Published online Aug 28, 2016. doi: 10.3748/wjg.v22.i32.7264
Peer-review started: March 28, 2016
First decision: May 30, 2016
Revised: June 27, 2016
Accepted: August 1, 2016
Article in press: August 1, 2016
Published online: August 28, 2016
Processing time: 149 Days and 9.7 Hours
Indonesia has a moderate to high endemicity of hepatitis B virus (HBV) infection. The risk for chronic HBV infection is highest among those infected during infancy. Since 1997, hepatitis B (HepB) vaccination of newborns has been fully integrated into the National Immunization Program. Although HBV infection has been reduced by the universal newborn HepB immunization program, it continues to occur in Indonesia. The low birth dose coverage and the presence of vaccine escape mutants might contribute to this endemicity among children. Although limited information is available for an analysis of occult HBV infection (OBI), several variations and substitutions in the pre-S/S region have been detected in Indonesian HBV strains. Additionally, persistent infection and disease progression of chronic hepatitis B are related to not only viral factors but also the host genome. Indonesia is one of the most ethnically heterogeneous nations, with Javanese and Sundanese as the two highest ethnic groups. This multi-ethnicity makes genomic research in Indonesia difficult. In this article, we focused on and reviewed the following aspects: the current hepatitis B immunization program and its efficacy, OBI, HBV infection among high-risk patients, such as hemodialysis patients, and research regarding the host genome in Indonesia.
Core tip: Hepatitis B virus (HBV) infection is still an important health problem in Indonesia. Although HBV infection has been reduced by the universal newborn hepatitis B immunization program, it continues to occur in Indonesia. The high prevalence of occult hepatitis B infection and HBV infection among hemodialysis patients also contributes to its endemicity. The association between human genetic variations and HBV infection in several Asian countries, including in Indonesia was also identified. We reviewed these important aspects of the current HBV infection situation in Indonesia.
- Citation: Lusida MI, Juniastuti, Yano Y. Current hepatitis B virus infection situation in Indonesia and its genetic diversity. World J Gastroenterol 2016; 22(32): 7264-7274
- URL: https://www.wjgnet.com/1007-9327/full/v22/i32/7264.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i32.7264
An estimated 240 million people are chronically infected with hepatitis B worldwide [defined as hepatitis B surface antigen (HBsAg) positivity for at least 6 mo]. A vaccine against hepatitis B has been available since 1982. Although the prevalence of hepatitis B virus (HBV) infection is relatively low in developed countries (e.g., as low as 0.4% in the United States), HBV infection is still quite prevalent in East Asia and Southeast Asia, including Indonesia (2.5%-10%)[1,2]. The endemicity of hepatitis B (marked by HBsAg positivity) in Indonesia is intermediate to high with geographical differences. HBV has been classified into at least 9 genotypes (A through H and J) and has been shown to have a distinct geographical distribution[3,4]. The most common HBV subgenotype in Indonesia is B3, followed by C1. Various novel HBV subgenotypes have been identified throughout Indonesia, and the novel HBV subgenotypes C6-C16 and D6 have been successfully isolated[2] in the general population.
The risk for chronic infection is related to the age at infection; for instance, approximately 90% of infected infants become chronically infected compared with 2%-6% of adults[1]. In addition to HBsAg, HBeAg is an important hepatitis B marker in the field of mother-to-child transmission. HBeAg is a small secretory antigen that can cross the placenta from the mother to the fetus[5]. The vaccine is generally effective in preventing infection[6]. A universal hepatitis B vaccination program for infants was adopted in Indonesia in 1997. What is the current HBV serological status and molecular profile among children in Indonesia fifteen years after the adoption of this universal infant vaccination program?
A specific community with maintenance hemodialysis (HD) is at high risk for blood-borne infections, especially HBV. However, few studies have been conducted on the prevalence of HBV among HD patients in Indonesia, and adequate databases on HBV infection in this population are still limited. Therefore, the HBV subgenotypes among HD patients in Indonesia is also an interesting subject for discussion.
Undetectable HBsAg is generally considered to indicate a lack of HBV infection or the disappearance of viremia and disease remission[7,8]. This belief may result in misinterpretation among patients with occult HBV infection (OBI), which is an HBV infection that lacks detectable HBsAg. Considering the importance of OBIs, the purpose of this review is to provide comprehensive information on OBIs in Indonesia, including infections among HD patients.
In addition to viral factors (e.g., HBV DNA levels, genotypes, and genomic mutations), host factors (e.g., age, gender, race, and immune status) might contribute to the progression of liver diseases[9,10]. Genome-wide association studies have identified associations of genetic variations with diseases related to HBV, including HBV-related hepatocellular carcinoma (HCC).
All of the topics listed above are among the HBV subtopics in Indonesia that will be discussed here.
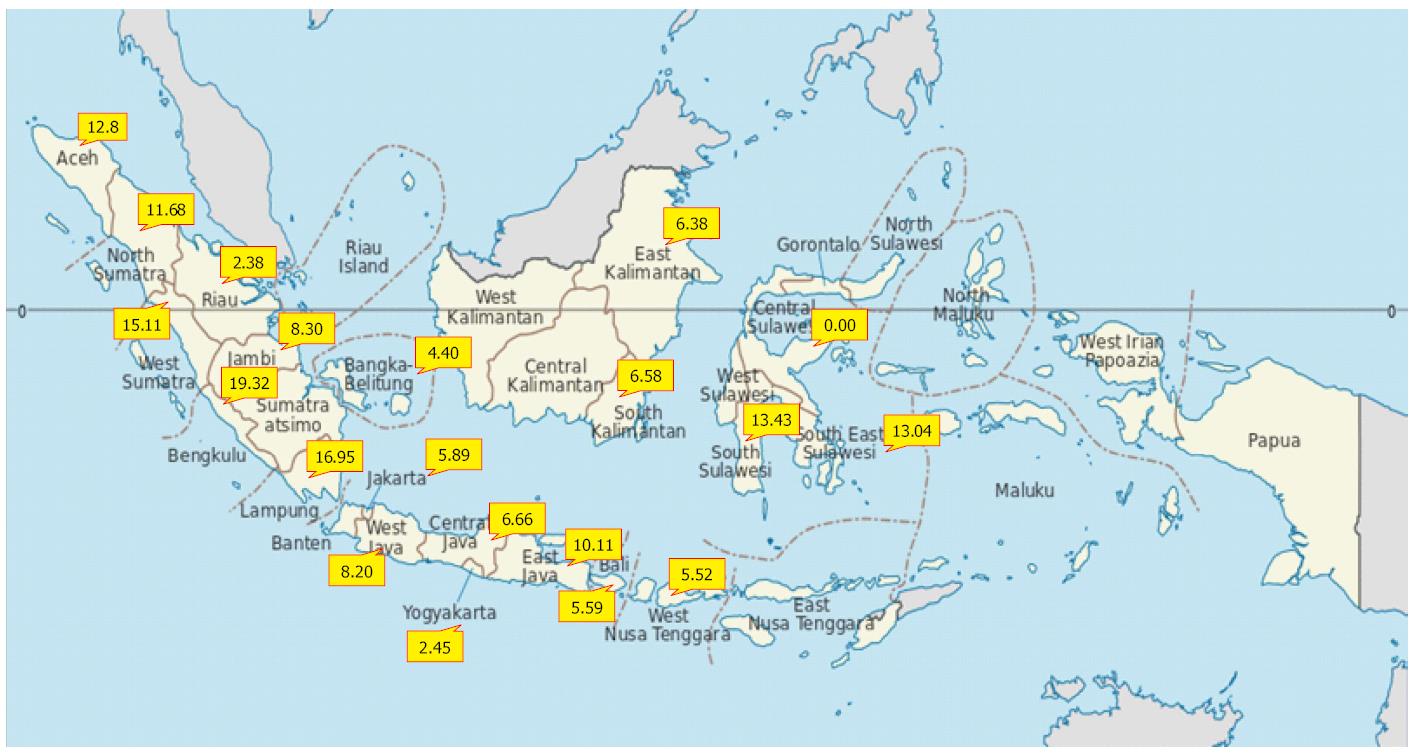
Indonesia experiences intermediate to high hepatitis B endemicity that varies between provinces[11] (Figure 1). In countries with a high prevalence of chronic hepatitis B infection, a higher proportion of carriers are infected during infancy or early childhood; historically, 25%-50% of chronic infections in these countries are caused by vertical mother-to-infant perinatal transmission. Surveys of pregnant women in Indonesia have shown prevalence rates between 3% and 8%. This phenomenon generates high potential for perinatal transmission from carrier mothers to their babies[12].
