mo after the surgical intervention |
| Dhere et al[52] (2016) | Phase 1 safety trial | 18-52 | Established CD for at least 3 mo refractory to conventional therapies (lack of response to immunomodulators and/or biologics for at least 3 mo) | 1, 5 and 9 wk after infusion | Autologous BM-MSC | 2, 5 and 10 × 106 | Changes in respiratory or cardiovascular parameters during the 1 h infusion and for 4 h after. Temperature, heart rate, mean arterial pressure and respiratory rate assessed at 15 min, 30 min, 1 h, 2 h, 3 h and 4 h | No patient developed significant infusion reaction. SAEs in 7 patients, of which 2 likely to be related to MSC administration: appendicitis (with appendectomy 9 d after infusion and complete colectomy for medically refractory CD after 120 d) and C. difficile colitis (30 d after infusion) |
| Panés et al[63] (2016) | Phase 3, randomized, placebo-controlled trial | 107 patients, mean age = 39 | Actively draining perianal fistulizing CD refractory to conventional therapies | 24 wk after local injection | Allogeneic ASC | 12 × 106 | TEAEs (MedDRA version 17.0) | 18 patients of the ASC treated group vs 30 of 107 in the placebo group developed treatment-related adverse events, (anal abscess and proctalgia) |
The body of evidence on the potential of MSCs is remarkable, but with conflicting results in terms of efficacy, especially for systemic administration in luminal IBD[39]. In this context, there are some unresolved clinical issues.
First, safety is still a matter of debate, especially in the long term. The primary concern is the potential malignant transformation of the administered cells. However, a recent meta-analysis partly reassured the scientific community by showing that malignancies occurred only in patients with previous or current malignancies, with no formation of de novo tumors[65]. Table 2 provides a synopsis of published clinical studies using MSCs in refractory CD or perianal fistulizing CD, with a focus on safety aspects.
Second, MSC administration, in terms of route, dose and type, deserves optimization. In particular, dose selection is crucial to find the right balance between efficacy and safety. Initial open-label trials used doses up to 3 × 107 cells. Subsequently, the amount of cells increased aiming at improving outcomes on the basis of the portion of intestinal tract to be treated (107 cells). Notably, in the study by Molendijk et al[66], patients were randomized to receive 3 different doses (1 × 107, 3 × 107, 9 × 107) or placebo, with those assigned to the 3 × 107 arm experiencing the best response rate. This further emphasizes the complex nature of MSCs as biotechnological products, which do not strictly follow the general pharmacokinetic rules in terms of dose-response. In addition, there is an urgent need to share uniform protocols and increase reproducibility and consistency of data.
The purpose of investigating the extracellular products of MSCs is twofold: to clarify their mechanism of action and, more importantly, to evaluate the efficacy of MSC mediators per se, so as to avoid whole-cell infusion. Apart from the importance of deepening the knowledge on MSC nature, an emerging interest surrounds MSC-secreted micro-mRNA (miRNA)[67] as a possible therapeutic option in pulmonary hypertension[68]. The same molecules regulate toll-like receptors (TLR) expression in MSC stimulated with bacterial derived lipopolysaccharide[69]. Further studies are warranted to clarify mechanisms of TLR4 expression of MSCs[70], especially in light of its role in the tolerogenic pathways of the intestinal immune homeostasis.
The use of MSC exosomes/extracellular vesicles is increasingly under study. These were proven to be effective in reducing NF-κB activity and the level of pro-inflammatory cytokines in a TNBS rat model[6]. Furthermore, MSC supernatants evoked an anti-inflammatory response and an overall improvement of bowel wall architecture in both DSS and TNBS rat models[71]. The same study suggested the intraperitoneal route as more effective, and a panel of candidate bioactive compounds derived from MSCs.
