Published online Jul 14, 2016. doi: 10.3748/wjg.v22.i26.5888
Peer-review started: March 11, 2016
First decision: April 14, 2016
Revised: April 30, 2016
Accepted: June 2, 2016
Article in press: June 2, 2016
Published online: July 14, 2016
Processing time: 117 Days and 22.6 Hours
Surgery used to be the only therapy for gastric cancer, and since its ability to cure gastric cancer was the focus of attention, less attention was paid to function-preserving surgery in gastric cancer, though it was studied for gastroduodenal ulcer. Maki et al developed pylorus-preserving gastrectomy for gastric ulcer in 1967. At the same time, the definition of early gastric cancer (EGC) was being considered, histopathological investigations of EGC were carried out, and the validity of modified surgery was sustained. After the development of H2-blockers, the number of operations for gastroduodenal ulcers decreased, and the number of EGC patients increased simultaneously. As a result, the indications for pylorus-preserving gastrectomy for EGC in the middle third of the stomach extended, and various alterations were added. Since then, many kinds of function-preserving gastrectomies have been performed and studied in other fields of gastric cancer, and proximal gastrectomy, jejunal pouch interposition, segmental gastrectomy, and local resection have been performed. On the other hand, from the overall perspective, it can be said that endoscopic resection, which was launched at almost the same time, is the ultimate function-preserving surgery under the current circumstances. The current function-preserving gastrectomies that are often performed and studied are pylorus-preserving gastrectomy and proximal gastrectomy. The reasons for this are that these procedures that can be performed with systemic lymph node dissection, and they include three important elements: (1) reduction of the extent of gastrectomy; (2) preservation of the pylorus; and (3) preservation of the vagal nerve. In addition, these operations are more likely to be performed with a laparoscopic approach as minimally invasive surgery. Of the above-mentioned three elements, reduction of the extent of gastrectomy is the most important in our view. Therefore, we should try to reduce the extent of gastrectomy if curability of the gastric cancer can still be achieved. However, if we preserve a wider residual stomach in function-preserving gastrectomy, we should pay attention to the development of metachronous gastric cancer.
Core tip: Current surgical function-preserving gastrectomies include pylorus-preserving gastrectomy, proximal gastrectomy, jejunal pouch interposition, segmental gastrectomy, and local resection. The procedures that include systemic lymph node dissection and the three elements that preserve function are pylorus-preserving gastrectomy and proximal gastrectomy.
- Citation: Nomura E, Okajima K. Function-preserving gastrectomy for gastric cancer in Japan. World J Gastroenterol 2016; 22(26): 5888-5895
- URL: https://www.wjgnet.com/1007-9327/full/v22/i26/5888.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i26.5888
Standard gastrectomy is defined in the Japanese gastric cancer treatment guidelines as the resection of at least two-thirds of the stomach with a D2 lymph node dissection. Modified surgery (limited surgery) is defined as a reduced extent of gastric resection and/or lymphadenectomy compared to standard surgery and includes optional procedures that preserve the bursa, omentum, pylorus, and vagal nerve[1]. This modified surgery was started for early gastric cancer (EGC) cases with favorable prognoses to reduce their surgical invasiveness, and it has overlapped with the indications for laparoscopic gastrectomy, which is regarded as one form of minimally invasive surgery; in fact, modified surgery has often been performed laparoscopically[2,3]. On the other hand, new concepts such as function-preserving surgery (FPS) that preserves gastric function, which has been sacrificed in gastric cancer surgery, were generated from the perspective of patients’ postoperative quality of life (QOL)[4,5]. Namely, an operation that is performed with the intent of achieving a better postoperative condition is thought to be FPS. Although modified surgery is apt to be used synonymously for FPS, modified surgery is not always function-preserving, and FPS does not always involve a modified procedure. However, because FPS has been derived from modified surgery, most FPS methods are currently considered modified surgery. These issues are reviewed, while providing a historical perspective.
In Japan, there used to be many gastroduodenal ulcer patients, and surgery was the most effective and certain therapy until the appearance of H2-blockers[6-9]. Therefore, it was necessary to analyze gastric physiological motor function[10,11], control of the autonomic nervous system[12], and the dynamics of acid secretion and hormonal secretion[13,14], and to investigate how these changed after gastrectomy[15-17]. The incidence rate of gastric cancer was similarly high, and too much attention was paid to the ability of surgery to cure gastric cancer, so that less attention was paid to FPS in gastric cancer, though it was studied for gastroduodenal ulcer. In particular, there was much research on the relationship between the vagus nerve and acid secretion[18]. Maki et al[19] developed pylorus-preserving gastrectomy (PPG) for gastric ulcer in 1967. They found that pyloric motor function changed according to the distance of the transection line from the pyloric ring in canine experiments, and they advocated that the transection line should be placed 1.5 cm proximal to the pyloric ring. Meanwhile, the definition of EGC was investigated[1,20] from the 1960s. Then, from the 1970s, histopathological investigations of EGC were carried out in an active manner[21-23], and the validity of modified surgery was confirmed under specific indications[24-26]. Because the number of operations for gastroduodenal ulcer decreased after the development of H2-blockers, and the number of EGC patients simultaneously increased, modified surgery for EGC was gradually started. In the late 1980s, PPG for EGC in the middle third of the stomach had come to be performed[27]. Thereafter, many kinds of function-preserving gastrectomies (FPGs) came to be performed and studied in other fields of gastric cancer[28-33]. During the same period of time, endoscopists took the initiative to start endoscopic mucosal resection (EMR) for EGC, and endoscopic submucosal dissection (ESD) was developed with improved instruments and techniques. Originally, radicality and QOL conflicted with each other, but FPS tried to improve postoperative QOL while maintaining radicality. After modified surgery began, the ability of FPS to preserve gastric function and physical condition was studied.