The Lombok Hepatitis B Model Immunization Project (1987-1991) was the first universal infant hepatitis B immunization project[13,14] in Indonesia. This project aimed to integrate the HepB vaccine into the National Immunization Program, including a birth dose targeted as early as possible within the first week after birth. This project achieved > 90% coverage for the administration of the HepB vaccine and was able to demonstrate a decrease in the prevalence of hepatitis B carriage among children under 4 years of age who had received three doses of the vaccine. The carriage rates dropped from 6.2% to 3.0% for infants who received the first dose later than 7 d after birth and to 1.4% in infants who received the first dose within 7 d of birth.
Following the Lombok Hepatitis B project, in 1991, routine HepB immunization was implemented in 4 provinces (West Nusa Tenggara, Bali, Yogyakarta and 5 districts in East Java). In that year, immunization for newborns (birth dose immunization) was recommended. During the period from 1992-1995, routine HepB immunization was expanded to 6 other provinces (Lampung, DKI Jakarta, West Java, Central Java, West Sumatera, and West Kalimantan)[13,15]. Finally, in 1997, HepB vaccination of newborns was fully integrated into the National Immunization Program[15]. A plasma-derived vaccine was produced in Indonesia until 1997, when it was replaced by a recombinant hepatitis B vaccine[16]. By 2000, Indonesia was able to use the HB-Uniject, which is a pre-filled single-use injection device for the hepatitis B vaccine that is stable outside of the cold chain, for the birth dose in seven of the provinces in the program. By 2003, the program expanded to target all of Indonesia’s five million annual births[17]. According to the National Immunization Program in Indonesia, the birth dose of the hepatitis B vaccine should be given within 7 d after birth and should be followed by three doses of combination vaccines, including Diphtheria, Pertussis, Tetanus and Hepatitis B, within the second, third and fourth months[17,18]. Parents should bring their babies to the Primary Health Center to receive the vaccine; however, many people in remote areas in Indonesia have difficulty reaching these centers due to geographic isolation[12]. Moreover, because screening for hepatitis B in all pregnant women has not been implemented as a national program in Indonesia, not all infants born to women with hepatitis B receive the hepatitis B immunoglobulin (HBIG)[19]. If the mother is infected and transmits the virus before the child is vaccinated and no HBIG is simultaneously administered within 24 h of birth, vaccination will not protect the child[20].
HBV infection has been reduced by the universal newborn HepB immunization program; nevertheless, it continues to occur in endemic countries[21]. Even with proper immunization, 5%-10% of infants delivered by hepatitis B e-antigen (HBeAg)-positive women become infected[22]. Because HBeAg and hepatitis B core antigen (HBcAg) are highly cross-reactive in terms of helper T cell recognition, transplacental HBeAg from the mother can induce specific helper T cell unresponsiveness to HBeAg and HBcAg in neonates born to HBeAg-positive carrier mothers, who become chronic carriers[5,23]. The maternal HBV DNA load is also strongly associated with HBV intrauterine transmission[24-26]. Up to 90% of infants infected during the first year of life will develop chronic HBV infection[27]. However, more than 90% of perinatal infections can be prevented if HBsAg-positive mothers are identified[28]. Although screening of pregnant women is necessary to prevent further spread of HBV, especially through perinatal transmission, data on the hepatitis B prevalence in pregnant women in Indonesia are limited in number and study coverage[15]. Previous studies reported HBsAg-positive rates of 2.6%, 4.7%, and 5.2% in Bali, West Java and Jakarta, respectively[15,29,30]. In 2003, HBsAg was detected in 1.9% of pregnant women in Bali, which was significantly lower than previously reported data. The prevalence of HBeAg also decreased from 50% to 28% within 10 years[31]. In 2009, the HBsAg prevalence was also significantly lower (2.2%) in Jakarta, with the peak prevalence occurring in women aged < 20 years and between 36-40 years[15].
In Indonesia, a screening test for HepB in pregnant women is not routinely performed, and the price of HBIG, which is unaffordable for many people, makes it difficult to provide for high-risk babies. In 2010, Fitria et al[32] reported that the effectiveness of HepB immunization in preventing vertical transmission was 70%-90%. Fourteen of 15 infants born to HBsAg-positive mothers were not infected by HBV, although some of them received their first immunization at more than 24 h of age. The infants were delivered per vaginam and did not receive HBIG. The authors suggested that HepB immunization could prevent vertical transmission in infants born to HBsAg-positive mothers even without HBIG administration. They also found that no infants who had HBsAg-positive umbilical cord blood were infected with HBV (negative HBsAg after HepB immunization). This result is in accordance with other findings[33,34] that transplacental transmission does not play a role in vertical transmission. However, these studies are in contrast with other studies. Beasley et al[35,36] found that HepB immunization alone prevented the development of the persistent carrier state in at least 75% of infants born to HBeAg-positive mothers; concurrent use of HBIG and HepB immunization in these infants appears to increase the rate of prevention to as high as 95%. Lavanchy[37] reported that combined HBIG and HepB immunization after birth reduced the risk of transmission from 70%-90% to less than 10% among infants of HBsAg-positive and HBeAg-positive mothers. Zhang et al[38] also reported that the immunoprophylaxis failure rate was 3.3% among infants of HBsAg-positive mothers and that the infection rate reached 9.3% in infants of both HBsAg- and HBeAg-positive mothers. Infants born to HBeAg-positive mothers who only received HepB immunization were more vulnerable to HBV infection compared with infants who received HepB immunization plus HBIG, with an immunoprophylaxis failure rate of 16.9% vs 7.9%. However, another finding showed that fulminant hepatitis B could occur in infants born to HBeAg-negative and HBsAg-positive mothers if they were not given HBIG, although these mothers were reportedly HBV DNA-negative. Thus, the etiology is most likely unrelated to maternal HBV infection, and the cause is unknown[39].