In conclusion, MSC-based therapies can make a step forward through strategies that: (1) enhance immunomodulatory phenotypes and cellular yields for large-scale production (for heterologous MSC-based therapy); (2) use animal models showing phylogenetic consistency for proof of concept studies on MSC mechanisms of action; (3) prefer a route of administration with no pulmonary or kidney MSC retention; (4) enhance MSC engraftment at the intestinal injured site, especially for those pathological conditions requiring cell replacement and mucosal/whole tissue healing; and (5); maintain standard cell markers favoring application of heterologous therapy. Finally, deepening the knowledge on MSC physiology could pave the way for novel pharmacological strategies based on MSC mediators.
| 1. | Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11055] [Cited by in RCA: 13053] [Article Influence: 687.0] [Reference Citation Analysis (12)] |
| 2. | Clinicaltrials gov 2016. Available from: https://clinicaltrials.gov/ct2/results/details?term=mesenchymal stromal cells OR mesenchymal stem cells AND Stem Cell Therapy. |
| 3. | Nam YS, Kim N, Im KI, Lim JY, Lee ES, Cho SG. Negative impact of bone-marrow-derived mesenchymal stem cells on dextran sulfate sodium-induced colitis. World J Gastroenterol. 2015;21:2030-2039. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Duijvestein M, Wildenberg ME, Welling MM, Hennink S, Molendijk I, van Zuylen VL, Bosse T, Vos AC, de Jonge-Muller ES, Roelofs H. Pretreatment with interferon-γ enhances the therapeutic activity of mesenchymal stromal cells in animal models of colitis. Stem Cells. 2011;29:1549-1558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 280] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 5. | Wang Y, Chen X, Cao W, Shi Y. Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications. Nat Immunol. 2014;15:1009-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 830] [Cited by in RCA: 1109] [Article Influence: 100.8] [Reference Citation Analysis (1)] |
| 6. | Yang J, Liu XX, Fan H, Tang Q, Shou ZX, Zuo DM, Zou Z, Xu M, Chen QY, Peng Y. Extracellular Vesicles Derived from Bone Marrow Mesenchymal Stem Cells Protect against Experimental Colitis via Attenuating Colon Inflammation, Oxidative Stress and Apoptosis. PLoS One. 2015;10:e0140551. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 135] [Cited by in RCA: 190] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 7. | Tayman C, Uckan D, Kilic E, Ulus AT, Tonbul A, Murat Hirfanoglu I, Helvacioglu F, Haltas H, Koseoglu B, Tatli MM. Mesenchymal stem cell therapy in necrotizing enterocolitis: a rat study. Pediatr Res. 2011;70:489-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 8. | Sun L, Xu R, Sun X, Duan Y, Han Y, Zhao Y, Qian H, Zhu W, Xu W. Safety evaluation of exosomes derived from human umbilical cord mesenchymal stromal cell. Cytotherapy. 2016;18:413-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 144] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 9. | Sohni A, Verfaillie CM. Mesenchymal stem cells migration homing and tracking. Stem Cells Int. 2013;2013:130763. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 248] [Cited by in RCA: 308] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 10. | Eggenhofer E, Benseler V, Kroemer A, Popp FC, Geissler EK, Schlitt HJ, Baan CC, Dahlke MH, Hoogduijn MJ. Mesenchymal stem cells are short-lived and do not migrate beyond the lungs after intravenous infusion. Front Immunol. 2012;3:297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 439] [Cited by in RCA: 619] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 11. | European Commission. Consultation Document - Good Manufacturing Practices for Advanced Therapy Medical Product. Available from: http://ec.europa.eu/health/files/advtherapies/2016_06_pc/2016_06_draft_guideline.pdf. |
| 12. | Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8:726-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2360] [Cited by in RCA: 2773] [Article Influence: 163.1] [Reference Citation Analysis (0)] |