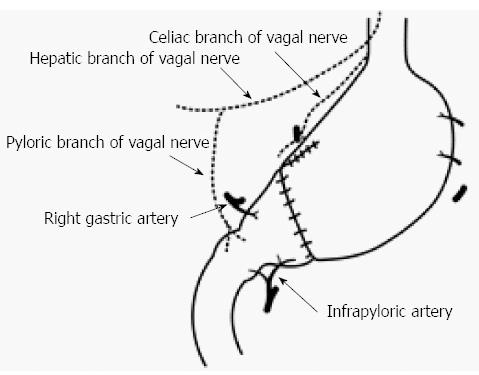
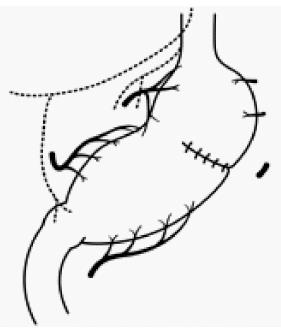
Maki et al[19] decided that pylorus-preserving gastrectomy (PPG) was indicated for gastric benign disease when the distal transection line could be made 1.5 cm proximal to the pyloric ring, and the therapeutic purpose could be achieved through resection of 1/2 to 2/3 of the stomach (Figure 1). It was initially expected that PPG would decrease dumping symptoms compared to the Billroth I method, with the later advantages of reservoir function and prevention of regurgitation of bile juice[28]. However, meal stasis was common, so that the kinetics of gastric emptying were studied, and the length of the pyloric cuff was gradually elongated[34,35]. Suprapyloric lymph node dissection has come to be omitted to preserve the pyloric branch of the vagal nerve and right gastric vessels[34]. Furthermore, there has been a tendency to preserve the infrapyloric vessels[36]. Thus, from the balance between radicality and functional preservation, PPG has become a procedure that preserves the upper third of the stomach and a 3 to 4-cm pyloric cuff for cN0, cT1 gastric cancer, and it preserves the hepatic branch, pyloric branch, and celiac branch as much as possible[1]. As a result, in a large-scale postgastrectomy syndrome assessment study, Fujita et al[37] reported that better postoperative QOL was observed in PPG, including a lower incidence of diarrhea, dumping symptoms, and frequency of additional meals compared to the Billroth I procedure. Furthermore, Namikawa et al[38] reported that the size of the proximal gastric remnant significantly affected the change in body weight, scores for dissatisfaction at meals, and the dissatisfaction for daily life subscale, and preservation of a sufficient proximal gastric remnant is recommended when using PPG as FPS.
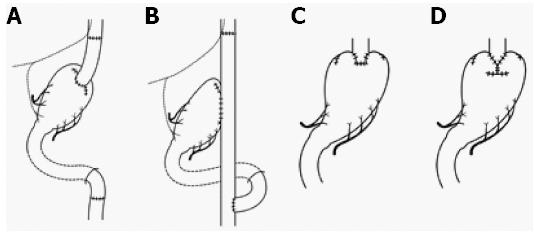
Proximal gastrectomy (PG) began as modified surgery for gastric cancer, and Papachristou and Fortner[39] reported that PG for adenocarcinoma of the cardia was curative only in cases of stage I and II disease (Figure 2). It turned out that the incidence rate of lymph node metastases for EGC in the upper third of the stomach was low[29], and proximal gastrectomy is currently performed for cN0, cT1 tumors where more than half of the distal stomach can be preserved[1]. Furthermore, the hepatic branch, pyloric branch, and celiac branch of the vagal nerve are preserved as much as possible. The reconstructive procedures need to be considered: jejunal interposition (Figure 2A), double tract (Figure 2B), and esophagogastrostomy (Figure 2C and D). The first two methods involve reconstruction with 8-15 cm of interposed jejunum between the esophagus and the remnant stomach to prevent reflux esophagitis and to observe the remnant stomach for follow-up of neoplastic tumor[40-42]. The third method involves reconstruction by fundoplication, wrapping the remnant stomach around the circumference of the esophagus[43] (Figure 2C) by double-flap technique, embedding the lower edge of the esophagus to the gastric submucosal layer, etc[44] (Figure 2D). For each reconstruction, QOL has been evaluated. Takiguchi et al[45] reported that PG was better than total gastrectomy in terms of weight loss, necessity of additional meals, diarrhea, and dumping symptoms in a multi-institutional study. Especially in esophagogastrostomy after PG, Inada et al[46] reported that diarrhea scores and the necessity for additional meals were lower in the group with more than three-quarters of a remnant stomach compared to patients with a remnant stomach two-thirds the preoperative size. Procedures to prevent gastroesophageal reflux and the use of a pyloric bougie were considered effective ways to reduce the deterioration of QOL.