Primary HepB immunization coverage among Indonesian infants gradually increased from 28% in 1992 to 78% in 2008 and then to 93% in 2009[40]. In 2012, the three doses of HepB vaccine coverage were 73.9%-94.1%, although the birth dose coverage was less than 50% according to the local health office data in 5 provinces in Indonesia (Central Kalimantan, West Timor, West Papua, South East Sulawesi and East Java)[41]. After 15 years of implementation of universal HepB immunization, the HBsAg-positive rates in pre-school- and school-aged children ranged from 2.1%-4.2% and 0%-5.9%, respectively. The anti-HBs seropositivity prevalence among pre-school-aged children was higher (61.4%-65.8%) than among school-aged children (20.9%-40.4%)[16,41] (Table 1). The antibody titer gradually fell to less than 10 mIU/mL by 10 to 15 years of age[42,43]. This decrease may reflect a decline in the anti-HBs levels; however, the lower immunization coverage in Indonesia during the earlier years of its implementation (before 2003)[17] may have contributed to these results. Utsumi et al[16] and Purwono et al[41] also found some substitutions (P120S, T126I, M133T/L, T140I, C147S, and S155F) in the S region of HBV isolates from vaccinated children. The T126I substitution involves the largest change in chemical properties and is the most likely substitution to cause structural changes in HBsAg[44-46]. T140I has also been potentially suggested as a vaccine escape mutant. The low birth dose coverage and the presence of a vaccine escape mutant may cause HBV infection among children to remain endemic in Indonesia[41]. The triple-antigen vaccine that includes regions other than the S region may be considered in regions where the anti-HBs prevalence remains insufficient among vaccinated children[16].
| Year | Population group | HBsAg (%) | Anti-HBc (%) | Anti-HBs (%) |
| 2007 | In all provinces[11] | |||
| General population: | ||||
| Male | 9.68 | 36.39 | 34.37 | |
| Female | 9.28 | 30.14 | 28.81 | |
| Age groups: | ||||
| 1-4 yr | 7.32 | 10.14 | 50.78 | |
| 5-9 yr | 6.92 | 11.56 | 34.50 | |
| 10-14 yr | 10.14 | 14.79 | 23.30 | |
| In East Java province[16] | ||||
| 6-12 yr | 3.10 | 23.80 | 23.60 | |
| 2012 | In Central Kalimantan, West Timor, South East Sulawesi, provinces[41] | |||
| 1-5 yr | 2.1-4.2 | 3.5-4.8 | 61.4-65.8 | |
| In Central Kalimantan, West Timor, West Papua provinces[41] | ||||
| 6-12 yr | 0-5.9 | 5.2-50.4 | 20.9-40.4 | |
Few studies have investigated pre-S/S variations in Indonesia. In 2011, Utama et al[47] reported that the prevalence of pre-S mutations was 2.7% (2/75), 12.9% (8/62), 16.7% (11/66), and 17.7% (11/62) in the asymptomatic carrier, chronic hepatitis, liver cirrhosis, and HCC groups, respectively. The authors concluded that the prevalence of HBV pre-S mutations was relatively low in Indonesian patients compared with patients from Taiwan, Japan, and other Asian countries and that there was a weak association between the pre-S deletion mutation and progressive liver disease. Conversely, Utama et al[48] found a mutation in the pre-S2 start codon in 59 samples from 268 subjects (22.0%) with a higher prevalence in patients with cirrhosis (27/66, 40.9%), followed by HCC (18/63, 28.6%), chronic hepatitis (12/66, 18.2%) and asymptomatic carriers (2/73, 2.7%). They reported that the pre-S2 start codon mutation was more common in Indonesian patients than in patients from other Asian countries and that its prevalence was associated with advanced liver disease. In 2015, Yamani et al[49] analyzed HBV-infected patients using a deep sequencing method and reported that the accumulation of variations in the major hydrophilic region was associated with a decrease in the HBsAg titer.
OBI is diagnosed by the detection of HBV DNA and the lack of HBsAg detection with or without anti-HBc or anti-HBs outside of the pre-seroconversion window period[50,51]. OBI usually depends on the difference in the sensitivity of the screening assay; for instance, detection by the HBsAg assay is less sensitive than the detection of HBV DNA by the PCR assay[52,53]. Recent epidemiological studies have detected occult HBV infection worldwide[54]. In 2015, Darmawan et al[55] examined 195 healthy young adults who received universal infant hepatitis B vaccination in Banjarmasin and reported that the prevalence of HBsAg, anti-HBc, and anti-HBs was 9 (4.6%), 62 (31.8%), and 96 (49.2%), respectively. Additionally, the authors detected HBV DNA and confirmed occult HBV infection in 9 HBsAg-negative and anti-HBc-positive individuals. Generally, clearance of HBsAg is considered to represent a disappearance of viremia and disease remission[7,8]. However, OBI is reportedly associated with severe liver damage and the development of liver cancer[50,56,57]. Many studies reported that OBI was associated with advanced liver diseases, such as cirrhosis and HCC[58,59]. However, references regarding this information are scarce in Indonesia, and further studies will be necessary.
OBI is sometimes related to the decreased activity of viral replication and mutations in the α determinant region of the S gene, which encodes amino acid residues 124-147 of HBsAg. Utsumi et al[16] examined 229 healthy children in East Java and reported that the prevalence of HBsAg positivity was 3.1%; occult HBV infection was detected in 5 out of 222 HBsAg-negative individuals. The authors reported that the T126I amino acid substitution was frequently found. In Indonesia, universal vaccination was introduced in the 1990s, but the efficacy has not been fully investigated. A follow up study is necessary to consider booster immunization. Thedja et al[60] examined 309 HBsAg-negative blood donors and reported that the prevalence of anti-HBc and HBV DNA was 134 (43.4%) and 25 (8.1%), respectively. They also examined amino acid substitutions in the α determinant region in HBsAg and reported that several amino acid substitutions, such as T123A, M133L, and T143M, might change the HBs antigenicity.
Occult HBV infection is sometimes related to high-risk patients, such as HD patients, HIV patients and immunosuppressed patients. Utsumi et al[61] examined 118 HIV-infected patients in Surabaya and reported that the prevalence of HBsAg and HBV DNA was 15.3% and 27.1%, respectively. HBV reactivation is especially critical in immunosuppressed OBI patients, and many clinicians should take precautions[62]. Although reported in relation to HBV, reactivation in Indonesia is still rare, and the potential risk for reactivation is considered to be high[63,64].
Patients on maintenance HD are among the group at highest risk for HBV infection. Most HBV infection outbreaks in patients in HD units are caused by cross-contamination via the following factors: (1) environmental surfaces, supplies (e.g., hemostats and clamps), or equipment that is not routinely disinfected after each use; (2) multiple dose medication vials and intravenous solutions that are not used exclusively for one patient; (3) medications for injection that are prepared in areas adjacent to areas where blood samples are handled; and (4) staff members who simultaneously care for both HBV-infected and susceptible patients[2,65-68].The risk of HBV transmission from blood-contaminated items in this setting is greater and more serious than would be expected for other common bloodborne viruses[66].
The prevalence of infection is generally lower in developed countries, which experience occasional outbreaks, than in developing countries; this difference might reflect the prevalence of the infection in the general population[65,69,70]. A study in 2003 in Manitoba, Canada, showed that 0.8% of HD patients were positive for the HBsAg[52]. A systematic review of HBV outbreaks in the dialysis units of developed and less-developed countries published between 1992 and 2014 showed fewer European outbreaks compared with other countries (P = 0.0046). Moreover, multiple deficiencies in standard or HD-specific procedures were the most common routes of patient-to-patient HBV transmission (80%). A recent multicenter prospective cohort study among dialysis patients in Korea revealed that 7.1% were HBsAg-positive[71]. In Vietnam, 7% of HD patients tested positive for HBsAg[72]. Several studies have also been performed in Indonesia. In a study conducted in West Java, the rates of HBsAg and anti-hepatitis C virus (anti-HCV) seropositivity among HD patients were 6.8% and 73.5%, respectively[73], whereas those in Yogyakarta were 7% and 81%, respectively[74]. After almost 20 years, more recent data on the rates of infection in Yogyakarta showed 11.2% seropositivity for HBsAg and 80.7% for anti-HCV. Our previous studies in Surabaya showed that the anti-HCV prevalence was between 76.3% and 88%[75-77], whereas our recent study in private hemodialysis units (HDUs) in Surabaya showed that the hepatitis B infection prevalence was 0-8.1%. Interestingly, no HBV- or HCV-infected HD patients were detected in one private HDU that strictly complied with the adherence to standard and dialysis-specific infection control precautions, whereas 24.2% to 60.6% of patients tested positive for anti-HCV in other private HDUs[78].