| 13. | Su J, Chen X, Huang Y, Li W, Li J, Cao K, Cao G, Zhang L, Li F, Roberts AI. Phylogenetic distinction of iNOS and IDO function in mesenchymal stem cell-mediated immunosuppression in mammalian species. Cell Death Differ. 2014;21:388-396. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 198] [Cited by in RCA: 198] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 14. | Frumento G, Rotondo R, Tonetti M, Damonte G, Benatti U, Ferrara GB. Tryptophan-derived catabolites are responsible for inhibition of T and natural killer cell proliferation induced by indoleamine 2,3-dioxygenase. J Exp Med. 2002;196:459-468. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 804] [Cited by in RCA: 905] [Article Influence: 37.7] [Reference Citation Analysis (0)] |
| 15. | Terness P, Bauer TM, Röse L, Dufter C, Watzlik A, Simon H, Opelz G. Inhibition of allogeneic T cell proliferation by indoleamine 2,3-dioxygenase-expressing dendritic cells: mediation of suppression by tryptophan metabolites. J Exp Med. 2002;196:447-457. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 709] [Cited by in RCA: 755] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 16. | Ciccocioppo R, Cangemi GC, Kruzliak P, Gallia A, Betti E, Badulli C, Martinetti M, Cervio M, Pecci A, Bozzi V. Ex vivo immunosuppressive effects of mesenchymal stem cells on Crohn’s disease mucosal T cells are largely dependent on indoleamine 2,3-dioxygenase activity and cell-cell contact. Stem Cell Res Ther. 2015;6:137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 17. | Ren G, Su J, Zhang L, Zhao X, Ling W, L’huillie A, Zhang J, Lu Y, Roberts AI, Ji W. Species variation in the mechanisms of mesenchymal stem cell-mediated immunosuppression. Stem Cells. 2009;27:1954-1962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 481] [Cited by in RCA: 468] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 18. | Prockop DJ, Oh JY. Mesenchymal stem/stromal cells (MSCs): role as guardians of inflammation. Mol Ther. 2012;20:14-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 578] [Cited by in RCA: 647] [Article Influence: 43.1] [Reference Citation Analysis (1)] |
| 19. | Sala E, Genua M, Petti L, Anselmo A, Arena V, Cibella J, Zanotti L, D’Alessio S, Scaldaferri F, Luca G. Mesenchymal Stem Cells Reduce Colitis in Mice via Release of TSG6, Independently of Their Localization to the Intestine. Gastroenterology. 2015;149:163-176.e20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 214] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 20. | Milner CM, Higman VA, Day AJ. TSG-6: a pluripotent inflammatory mediator? Biochem Soc Trans. 2006;34:446-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 167] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 21. | Kim DK, Choi H, Nishida H, Oh JY, Gregory C, Lee RH, Yu JM, Watanabe J, An SY, Bartosh TJ. Scalable Production of a Multifunctional Protein (TSG-6) That Aggregates with Itself and the CHO Cells That Synthesize It. PLoS One. 2016;11:e0147553. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Manieri NA, Stappenbeck TS. Mesenchymal stem cell therapy of intestinal disease: are their effects systemic or localized? Curr Opin Gastroenterol. 2011;27:119-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 23. | Karp JM, Leng Teo GS. Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell. 2009;4:206-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1012] [Cited by in RCA: 1109] [Article Influence: 65.2] [Reference Citation Analysis (0)] |
| 24. | Cheng Z, Ou L, Zhou X, Li F, Jia X, Zhang Y, Liu X, Li Y, Ward CA, Melo LG. Targeted migration of mesenchymal stem cells modified with CXCR4 gene to infarcted myocardium improves cardiac performance. Mol Ther. 2008;16:571-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 337] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 25. | Fiarresga A, Mata MF, Cavaco-Gonçalves S, Selas M, Simões IN, Oliveira E, Carrapiço B, Cardim N, Cabral JM, Ferreira RC. Intracoronary Delivery of Human Mesenchymal/Stromal Stem Cells: Insights from Coronary Microcirculation Invasive Assessment in a Swine Model. PLoS One. 2015;10:e0139870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 26. | Freyman T, Polin G, Osman H, Crary J, Lu M, Cheng L, Palasis M, Wilensky RL. A quantitative, randomized study evaluating three methods of mesenchymal stem cell delivery following myocardial infarction. Eur Heart J. 2006;27:1114-1122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 462] [Cited by in RCA: 461] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 27. | Wang N, Shao Y, Mei Y, Zhang L, Li Q, Li D, Shi S, Hong Q, Lin H, Chen X. Novel mechanism for mesenchymal stem cells in attenuating peritoneal adhesion: accumulating in the lung and secreting tumor necrosis factor α-stimulating gene-6. Stem Cell Res Ther. 2012;3:51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 28. | da Silva Meirelles L, Caplan AI, Nardi NB. In search of the in vivo identity of mesenchymal stem cells. Stem Cells. 