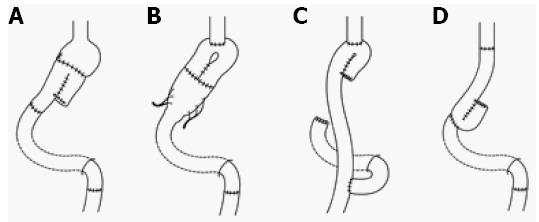
To increase the smaller gastric capacity after gastrectomy, distal gastrectomy[30] (Figure 3A), PG[31] (Figure 3B), and total gastrectomy[47] (Figure 3C and D) with an interposed jejunal pouch were performed throughout Japan[48]. Because this procedure was intended to recover the gastric reservoir function that was taken away by gastrectomy and to prevent the occurrence of reflux esophagitis, jejunal pouch interposition (JPI) was thought to be FPS. On the other hand, JPI was often added to the conventional operation (standard gastrectomy), so that it was not often a modified operation. However, Fukuhara[49] reported that, when jejunojejunostomy was performed, disappearance of systemic intestinal peristalsis due to the division of circular muscle resulted in the occurrence of meal stasis in the jejunal pouch. Mochiki et al[50] reported that the interposed jejunum with a pouch showed motor abnormalities. Katsube et al[51] reported a case with severe dilatation of the jejunal pouch and reflux esophagitis. Finally, Namikawa et al[52] reported that the better short-term QOL of JPI than of Roux-en-Y reconstruction decreases with time. As a result, the number of institutes performing surgery with JPI has been decreasing.
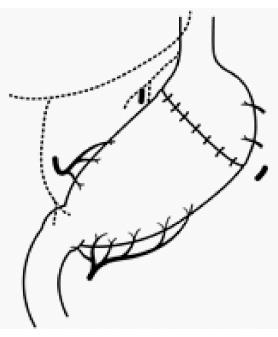
Segmental gastrectomy (SG) is defined as a relatively small circumferential gastric resection preserving the cardia and pylorus, excluding PPG (Figure 4). Local gastrectomy (LG) is defined as a non-circumferential gastric resection (Figure 5)[1]. If these operative procedures could achieve radicality, they might be the ultimate FPS. Although some institutes have performed these operations under strict indications[32,53], systemic lymph node dissection cannot be performed. Therefore, in order to assure radicality in these operations, the number of institutes that perform these operations using sentinel node navigation has been increasing[54]. In the original concept of sentinel node navigation surgery (SNNS), detected sentinel nodes were histologically examined intraoperatively, and if no lymph node metastasis was detected, further lymphadenectomy was omitted[55]. The feasibility and accuracy of diagnosis using sentinel node biopsy in T1 gastric cancer were evaluated in a multicenter trial (JCOG0302)[56]. The primary endpoint was to determine the proportion of false negatives, which was defined as the number of patients with negative stained nodes by frozen section divided by those with positive stained nodes and/or positive non-stained nodes by paraffin section. It was found that the proportion of false negatives was much higher (14%) than expected (10%), and further accrual was suspended at semiannual monitoring. Thereafter, several clinical studies of lymphatic basin dissection, which is a selective lymphadenectomy to dissect stained areas, so-called lymphatic basins, containing lymph nodes and lymphatic vessels stained with dye or a radioisotope or both used as a tracer for sentinel node mapping in EGC, were conducted[57].
FPS was concurrent with the beginning of laparoscopic gastrectomy and interest shifted to minimally invasive surgery. For this reason, the number of institutes where PPG[58,59] or PG[41,42] is performed using the laparoscopic approach has been increasing. Of course, if the efficacy of SNNS could be proven, it was thought that SG and LG would become the FPS performed under the laparoscopic approach. However, laparoscopic surgery is a kind of approach that is thoroughly minimally invasive surgery, not FPS.
Generally, it may be thought that endoscopic resection is not a surgical procedure, namely FPG. However, as the techniques and instruments of endoscopic resection have developed, and its indications have expanded, the borderline between usual surgical operations and recent endoscopic resection has become unclear. Therefore, endoscopic resection was treated as an FPS in this article.
