Published online Aug 28, 2016. doi: 10.3748/wjg.v22.i32.7252
Peer-review started: April 9, 2016
First decision: May 12, 2016
Revised: June 7, 2016
Accepted: July 21, 2016
Article in press: July 21, 2016
Published online: August 28, 2016
Processing time: 137 Days and 22.4 Hours
Hepatocellular carcinoma (HCC) is one of the deadliest cancers in the world and is associated with a high risk of recurrence. The development of a wide range of new therapies is therefore essential. In this study, from the perspective of supportive therapy for the prevention of HCC recurrence and preservation of liver function in HCC patients, we surveyed a variety of different therapeutic agents. We show that branched chain amino acids (BCAA) supplementation and late evening snack with BCAA, strategies that address issues of protein-energy malnutrition, are important for liver cirrhotic patients with HCC. For chemoprevention of HCC recurrence, we show that viral control after radical treatment is important. We also reviewed the therapeutic potential of antiviral drugs, sorafenib, peretinoin, iron chelators. Sorafenib is a kinase inhibitor and a standard therapy in the treatment of advanced HCC. Peretinoin is a vitamin A-like molecule that targets the retinoid nuclear receptor to induce apoptosis and inhibit tumor growth in HCC cells. Iron chelators, such as deferoxamine and deferasirox, act to prevent cancer cell growth. These chelators may have potential as combination therapies in conjunction with peretinoin. Finally, we review the potential inhibitory effect of bone marrow cells on hepatocarcinogenesis.
Core tip: Hepatocellular carcinoma (HCC) is one of the deadliest cancers in the world and is associated with a high risk of recurrence. Because liver function worsens upon repeated treatment for HCC recurrence, therapies that preserve liver function are essential. Here, we survey a variety of different therapeutic agents and then review the current status and prospects for prevention of HCC recurrence, particularly from the perspective of supportive therapy to preserve liver function. The agents included branched-chain amino acids (BCAA) supplementation, late evening snacking with BCAA, antiviral drugs, sorafenib, peretinoin, iron chelators, and bone marrow cells.
- Citation: Takami T, Yamasaki T, Saeki I, Matsumoto T, Suehiro Y, Sakaida I. Supportive therapies for prevention of hepatocellular carcinoma recurrence and preservation of liver function. World J Gastroenterol 2016; 22(32): 7252-7263
- URL: https://www.wjgnet.com/1007-9327/full/v22/i32/7252.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i32.7252
Hepatocellular carcinoma (HCC) is the fifth most common cancer and the second leading cause of deaths due to cancer in the world[1]. The prognosis of HCC has improved recently as a result of progress in a variety of therapies. These therapies include surgical resection, percutaneous-ethanol-injection (PEI), radiofrequency ablation (RFA), trans-arterial chemoembolization (TACE), administration of the drug, sorafenib, and liver transplantation[2-7]. The greatest problem with HCC is a high risk of recurrence, even when radical treatment is conducted. Recurrent HCC results in a fatal outcome for many patients with hepatic dysfunction. Antiviral therapies, such as nucleic acid analogs and interferon (IFN), have the potential to inhibit HCC recurrence after radical treatment of patients who have hepatitis B virus (HBV)- or hepatitis C virus (HCV)-related liver diseases[8,9]. Recently, however, the occurrence of both HBs antigen-negative and HCV antibody-negative HCC has actually increased in Japan as the development of antiviral agent and IFN treatments has progressed[10,11]. It is therefore necessary to develop other therapies to prevent HCC recurrence. Because liver function worsens upon repeated treatment for HCC recurrence, therapies that preserve liver function are essential. In this paper, we review the current status and prospects for prevention of HCC recurrence, particularly from the perspective of supportive therapy to preserve liver function.
First, we review the use of branched chain amino acids and late evening snack (LES), particularly for HCC patients with liver cirrhosis. These treatments are generally intended to address issues of protein-energy malnutrition (PEM) in these patients. We then review a range of chemoprevention options. These include antiviral therapies (nucleic acid analogs, IFN) for the treatment of hepatitis virus-related HCC, as well as treatment options such sorafenib, peretinoin, and iron chelators. Finally, we review the potential inhibitory effect of bone marrow cells (BMCs) on hepatocarcinogenesis.
Most patients with HCC have liver cirrhosis. Generally, liver cirrhotic patients suffer from PEM. These patients commonly exhibit decreased nutrient intake, hyper-metabolism, and increased branched-chain amino acids (BCAA) consumption associated with ammonia metabolism in the skeletal muscle, leading to a decrease in plasma BCAA levels[12-14]. This decrease in plasma BCAA levels reduces protein synthesis in the liver and causes proteolysis in the muscle, which leads to edema and ascites with hypoalbuminemia and decrease in skeletal muscle mass.
BCAA granules consist of leucine, isoleucine, and valine, which are essential amino acids in humans. The Japanese Nutritional Study Group recommends administration of BCAA to liver cirrhotic patients who have serum albumin levels of 3.5 g/dL or less, a Fisher ratio of 1.8 or less, and a BCAA-to-tyrosine ratio of 3.5 or less[15]. In contrast, in the guidelines set by the American Society for Parenteral and Enteral Nutrition (ASPEN) and the European Society for Clinical Nutrition and Metabolism (ESPEN), oral administration of BCAA is recommended only in patients with liver cirrhosis who have hepatic encephalopathy[16,17].
A wide variety of effects of BCAA on chronic liver disease has been confirmed in previous fundamental research and clinical studies. Improvement of insulin resistance[18,19], improvement of hypoalbuminemia and reduction of oxidative stress[20,21], activation of immune function[22,23], promotion of liver regeneration[24,25], and inhibitory effects on hepatocarcinogenesis[26-28] have been reported. With regards to the mechanism of action, it has been reported that BCAA activates the insulin signal cascade through upregulation of phosphatidylinositol 3-kinase[29]. This serves to decrease circulating insulin levels and reduce the expression of insulin-like growth factors (IGF)-1 and IGF-2 as well as IGF-1 receptors to inhibit the IGF/IGF-1 receptor axis[29].
Given the biological properties referred to above, the following effects of BCAA on HCC can be anticipated: (1) inhibition of hepatocarcinogenesis associated with chronic liver disease; (2) prevention of a reduction in residual liver function caused by HCC treatment; and (3) prevention of recurrence after HCC treatment.
Muto et al[26] performed a multicenter, randomized controlled trial (RCT) that included 622 decompensated liver cirrhotic patients. They reported that oral administration of BCAA inhibited hepatocarcinogenesis in obese patients [≥ body mass index (BMI) 25 kg/m2] who had HCV-related liver cirrhosis.
Currently, there are many options for the treatment of HCC. Appropriate treatments such as surgical resection, PEI, RFA, TACE, hepatic arterial infusion chemotherapy (HAIC), molecular target-based therapy by sorafenib, and radiation therapy may be chosen depending on residual liver function and tumor stage. Improvements in prognosis have been observed using these approaches[1,30-40]. However, because recurrence of HCC can occur in liver cirrhosis patients after radical treatment, it is important to maintain residual liver function and to seek ways to inhibit the recurrence.