2008;26:2287-2299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 778] [Cited by in RCA: 722] [Article Influence: 40.1] [Reference Citation Analysis (0)] |
| 29. | Barbash IM, Chouraqui P, Baron J, Feinberg MS, Etzion S, Tessone A, Miller L, Guetta E, Zipori D, Kedes LH. Systemic delivery of bone marrow-derived mesenchymal stem cells to the infarcted myocardium: feasibility, cell migration, and body distribution. Circulation. 2003;108:863-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 916] [Cited by in RCA: 902] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 30. | Bazhanov N, Ylostalo JH, Bartosh TJ, Tiblow A, Mohammadipoor A, Foskett A, Prockop DJ. Intraperitoneally infused human mesenchymal stem cells form aggregates with mouse immune cells and attach to peritoneal organs. Stem Cell Res Ther. 2016;7:27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 31. | Castelo-Branco MT, Soares ID, Lopes DV, Buongusto F, Martinusso CA, do Rosario A, Souza SA, Gutfilen B, Fonseca LM, Elia C. Intraperitoneal but not intravenous cryopreserved mesenchymal stromal cells home to the inflamed colon and ameliorate experimental colitis. PLoS One. 2012;7:e33360. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 111] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 32. | Chen QQ, Yan L, Wang CZ, Wang WH, Shi H, Su BB, Zeng QH, Du HT, Wan J. Mesenchymal stem cells alleviate TNBS-induced colitis by modulating inflammatory and autoimmune responses. World J Gastroenterol. 2013;19:4702-4717. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 84] [Cited by in RCA: 103] [Article Influence: 7.9] [Reference Citation Analysis (1)] |
| 33. | Wang H, Liang X, Xu ZP, Crawford DH, Liu X, Roberts MS. A physiologically based kinetic model for elucidating the in vivo distribution of administered mesenchymal stem cells. Sci Rep. 2016;6:22293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 34. | Kang MH, Park HM. Evaluation of adverse reactions in dogs following intravenous mesenchymal stem cell transplantation. Acta Vet Scand. 2014;56:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 35. | Weeks S, Kulkarni A, Smith H, Whittall C, Yang Y, Middleton J. The effects of chemokine, adhesion and extracellular matrix molecules on binding of mesenchymal stromal cells to poly(l-lactic acid). Cytotherapy. 2012;14:1080-1088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 36. | Leibacher J, Henschler R. Biodistribution, migration and homing of systemically applied mesenchymal stem/stromal cells. Stem Cell Res Ther. 2016;7:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 211] [Cited by in RCA: 266] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 37. | Chavakis E, Urbich C, Dimmeler S. Homing and engraftment of progenitor cells: a prerequisite for cell therapy. J Mol Cell Cardiol. 2008;45:514-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 244] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 38. | Brenner S, Whiting-Theobald N, Kawai T, Linton GF, Rudikoff AG, Choi U, Ryser MF, Murphy PM, Sechler JM, Malech HL. CXCR4-transgene expression significantly improves marrow engraftment of cultured hematopoietic stem cells. Stem Cells. 2004;22:1128-1133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 89] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 39. | Ciccocioppo R, Cangemi GC, Kruzliak P, Corazza GR. Concise Review: Cellular Therapies: The Potential to Regenerate and Restore Tolerance in Immune-Mediated Intestinal Diseases. Stem Cells. 2016;34:1474-1486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 40. | Chinnadurai R, Ng S, Velu V, Galipeau J. Challenges in animal modelling of mesenchymal stromal cell therapy for inflammatory bowel disease. World J Gastroenterol. 