Endoscopic resection was developed as the endoscopic resection method for tumors of the colon by Rosenberg et al[60] and Deyhle et al[61] in Western countries. In Japan, there was a report of its use for gastric cancer by Hirao et al[62]. The indication for endoscopic resection was based on investigation of a large number of EGC cases who underwent open gastrectomy[63]. Endoscopic mucosal resection (EMR) for selected intramucosal EGC cases, for which the possibility of lymph node metastasis is almost zero, has been widely accepted as a curative therapeutic strategy. The accepted indications for EMR are: (1) well differentiated elevated lesions less than 20 mm in size; and (2) small (≤ 10 mm), depressed, well-differentiated tumors without ulceration[1]. From further investigation of many EGC cases and the development of instruments for tissue detachment and dissection, EMR has been evolving to endoscopic submucosal dissection (ESD)[64,65]. Currently, tumors indicated for endoscopic resection as an investigational treatment (expanded indication) are as follows: tumors clinically diagnosed as T1a and: (1) of differentiated type, UL(-), but > 2 cm in diameter; (2) of differentiated-type, UL(+), and ≤ 3 cm in diameter; and (3) of undifferentiated-type, UL(-), and ≤ 2 cm in diameter[1]. After non-curative resection by EMR or ESD, additional surgical treatment should be performed; in fact, it can be said that surgical treatment could be easily added. Gastric mucosal resection as intra-gastric surgery had been performed using laparoscopic instruments through the abdominal and gastric walls[66] and seemed to be replaced by endoscopic resection. Although the indication is restricted, endoscopic resection can be said to be the ultimate FPS with respect to reduction of invasiveness and maintenance of QOL.
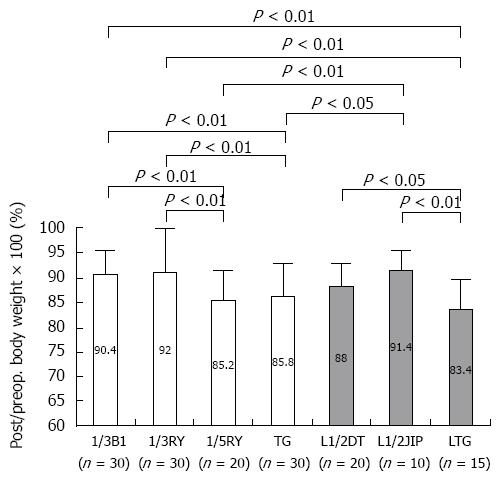
Given the view that function-preserving gastrectomy (FPG) preserves the autonomic nerves and maintains physiological gastrointestinal hormonal secretion, we evaluated the postoperative physical conditions of patients who had undergone various kinds of operating methods incorporating three elements: (1) reduction of the extent of gastrectomy; (2) preservation of the pylorus; and (3) preservation of the vagal nerve[67]. It was found that the operating methods incorporating more than two elements maintained postoperative function and QOL. In fact, PPG and PG are thought to be the ideal methods to fulfill all elements. Saito et al[68] discussed PPG and PG as FPG, and they described their oncological safety under the rigid indications and their several advantages with respect to postoperative QOL. Furthermore, in order to investigate the most important of these 3 elements, the following studies were performed. The functional outcomes of EGC patients treated by laparoscopic distal gastrectomy were compared with respect to size of the remnant stomach (1/2 vs 1/3) and the type of reconstruction (Billroth I vs Roux-en-Y). It was found that patients actually benefited from 1/2 gastrectomy rather than the typical 2/3 gastrectomy, irrespective of reconstruction method[69]. Similar results were seen in the investigation of advanced gastric cancer patients; better functional outcomes were observed in patients with a large remnant stomach (1/3) compared to a small one (1/5), regardless of the reconstruction[70] (Figure 6). However, a large remnant stomach sometimes shows gastric stasis, so that appropriate selection of the reconstruction method with smooth gastric emptying is needed, such as avoiding the Roux-en-Y reconstruction[69]. Furthermore, we compared functional outcomes between different types of reconstructions (jejunal interposition method, double tract method) following open or laparoscopic 1/2- or 2/3-PG for gastric cancer. Better functional outcomes were observed in patients with a large remnant stomach and with easy flow of food into the remnant stomach regardless of whether they underwent open or laparoscopic procedures[71]. In laparoscopic 1/2-PG with as much vagal nerve preservation as possible, the postoperative/preoperative body weight ratio was significantly higher in the jejunal interposition group in which all meals passed through the remnant stomach than in the double tract group[41,71]. Figure 6 shows the comparison of the postoperative/preoperative body weight ratio between the open distal gastrectomy without preservation of the vagal nerve group and the laparoscopic PG with preservation of the vagal nerve group. Of the above mentioned three elements, we think that reduction of the extent of gastrectomy and passage through the stomach are the most important, although the proof for preservation of the vagal nerve is difficult. Therefore, we should try to reduce the extent of gastrectomy if curability of the gastric cancer can be achieved. However, Miwa et al[72] stated that FPG carries the risk of metachronous gastric cancer. In fact, since 1995, 160 EGC patients with negative sentinel nodes underwent FPG, which consisted of local resection, SG, and limited distal gastrectomy. Of these 160 patients, 5 developed metachronous gastric cancer. The incidence of metachronous gastric cancer at 5 years after surgery was 2.8%, which was less than that for EMR and almost the same as that for conventional D2 distal gastrectomy[73]. Consequently, if we could preserve a wider residual stomach as in FPG, we should pay attention to the development of metachronous gastric cancer. Specifically, regular follow-up with endoscopic examination is needed. Furthermore, for the surgeon, especially following PG, it is most important to select the reconstruction method that is appropriate for observation of the remnant stomach through endoscopy. Of course, eradication of Helicobacter pylori should be considered.