To our knowledge, there have been eight reports on the efficacy of BCAA granules in patients being treated for HCC; the HCC treatment in these reports was surgical operation (2 reports), RFA (3 reports), TACE (1 report), and molecular target-based therapy (2 reports). In terms of study design, both cohort studies (5 reports) and RCTs (3 reports) were available (Table 1)[41-48].
| Ref. | Group | Patient number | Therapy for HCC | Male/Female | Age (yr) | Child-Pugh A/B/C | Maximum tumor size (mm) | Study design |
| Togo et al[41] | BCAA | 21 | Surgery | 17/4 | 66.5 ± 4.5 | 15/7/0 | ND | RCT |
| 2005 | control | 22 | 17/5 | 64.3 ± 9.1 | 17/5/0 | ND | ||
| Ichikawa et al[42] | BCAA | 26 | Surgery | 18/8 | 64.7 ± 9.8 | 21/5/0 | ND | RCT |
| 2012 | control | 30 | 20/10 | 64.5 ± 11.4 | 25/5/0 | ND | ||
| Nishikawa et al[43] | BCAA | 115 | RFA | 64/51 | 69.3 ± 9.4 | 83/30/2 | 19.5 ± 6.0 | cohort |
| 2013 | control | 141 | 85/58 | 70.9 ± 7.8 | 88/52/1 | 19.6 ± 6.6 | ||
| Yoshiji et al[44] | BCAA | 51 | RFA | 32/19 | 63.6 ± 15.3 | 41/10/0 | ND | RCT |
| 2013 | control | 42 | 25/17 | 62.2 ± 14.8 | 33/9/0 | ND | ||
| Saito et al[45] | BCAA | 13 | RFA | 8/5 | 73.4 ± 2.2 | 83/30/2 | ND | cohort |
| 2014 | control | 27 | 16/11 | 70.0 ± 1.9 | 88/52/1 | ND | ||
| Nishikawa et al[46] | BCAA | 40 | TACE | 27/13 | 69.9 ± 8.8 | 6.4 ± 0.4 | 33.4 ± 16.7 | cohort |
| 2012 | control | 59 | 32/27 | 73.2 ± 10.1 | 5.4 ± 0.1 | 35.9 ± 14.7 | ||
| (score) | ||||||||
| Takeda et al[47] | BCAA | 34 | Sorafenib | 27/7 | 72 (55-88) | 16/18/0 | ND | cohort |
| 2014 | control | 44 | 37/7 | 68 (46-89) | 30/14/0 | ND | ||
| Imanaka et al[48] | BCAA | 55 | Sorafenib | 45/10 | 72.2 ± 7.8/73.1 ± 6.4 | 37/18/0 | ND | cohort |
| 2015 | control | 201 | 167/34 | 72.4 ± 8.8/67.2 ± 13.0 | 179/22/0 | ND | ||
| (Child-Pugh A/B) |
Early recovery of protein metabolism after hepatectomy can be achieved by administering BCAA granules[41]. Ichikawa et al[42] also reported that BCAA granules were effective in inhibiting early relapse after hepatectomy.
Reduction in the cumulative relapse rate and improvement in survival rate were observed after long-term oral administration of BCAA granules to patients who had received RFA[43-45]. Nishikawa et al[43] performed a retrospective study of 256 patients who had received RFA and had serum albumin levels of 3.5 g/dL or less. They reported improvements in overall survival (OS) and recurrence-free survival after oral administration of BCAA granules. The study also reported an improvement in OS in patients with HCC who suffered from obesity (≥ BMI 25 kg/m2) and diabetes. Yoshiji et al[44] performed a RCT involving 93 patients who had received RFA and reported improvement of insulin resistance after oral administration of BCAA granules. They also found a decrease in levels of the plasma soluble form of vascular endothelial growth factor receptor 2 (VEGFR2) and a reduction in the cumulative relapse rate after RFA in liver cancer patients who had insulin resistance (≥ homeostasis model assessment for insulin resistance of 2.5) Thus, BCAA can be considered to inhibit recurrence of HCC and to improve survival rates through improved insulin resistance and an anti-angiogenic effect. This may be the same mechanism by which BCAA inhibits hepatocarcinogenesis in liver cirrhotic patients suffering from obesity.
For unresectable HCC, it is common to perform TACE repeatedly, but it is necessary to pay attention to the liver function after TACE. In this respect, it has been reported that administration of BCAA granules prior to TACE inhibited reduction of serum albumin levels measured three and six months after TACE, and helped maintain residual liver function in patients with Child-Pugh A/B[46].
In molecular target-based therapy using sorafenib for treatment of unresectable HCC, it is important to maintain residual liver function, as any reduction could lead to discontinuation of treatment and a poor prognosis. In patients with Child-Pugh A (but not in patients with Child-Pugh B), administration of BCAA granules when sorafenib is used inhibits reduction of serum albumin levels. The dosing period of sorafenib and the survival period are also prolonged[47,48].
These observations suggest that BCAA is effective in inhibiting hepatocarcinogenesis, maintaining residual liver function after HCC treatment, and preventing recurrence of HCC in patients with chronic liver disease. Early administration of BCAA granules is expected to be useful for patients, with or without HCC, whose plasma BCAA levels have decreased. However, many of the findings above are based on reports from retrospective studies. Further evaluation of data from RCTs will be required in the future to corroborate these results.
Patients with liver cirrhosis enter a nocturnal starvation state, LES is recommended in the guidelines of both the ASPEN and ESPEN[16,17]. LES with BCAA nutrients improves serum albumin and energy metabolism more than LES with ordinary food; LES with BCAA nutrients is therefore typical[49]. Therefore, BCAA nutrients has been used in a LES. We have reported previously that LES with BCAA nutrients improve energy malnutrition, amino acid imbalance, and glucose intolerance in liver cirrhotic patients[50-52]. However, at present, there are no guidelines for nutrition care in the treatment of HCC[53-55].
To our knowledge, there are as few as five reports on the effects of LES with BCAA nutrients on liver cirrhotic patients with HCC; the HCC treatment in the studies included surgical resection (1 report), RFA (2 reports), TACE (1 report), and HAIC (1 report). The study designs included cohort studies (2 reports) and RCTs (3 reports) (Table 2)[56-60].
| Ref. | Group | Patient number | Therapy for HCC | Male/Female | Age (yr) | Child-Pugh A/B/C | Maximum tumor size (mm) | Study design |
| Okabayashi et al[56] | LES-BCAA | 40 | surgery | 29/11 | 65.7 ± 8.6 | 33/7/0 | ND | cohort |
| 2008 | control | 72 | 55/17 | 68.3 ± 8.1 | 62/10/0 | ND | ||
| Kuroda et al[57] | LES-BCAA | 20 | RFA | 13/7 | 65.6 ± 7.0 | 8/11/1 | 20.2 (median) | cohort |
| 2010 | control | 15 | 9/6 | 66.0 ± 8.1 | 6/8/1 | 19.8 (median) | ||
| Morihara et al[58] | LES-BCAA | 10 | RFA | 8/2 | 73.5 ± 8.5 | 9/1/0 | 20.0 ± 10.7 | RCT |
| 2012 | Morning-BCAA | 10 | 8/2 | 66.9 ± 9.7 | 7/3/0 | 24.3 ± 7.7 | ||
| control | 10 | 7/3 | 69.3 ± 8.0 | 7/3/0 | 24.4 ± 7.7 | |||
| Takeshita et al[59] | LES-BCAA | 28 | TACE | 19/9 | 69.1 ± 8.231 | 6.107 ± 1.315 (score) | ND | RCT |
| 2009 | control | 28 | 21/7 | 70.6 ± 9.745 | 5.53 ± 0.516 (score) | ND | ||
| Harima et al[60] | LES-BCAA | 13 | HAIC | 11/2 | 64.5 ± 9.5 | 6/7/0 | 77.7 ± 50.5 | RCT |
| 2010 | control | 10 | 8/2 | 66.4 ± 12.8 | 6/4/0 | 88.0 ± 39.7 |
LES with BCAA nutrients prior to surgical resection was shown to significantly improve postoperative liver function, significantly reduce postoperative complications, and significantly shorten hospitalization[56]. In patients treated with RFA, LES with BCAA nutrients improved liver function[57,58], nutritional status, and quality of life (QOL)[57]. Takeshita et al[59] reported that LES with BCAA nutrients for two weeks caused a reduction in decreased liver function in patients treated with TACE.