In general hospitals in Indonesia, dialyzers are commonly reused up to a maximum of eight times for all patients. Following the recommendations for HBV and HCV infection control issued by the Indonesian Society of Nephrology, separate rooms are only available for patients who are HBsAg seropositive but not for anti-HCV-positive patients. Based on the slightly higher prevalence of HBV infection compared with a markedly higher prevalence of HCV infection among HD patients than the general population (2.5%-10% and 2.1%-2.3%, respectively) in Indonesia[2,75,79] and the practice by which patients with hepatitis B but not hepatitis C are isolated in separate rooms, there is a strong possibility that the prevalence of HBV and/or HCV infections among HD patients is caused by nosocomial infections. Our previous study also showed that the HD duration and number of blood transfusions were significantly associated with HCV infection but not with HBV infection[68]. A study in 4 private HDUs in which serological tests were conducted every 3 mo for 9 mo to investigate the new incidence of hepatitis virus infections found no new incidence of HBV in any HDU, whereas the new incidence of HCV was 5.6% during the third sampling in HDU-C and 11.1% and 13.3% during the second and third samplings in HDU-D, respectively[78]. Due to resource limitations, only 5% of the patients in the general hospital and 8.6%-83.3% of patients in the private HDUs with HBsAg seronegativity were vaccinated for HBV[68,78], which made the HD patients more susceptible.
Subgenotype B3 is the most prominent because this genotype is commonly found among the Javanese ethnic group; the Javanese group is the main ethnic group in Indonesia and has mostly settled on Java Island, which is the Indonesian mainland[68,78]. HBV subgenotypes A2, B2-3, B7-9, C1-2, C5-8, C10-16, D6, F, and J are unique to Indonesia, with specific geographic and ethnic distributions[80-86]. Our studies on HBV infection among HD patients showed that all of the HBV/B strains were classified as HBV/B3[68,78]. These studies were performed in Yogyakarta and Surabaya, which are located on Java Island. We presume that a study conducted on HBV infection among HD patients in other parts of Indonesia, especially in East Indonesia where we found several other unique HBV subgenotypes, may result in different prominent subgenotypes.
Patients undergoing dialysis potentially have an increased risk of OBI. OBI harbors a potential risk of HBV transmission through HD[87]. Inadequate data are available concerning OBIs among Indonesian chronic HD patients. In 2013, Rinonce et al[68] reported that OBIs were detected in 21 (14.7%) of 143 HBsAg-negative patients, and 7 (33.3%) of these 21 patients tested positive for anti-HBc in Yogyakarta. Most patients who were co-infected with HBV and HCV had lower HBsAg titers than patients with only HBV infection, suggesting that HBV infection was suppressed by HCV co-infection[88]. However, Kanbay et al[89] reported in 2006 that HCV positivity was not a contributing factor to OBIs in HD patients. Rinonce et al[68] also found that 15 (52%) of 29 HBV DNA-positive patients co-infected with HCV (anti-HCV or HCV RNA-positive) were HBsAg-positive and that 14 (48%) had occult HBV infections.
Indonesia consists of five major islands and is the largest archipelago in the world. Archaeological evidence revealed that Central and East Java were occupied by the ancestors of modern humans as early as 1.9 million years ago. Currently, Indonesia consists of more than 300 ethnic groups, more than 95% of which are of native Indonesian ancestry. The largest ethnic group in Indonesia is the Javanese, who primarily live on Java Island and make up approximately 40% of the total population.
Although single nucleotide polymorphisms (SNPs) near the interleukin 28B gene (IL28B; IFN-λ-3) are the strongest genetic predictors of the response to interferon-based therapy for chronic hepatitis C patients, whether SNPs are associated with the therapeutic outcome for chronic hepatitis B patients is controversial[90,91]. These SNPs were also reported to be associated with spontaneous clearance of HBV infection, although the data are still limited and have not been confirmed[92,93]. The human leukocyte antigen (HLA) gene is located in region 6p21.3 and plays an important role in antigen presentation. Although technological advancement for sequencing of the human genome has made the analysis of many diseases easier, genomic studies in Indonesia are still limited. Several studies on novel HLA alleles in Indonesian populations have been conducted[94,95]. However, whether these alleles are associated with disease is unclear. Yuliwulandari et al[96] examined HLA alleles of 237 Javanese and Sundanese-Javanese ethnic groups and reported that the Western Javanese population was closer to Southwest Asian populations than Northeast Asian populations. These studies would be helpful for future studies in anthropology, organ transplantation, and disease associations in Indonesian populations.
Several studies, especially in Asian countries, revealed that host factors were associated with HBV infection. Host factors, such as age, gender, obesity, diabetes, and genetic variants, are associated with persistent infection and disease progression among HBV-infected individuals. In 2009, Kamatani et al[97] first reported that SNPs in the HLA-DP region were associated with chronic HBV in a study of 188 Japanese patients with chronic HBV infection and 934 controls. Thereafter, a large number of studies concerning HLA regions susceptible to HBV infection have been reported from Asian countries (Table 2)[97-109]. A study from the Netherlands reported that several SNPs, including HLA-DR (rs3135363), HLA-DP (rs9277535), and a gene-rich HLA Class III interval (rs9267665), were independent risk variants in Indonesian vaccine recipients[106]. For instance, rs3135363 was revealed to be the most significant contributor to the antibody response in Indonesian populations. This result supported the finding that the HLA-DR allele was associated with the host response to HBsAg vaccination[110,111]. More recently, Wasityastuti et al[101] reported that the HLA-DPA1 rs3077 variant was associated with a protective effect by increasing spontaneously resolved HBV infection and that combinations of haplotype markers (CA for rs3077-rs9277535 and GA for rs3135021-rs9277535) were associated with HBV susceptibility.
| SNP region | rs | Ethnics | Ref. |
| HLA-DPA1 | rs3077 | Chinese, Japanese, Thai | [97-101] |
| Korean, Indonesian | |||
| HLA-DPB1 | rs9277378 | Thai | [102] |
| rs9277535 | Chinese, Japanese, Indonesian, Taiwanese | [97-99,101,103-106] | |
| rs9277542 | Chinese, Japanese, Korean, Thai | [100] | |
| rs7770370 | Korean | [107] | |
| HLA-DQ | rs9275319 | Chinese | [108] |
| HLA-DQA2 | rs9276370 | Taiwanese | [104] |
| HLA-DQB1 | rs2856718 | Chinese, Japanese | [98,99] |
| HLA-DQB2 | rs7453920 | Chinese, Japanese, Taiwanese | [98,99,103,104] |
| HLA-DQ/DR | rs9272105 | Chinese | [108] |
| HLA-DR | rs3135363 | Indonesian | [106] |
| HLA-DRB1 | Chinese | [109] |
The HBV infection rate has been reduced by a universal newborn HepB vaccination program, but the low birth dose coverage and the presence of a vaccine escape mutation might cause HBV infection among children to remain endemic in Indonesia. OBIs have also been reported among the general population, patients with chronic liver disease and patients with immunosuppressive conditions, such as HD. Additionally, some mutations in the pre-S/S region play an important role. Genetic and other data show possible cross-HBV infections among patients in HDUs. Occult hepatitis B cases might also play an important role in HBV transmission in Indonesian HDUs.