Although the above mentioned 3 elements should be considered in FPG, further randomized, clinical trials are needed to identify the most important element.
Current surgical FPGs are thought to include PPG, PG, JPI, SG, and LG. Of these operations, the procedures that include systemic lymph node dissection and three important elements (reduction of the extent of gastrectomy, preservation of the pylorus, and preservation of the vagal nerve) are thought to be PPG and PG. Recently, the number of institutes that perform these operations with laparoscopic approaches has been increasing. Furthermore, with diagnostic examinations such as SNNS, SG and LG will become conventional as FPS in the near future.
| 1. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer. 2011;14:113-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1723] [Cited by in RCA: 1911] [Article Influence: 127.4] [Reference Citation Analysis (0)] |
| 2. | Katai H, Sasako M, Fukuda H, Nakamura K, Hiki N, Saka M, Yamaue H, Yoshikawa T, Kojima K. Safety and feasibility of laparoscopy-assisted distal gastrectomy with suprapancreatic nodal dissection for clinical stage I gastric cancer: a multicenter phase II trial (JCOG 0703). Gastric Cancer. 2010;13:238-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 266] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 3. | Lee SW, Nomura E, Bouras G, Tokuhara T, Tsunemi S, Tanigawa N. Long-term oncologic outcomes from laparoscopic gastrectomy for gastric cancer: a single-center experience of 601 consecutive resections. J Am Coll Surg. 2010;211:33-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 101] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 4. | Hioki K, Nakane Y, Yamamoto M. Surgical strategy for early gastric cancer. Br J Surg. 1990;77:1330-1334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 67] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 5. | Sawai K, Takahashi T, Suzuki H. New trends in surgery for gastric cancer in Japan. J Surg Oncol. 1994;56:221-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 6. | Thomas JM, Misiewicz G. Histamine H2-receptor antagonists in short- and long-term treatment of duodenal ulcer. Peptic ulcer clinics in gastroenterology. lst ed. London: Saunders 1984; 501-542. |
| 7. | Dlille W. Histamine HZ-receptor antagonists in short- and long-term treatment of gastric ulcer. Advances in ulcer research. 11st ed. Amsterdam: Excerpta Medica 1988; 330-335. |
| 8. | Gorey TF, Lennon F, Heffernan SJ. Highly selective vagotomy in duodenal ulceration and its complications. A 12-year review. Ann Surg. 1984;200:181-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Amdrup E, Andersen D, Høstrup H. The Aarhus County vagotomy trial. I. An interim report on primary results and incidence of sequelae following parietal cell vagotomy and selective gastric vagotomy in 748 patients. World J Surg. 1978;2:85-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 53] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Edwards DAW, Rowlands EN. Physiology of the gastroduodenal junction. Handbook of physiology, sect. 6: Alimentary Canal, 4, Edit Code CF. Washington DC: American Physiological Society 1968; 1985-2000. |
| 11. | Magee DF. Gastro-intestinal physiology. Springfield: Charles C Thomas Publisher 1962; 39-40. |
| 12. | Edin R, Lundberg J, Terenius L, Dahlström A, Hökfelt T, Kewenter J, Ahlman H. Evidence for vagal enkephalinergic neural control of the feline pylorus and stomach. Gastroenterology. 1980;78:492-497. [PubMed] |
| 13. | Sachs G, Prinz C, Loo D, Bamberg K, Besancon M, Shin JM. Gastric acid secretion: activation and inhibition. Yale J Biol Med. 1994;67:81-95. [PubMed] |
| 14. | Fisher RS, Lipshutz W, Cohen S. The hormonal regulation of pyloric sphincter function. J Clin Invest. 1973;52:1289-1296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 76] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Wangensteen OH. Segmental gastric resection for peptic ulcer; method permitting restoration of anatomic continuity. J Am Med Assoc. 1952;149:18-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 16. | Ferguson DJ, Billings H, Swensen D, Hoover G. Segmental gastrectomy with innervated antrum for duodenal ulcer. Surgery. 1960;47:548-556. [PubMed] |
| 17. | Shiratori T, Kanaizumi T, Murata S. Gastric motility and emptying in health and after vagotomy and after gastric resection. Nihon Heikatsukin Gakkai Zasshi. 1985;21 Suppl:127-134. [PubMed] |
| 18. | Jordan PH, Thornby J. Twenty years after parietal cell vagotomy or selective vagotomy antrectomy for treatment of duodenal ulcer. Final report. Ann Surg. 1994;220:283-93; discussion 293-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Maki T, Shiratori T, Hatafuku T, Sugawara K. Pylorus-preserving gastrectomy as an improved operation for gastric ulcer. Surgery. 1967;61:838-845. [PubMed] |