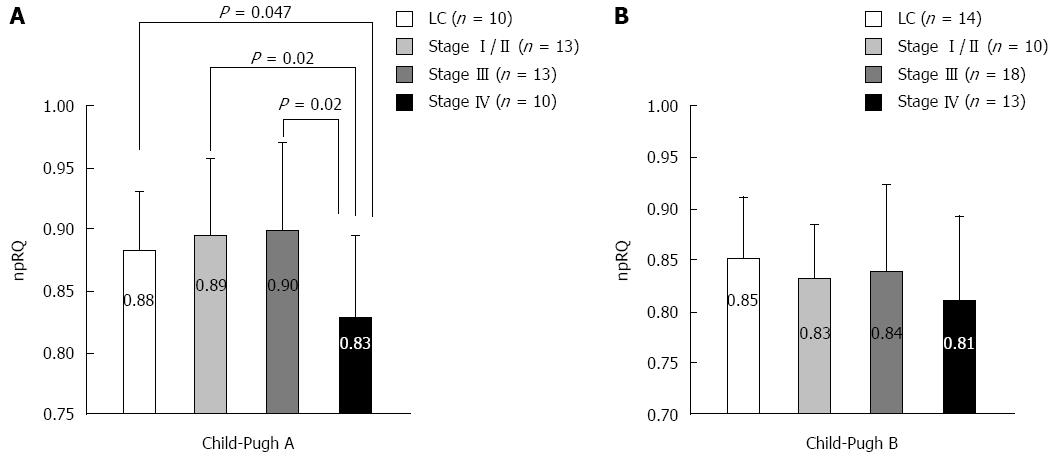
We measured energy metabolism using indirect calorimetry (Figure 1) in liver cirrhosis patients without HCC and in liver cirrhosis patients with HCC at different stages as classified by the Liver Cancer Study Group of Japan criteria. In the Child-Pugh A score, the non-protein respiratory quotient (npRQ) significantly decreased in patients with advanced HCC at stage IV[60]. In the Child-Pugh B score, nutritional status was generally poor, and npRQ decreased in all patient groups; there were no significant differences between the groups (unpublished data). Therefore, in patients with advanced HCC, LES with BCAA nutrients may be necessary depending on the Child-Pugh A score. In fact, LES with BCAA nutrients (LES group) improved the energy metabolism in advanced HCC patients undergoing HAIC compared with ordinary food (control group)[60]. In the 75-g oral glucose tolerance test (75-g OGTT), the area under the concentration curve for glucose (AUC glucose) showed an improvement in the LES group (P = 0.055). No significant difference in survival was identified between the groups (P = 0.667). However, the survival time of the patients whose therapeutic effect of HAIC was stable disease (SD) or progressive disease (PD) tended to be longer in the LES group (P = 0.156) than in the control group. For patients with SD or PD, a significant improvement in npRQ was observed in the LES group, whereas significant reductions in cholinesterase and natural killer cell activity were observed in the control group[61].
Thus, we consider that nutritional therapy tailored to tumor stage and residual liver capacity is required for HCC patients. However, further investigations are necessary because the previous reports examined only a small number of HCC patients.
In HCC patients, the recurrence rate is approximately 50% even after radical treatment[62,63]. Notably, it is approximately 70% in patients with HCV-related HCC[64]. Therefore, various studies on the inhibition of recurrence after radical treatment have been conducted. However, prevention of recurrence should be addressed based on the causative diseases of HCC. This chapter describes this issue with respect to antiviral treatment for viral (e.g., HBV and HCV) hepatitis and other cancer inhibitors.
For HBV- and/or HCV-related HCC, it has been suggested that antiviral treatment for inhibition of HCC recurrence is best administered after radical treatment rather than before. The efficacy of IFNs as antiviral treatments in viral-related HCC has been reported in six meta-analyses[65-70]. However, these meta-analyses had limitations. In the meta-analyses of Zhang et al[65] and Breitenstein et al[70], HBV and HCV patients were examined together and only IFN-α was evaluated. In the study of Miao et al[68], HBV patients and HCV patients were also examined together and various IFNs were assessed. In the study by Singal et al[66], only HCV patients were examined, a cohort study was also included, and various IFNs (IFN-α, α-2b, PEGylated IFN, and IFN-β) were evaluated. In the study by Shen et al[67], both HBV and HCV patients were examined together, a cohort study was also included, and various IFNs were evaluated. In the study by Miyake et al[69], only patients with HCV-related HCC were examined and tumor factors were limited. In any case, as an overall conclusion, it was reported that IFN-α may inhibit postoperative recurrence within the Milan criteria (a generally accepted set of criteria used to assess suitability of patients with cirrhosis and hepatocellular carcinoma for liver transplantation.). Sustained virological response was particularly associated with inhibition of recurrence.
Lee et al[71] reported that scores calculated from age, sex, alanine aminotransferase, HBe antigen, content of HBV-DNA, and HBV genotype were informative as predictors of cancer associated with HBV. There are many reports on the cancer inhibition effect of IFN and nucleic acid analogs administered to patients with HBV-related chronic liver disease[69,72]. Hosaka et al[73] also conducted a propensity score matching analysis after classifying patients with HBV-related chronic liver disease into an entecavir (a deoxyguanosine analog)-therapy group and a non-therapy group. They reported that the 5-year cancer incidence rate was significantly reduced (3.7% vs 13.7%; HR = 0.37, P = 0.030) in the therapy group. Sohn et al[74] reported that higher amounts of HBV-DNA were associated with higher risk of early recurrence and that a higher amount of HBs antigen was associated with higher risk of late recurrence. These data strongly support the value of antiviral therapy after HCC treatment, and meta-analyses have shown that the use of nucleic acid analogs after HCC treatment can help inhibit HCC recurrence and improve prognosis[75,76]. Although results from an RCT suggested that IFN can inhibit HCC, no firm conclusion was reached, and further investigation will be required[76]. Recently, Lee et al[77] reported on the beneficial effect of a nucleic acid analog on inhibition of HCC recurrence after radical treatment with RFA.
Thus, it is suggested that viral control after radical treatment is important for inhibiting HCC recurrence in patients with HBV-related HCC.
The effect of IFN on cancer inhibition in patients with HCV-related chronic liver disease has been reported in many previous studies[78-80]. Miyake et al[81] reported in a meta-analysis that IFN can decrease the carcinogenic risk. In addition, many studies have mentioned the value of IFN even after radical treatment for HCV-related HCC. In these studies, the effect of IFN on prognosis was reported; a trend in inhibiting recurrence was noted but the results did not reach statistical significance[82,83]. However, several studies have reported that IFN-α after radical treatment for HCC inhibited later successive recurrences after a second recurrence[64,84,85]. In addition, the effect of low dosages of IFN in long-term therapy on inhibition of recurrence has been reported. Thus, Kudo et al[86] reported that a small amount of IFN-α2b inhibited the first, second, and third recurrence after radical treatment with RFA and contributed to survival (HR = 0.21)[87]. It has also been reported that IFN-β inhibited recurrence after radical treatment for HCV-related HCC (P = 0.0004).
Several direct-acting antivirals (DAAs) have emerged recently as treatments for the safe elimination of viral infections, even in cirrhotic patients. Recently, Reig et al[88] administered DAAs to the patients after curative treatment of HCC and investigated subsequent recurrence rate. Although they reported high rate of recurrence after the viral elimination by DAAs, it is a small cohort retrospective study and the reliable opinion is not obtained. In addition, Pol S conducted a multicenter prospective study, and he concluded that there was no evidence that DAAs promote an HCC recurrence[89]. It is still needed future analysis.
This section describes the current status and prospects of these three agents in the treatment of advanced HCC.
Sorafenib is a standard therapeutic drug for advanced HCC that was developed as a C-Raf and B-Raf serine/threonine kinase activity inhibitor[7]. It affects both the Raf/MEK/ERK signaling pathway, which influences cell proliferation, and VEGFR, which is associated with neovascularization. Sorafenib is also known to inhibit the tyrosine kinase activity of the platelet-derived growth factor receptor[7].
In 2008, international cooperative group clinical trials involving patients with advanced HCC demonstrated that sorafenib offered a significant prolongation of OS when compared with placebo[7]. To test the hypothesis that sorafenib could prevent HCC recurrence, a RCT targeting patients who had received HCC radical curative treatment (hepatectomy/RFA/PEI) was conducted[88]. This trial (termed the STORM trial) comprised two groups: one that received sorafenib at 800 mg/d and the placebo group. Progression-free survival was set as the primary endpoint. However, sorafenib offered no significant prolongation effect. A major issue in the STORM trial was that long-term oral administration of sorafenib was not possible because of the high incidence of adverse side effects associated with this treatment[90].