2015;21:4779-4787. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 34] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 41. | Wang Y, Huso DL, Harrington J, Kellner J, Jeong DK, Turney J, McNiece IK. Outgrowth of a transformed cell population derived from normal human BM mesenchymal stem cell culture. Cytotherapy. 2005;7:509-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 198] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 42. | Moll G, Alm JJ, Davies LC, von Bahr L, Heldring N, Stenbeck-Funke L, Hamad OA, Hinsch R, Ignatowicz L, Locke M. Do cryopreserved mesenchymal stromal cells display impaired immunomodulatory and therapeutic properties? Stem Cells. 2014;32:2430-2442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 291] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 43. | Luetzkendorf J, Nerger K, Hering J, Moegel A, Hoffmann K, Hoefers C, Mueller-Tidow C, Mueller LP. Cryopreservation does not alter main characteristics of Good Manufacturing Process-grade human multipotent mesenchymal stromal cells including immunomodulating potential and lack of malignant transformation. Cytotherapy. 2015;17:186-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 71] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 44. | Wuchter P, Bieback K, Schrezenmeier H, Bornhäuser M, Müller LP, Bönig H, Wagner W, Meisel R, Pavel P, Tonn T. Standardization of Good Manufacturing Practice-compliant production of bone marrow-derived human mesenchymal stromal cells for immunotherapeutic applications. Cytotherapy. 2015;17:128-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 104] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 45. | Hervy M, Weber JL, Pecheul M, Dolley-Sonneville P, Henry D, Zhou Y, Melkoumian Z. Long term expansion of bone marrow-derived hMSCs on novel synthetic microcarriers in xeno-free, defined conditions. PLoS One. 2014;9:e92120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 46. | Rafiq QA, Coopman K, Nienow AW, Hewitt CJ. Systematic microcarrier screening and agitated culture conditions improves human mesenchymal stem cell yield in bioreactors. Biotechnol J. 2016;11:473-486. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 116] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 47. | Tsai AC, Ma T. Expansion of Human Mesenchymal Stem Cells in a Microcarrier Bioreactor. Methods Mol Biol. 2016;1502:77-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 48. | Baghbaderani BA, Mukhida K, Hong M, Mendez I, Behie LA. A review of bioreactor protocols for human neural precursor cell expansion in preparation for clinical trials. Curr Stem Cell Res Ther. 2011;6:229-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 49. | Jossen V, Schirmer C, Mostafa Sindi D, Eibl R, Kraume M, Pörtner R, Eibl D. Theoretical and Practical Issues That Are Relevant When Scaling Up hMSC Microcarrier Production Processes. Stem Cells Int. 2016;2016:4760414. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 50. | Sart S, Agathos SN. Large-Scale Expansion and Differentiation of Mesenchymal Stem Cells in Microcarrier-Based Stirred Bioreactors. Methods Mol Biol. 2016;1502:87-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 51. | Martin I, De Boer J, Sensebe L. A relativity concept in mesenchymal stromal cell manufacturing. Cytotherapy. 2016;18:613-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 52. | Dhere T, Copland I, Garcia M, Chiang KY, Chinnadurai R, Prasad M, Galipeau J, Kugathasan S. The safety of autologous and metabolically fit bone marrow mesenchymal stromal cells in medically refractory Crohn’s disease - a phase 1 trial with three doses. Aliment Pharmacol Ther. 2016;44:471-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 87] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 53. | Kim HS, Shin TH, Lee BC, Yu KR, Seo Y, Lee S, Seo MS, Hong IS, Choi SW, Seo KW. Human umbilical cord blood mesenchymal stem cells reduce colitis in mice by activating NOD2 signaling to COX2. Gastroenterology. 