| 20. | Japanese Research Society for Gastric Cancer. Japanese classification of gastric carcinoma 3rd ed. Tokyo: Kanehara 1964; . |
| 21. | Mori M, Sugimachi K, Ohiwa T, Okamura T, Tamura S, Inokuchi K. Early gastric carcinoma in Japanese patients under 30 years of age. Br J Surg. 1985;72:289-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Korenaga D, Haraguchi M, Tsujitani S, Okamura T, Tamada R, Sugimachi K. Clinicopathological features of mucosal carcinoma of the stomach with lymph node metastasis in eleven patients. Br J Surg. 1986;73:431-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 73] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 23. | Mori M, Kitagawa S, Iida M, Sakurai T, Enjoji M, Sugimachi K, Ooiwa T. Early carcinoma of the gastric cardia. A clinicopathologic study of 21 cases. Cancer. 1987;59:1758-1766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 24. | Yoshino K, Hirahata S. Limited operation for early gastric cancer (in Japanese). Pharma Medica. 1989;7:35-40. |
| 25. | Iriyama K, Asakawa T, Koike H, Nishiwaki H, Suzuki H. Is extensive lymphadenectomy necessary for surgical treatment of intramucosal carcinoma of the stomach? Arch Surg. 1989;124:309-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 64] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 26. | Sowa M, Kato Y, Nishimura M, Kubo T, Maekawa H, Umeyama K. Surgical approach to early gastric cancer with lymph node metastasis. World J Surg. 1989;13:630-635; discussion 635-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 27. | Kodama M, Koyama K. Indications for pylorus preserving gastrectomy for early gastric cancer located in the middle third of the stomach. World J Surg. 1991;15:628-633; discussion 633-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 63] [Article Influence: 1.8] [Reference Citation Analysis (1)] |
| 28. | Isozaki H, Okajima K, Momura E, Ichinona T, Fujii K, Izumi N, Takeda Y. Postoperative evaluation of pylorus-preserving gastrectomy for early gastric cancer. Br J Surg. 1996;83:266-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 49] [Article Influence: 1.6] [Reference Citation Analysis (1)] |
| 29. | Isozaki H, Okajima K, Yamada S, Nakata E, Nishimura J, Ichinona T, Tanimura M, Takeda Y. Proximal subtotal gastrectomy for the treatment of carcinoma of the upper third of the stomach: its indications based on lymph node metastasis and perigastric lymphatic flow. Surg Today. 1995;25:21-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 30. | Miwa K, Kinami S, Sahara H, Matsumoto H, Segawa M, Michiwa Y, Miyazaki I. [Jejunal pouch interposition and distal gastrectomy]. Nihon Geka Gakkai Zasshi. 1997;98:560-564. [PubMed] |
| 31. | Kameyama J, Ishida H, Yasaku Y, Suzuki A, Kuzu H, Tsukamoto M. Proximal gastrectomy reconstructed by interposition of a jejunal pouch. Surgical technique. Eur J Surg. 1993;159:491-493. [PubMed] |
| 32. | Shinohara T, Ohyama S, Muto T, Kato Y, Yanaga K, Yamaguchi T. Clinical outcome of high segmental gastrectomy for early gastric cancer in the upper third of the stomach. Br J Surg. 2006;93:975-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 33. | Seto Y, Nagawa H, Muto Y, Kaizaki S, Kitayama J, Muto T. Preliminary report on local resection with lymphadenectomy for early gastric cancer. Br J Surg. 1999;86:526-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 34. | Nakane Y, Michiura T, Inoue K, Sato M, Nakai K, Yamamichi K. Length of the antral segment in pylorus-preserving gastrectomy. Br J Surg. 2002;89:220-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 35. | Morita S, Sasako M, Saka M, Fukagawa T, Sano T, Katai H. Correlation between the length of the pyloric cuff and postoperative evaluation after pylorus-preserving gastrectomy. Gastric Cancer. 2010;13:109-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 36. | Shibata C, Saijo F, Kakyo M, Kinouchi M, Tanaka N, Sasaki I, Aikou T. Current status of pylorus-preserving gastrectomy for the treatment of gastric cancer: a questionnaire survey and review of literatures. World J Surg. 2012;36:858-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (1)] |
| 37. | Fujita J, Takahashi M, Urushihara T, Tanabe K, Kodera Y, Yumiba T, Matsumoto H, Takagane A, Kunisaki C, Nakada K. Assessment of postoperative quality of life following pylorus-preserving gastrectomy and Billroth-I distal gastrectomy in gastric cancer patients: results of the nationwide postgastrectomy syndrome assessment study. Gastric Cancer. 2016;19:302-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (1)] |