Peretinoin is an orally administered acyclic retinoid with a vitamin A-like structure that targets the retinoid nuclear receptor[91]. It induces apoptosis and inhibits tumor growth in HCC cells[91]. Recently, it has been reported that acyclic retinoids increase the expression of intra-nuclear transglutaminase-2 in JHH-7 cells and induce apoptosis in HCC[92]. Muto et al[93] performed a small-scale RCT to determine the effect of peretinoin on inhibition of HCC recurrence after radical treatment (hepatectomy/PEI). They reported that pereretinoin inhibited the second recurrence (adjusted relative risk 0.31; 95%CI: 0.12-0.78)[93]. Based on these results, Okita et al[94,95]. performed a randomized double-blind placebo-controlled study in patients after radical treatment for HCV-related HCC (operation/RFA). Recurrence was significantly inhibited in the peretinoin (600 mg/d) group (P = 0.023; multiplicity-adjusted P = 0.048)[94,95]. A double-blind, placebo-controlled, multicenter, randomized, parallel intergroup trial is currently under way (NCT01640808) to verify these findings.
Iron is necessary for oxygen transport, energy production, and cell metabolism and growth[96,97]. It is especially important in cells with active growth, including cancer cells[98]. A clinical study on hepatocarcinogenesis and iron overload has been conducted; Kato et al[99] reported that reduction of iron levels through phlebotomy therapy might significantly inhibit hepatocarcinogenesis. Iron metabolism control may thus become a target for cancer inhibition. An antitumor effect of deferoxamine (DFO) in HCC patients has been reported[100,101]. We have also reported on the antitumor effect of arterial DFO administration in patients with advanced HCC[102]. In a fundamental experiment, we reported that DFO inhibited liver fibrosis and pre-neoplastic lesions in a rat model of hepato-carcinogenesis[103]. Deferasirox (DFX) has also recently emerged as an orally administered iron chelator, and a strong antiproliferative effect associated with DFX has been reported in vitro. The effect of DFX on cancer inhibition in combination with losartan has also been reported in an in vivo study[104]. By combining DFX and sorafenib, we confirmed not only a therapeutic effect against liver fibrosis and cancer but also a reduction in adverse side effects that were associated with treatment with sorafenib alone. As noted above, long-term administration of sorafenib alone was not possible in the STORM trial because of the high incidence of adverse side effects. In this respect, combined treatment of DFX and sorafenib may prove to be a new therapy to prevent recurrence of HCC.
We have reported that infusion of BMCs decreased livers fibrosis and improved liver function in mice[105,106]. Based on these results, we initiated an autologous BMC infusion therapy for liver cirrhosis in 2003. The safety and efficacy of this therapy have been confirmed in clinical studies[107-110]. Short-term results to date indicate no serious complications associated with reproduction therapy using BMCs. However, longer-term evaluation, particularly evaluation of the potential for hepatocarcinogenesis, is still required.
Ishikawa et al[111] generated a rodent model of chemical carcinogenesis by injecting mice with diethylnitrosamine and phenobarbital. They then infused BMCs into these mice. No tumorigenesis associated with the BMC infusion was observed, and the authors reported that the potential for carcinogenesis was low. We examined the influence of BMC infusion on hepatocarcinogenesis using a highly oncogenic cirrhotic murine model. The influence of BMCs on hepatocarcinogenesis was evaluated histologically. The number of liver tumors was smaller and liver fibrosis was inhibited in mice treated with repeated doses of BMCs[112]. This confirmed that BMC infusion contributed to inhibition of hepatocarcinogenesis. Most of the BMCs that engrafted into the damaged liver expressed superoxide dismutase 3, which is an antioxidant protein[112]. It is therefore considered that BMCs inhibit hepatocarcinogenesis by regulating redox homeostasis.
Although it is known that bone marrow-derived mesenchymal stem cells (MSCs) migrate to tumor tissues, their role is mostly unclear. As MSCs secrete a variety of growth factors, there is concern about their effects on tumor progression[113]. In previous studies, it has been reported that growth, invasion, and metastasis of lung cancer and neovascularization are promoted by MSC secretion of factors such as IL-6, VEGF, and IGF-1[114]; that tumor cells cause epithelial-mesenchymal transition[115]; and that tumors are activated as MSCs are differentiated into carcinoma-associated fibroblasts comprising the tumor microenvironment[116].
Conversely, it has also been reported that MSCs inhibit tumor proliferation by controlling WNT signaling and PARP cleavage of tumor cells, thus promoting apoptosis[117,118]. Furthermore, in a clinical study, carcinogenesis was not observed in follow-up at 11 years and five months after cultured MSC was used to reproduce cartilage[119].
Thus, we consider that the potential for carcinogenesis associated with BMCs is low, and that these cells are likely play a minimal role in any newly occurring carcinogenesis. However, the potential for tumor formation through neovascularization or secretion by BMCs of various humoral factors also cannot be neglected. Therefore, it is important to generate further relevant data to determine whether or not tumorigenesis potentially associated with regenerative medicine using BMCs is a realistic concern, or whether BMCs can be developed as a safe and efficacious therapy.
We noted that BCAA and LES with BCAA, which address nutritional issues, were important for liver cirrhotic patients with HCC. We also emphasized that antiviral agents, including nucleic acid analogs and IFNs, were effective in the treatment of HCC. In addition, we described the potential of peretinoin, progress in the development of iron chelators, and the promise of BMCs to suppress hepato-carcinogenesis. We showed results on some positive trials supporting the prevention of HCC-recurrence and the preservation of liver function. Therefore, by generating further data and evidence, it is expected that new HCC strategies can be developed by combining the therapies above alongside treatments with anticancer drugs.
| 1. | GLOBOCAN 2012: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012. Accessed March 12, 2015. Available from: http://globocan.iarc.fr/. |
| 2. | El-Serag HB, Marrero JA, Rudolph L, Reddy KR. Diagnosis and treatment of hepatocellular carcinoma. Gastroenterology. 2008;134:1752-1763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 768] [Cited by in RCA: 834] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 3. | Ebara M, Okabe S, Kita K, Sugiura N, Fukuda H, Yoshikawa M, Kondo F, Saisho H. Percutaneous ethanol injection for small hepatocellular carcinoma: therapeutic efficacy based on 20-year observation. J Hepatol. 2005;43:458-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 127] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 4. | Matsui O, Kadoya M, Yoshikawa J, Gabata T, Arai K, Demachi H, Miyayama S, Takashima T, Unoura M, Kogayashi K. Small hepatocellular carcinoma: treatment with subsegmental transcatheter arterial embolization. Radiology. 1993;188:79-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 312] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 5. | Yamasaki T, Kurokawa F, Shirahashi H, Kusano N, Hironaka K, Okita K. Percutaneous radiofrequency ablation therapy with combined angiography and computed tomography assistance for patients with hepatocellular carcinoma. Cancer. 2001;91:1342-1348. [PubMed] |
| 6. | Todo S, Furukawa H; Japanese Study Group on Organ Transplantation. Living donor liver transplantation for adult patients with hepatocellular carcinoma: experience in Japan. Ann Surg. 2004;240:451-459; discussion 459-461. [PubMed] |
| 7. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9016] [Cited by in RCA: 10523] [Article Influence: 584.6] [Reference Citation Analysis (9)] |