2013;145:1392-403.e1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 150] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 54. | Tanaka F, Tominaga K, Ochi M, Tanigawa T, Watanabe T, Fujiwara Y, Ohta K, Oshitani N, Higuchi K, Arakawa T. Exogenous administration of mesenchymal stem cells ameliorates dextran sulfate sodium-induced colitis via anti-inflammatory action in damaged tissue in rats. Life Sci. 2008;83:771-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 106] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 55. | Markel TA, Crafts TD, Jensen AR, Hunsberger EB, Yoder MC. Human mesenchymal stromal cells decrease mortality after intestinal ischemia and reperfusion injury. J Surg Res. 2015;199:56-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 56. | Xie M, Qin H, Luo Q, He X, He X, Lan P, Lian L. Comparison of Adipose-Derived and Bone Marrow Mesenchymal Stromal Cells in a Murine Model of Crohn’s Disease. Dig Dis Sci. 2016; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 57. | te Velde AA, Verstege MI, Hommes DW. Critical appraisal of the current practice in murine TNBS-induced colitis. Inflamm Bowel Dis. 2006;12:995-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 152] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 58. | Podolsky DK. Inflammatory bowel disease (1). N Engl J Med. 1991;325:928-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 762] [Cited by in RCA: 780] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 59. | Flores AI, Gómez-Gómez GJ, Masedo-González Á, Martínez-Montiel MP. Stem cell therapy in inflammatory bowel disease: A promising therapeutic strategy? World J Stem Cells. 2015;7:343-351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 60. | Martínez-Montiel Mdel P, Gómez-Gómez GJ, Flores AI. Therapy with stem cells in inflammatory bowel disease. World J Gastroenterol. 2014;20:1211-1227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 51] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (2)] |
| 61. | Jauregui-Amezaga A, Rovira M, Marín P, Salas A, Pinó-Donnay S, Feu F, Elizalde JI, Fernández-Avilés F, Martínez C, Gutiérrez G. Improving safety of autologous haematopoietic stem cell transplantation in patients with Crohn’s disease. Gut. 2016;65:1456-1462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 62. | Garcia-Olmo D, Guadalajara H, Rubio-Perez I, Herreros MD, de-la-Quintana P, Garcia-Arranz M. Recurrent anal fistulae: limited surgery supported by stem cells. World J Gastroenterol. 2015;21:3330-3336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 44] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 63. | Panés J, García-Olmo D, Van Assche G, Colombel JF, Reinisch W, Baumgart DC, Dignass A, Nachury M, Ferrante M, Kazemi-Shirazi L. Expanded allogeneic adipose-derived mesenchymal stem cells (Cx601) for complex perianal fistulas in Crohn’s disease: a phase 3 randomised, double-blind controlled trial. Lancet. 2016;388:1281-1290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 628] [Cited by in RCA: 778] [Article Influence: 77.8] [Reference Citation Analysis (1)] |
| 64. | Clinicaltrials gov 2016. Available from: https://clinicaltrials.gov/ct2/show/NCT00482092. |
| 65. | Lalu MM, McIntyre L, Pugliese C, Fergusson D, Winston BW, Marshall JC, Granton J, Stewart DJ. Safety of cell therapy with mesenchymal stromal cells (SafeCell): a systematic review and meta-analysis of clinical trials. PLoS One. 2012;7:e47559. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 901] [Cited by in RCA: 873] [Article Influence: 62.4] [Reference Citation Analysis (0)] |
| 66. | Molendijk I, Bonsing BA, Roelofs H, Peeters KC, Wasser MN, Dijkstra G, van der Woude CJ, Duijvestein M, Veenendaal RA, Zwaginga JJ. Allogeneic Bone Marrow-Derived Mesenchymal Stromal Cells Promote Healing of Refractory Perianal Fistulas in Patients With Crohn’s Disease. Gastroenterology. 2015;149:918-927.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 254] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 67. | Chen TS, Lim SK. Measurement of precursor miRNA in exosomes from human ESC-derived mesenchymal stem cells. Methods Mol Biol. 2013;1024:69-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 68. | Zhu Z, Fang Z, Hu X, Zhou S. MicroRNAs and mesenchymal stem cells: hope for pulmonary hypertension. Rev Bras Cir Cardiovasc. 2015;30:380-385. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 69. | Wang X, Zhu Y, Xu B, Wang J, Liu X. Identification of TLR2 and TLR4induced microRNAs in human mesenchymal stem cells and their possible roles in regulating TLR signals. Mol Med Rep. 2016;13:4969-4980. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 70. | Zeuner M, Bieback K, Widera D. Controversial Role of Toll-like Receptor 4 in Adult Stem Cells. Stem Cell Rev. 2015;11:621-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 71. | Watanabe S, Arimura Y, Nagaishi K, Isshiki H, Onodera K, Nasuno M, Yamashita K, Idogawa M, Naishiro Y, Murata M. Conditioned mesenchymal stem cells produce pleiotropic gut trophic factors. J Gastroenterol. 2014;49:270-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 72. | Prockop DJ. Inflammation, fibrosis, and modulation of the process by mesenchymal stem/stromal cells. Matrix Biol. 2016;51:7-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 79] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 73. | Lee RH, Yu JM, Foskett AM, Peltier G, Reneau JC, Bazhanov N, Oh JY, Prockop DJ. TSG-6 as a biomarker to predict efficacy of human mesenchymal stem/progenitor cells (hMSCs) in modulating sterile inflammation in vivo. Proc Natl Acad Sci USA. 2014;111:16766-16771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 159] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 74. | Torihashi S, Ho M, Kawakubo Y, Komatsu K, Nagai M, Hirayama Y, Kawabata Y, Takenaka-Ninagawa N, Wanachewin O, Zhuo L. Acute and temporal expression of tumor necrosis factor (TNF)-α-stimulated gene 6 product, TSG6, in mesenchymal stem cells creates microenvironments required for their successful transplantation into muscle tissue. J Biol Chem. 2015;290:22771-22781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 75. | Liu L, Yu Y, Hou Y, Chai J, Duan H, Chu W, Zhang H, Hu Q, Du J. Human umbilical cord mesenchymal stem cells transplantation promotes cutaneous wound healing of severe burned rats. PLoS One. 2014;9:e88348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 159] [Cited by in RCA: 155] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 76. | Qi Y, Jiang D, Sindrilaru A, Stegemann A, Schatz S, Treiber N, Rojewski M, Schrezenmeier H, Vander Beken S, Wlaschek M. TSG-6 released from intradermally injected mesenchymal stem cells accelerates wound healing and reduces tissue fibrosis in murine full-thickness skin wounds. J Invest Dermatol. 2014;134:526-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 196] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 77. | Meisel R, Zibert A, Laryea M, Göbel U, Däubener W, Dilloo D. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood. 2004;103:4619-4621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1243] [Cited by in RCA: 1265] [Article Influence: 57.5] [Reference Citation Analysis (0)] |
| 78. | Németh K, Leelahavanichkul A, Yuen PS, Mayer B, Parmelee A, Doi K, Robey PG, Leelahavanichkul K, Koller BH, Brown JM. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15:42-49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1919] [Cited by in RCA: 1847] [Article Influence: 108.6] [Reference Citation Analysis (1)] |
| 79. | Voswinkel J, Francois S, Simon JM, Benderitter M, Gorin NC, Mohty M, Fouillard L, Chapel A. Use of mesenchymal stem cells (MSC) in chronic inflammatory fistulizing and fibrotic diseases: a comprehensive review. Clin Rev Allergy Immunol. 2013;45:180-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 95] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 80. | Spaggiari GM, Capobianco A, Abdelrazik H, Becchetti F, Mingari MC, Moretta L. Mesenchymal stem cells inhibit natural killer-cell proliferation, cytotoxicity, and cytokine production: role of indoleamine 2,3-dioxygenase and prostaglandin E2. Blood. 2008;111:1327-1333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 799] [Cited by in RCA: 826] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 81. | Stavely R, Robinson AM, Miller S, Boyd R, Sakkal S, Nurgali K. Allogeneic guinea pig mesenchymal stem cells ameliorate neurological changes in experimental colitis. Stem Cell Res Ther. 2015;6:263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 82. | Hayashi Y, Tsuji S, Tsujii M, Nishida T, Ishii S, Iijima H, Nakamura T, Eguchi H, Miyoshi E, Hayashi N. Topical implantation of mesenchymal stem cells has beneficial effects on healing of experimental colitis in rats. J Pharmacol Exp Ther. 2008;326:523-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 106] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 83. | Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, Grisanti S, Gianni AM. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99:3838-3843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2455] [Cited by in RCA: 2378] [Article Influence: 99.1] [Reference Citation Analysis (0)] |