| 38. | Namikawa T, Hiki N, Kinami S, Okabe H, Urushihara T, Kawahira H, Fukushima N, Kodera Y, Yumiba T, Oshio A. Factors that minimize postgastrectomy symptoms following pylorus-preserving gastrectomy: assessment using a newly developed scale (PGSAS-45). Gastric Cancer. 2015;18:397-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (4)] |
| 39. | Papachristou DN, Fortner JG. Adenocarcinoma of the gastric cardia. The choice of gastrectomy. Ann Surg. 1980;192:58-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 89] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 40. | Nozaki I, Hato S, Kobatake T, Ohta K, Kubo Y, Kurita A. Long-term outcome after proximal gastrectomy with jejunal interposition for gastric cancer compared with total gastrectomy. World J Surg. 2013;37:558-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 41. | Nomura E, Lee SW, Kawai M, Yamazaki M, Nabeshima K, Nakamura K, Uchiyama K. Functional outcomes by reconstruction technique following laparoscopic proximal gastrectomy for gastric cancer: double tract versus jejunal interposition. World J Surg Oncol. 2014;12:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 79] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 42. | Kinoshita T, Gotohda N, Kato Y, Takahashi S, Konishi M, Kinoshita T. Laparoscopic proximal gastrectomy with jejunal interposition for gastric cancer in the proximal third of the stomach: a retrospective comparison with open surgery. Surg Endosc. 2013;27:146-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 43. | Nakamura M, Nakamori M, Ojima T, Katsuda M, Iida T, Hayata K, Matsumura S, Kato T, Kitadani J, Iwahashi M. Reconstruction after proximal gastrectomy for early gastric cancer in the upper third of the stomach: an analysis of our 13-year experience. Surgery. 2014;156:57-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 89] [Article Influence: 7.4] [Reference Citation Analysis (1)] |
| 44. | Mine S, Nunobe S, Watanabe M. A Novel Technique of Anti-reflux Esophagogastrostomy Following Left Thoracoabdominal Esophagectomy for Carcinoma of the Esophagogastric Junction. World J Surg. 2015;39:2359-2361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (1)] |
| 45. | Takiguchi N, Takahashi M, Ikeda M, Inagawa S, Ueda S, Nobuoka T, Ota M, Iwasaki Y, Uchida N, Kodera Y. Long-term quality-of-life comparison of total gastrectomy and proximal gastrectomy by postgastrectomy syndrome assessment scale (PGSAS-45): a nationwide multi-institutional study. Gastric Cancer. 2015;18:407-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 178] [Article Influence: 16.2] [Reference Citation Analysis (1)] |
| 46. | Inada T, Yoshida M, Ikeda M, Yumiba T, Matsumoto H, Takagane A, Kunisaki C, Fukushima R, Yabusaki H, Nakada K. Evaluation of QOL after proximal gastrectomy using a newly developed assessment scale (PGSAS-45). World J Surg. 2014;38:3152-3162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 47. | Nakane Y, Akehira K, Okumura S, Okamura S, Boku T, Okusa T, Hioki K. Jejunal pouch and interposition reconstruction after total gastrectomy for cancer. Surg Today. 1997;27:696-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 48. | Nomura E, Shinohara H, Mabuchi H, Sang-Woong L, Sonoda T, Tanigawa N. Postoperative evaluation of the jejunal pouch reconstruction following proximal and distal gastrectomy for cancer. Hepatogastroenterology. 2004;51:1561-1566. [PubMed] |
| 49. | Fukuhara T. Control mechanism of the gastrointestinal peristalsis through intramural nerve (in Japanese). Jap J Smooth Muscle Res. 1967;3:1-18. |
| 50. | Mochiki E, Kamiyama Y, Aihara R, Nakabayashi T, Kamimura H, Asao T, Kuwano H. Postoperative functional evaluation of jejunal interposition with or without a pouch after a total gastrectomy for gastric cancer. Am J Surg. 2004;187:728-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 51. | Katsube T, Konno S, Hamaguchi K, Shimakawa T, Naritaka Y, Ogawa K. Complications after proximal gastrectomy with jejunal pouch interposition: report of a case. Eur J Surg Oncol. 2005;31:1036-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 52. | Namikawa T, Oki T, Kitagawa H, Okabayashi T, Kobayashi M, Hanazaki K. Impact of jejunal pouch interposition reconstruction after proximal gastrectomy for early gastric cancer on quality of life: short- and long-term consequences. Am J Surg. 2012;204:203-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (1)] |