| 1. | Dunkelberg JC, Berkley EM, Thiel KW, Leslie KK. Hepatitis B and C in pregnancy: a review and recommendations for care. J Perinatol. 2014;34:882-891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 2. | Yano Y, Utsumi T, Lusida MI, Hayashi Y. Hepatitis B virus infection in Indonesia. World J Gastroenterol. 2015;21:10714-10720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 3. | Norder H, Couroucé AM, Coursaget P, Echevarria JM, Lee SD, Mushahwar IK, Robertson BH, Locarnini S, Magnius LO. Genetic diversity of hepatitis B virus strains derived worldwide: genotypes, subgenotypes, and HBsAg subtypes. Intervirology. 2004;47:289-309. [PubMed] |
| 4. | Locarnini S, Littlejohn M, Aziz MN, Yuen L. Possible origins and evolution of the hepatitis B virus (HBV). Semin Cancer Biol. 2013;23:561-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 74] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 5. | Milich D, Liang TJ. Exploring the biological basis of hepatitis B e antigen in hepatitis B virus infection. Hepatology. 2003;38:1075-1086. [PubMed] |
| 6. | World Health Organization. Hepatitis B. Fact sheet N°204. Updated July 2015. Available from: http://www.who.int/mediacentre/factsheets/fs204/en/. |
| 7. | Gitlin N. Hepatitis B: diagnosis, prevention, and treatment. Clin Chem. 1997;43:1500-1506. [PubMed] |
| 8. | Lee WM. Hepatitis B virus infection. N Engl J Med. 1997;337:1733-1745. [PubMed] |
| 9. | Nishida N, Tokunaga K, Mizokami M. Genome-Wide Association Study Reveals Host Genetic Factors for Liver Diseases. J Clin Transl Hepatol. 2013;1:45-50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Yano Y, Seo Y, Azuma T, Hayashi Y. Hepatitis B virus and host factors. Hepatobiliary Surg Nutr. 2013;2:121-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 11. | Basic Health Research - Biomedical Group. Report of Basic Health Research in Biomedics in 2007. National Institute of Health Research and Development, Ministry of Health of Republic of Indonesia. 2010;38-43. |
| 12. | Creati M, Saleh A, Ruff TA, Stewart T, Otto B, Sutanto A, Clements CJ. Implementing the birth dose of hepatitis B vaccine in rural Indonesia. Vaccine. 2007;25:5985-5993. [PubMed] |
| 13. | Ruff TA, Gertig DM, Otto BF, Gust ID, Sutanto A, Soewarso TI, Kandun N, Marschner IC, Maynard JE. Lombok Hepatitis B Model Immunization Project: toward universal infant hepatitis B immunization in Indonesia. J Infect Dis. 1995;171:290-296. [PubMed] |
| 14. | Ruff TA, Muller N, Gust ID. Hepatitis B control: lessons from the International Task Force on Hepatitis B Immunization and the Lombok Hepatitis B Model Immunization Project. Prog Liver Dis. 1993;11:179-201. [PubMed] |
| 15. | Gunardi H, Zaimi LF, Soedjatmiko AR, Muljono DH. Current prevalence of hepatitis B infection among parturient women in Jakarta, Indonesia. Acta Med Indones. 2014;46:3-9. [PubMed] |
| 16. | Utsumi T, Yano Y, Lusida MI, Amin M, Soetjipto H, Hayashi Y. Serologic and molecular characteristics of hepatitis B virus among school children in East Java, Indonesia. Am J Trop Med Hyg. 2010;83:189-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | Levin CE, Nelson CM, Widjaya A, Moniaga V, Anwar C. The costs of home delivery of a birth dose of hepatitis B vaccine in a prefilled syringe in Indonesia. Bull World Health Organ. 2005;83:456-461. [PubMed] |
| 18. | Ministry of Health of Republic of Indonesia. National Guideline of Vaccination Implementation, 2004. Available from: http://www.hukor.depkes.go.id. |
| 19. | World Health Organization. Global policy report on the prevention and control of viral hepatitis. Geneve, Swiss: World Health Organization 2013; 194-197. |
| 20. | World Health Organization, Regional Office for South-East Asia. Regional strategy for the prevention and control of viral hepatitis. New Delhi, India: World Health Organization 2013; 2-18. |
| 21. | Merican I, Guan R, Amarapuka D, Alexander MJ, Chutaputti A, Chien RN, Hasnian SS, Leung N, Lesmana L, Phiet PH. Chronic hepatitis B virus infection in Asian countries. J Gastroenterol Hepatol. 2000;15:1356-1361. [PubMed] |
| 22. | Lee C, Gong Y, Brok J, Boxall EH, Gluud C. Effect of hepatitis B immunisation in newborn infants of mothers positive for hepatitis B surface antigen: systematic review and meta-analysis. BMJ. 2006;332:328-336. [PubMed] |
| 23. | Hsu HY, Chang MH, Hsieh KH, Lee CY, Lin HH, Hwang LH, Chen PJ, Chen DS. Cellular immune response to HBcAg in mother-to-infant transmission of hepatitis B virus. Hepatology. 1992;15:770-776. [PubMed] |
| 24. | Burk RD, Hwang LY, Ho GY, Shafritz DA, Beasley RP. Outcome of perinatal hepatitis B virus exposure is dependent on maternal virus load. J Infect Dis. 1994;170:1418-1423. [PubMed] |
| 25. | Xu DZ, Yan YP, Choi BC, Xu JQ, Men K, Zhang JX, Liu ZH, Wang FS. Risk factors and mechanism of transplacental transmission of hepatitis B virus: a case-control study. J Med Virol. 2002;67:20-26. [PubMed] |
| 26. | Wang Z, Zhang J, Yang H, Li X, Wen S, Guo Y, Sun J, Hou J. Quantitative analysis of HBV DNA level and HBeAg titer in hepatitis B surface antigen positive mothers and their babies: HBeAg passage through the placenta and the rate of decay in babies. J Med Virol. 2003;71:360-366. [PubMed] |
| 27. | Shepard CW, Simard EP, Finelli L, Fiore AE, Bell BP. Hepatitis B virus infection: epidemiology and vaccination. Epidemiol Rev. 2006;28:112-125. [PubMed] |
| 28. | Mahamat A, Louvel D, Vaz T, Demar M, Nacher M, Djossou F. High prevalence of HBsAg during pregnancy in Asian communities at Cayenne Hospital, French Guiana. Am J Trop Med Hyg. 2010;83:711-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 29. | Wiharta AS, Sulaiman A, Sjaifoellah Noer HM, Syahbudin S, Marzuki K, Mayumi M. The prevalence of HBsAg and anti HBs in pregnant women and young generation in Jakarta, Indonesia. Paediatr Indones. 1986;26:156-160. [PubMed] |
| 30. | Reniers J, Vranckx R, Ngantung W, Sugita E, Meheus A. Prevalence and determinants of hepatitis B virus markers in pregnant women in West Java, Indonesia. J Trop Med Hyg. 1987;90:249-253. [PubMed] |
| 31. | Surya IG, Kornia K, Suwardewa TG, Mulyanto F, Mishiro S. Serological markers of hepatitis B, C, and E viruses and human immunodeficiency virus type-1 infections in pregnant women in Bali, Indonesia. J Med Virol. 2005;75:499-503. [PubMed] |
| 32. | Fitria L, Gunaardi H, Akib AAP. Influence of hepatitis B immunization to prevent vertical transmission of Hep-B virus in infants born form Hep-B positive mother. Paediatr Indones. 2010;50:321-325. |
| 33. | Lee AK, Ip HM, Wong VC. Mechanisms of maternal-fetal transmission of hepatitis B virus. J Infect Dis. 1978;138:668-671. [PubMed] |
| 34. | Wong VC, Lee AK, Ip HM. Transmission of hepatitis B antigens from symptom free carrier mothers to the fetus and the infant. Br J Obstet Gynaecol. 1980;87:958-965. [PubMed] |
| 35. | Beasley RP, Hwang LY, Lee GC, Lan CC, Roan CH, Huang FY, Chen CL. Prevention of perinatally transmitted hepatitis B virus infections with hepatitis B immune globulin and hepatitis B vaccine. Lancet. 1983;2:1099-1102. [PubMed] |