| 8. | Tan ZM, Sun BC. Effects of antiviral therapy on preventing liver tumorigenesis and hepatocellular carcinoma recurrence. World J Gastroenterol. 2013;19:8895-8901. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Sun P, Yang X, He RQ, Hu QG, Song ZF, Xiong J, Zheng QC. Antiviral therapy after curative treatment of hepatitis B/C virus-related hepatocellular carcinoma: A systematic review of randomized trials. Hepatol Res. 2014;44:259-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Nagaoki Y, Hyogo H, Aikata H, Tanaka M, Naeshiro N, Nakahara T, Honda Y, Miyaki D, Kawaoka T, Takaki S. Recent trend of clinical features in patients with hepatocellular carcinoma. Hepatol Res. 2012;42:368-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 75] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 11. | Tateishi R, Okanoue T, Fujiwara N, Okita K, Kiyosawa K, Omata M, Kumada H, Hayashi N, Koike K. Clinical characteristics, treatment, and prognosis of non-B, non-C hepatocellular carcinoma: a large retrospective multicenter cohort study. J Gastroenterol. 2015;50:350-360. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 135] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 12. | Lautz HU, Selberg O, Körber J, Bürger M, Müller MJ. Protein-calorie malnutrition in liver cirrhosis. Clin Investig. 1992;70:478-486. [PubMed] |
| 13. | Tajika M, Kato M, Mohri H, Miwa Y, Kato T, Ohnishi H, Moriwaki H. Prognostic value of energy metabolism in patients with viral liver cirrhosis. Nutrition. 2002;18:229-234. [PubMed] |
| 14. | Müller MJ, Böttcher J, Selberg O, Weselmann S, Böker KH, Schwarze M, von zur Mühlen A, Manns MP. Hypermetabolism in clinically stable patients with liver cirrhosis. Am J Clin Nutr. 1999;69:1194-1201. [PubMed] |
| 15. | Suzuki K, Endo R, Kohgo Y, Ohtake T, Ueno Y, Kato A, Suzuki K, Shiraki R, Moriwaki H, Habu D. Guidelines on nutritional management in Japanese patients with liver cirrhosis from the perspective of preventing hepatocellular carcinoma. Hepatol Res. 2012;42:621-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 16. | ASPEN Board of Directors and the Clinical Guidelines Task Force. Guidelines for the use of parenteral and enteral nutrition in adult and pediatric patients. JPEN J Parenter Enteral Nutr. 2002;26:1SA-138SA. [PubMed] |
| 17. | Plauth M, Merli M, Kondrup J, Weimann A, Ferenci P, Müller MJ. ESPEN guidelines for nutrition in liver disease and transplantation. Clin Nutr. 1997;16:43-55. [PubMed] |
| 18. | Miyake T, Abe M, Furukawa S, Tokumoto Y, Toshimitsu K, Ueda T, Yamamoto S, Hirooka M, Kumagi T, Hiasa Y. Long-term branched-chain amino acid supplementation improves glucose tolerance in patients with nonalcoholic steatohepatitis-related cirrhosis. Intern Med. 2012;51:2151-2155. [PubMed] |
| 19. | Yoshiji H, Noguchi R, Kitade M, Kaji K, Ikenaka Y, Namisaki T, Yoshii J, Yanase K, Yamazaki M, Tsujimoto T. Branched-chain amino acids suppress insulin-resistance-based hepatocarcinogenesis in obese diabetic rats. J Gastroenterol. 2009;44:483-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 51] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 20. | Ohno T, Tanaka Y, Sugauchi F, Orito E, Hasegawa I, Nukaya H, Kato A, Matunaga S, Endo M, Tanaka Y. Suppressive effect of oral administration of branched-chain amino acid granules on oxidative stress and inflammation in HCV-positive patients with liver cirrhosis. Hepatol Res. 2008;38:683-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 21. | Iwasa M, Kobayashi Y, Mifuji-Moroka R, Hara N, Miyachi H, Sugimoto R, Tanaka H, Fujita N, Gabazza EC, Takei Y. Branched-chain amino acid supplementation reduces oxidative stress and prolongs survival in rats with advanced liver cirrhosis. PLoS One. 2013;8:e70309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 22. | Nakamura I. Impairment of innate immune responses in cirrhotic patients and treatment by branched-chain amino acids. World J Gastroenterol. 2014;20:7298-7305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 23. | Kakazu E, Ueno Y, Kondo Y, Fukushima K, Shiina M, Inoue J, Tamai K, Ninomiya M, Shimosegawa T. Branched chain amino acids enhance the maturation and function of myeloid dendritic cells ex vivo in patients with advanced cirrhosis. Hepatology. 2009;50:1936-1945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 58] [Article Influence: 3.4] [Reference Citation Analysis (1)] |
| 24. | Beppu T, Nitta H, Hayashi H, Imai K, Okabe H, Nakagawa S, Hashimoto D, Chikamoto A, Ishiko T, Yoshida M. Effect of branched-chain amino acid supplementation on functional liver regeneration in patients undergoing portal vein embolization and sequential hepatectomy: a randomized controlled trial. J Gastroenterol. 2015;50:1197-1205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 25. | Kim SJ, Kim DG, Lee MD. Effects of branched-chain amino acid infusions on liver regeneration and plasma amino acid patterns in partially hepatectomized rats. Hepatogastroenterology. 2011;58:1280-1285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Muto Y, Sato S, Watanabe A, Moriwaki H, Suzuki K, Kato A, Kato M, Nakamura T, Higuchi K, Nishiguchi S. Overweight and obesity increase the risk for liver cancer in patients with liver cirrhosis and long-term oral supplementation with branched-chain amino acid granules inhibits liver carcinogenesis in heavier patients with liver cirrhosis. Hepatol Res. 2006;35:204-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 77] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 27. | Cha JH, Bae SH, Kim HL, Park NR, Choi ES, Jung ES, Choi JY, Yoon SK. Branched-chain amino acids ameliorate fibrosis and suppress tumor growth in a rat model of hepatocellular carcinoma with liver cirrhosis. PLoS One. 2013;8:e77899. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 28. | Hagiwara A, Nishiyama M, Ishizaki S. Branched-chain amino acids prevent insulin-induced hepatic tumor cell proliferation by inducing apoptosis through mTORC1 and mTORC2-dependent mechanisms. J Cell Physiol. 2012;227:2097-2105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 29. | Kawaguchi T, Izumi N, Charlton MR, Sata M. Branched-chain amino acids as pharmacological nutrients in chronic liver disease. Hepatology. 2011;54:1063-1070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 269] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 30. | Rahimi RS, Trotter JF. Liver transplantation for hepatocellular carcinoma: outcomes and treatment options for recurrence. Ann Gastroenterol. 2015;28:323-330. [PubMed] |
| 31. | Kang TW, Kim JM, Rhim H, Lee MW, Kim YS, Lim HK, Choi D, Song KD, Kwon CH, Joh JW. Small Hepatocellular Carcinoma: Radiofrequency Ablation versus Nonanatomic Resection--Propensity Score Analyses of Long-term Outcomes. Radiology. 2015;275:908-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 129] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 32. | Song KD, Lim HK, Rhim H, Lee MW, Kim YS, Lee WJ, Paik YH, Gwak GY, Kim JM, Kwon CH. Repeated Hepatic Resection versus Radiofrequency Ablation for Recurrent Hepatocellular Carcinoma after Hepatic Resection: A Propensity Score Matching Study. Radiology. 2015;275:599-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 86] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 33. | Khan AS, Fowler KJ, Chapman WC. Current surgical treatment strategies for hepatocellular carcinoma in North America. World J Gastroenterol. 2014;20:15007-15017. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 34. | Lee DH, Lee JM, Lee JY, Kim SH, Yoon JH, Kim YJ, Han JK, Choi BI. Radiofrequency ablation of hepatocellular carcinoma as first-line treatment: long-term results and prognostic factors in 162 patients with cirrhosis. Radiology. 2014;270:900-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 261] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 35. | Takuma Y, Takabatake H, Morimoto Y, Toshikuni N, Kayahara T, Makino Y, Yamamoto H. Comparison of combined transcatheter arterial chemoembolization and radiofrequency ablation with surgical resection by using propensity score matching in patients with hepatocellular carcinoma within Milan criteria. Radiology. 2013;269:927-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 74] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 36. | Lencioni R, de Baere T, Soulen MC, Rilling WS, Geschwind JF. Lipiodol transarterial chemoembolization for hepatocellular carcinoma: A systematic review of efficacy and safety data. Hepatology. 2016;64:106-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 552] [Cited by in RCA: 553] [Article Influence: 55.3] [Reference Citation Analysis (0)] |