| 84. | García-Olmo D, García-Arranz M, Herreros D, Pascual I, Peiro C, Rodríguez-Montes JA. A phase I clinical trial of the treatment of Crohn’s fistula by adipose mesenchymal stem cell transplantation. Dis Colon Rectum. 2005;48:1416-1423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 604] [Cited by in RCA: 576] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 85. | Garcia-Olmo D, Herreros D, Pascual I, Pascual JA, Del-Valle E, Zorrilla J, De-La-Quintana P, Garcia-Arranz M, Pascual M. Expanded adipose-derived stem cells for the treatment of complex perianal fistula: a phase II clinical trial. Dis Colon Rectum. 2009;52:79-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 598] [Cited by in RCA: 572] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 86. | Ciccocioppo R, Bernardo ME, Sgarella A, Maccario R, Avanzini MA, Ubezio C, Minelli A, Alvisi C, Vanoli A, Calliada F. Autologous bone marrow-derived mesenchymal stromal cells in the treatment of fistulising Crohn’s disease. Gut. 2011;60:788-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 421] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 87. | Guadalajara H, Herreros D, De-La-Quintana P, Trebol J, Garcia-Arranz M, Garcia-Olmo D. Long-term follow-up of patients undergoing adipose-derived adult stem cell administration to treat complex perianal fistulas. Int J Colorectal Dis. 2012;27:595-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 135] [Article Influence: 9.6] [Reference Citation Analysis (2)] |
| 88. | Herreros MD, Garcia-Arranz M, Guadalajara H, De-La-Quintana P, Garcia-Olmo D. Autologous expanded adipose-derived stem cells for the treatment of complex cryptoglandular perianal fistulas: a phase III randomized clinical trial (FATT 1: fistula Advanced Therapy Trial 1) and long-term evaluation. Dis Colon Rectum. 2012;55:762-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 208] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 89. | Lee WY, Park KJ, Cho YB, Yoon SN, Song KH, Kim DS, Jung SH, Kim M, Yoo HW, Kim I. Autologous adipose tissue-derived stem cells treatment demonstrated favorable and sustainable therapeutic effect for Crohn’s fistula. Stem Cells. 2013;31:2575-2581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 212] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 90. | de la Portilla F, Alba F, García-Olmo D, Herrerías JM, González FX, Galindo A. Expanded allogeneic adipose-derived stem cells (eASCs) for the treatment of complex perianal fistula in Crohn’s disease: results from a multicenter phase I/IIa clinical trial. Int J Colorectal Dis. 2013;28:313-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 265] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 91. | Ciccocioppo R, Gallia A, Sgarella A, Kruzliak P, Gobbi PG, Corazza GR. Long-Term Follow-Up of Crohn Disease Fistulas After Local Injections of Bone Marrow-Derived Mesenchymal Stem Cells. Mayo Clin Proc. 2015;90:747-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 75] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 92. | Cho YB, Lee WY, Park KJ, Kim M, Yoo HW, Yu CS. Autologous adipose tissue-derived stem cells for the treatment of Crohn’s fistula: a phase I clinical study. Cell Transplant. 2013;22:279-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 156] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 93. | Cho YB, Park KJ, Yoon SN, Song KH, Kim DS, Jung SH, Kim M, Jeong HY, Yu CS. Long-term results of adipose-derived stem cell therapy for the treatment of Crohn’s fistula. Stem Cells Transl Med. 2015;4:532-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 139] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Eder P, Strom SC, Yao CL S- Editor: Gong ZM L- Editor: A E- Editor: Wang CH