| 36. | Beasley RP, Hwang LY, Stevens CE, Lin CC, Hsieh FJ, Wang KY, Sun TS, Szmuness W. Efficacy of hepatitis B immune globulin for prevention of perinatal transmission of the hepatitis B virus carrier state: final report of a randomized double-blind, placebo-controlled trial. Hepatology. 1983;3:135-141. [PubMed] |
| 37. | Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepat. 2004;11:97-107. [PubMed] |
| 38. | Zhang L, Gui XE, Teter C, Zhong H, Pang Z, Ding L, Li F, Zhou Y, Zhang L. Effects of hepatitis B immunization on prevention of mother-to-infant transmission of hepatitis B virus and on the immune response of infants towards hepatitis B vaccine. Vaccine. 2014;32:6091-6097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 39. | Chang MH, Lee CY, Chen DS, Hsu HC, Lai MY. Fulminant hepatitis in children in Taiwan: the important role of hepatitis B virus. J Pediatr. 1987;111:34-39. [PubMed] |
| 40. | Ministry of Health of Republic of Indonesia. Health Profile in Indonesia in 2009. Jakarta: Ministry of Health Republic of Indonesia 2010; . |
| 41. | Purwono PB, Juniastuti , Amin M, Bramanthi R, Nursidah , Resi EM, Wahyuni RM, Yano Y, Soetjipto , Hotta H. Hepatitis B Virus Infection in Indonesia 15 Years after Adoption of a Universal Infant Vaccination Program: Possible Impacts of Low Birth Dose Coverage and a Vaccine-Escape Mutant. Am J Trop Med Hyg. 2016; Epub ahead of print. [PubMed] |
| 42. | Arauz-Ruiz P, Norder H, Robertson BH, Magnius LO. Genotype H: a new Amerindian genotype of hepatitis B virus revealed in Central America. J Gen Virol. 2002;83:2059-2073. [PubMed] |
| 43. | National Center for Statistics of the Republic Indonesia. Life Time Migration: 1971-2010. Available from: http://www.bps.go.id/linkTableDinamis/view/id/ 855. |
| 44. | Ren F, Tsubota A, Hirokawa T, Kumada H, Yang Z, Tanaka H. A unique amino acid substitution, T126I, in human genotype C of hepatitis B virus S gene and its possible influence on antigenic structural change. Gene. 2006;383:43-51. [PubMed] |
| 45. | Ruiz-Tachiquín ME, Valdez-Salazar HA, Juárez-Barreto V, Dehesa-Violante M, Torres J, Muñoz-Hernández O, Alvarez-Muñoz MT. Molecular analysis of hepatitis B virus “a” determinant in asymptomatic and symptomatic Mexican carriers. Virol J. 2007;4:6. [PubMed] |
| 46. | Katsoulidou A, Paraskevis D, Magiorkinis E, Moschidis Z, Haida C, Hatzitheodorou E, Varaklioti A, Karafoulidou A, Hatzitaki M, Kavallierou L. Molecular characterization of occult hepatitis B cases in Greek blood donors. J Med Virol. 2009;81:815-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 47. | Utama A, Siburian MD, Fanany I, Intan MD, Dhenni R, Kurniasih TS, Lelosutan SA, Achwan WA, Arnelis B, Yusuf I. Low prevalence of hepatitis B virus pre-S deletion mutation in Indonesia. J Med Virol. 2011;83:1717-1726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 48. | Utama A, Siburian MD, Fanany I, Intan MD, Dhenni R, Kurniasih TS, Lelosutan SA, Achwan WA, Zubir N, Arnelis B. Hepatitis B virus pre-S2 start codon mutations in Indonesian liver disease patients. World J Gastroenterol. 2012;18:5418-5426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 49. | Yamani LN, Yano Y, Utsumi T, Juniastuti H, Widjanarko D, Triantanoe A, Wasityastuti W, Liang Y, Okada R, Tanahashi T. Ultradeep Sequencing for Detection of Quasispecies Variants in the Major Hydrophilic Region of Hepatitis B Virus in Indonesian Patients. J Clin Microbiol. 2015;53:3165-3175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 50. | Bréchot C, Thiers V, Kremsdorf D, Nalpas B, Pol S, Paterlini-Bréchot P. Persistent hepatitis B virus infection in subjects without hepatitis B surface antigen: clinically significant or purely “occult”? Hepatology. 2001;34:194-203. [PubMed] |
| 51. | Hu KQ. Occult hepatitis B virus infection and its clinical implications. J Viral Hepat. 2002;9:243-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 222] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 52. | Biswas R, Tabor E, Hsia CC, Wright DJ, Laycock ME, Fiebig EW, Peddada L, Smith R, Schreiber GB, Epstein JS. Comparative sensitivity of HBV NATs and HBsAg assays for detection of acute HBV infection. Transfusion. 2003;43:788-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 199] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 53. | Reesink HW, Engelfriet CP, Henn G, Mayr WR, Delage G, Bernier F, Krusius T, Assal A, Gallian P, Corbi C. Occult hepatitis B infection in blood donors. Vox Sang. 2008;94:153-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 54. | Minuk GY, Sun DF, Uhanova J, Zhang M, Caouette S, Nicolle LE, Gutkin A, Doucette K, Martin B, Giulivi A. Occult hepatitis B virus infection in a North American community-based population. J Hepatol. 2005;42:480-485. [PubMed] |
| 55. | Darmawan E, Turyadi KE, Nursanty NK, Thedja MD, Muljono DH. Seroepidemiology and occult hepatitis B virus infection in young adults in Banjarmasin, Indonesia. J Med Virol. 2015;87:199-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 56. | Pollicino T, Squadrito G, Cerenzia G, Cacciola I, Raffa G, Craxi A, Farinati F, Missale G, Smedile A, Tiribelli C. Hepatitis B virus maintains its pro-oncogenic properties in the case of occult HBV infection. Gastroenterology. 2004;126:102-110. [PubMed] |
| 57. | Cacciola I, Pollicino T, Squadrito G, Cerenzia G, Orlando ME, Raimondo G. Occult hepatitis B virus infection in patients with chronic hepatitis C liver disease. N Engl J Med. 1999;341:22-26. [PubMed] |
| 58. | Pollicino T, Saitta C. Occult hepatitis B virus and hepatocellular carcinoma. World J Gastroenterol. 2014;20:5951-5961. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 47] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 59. | Saitta C, Tripodi G, Barbera A, Bertuccio A, Smedile A, Ciancio A, Raffa G, Sangiovanni A, Navarra G, Raimondo G. Hepatitis B virus (HBV) DNA integration in patients with occult HBV infection and hepatocellular carcinoma. Liver Int. 2015;35:2311-2317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 86] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 60. | Thedja MD, Roni M, Harahap AR, Siregar NC, Ie SI, Muljono DH. Occult hepatitis B in blood donors in Indonesia: altered antigenicity of the hepatitis B virus surface protein. Hepatol Int. 2010;4:608-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 61. | Utsumi T, Yano Y, Lusida MI, Nasronudin M, Juniastuti H, Hayashi Y. Detection of highly prevalent hepatitis B virus co-infection with HIV in Indonesia. Hepatol Res. 2013;43:1032-1039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 62. | Raimondo G, Filomia R, Maimone S. Therapy of occult hepatitis B virus infection and prevention of reactivation. Intervirology. 2014;57:189-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 63. | Wijaya I, Hasan I. Reactivation of hepatitis B virus associated with chemotherapy and immunosuppressive agent. Acta Med Indones. 2013;45:61-66. [PubMed] |