| 37. | Song MJ. Hepatic artery infusion chemotherapy for advanced hepatocellular carcinoma. World J Gastroenterol. 2015;21:3843-3849. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 62] [Cited by in RCA: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 38. | Sohn W, Paik YH, Cho JY, Lim HY, Ahn JM, Sinn DH, Gwak GY, Choi MS, Lee JH, Koh KC. Sorafenib therapy for hepatocellular carcinoma with extrahepatic spread: treatment outcome and prognostic factors. J Hepatol. 2015;62:1112-1121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 39. | Kondo Y, Kimura O, Shimosegawa T. Radiation therapy has been shown to be adaptable for various stages of hepatocellular carcinoma. World J Gastroenterol. 2015;21:94-101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 40. | Hanazaki K, Kajikawa S, Shimozawa N, Mihara M, Shimada K, Hiraguri M, Koide N, Adachi W, Amano J. Survival and recurrence after hepatic resection of 386 consecutive patients with hepatocellular carcinoma. J Am Coll Surg. 2000;191:381-388. [PubMed] |
| 41. | Togo S, Tanaka K, Morioka D, Sugita M, Ueda M, Miura Y, Kubota T, Nagano Y, Matsuo K, Endo I. Usefulness of granular BCAA after hepatectomy for liver cancer complicated with liver cirrhosis. Nutrition. 2005;21:480-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 42. | Ichikawa K, Okabayashi T, Maeda H, Namikawa T, Iiyama T, Sugimoto T, Kobayashi M, Mimura T, Hanazaki K. Oral supplementation of branched-chain amino acids reduces early recurrence after hepatic resection in patients with hepatocellular carcinoma: a prospective study. Surg Today. 2013;43:720-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 43. | Nishikawa H, Osaki Y, Iguchi E, Koshikawa Y, Ako S, Inuzuka T, Takeda H, Nakajima J, Matsuda F, Sakamoto A. The effect of long-term supplementation with branched-chain amino acid granules in patients with hepatitis C virus-related hepatocellular carcinoma after radiofrequency thermal ablation. J Clin Gastroenterol. 2013;47:359-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 44. | Yoshiji H, Noguchi R, Namisaki T, Moriya K, Kitade M, Aihara Y, Douhara A, Yamao J, Fujimoto M, Toyohara M. Branched-chain amino acids suppress the cumulative recurrence of hepatocellular carcinoma under conditions of insulin-resistance. Oncol Rep. 2013;30:545-552. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 45. | Saito M, Yano Y, Minami A, Hirano H, Momose K, Sugimoto M, Yoshida M, Azuma T. Branched-chain amino acid granules improve the non-protein respiratory quotient after radiofrequency ablation. Intern Med. 2014;53:1469-1475. [PubMed] |
| 46. | Nishikawa H, Osaki Y, Inuzuka T, Takeda H, Nakajima J, Matsuda F, Henmi S, Sakamoto A, Ishikawa T, Saito S. Branched-chain amino acid treatment before transcatheter arterial chemoembolization for hepatocellular carcinoma. World J Gastroenterol. 2012;18:1379-1384. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (2)] |
| 47. | Takeda H, Nishikawa H, Iguchi E, Ohara Y, Sakamoto A, Saito S, Nishijima N, Nasu A, Komekado H, Kita R. Effect of treatment with branched-chain amino acids during sorafenib therapy for unresectable hepatocellular carcinoma. Hepatol Res. 2014;44:302-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 48. | Imanaka K, Ohkawa K, Tatsumi T, Katayama K, Inoue A, Imai Y, Oshita M, Iio S, Mita E, Fukui H. Impact of branched-chain amino acid supplementation on the survival in patients with advanced hepatocellular carcinoma treated with sorafenib; a multicenter retrospective cohort study. Hepatol Res. 2015; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 49. | Nakaya Y, Okita K, Suzuki K, Moriwaki H, Kato A, Miwa Y, Shiraishi K, Okuda H, Onji M, Kanazawa H. BCAA-enriched snack improves nutritional state of cirrhosis. Nutrition. 2007;23:113-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 162] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 50. | Okamoto M, Sakaida I, Tsuchiya M, Suzuki C, Okita K. Effect of a late evening snack on the blood glucose level and energy metabolism in patients with liver cirrhosis. Hepatol Res. 2003;27:45-50. [PubMed] |
| 51. | Sakaida I, Tsuchiya M, Okamoto M, Okita K. Late evening snack and the change of blood glucose level in patients with liver cirrhosis. Hepatol Res. 2004;30S:67-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 52. | Tsuchiya M, Sakaida I, Okamoto M, Okita K. The effect of a late evening snack in patients with liver cirrhosis. Hepatol Res. 2005;31:95-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 44] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 53. | European Association For The Study Of The Liver; European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4059] [Cited by in RCA: 4562] [Article Influence: 325.9] [Reference Citation Analysis (4)] |
| 54. | Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5972] [Cited by in RCA: 6629] [Article Influence: 441.9] [Reference Citation Analysis (1)] |
| 55. | Omata M, Lesmana LA, Tateishi R, Chen PJ, Lin SM, Yoshida H, Kudo M, Lee JM, Choi BI, Poon RT. Asian Pacific Association for the Study of the Liver consensus recommendations on hepatocellular carcinoma. Hepatol Int. 2010;4:439-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 797] [Cited by in RCA: 844] [Article Influence: 52.8] [Reference Citation Analysis (2)] |
| 56. | Okabayashi T, Nishimori I, Sugimoto T, Maeda H, Dabanaka K, Onishi S, Kobayashi M, Hanazaki K. Effects of branched-chain amino acids-enriched nutrient support for patients undergoing liver resection for hepatocellular carcinoma. J Gastroenterol Hepatol. 2008;23:1869-1873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 53] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 57. | Kuroda H, Ushio A, Miyamoto Y, Sawara K, Oikawa K, Kasai K, Endo R, Takikawa Y, Kato A, Suzuki K. Effects of branched-chain amino acid-enriched nutrient for patients with hepatocellular carcinoma following radiofrequency ablation: a one-year prospective trial. J Gastroenterol Hepatol. 2010;25:1550-1555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 58. | Morihara D, Iwata K, Hanano T, Kunimoto H, Kuno S, Fukunaga A, Yotsumoto K, Takata K, Tanaka T, Sakurai K. Late-evening snack with branched-chain amino acids improves liver function after radiofrequency ablation for hepatocellular carcinoma. Hepatol Res. 2012;42:658-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 59. | Takeshita S, Ichikawa T, Nakao K, Miyaaki H, Shibata H, Matsuzaki T, Muraoka T, Honda T, Otani M, Akiyama M. A snack enriched with oral branched-chain amino acids prevents a fall in albumin in patients with liver cirrhosis undergoing chemoembolization for hepatocellular carcinoma. Nutr Res. 2009;29:89-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 46] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 60. | Harima Y, Yamasaki T, Hamabe S, Saeki I, Okita K, Terai S, Sakaida I. Effect of a late evening snack using branched-chain amino acid-enriched nutrients in patients undergoing hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma. Hepatol Res. 2010;40:574-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 61. | Yamasaki T, Sakaida I. Hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma and future treatments for the poor responders. Hepatol Res. 2012;42:340-348. [PubMed] |
| 62. | Castells A, Bruix J, Bru C, Fuster J, Vilana R, Navasa M, Ayuso C, Boix L, Visa J, Rodés J. Treatment of small hepatocellular carcinoma in cirrhotic patients: a cohort study comparing surgical resection and percutaneous ethanol injection. Hepatology. 1993;18:1121-1126. [PubMed] |
| 63. | Llovet JM, Schwartz M, Mazzaferro V. Resection and liver transplantation for hepatocellular carcinoma. Semin Liver Dis. 2005;25:181-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 662] [Cited by in RCA: 669] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 64. | Shiratori Y, Shiina S, Teratani T, Imamura M, Obi S, Sato S, Koike Y, Yoshida H, Omata M. Interferon therapy after tumor ablation improves prognosis in patients with hepatocellular carcinoma associated with hepatitis C virus. Ann Intern Med. 2003;138:299-306. [PubMed] |