| 64. | Wijaya I, Sanityoso A, Lesmana LA. Acute liver failure related to chemotherapy. Acta Med Indones. 2012;44:145-149. [PubMed] |
| 65. | Rahnavardi M, Hosseini Moghaddam SM, Alavian SM. Hepatitis C in hemodialysis patients: current global magnitude, natural history, diagnostic difficulties, and preventive measures. Am J Nephrol. 2008;28:628-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 59] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 66. | Edey M, Barraclough K, Johnson DW. Review article: Hepatitis B and dialysis. Nephrology (Carlton). 2010;15:137-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 97] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 67. | Centers for Disease Control and Prevention. Recommendations for preventing transmission of infections among chronic hemodialysis patients. MMWR Recomm Rep. 2001;50:1-43. [PubMed] |
| 68. | Rinonce HT, Yano Y, Utsumi T, Heriyanto DS, Anggorowati N, Widasari DI, Lusida MI, Soetjipto H, Hotta H, Hayashi Y. Hepatitis B and C virus infection among hemodialysis patients in Yogyakarta, Indonesia: Prevalence and molecular evidence for nosocomial transmission. J Med Virol. 2013;85:1348-1361. [PubMed] |
| 69. | Burdick RA, Bragg-Gresham JL, Woods JD, Hedderwick SA, Kurokawa K, Combe C, Saito A, LaBrecque J, Port FK, Young EW. Patterns of hepatitis B prevalence and seroconversion in hemodialysis units from three continents: the DOPPS. Kidney Int. 2003;63:2222-2229. [PubMed] |
| 70. | Johnson DW, Dent H, Yao Q, Tranaeus A, Huang CC, Han DS, Jha V, Wang T, Kawaguchi Y, Qian J. Frequencies of hepatitis B and C infections among haemodialysis and peritoneal dialysis patients in Asia-Pacific countries: analysis of registry data. Nephrol Dial Transplant. 2009;24:1598-1603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 120] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 71. | Kwon E, Cho JH, Jang HM, Kim YS, Kang SW, Yang CW, Kim NH, Kim HJ, Park JM, Lee JE. Differential Effect of Viral Hepatitis Infection on Mortality among Korean Maintenance Dialysis Patients: A Prospective Multicenter Cohort Study. PLoS One. 2015;10:e0135476. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 72. | Duong CM, Olszyna DP, McLaws ML. Hepatitis B and C virus infections among patients with end stage renal disease in a low-resourced hemodialysis center in Vietnam: a cross-sectional study. BMC Public Health. 2015;15:192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 73. | Saketi JR, Boland GJ, van Loon AM, van Hattum J, Abdurachman SA, Sukandar E. Prevalence of hepatitis C virus infection among haemodialysis patients in West Java, Indonesia. Adv Exp Med Biol. 2003;531:201-209. [PubMed] |
| 74. | Hadiwandowo S, Tsuda F, Okamoto H, Tokita H, Wang Y, Tanaka T, Miyakawa Y, Mayumi M. Hepatitis B virus subtypes and hepatitis C virus genotypes in patients with chronic liver disease or on maintenance hemodialysis in Indonesia. J Med Virol. 1994;43:182-186. [PubMed] |
| 75. | Soetjipto R, Lusida MI, Darmadi S, Adi P, Soemarto S, Katayama Y, Hotta H. Differential prevalence of hepatitis C virus subtypes in healthy blood donors, patients on maintenance hemodialysis, and patients with hepatocellular carcinoma in Surabaya, Indonesia. J Clin Microbiol. 1996;34:2875-2880. [PubMed] |
| 76. | Santoso D, Pranawa , Yogiantoro M, Widodo , Wardana A, Mardiana N, Irwanadi C, Soewanto , Shou I, Maeda K. Hepatitis C Virus in Hemodialysis Patients: Comparison of the Surabaya Dialysis Center and Juntendo University Hospital Dialysis Center. IJTID. 2010;1:105-109. |
| 77. | Amin M, Juniastuti , Utsumi T, Yano Y, Yusuf M, Thaha M, Purwono PB, Handajani R, Soetjipto , Hotta H. The prevalence and subtype distribution of hepatitis C virus infection among hemodialysis patients in a private hospital in Surabaya, Indonesia. Microbiol Indones. 2012;6:173-179. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 78. | Utsumi T, Lusida MI, Yano Y, Wahyuni RM, Istimagfiroh A, Amin M, Rinonce HT, Juniastuti , Wardana A, Tjempakasari A. The prevalence and risk factors of hepatitis B and C virus infections among hemodialysis patients from private units in Surabaya, Indonesia. Southeast Asian Journal of Tropical Medicine and Public Health. 2016;47:5. |
| 79. | Sulaiman HA, Julitasari A, Rustam M, Melani W, Corwin A, Jennings GB. Prevalence of hepatitis B and C viruses in healthy Indonesian blood donors. Trans R Soc Trop Med Hyg. 1995;89:167-170. [PubMed] |
| 80. | Lusida MI, Nugrahaputra VE, Soetjipto R, Nagano-Fujii M, Sasayama M, Utsumi T, Hotta H. Novel subgenotypes of hepatitis B virus genotypes C and D in Papua, Indonesia. J Clin Microbiol. 2008;46:2160-2166. [PubMed] |
| 81. | Utsumi T, Lusida MI, Yano Y, Nugrahaputra VE, Amin M, Juniastuti Y, Hotta H. Complete genome sequence and phylogenetic relatedness of hepatitis B virus isolates in Papua, Indonesia. J Clin Microbiol. 2009;47:1842-1847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 82. | Mulyanto SN, Surayah K, Tsuda F, Ichiyama K, Takahashi M, Okamoto H. A nationwide molecular epidemiological study on hepatitis B virus in Indonesia: identification of two novel subgenotypes, B8 and C7. Arch Virol. 2009;154:1047-1059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 83. | Mulyanto SN, Surayah K, Tjahyono AA, Jirintai S, Takahashi M, Okamoto H. Identification and characterization of novel hepatitis B virus subgenotype C10 in Nusa Tenggara, Indonesia. Arch Virol. 2010;155:705-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 84. | Utsumi T, Nugrahaputra VE, Amin M, Hayashi Y, Hotta H, Lusida MI. Another novel subgenotype of hepatitis B virus genotype C from papuans of Highland origin. J Med Virol. 2011;83:225-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 85. | Mulyanto SN, Wahyono A, Jirintai S, Takahashi M, Okamoto H. Analysis of the full-length genomes of novel hepatitis B virus subgenotypes C11 and C12 in Papua, Indonesia. J Med Virol. 2011;83:54-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 86. | Mulyanto P, Depamede SN, Wahyono A, Jirintai S, Nagashima S, Takahashi M, Nishizawa T, Okamoto H. Identification of four novel subgenotypes (C13-C16) and two inter-genotypic recombinants (C12/G and C13/B3) of hepatitis B virus in Papua province, Indonesia. Virus Res. 2012;163:129-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 87. | Aghakhani A, Banifazl M, Velayati AA, Eslamifar A, Ramezani A. Occult hepatitis B virus infection in hemodialysis patients: a concept for consideration. Ther Apher Dial. 2012;16:328-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 88. | Lusida MI, Surayah H, Nagano-Fujii M, Soetjipto R, Boediwarsono PB, Nidom CA, Ohgimoto S, Hotta H. Genotype and subtype analyses of hepatitis B virus (HBV) and possible co-infection of HBV and hepatitis C virus (HCV) or hepatitis D virus (HDV) in blood donors, patients with chronic liver disease and patients on hemodialysis in Surabaya, Indonesia. Microbiol Immunol. 2003;47:969-975. [PubMed] |
| 89. | Kanbay M, Gur G, Akcay A, Selcuk H, Yilmaz U, Arslan H, Boyacioglu S, Ozdemir FN. Is hepatitis C virus positivity a contributing factor to occult hepatitis B virus infection in hemodialysis patients? Dig Dis Sci. 2006;51:1962-1966. [PubMed] |
| 90. | Kim SU, Song KJ, Chang HY, Shin EC, Park JY, Kim do Y, Han KH, Chon CY, Ahn SH. Association between IL28B polymorphisms and spontaneous clearance of hepatitis B virus infection. PLoS One. 2013;8:e69166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 91. | Lee DH, Lee JH, Kim YJ, Park NH, Cho Y, Lee YB, Yoo JJ, Lee M, Cho YY, Choi WM. Relationship between polymorphisms near the IL28B gene and spontaneous HBsAg seroclearance: a systematic review and meta-analysis. J Viral Hepat. 2014;21:163-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 92. | Galmozzi E, Viganò M, Lampertico P. Systematic review with meta-analysis: do interferon lambda 3 polymorphisms predict the outcome of interferon-therapy in hepatitis B infection? Aliment Pharmacol Ther. 2014;39:569-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 93. | Sonneveld MJ, Wong VW, Woltman AM, Wong GL, Cakaloglu Y, Zeuzem S, Buster EH, Uitterlinden AG, Hansen BE, Chan HL. Polymorphisms near IL28B and serologic response to peginterferon in HBeAg-positive patients with chronic hepatitis B. Gastroenterology. 2012;142:513-520.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 131] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 94. | Gao X, Matheson B. A novel HLA-A*24 (A*2410) identified in a Javanese population. Tissue Antigens. 1996;48:711-713. [PubMed] |
| 95. | Panigoro R, Greville WD, Kennedy A, Tréjaut J, Dunckley H. New HLA class II alleles in the Indonesian population. Tissue Antigens. 1999;54:521-523. [PubMed] |
| 96. | Yuliwulandari R, Kashiwase K, Nakajima H, Uddin J, Susmiarsih TP, Sofro AS, Tokunaga K. Polymorphisms of HLA genes in Western Javanese (Indonesia): close affinities to Southeast Asian populations. Tissue Antigens. 2009;73:46-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 97. | Kamatani Y, Wattanapokayakit S, Ochi H, Kawaguchi T, Takahashi A, Hosono N, Kubo M, Tsunoda T, Kamatani N, Kumada H. A genome-wide association study identifies variants in the HLA-DP locus associated with chronic hepatitis B in Asians. Nat Genet. 2009;41:591-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 431] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 98. | Hu L, Zhai X, Liu J, Chu M, Pan S, Jiang J, Zhang Y, Wang H, Chen J, Shen H. Genetic variants in human leukocyte antigen/DP-DQ influence both hepatitis B virus clearance and hepatocellular carcinoma development. Hepatology. 2012;55:1426-1431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 144] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 99. | Mbarek H, Ochi H, Urabe Y, Kumar V, Kubo M, Hosono N, Takahashi A, Kamatani Y, Miki D, Abe H. A genome-wide association study of chronic hepatitis B identified novel risk locus in a Japanese population. Hum Mol Genet. 2011;20:3884-3892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 189] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 100. | Nishida N, Sawai H, Matsuura K, Sugiyama M, Ahn SH, Park JY, Hige S, Kang JH, Suzuki K, Kurosaki M. Genome-wide association study confirming association of HLA-DP with protection against chronic hepatitis B and viral clearance in Japanese and Korean. PLoS One. 2012;7:e39175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 130] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 101. | Wasityastuti W, Yano Y, Ratnasari N, Triyono T, Triwikatmani C, Indrarti F, Heriyanto DS, Yamani LN, Liang Y, Utsumi T. Protective effects of HLA-DPA1/DPB1 variants against Hepatitis B virus infection in an Indonesian population. Infect Genet Evol. 2016;41:177-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 102. | Posuwan N, Payungporn S, Tangkijvanich P, Ogawa S, Murakami S, Iijima S, Matsuura K, Shinkai N, Watanabe T, Poovorawan Y. Genetic association of human leukocyte antigens with chronicity or resolution of hepatitis B infection in thai population. PLoS One. 2014;9:e86007. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 103. | Liao Y, Cai B, Li Y, Chen J, Tao C, Huang H, Wang L. Association of HLA-DP/DQ and STAT4 polymorphisms with HBV infection outcomes and a mini meta-analysis. PLoS One. 2014;9:e111677. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 104. | Chang SW, Fann CS, Su WH, Wang YC, Weng CC, Yu CJ, Hsu CL, Hsieh AR, Chien RN, Chu CM. A genome-wide association study on chronic HBV infection and its clinical progression in male Han-Taiwanese. PLoS One. 2014;9:e99724. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 105. | Zhang XL, Ni XC, Jia JH, Dong JH, Yu FX, Ma N, Liu XH, Li M, Liu DW. Association of the rs3077 and rs9277535 polymorphisms in HLA-DP with hepatitis B virus infection and spontaneous clearance: a meta-analysis. Scand J Gastroenterol. 2013;48:736-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 106. | Png E, Thalamuthu A, Ong RT, Snippe H, Boland GJ, Seielstad M. A genome-wide association study of hepatitis B vaccine response in an Indonesian population reveals multiple independent risk variants in the HLA region. Hum Mol Genet. 2011;20:3893-3898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 99] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 107. | Roh EY, Yoon JH, In JW, Lee N, Shin S, Song EY. Association of HLA-DP variants with the responsiveness to Hepatitis B virus vaccination in Korean Infants. Vaccine. 2016;34:2602-2607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 108. | Wen J, Song C, Jiang D, Jin T, Dai J, Zhu L, An J, Liu Y, Ma S, Qin N. Hepatitis B virus genotype, mutations, human leukocyte antigen polymorphisms and their interactions in hepatocellular carcinoma: a multi-centre case-control study. Sci Rep. 2015;5:16489. [PubMed] |
| 109. | Li S, Qian J, Yang Y, Zhao W, Dai J, Bei JX, Foo JN, McLaren PJ, Li Z, Yang J. GWAS identifies novel susceptibility loci on 6p21.32 and 21q21.3 for hepatocellular carcinoma in chronic hepatitis B virus carriers. PLoS Genet. 2012;8:e1002791. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 143] [Cited by in RCA: 171] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 110. | Davila S, Froeling FE, Tan A, Bonnard C, Boland GJ, Snippe H, Hibberd ML, Seielstad M. New genetic associations detected in a host response study to hepatitis B vaccine. Genes Immun. 2010;11:232-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 70] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 111. | Milich DR, Leroux-Roels GG. Immunogenetics of the response to HBsAg vaccination. Autoimmun Rev. 2003;2:248-257. [PubMed] |
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Indonesia
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Chemin IA, Dugum M, Netter HJ, Sporea I, Toyoda T S- Editor: Yu J L- Editor: A E- Editor: Ma S