| 65. | Zhang CH, Xu GL, Jia WD, Ge YS. Effects of interferon alpha treatment on recurrence and survival after complete resection or ablation of hepatocellular carcinoma: a meta-analysis of randomized controlled trials. Int J Cancer. 2009;124:2982-2988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 66. | Singal AG, Waljee AK, Shiffman M, Bacon BR, Schoenfeld PS. Meta-analysis: re-treatment of genotype I hepatitis C nonresponders and relapsers after failing interferon and ribavirin combination therapy. Aliment Pharmacol Ther. 2010;32:969-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 67. | Shen YC, Hsu C, Chen LT, Cheng CC, Hu FC, Cheng AL. Adjuvant interferon therapy after curative therapy for hepatocellular carcinoma (HCC): a meta-regression approach. J Hepatol. 2010;52:889-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 118] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 68. | Miao RY, Zhao HT, Yang HY, Mao YL, Lu X, Zhao Y, Liu CN, Zhong SX, Sang XT, Huang JF. Postoperative adjuvant antiviral therapy for hepatitis B/C virus-related hepatocellular carcinoma: a meta-analysis. World J Gastroenterol. 2010;16:2931-2942. [PubMed] |
| 69. | Miyake Y, Kobashi H, Yamamoto K. Meta-analysis: the effect of interferon on development of hepatocellular carcinoma in patients with chronic hepatitis B virus infection. J Gastroenterol. 2009;44:470-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 70. | Breitenstein S, Dimitroulis D, Petrowsky H, Puhan MA, Müllhaupt B, Clavien PA. Systematic review and meta-analysis of interferon after curative treatment of hepatocellular carcinoma in patients with viral hepatitis. Br J Surg. 2009;96:975-981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 115] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 71. | Lee MH, Yang HI, Liu J, Batrla-Utermann R, Jen CL, Iloeje UH, Lu SN, You SL, Wang LY, Chen CJ. Prediction models of long-term cirrhosis and hepatocellular carcinoma risk in chronic hepatitis B patients: risk scores integrating host and virus profiles. Hepatology. 2013;58:546-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 262] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 72. | Yang YF, Zhao W, Zhong YD, Xia HM, Shen L, Zhang N. Interferon therapy in chronic hepatitis B reduces progression to cirrhosis and hepatocellular carcinoma: a meta-analysis. J Viral Hepat. 2009;16:265-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 106] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 73. | Hosaka T, Suzuki F, Kobayashi M, Seko Y, Kawamura Y, Sezaki H, Akuta N, Suzuki Y, Saitoh S, Arase Y. Long-term entecavir treatment reduces hepatocellular carcinoma incidence in patients with hepatitis B virus infection. Hepatology. 2013;58:98-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 519] [Cited by in RCA: 554] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 74. | Sohn W, Paik YH, Kim JM, Kwon CH, Joh JW, Cho JY, Gwak GY, Choi MS, Lee JH, Koh KC. HBV DNA and HBsAg levels as risk predictors of early and late recurrence after curative resection of HBV-related hepatocellular carcinoma. Ann Surg Oncol. 2014;21:2429-2435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 94] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 75. | Sun P, Dong X, Cheng X, Hu Q, Zheng Q. Nucleot(s)ide analogues for hepatitis B virus-related hepatocellular carcinoma after curative treatment: a systematic review and meta-analysis. PLoS One. 2014;9:e102761. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 76. | Liu GM, Huang XY, Shen SL, Hu WJ, Peng BG. Adjuvant antiviral therapy for hepatitis B virus-related hepatocellular carcinoma after curative treatment: A systematic review and meta-analysis. Hepatol Res. 2016;46:100-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 77. | Lee TY, Lin JT, Zeng YS, Chen YJ, Wu MS, Wu CY. Association between nucleos(t)ide analog and tumor recurrence in hepatitis B virus-related hepatocellular carcinoma after radiofrequency ablation. Hepatology. 2016;63:1517-1527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 70] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 78. | Nishiguchi S, Kuroki T, Nakatani S, Morimoto H, Takeda T, Nakajima S, Shiomi S, Seki S, Kobayashi K, Otani S. Randomised trial of effects of interferon-alpha on incidence of hepatocellular carcinoma in chronic active hepatitis C with cirrhosis. Lancet. 1995;346:1051-1055. [PubMed] |
| 79. | Nishiguchi S, Shiomi S, Nakatani S, Takeda T, Fukuda K, Tamori A, Habu D, Tanaka T. Prevention of hepatocellular carcinoma in patients with chronic active hepatitis C and cirrhosis. Lancet. 2001;357:196-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 179] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 80. | Shiratori Y, Ito Y, Yokosuka O, Imazeki F, Nakata R, Tanaka N, Arakawa Y, Hashimoto E, Hirota K, Yoshida H. Antiviral therapy for cirrhotic hepatitis C: association with reduced hepatocellular carcinoma development and improved survival. Ann Intern Med. 2005;142:105-114. [PubMed] |
| 81. | Miyake Y, Takaki A, Iwasaki Y, Yamamoto K. Meta-analysis: interferon-alpha prevents the recurrence after curative treatment of hepatitis C virus-related hepatocellular carcinoma. J Viral Hepat. 2010;17:287-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 70] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 82. | Kubo S, Nishiguchi S, Hirohashi K, Tanaka H, Shuto T, Kinoshita H. Randomized clinical trial of long-term outcome after resection of hepatitis C virus-related hepatocellular carcinoma by postoperative interferon therapy. Br J Surg. 2002;89:418-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 128] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 83. | Nishiguchi S, Tamori A, Kubo S. Effect of long-term postoperative interferon therapy on intrahepatic recurrence and survival rate after resection of hepatitis C virus-related hepatocellular carcinoma. Intervirology. 2005;48:71-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 58] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 84. | Jeong SC, Aikata H, Katamura Y, Azakami T, Kawaoka T, Saneto H, Uka K, Mori N, Takaki S, Kodama H. Effects of a 24-week course of interferon-alpha therapy after curative treatment of hepatitis C virus-associated hepatocellular carcinoma. World J Gastroenterol. 2007;13:5343-5350. [PubMed] |
| 85. | Mazzaferro V, Romito R, Schiavo M, Mariani L, Camerini T, Bhoori S, Capussotti L, Calise F, Pellicci R, Belli G. Prevention of hepatocellular carcinoma recurrence with alpha-interferon after liver resection in HCV cirrhosis. Hepatology. 2006;44:1543-1554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 295] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 86. | Kudo M, Sakaguchi Y, Chung H, Hatanaka K, Hagiwara S, Ishikawa E, Takahashi S, Kitai S, Inoue T, Minami Y. Long-term interferon maintenance therapy improves survival in patients with HCV-related hepatocellular carcinoma after curative radiofrequency ablation. A matched case-control study. Oncology. 2007;72 Suppl 1:132-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 87. | Ikeda K, Arase Y, Saitoh S, Kobayashi M, Suzuki Y, Suzuki F, Tsubota A, Chayama K, Murashima N, Kumada H. Interferon beta prevents recurrence of hepatocellular carcinoma after complete resection or ablation of the primary tumor-A prospective randomized study of hepatitis C virus-related liver cancer. Hepatology. 2000;32:228-232. [PubMed] |
| 88. | Reig M, Mariño Z, Perelló C, Iñarrairaegui M, Ribeiro A, Lens S, Díaz A, Vilana R, Darnell A, Varela M. Unexpected high rate of early tumor recurrence in patients with HCV-related HCC undergoing interferon-free therapy. J Hepatol. 2016; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 725] [Cited by in RCA: 818] [Article Influence: 81.8] [Reference Citation Analysis (0)] |
| 89. | Pol S. Lack of evidence of an effect of Direct Acting Antivirals on the recurrence of hepatocellular carcinoma: The ANRS collaborative study group on hepatocellular carcinoma (ANRS CO22 HEPATHER, CO12 CIRVIR and CO23 CUPILT cohorts). J Hepatol. 2016; Epub ahead of print. [RCA] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 336] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 90. | Bruix J, Takayama T, Mazzaferro V, Chau GY, Yang J, Kudo M, Cai J, Poon RT, Han KH, Tak WY. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2015;16:1344-1354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 558] [Cited by in RCA: 833] [Article Influence: 75.7] [Reference Citation Analysis (0)] |
| 91. | Nakamura N, Shidoji Y, Yamada Y, Hatakeyama H, Moriwaki H, Muto Y. Induction of apoptosis by acyclic retinoid in the human hepatoma-derived cell line, HuH-7. Biochem Biophys Res Commun. 1995;207:382-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 68] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 92. | Shrestha R, Tatsukawa H, Shrestha R, Ishibashi N, Matsuura T, Kagechika H, Kose S, Hitomi K, Imamoto N, Kojima S. Molecular mechanism by which acyclic retinoid induces nuclear localization of transglutaminase 2 in human hepatocellular carcinoma cells. Cell Death Dis. 2015;6:e2002. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 93. | Muto Y, Moriwaki H, Ninomiya M, Adachi S, Saito A, Takasaki KT, Tanaka T, Tsurumi K, Okuno M, Tomita E. Prevention of second primary tumors by an acyclic retinoid, polyprenoic acid, in patients with hepatocellular carcinoma. Hepatoma Prevention Study Group. N Engl J Med. 1996;334:1561-1567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 509] [Cited by in RCA: 479] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 94. | Okita K, Izumi N, Ikeda K, Osaki Y, Numata K, Ikeda M, Kokudo N, Imanaka K, Nishiguchi S, Kondo S. Survey of survival among patients with hepatitis C virus-related hepatocellular carcinoma treated with peretinoin, an acyclic retinoid, after the completion of a randomized, placebo-controlled trial. J Gastroenterol. 2015;50:667-674. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 95. | Okita K, Izumi N, Matsui O, Tanaka K, Kaneko S, Moriwaki H, Ikeda K, Osaki Y, Numata K, Nakachi K. Peretinoin after curative therapy of hepatitis C-related hepatocellular carcinoma: a randomized double-blind placebo-controlled study. J Gastroenterol. 2015;50:191-202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 96. | Gkouvatsos K, Papanikolaou G, Pantopoulos K. Regulation of iron transport and the role of transferrin. Biochim Biophys Acta. 2012;1820:188-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 366] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 97. | Takami T, Sakaida I. Iron regulation by hepatocytes and free radicals. J Clin Biochem Nutr. 2011;48:103-106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 98. | Andrews NC. Disorders of iron metabolism. N Engl J Med. 1999;341:1986-1995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1329] [Cited by in RCA: 1288] [Article Influence: 47.7] [Reference Citation Analysis (0)] |
| 99. | Kato J, Miyanishi K, Kobune M, Nakamura T, Takada K, Takimoto R, Kawano Y, Takahashi S, Takahashi M, Sato Y. Long-term phlebotomy with low-iron diet therapy lowers risk of development of hepatocellular carcinoma from chronic hepatitis C. J Gastroenterol. 2007;42:830-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 138] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 100. | Yu Y, Gutierrez E, Kovacevic Z, Saletta F, Obeidy P, Suryo Rahmanto Y, Richardson DR. Iron chelators for the treatment of cancer. Curr Med Chem. 2012;19:2689-2702. [PubMed] |
| 101. | Torti SV, Torti FM. Iron and cancer: more ore to be mined. Nat Rev Cancer. 2013;13:342-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 936] [Cited by in RCA: 1221] [Article Influence: 93.9] [Reference Citation Analysis (0)] |
| 102. | Yamasaki T, Terai S, Sakaida I. Deferoxamine for advanced hepatocellular carcinoma. N Engl J Med. 2011;365:576-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 126] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 103. | Sakaida I, Hironaka K, Uchida K, Okita K. Iron chelator deferoxamine reduces preneoplastic lesions in liver induced by choline-deficient L-amino acid-defined diet in rats. Dig Dis Sci. 1999;44:560-569. [PubMed] |
| 104. | Jin H, Terai S, Sakaida I. The iron chelator deferoxamine causes activated hepatic stellate cells to become quiescent and to undergo apoptosis. J Gastroenterol. 2007;42:475-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 1.5] [Reference Citation Analysis (1)] |
| 105. | Terai S, Sakaida I, Yamamoto N, Omori K, Watanabe T, Ohata S, Katada T, Miyamoto K, Shinoda K, Nishina H. An in vivo model for monitoring trans-differentiation of bone marrow cells into functional hepatocytes. J Biochem. 2003;134:551-558. [PubMed] |
| 106. | Sakaida I, Terai S, Yamamoto N, Aoyama K, Ishikawa T, Nishina H, Okita K. Transplantation of bone marrow cells reduces CCl4-induced liver fibrosis in mice. Hepatology. 2004;40:1304-1311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 423] [Cited by in RCA: 420] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 107. | Terai S, Ishikawa T, Omori K, Aoyama K, Marumoto Y, Urata Y, Yokoyama Y, Uchida K, Yamasaki T, Fujii Y. Improved liver function in patients with liver cirrhosis after autologous bone marrow cell infusion therapy. Stem Cells. 2006;24:2292-2298. [PubMed] |
| 108. | Kim JK, Park YN, Kim JS, Park MS, Paik YH, Seok JY, Chung YE, Kim HO, Kim KS, Ahn SH. Autologous bone marrow infusion activates the progenitor cell compartment in patients with advanced liver cirrhosis. Cell Transplant. 2010;19:1237-1246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 65] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 109. | Saito T, Okumoto K, Haga H, Nishise Y, Ishii R, Sato C, Watanabe H, Okada A, Ikeda M, Togashi H. Potential therapeutic application of intravenous autologous bone marrow infusion in patients with alcoholic liver cirrhosis. Stem Cells Dev. 2011;20:1503-1510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 110. | Takami T, Terai S, Sakaida I. Stem cell therapy in chronic liver disease. Curr Opin Gastroenterol. 2012;28:203-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 111. | Ishikawa H, Nakao K, Matsumoto K, Nishimura D, Ichikawa T, Hamasaki K, Eguchi K. Bone marrow engraftment in a rodent model of chemical carcinogenesis but no role in the histogenesis of hepatocellular carcinoma. Gut. 2004;53:884-889. [PubMed] |
| 112. | Maeda M, Takami T, Terai S, Sakaida I. Autologous bone marrow cell infusions suppress tumor initiation in hepatocarcinogenic mice with liver cirrhosis. J Gastroenterol Hepatol. 2012;27 Suppl 2:104-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 113. | Lazennec G, Jorgensen C. Concise review: adult multipotent stromal cells and cancer: risk or benefit? Stem Cells. 2008;26:1387-1394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 185] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 114. | Li M, Wu Y, Liu R, Guo L, Xu T, Chen J, Xu S. [Investigational Study of Mesenchymal Stem Cells on Lung Cancer Cell Proliferation and Invasion]. Zhongguo Fei Ai Zazhi. 2015;18:674-679. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 115. | So KA, Min KJ, Hong JH, Lee JK. Interleukin-6 expression by interactions between gynecologic cancer cells and human mesenchymal stem cells promotes epithelial-mesenchymal transition. Int J Oncol. 2015;47:1451-1459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 116. | Mishra PJ, Mishra PJ, Humeniuk R, Medina DJ, Alexe G, Mesirov JP, Ganesan S, Glod JW, Banerjee D. Carcinoma-associated fibroblast-like differentiation of human mesenchymal stem cells. Cancer Res. 2008;68:4331-4339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 715] [Cited by in RCA: 732] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 117. | Zhu Y, Sun Z, Han Q, Liao L, Wang J, Bian C, Li J, Yan X, Liu Y, Shao C. Human mesenchymal stem cells inhibit cancer cell proliferation by secreting DKK-1. Leukemia. 2009;23:925-933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 263] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 118. | Sun B, Roh KH, Park JR, Lee SR, Park SB, Jung JW, Kang SK, Lee YS, Kang KS. Therapeutic potential of mesenchymal stromal cells in a mouse breast cancer metastasis model. Cytotherapy. 2009;11:289-98, 1 p following 298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 171] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 119. | Wakitani S, Okabe T, Horibe S, Mitsuoka T, Saito M, Koyama T, Nawata M, Tensho K, Kato H, Uematsu K. Safety of autologous bone marrow-derived mesenchymal stem cell transplantation for cartilage repair in 41 patients with 45 joints followed for up to 11 years and 5 months. J Tissue Eng Regen Med. 2011;5:146-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 230] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Gong ZJ, Tasci I S- Editor: Qi Y L- Editor: A E- Editor: Ma S