| 53. | Seto Y, Yamaguchi H, Shimoyama S, Shimizu N, Aoki F, Kaminishi M. Results of local resection with regional lymphadenectomy for early gastric cancer. Am J Surg. 2001;182:498-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 54. | Mitsumori N, Nimura H, Takahashi N, Kawamura M, Aoki H, Shida A, Omura N, Yanaga K. Sentinel lymph node navigation surgery for early stage gastric cancer. World J Gastroenterol. 2014;20:5685-5693. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 42] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 55. | Morton DL, Wen DR, Wong JH, Economou JS, Cagle LA, Storm FK, Foshag LJ, Cochran AJ. Technical details of intraoperative lymphatic mapping for early stage melanoma. Arch Surg. 1992;127:392-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3238] [Cited by in RCA: 2946] [Article Influence: 86.6] [Reference Citation Analysis (4)] |
| 56. | Miyashiro I, Hiratsuka M, Sasako M, Sano T, Mizusawa J, Nakamura K, Nashimoto A, Tsuburaya A, Fukushima N. High false-negative proportion of intraoperative histological examination as a serious problem for clinical application of sentinel node biopsy for early gastric cancer: final results of the Japan Clinical Oncology Group multicenter trial JCOG0302. Gastric Cancer. 2014;17:316-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 114] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 57. | Fujimura T, Fushida S, Tsukada T, Kinoshita J, Oyama K, Miyashita T, Takamura H, Kinami S, Ohta T. A new stage of sentinel node navigation surgery in early gastric cancer. Gastric Cancer. 2015;18:210-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 58. | Shinohara H, Sonoda T, Niki M, Nomura E, Nishiguchi K, Tanigawa N. Laparoscopically-assisted pylorus-preserving gastrectomy with preservation of the vagus nerve. Eur J Surg. 2002;168:55-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 59. | Kumagai K, Hiki N, Nunobe S, Sekikawa S, Chiba T, Kiyokawa T, Jiang X, Tanimura S, Sano T, Yamaguchi T. Totally laparoscopic pylorus-preserving gastrectomy for early gastric cancer in the middle stomach: technical report and surgical outcomes. Gastric Cancer. 2015;18:183-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (1)] |
| 60. | Rosenberg N. Submucosal saline wheal as safety factor in fulguration or rectal and sigmoidal polypi. AMA Arch Surg. 1955;70:120-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 70] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 61. | Deyhle P, Jenny S, Fumagalli I. [Endoscopic polypectomy in the proximal colon. A diagnostic, therapeutic (and preventive?) intervention]. Dtsch Med Wochenschr. 1973;98:219-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 27] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 62. | Hirao M, Masuda K, Asanuma T, Naka H, Noda K, Matsuura K, Yamaguchi O, Ueda N. Endoscopic resection of early gastric cancer and other tumors with local injection of hypertonic saline-epinephrine. Gastrointest Endosc. 1988;34:264-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 268] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 63. | Tsujitani S, Oka S, Saito H, Kondo A, Ikeguchi M, Maeta M, Kaibara N. Less invasive surgery for early gastric cancer based on the low probability of lymph node metastasis. Surgery. 1999;125:148-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 106] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 64. | Gotoda T, Yanagisawa A, Sasako M, Ono H, Nakanishi Y, Shimoda T, Kato Y. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer. 2000;3:219-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1308] [Cited by in RCA: 1349] [Article Influence: 51.9] [Reference Citation Analysis (0)] |
| 65. | Hirasawa T, Gotoda T, Miyata S, Kato Y, Shimoda T, Taniguchi H, Fujisaki J, Sano T, Yamaguchi T. Incidence of lymph node metastasis and the feasibility of endoscopic resection for undifferentiated-type early gastric cancer. Gastric Cancer. 2009;12:148-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 370] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 66. | Ohashi S. Laparoscopic intraluminal (intragastric) surgery for early gastric cancer. A new concept in laparoscopic surgery. Surg Endosc. 1995;9:169-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 151] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 67. | Nomura E, Isozaki H, Fujii K, Toyoda M, Niki M, Sako S, Mabuchi H, Nishiguchi K, Tanigawa N. Postoperative evaluation of function-preserving gastrectomy for early gastric cancer. Hepatogastroenterology. 2003;50:2246-2250. [PubMed] |
| 68. | Saito T, Kurokawa Y, Takiguchi S, Mori M, Doki Y. Current status of function-preserving surgery for gastric cancer. World J Gastroenterol. 2014;20:17297-17304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (2)] |
| 69. | Nomura E, Lee SW, Bouras G, Tokuhara T, Hayashi M, Hiramatsu M, Okuda J, Tanigawa N. Functional outcomes according to the size of the gastric remnant and type of reconstruction following laparoscopic distal gastrectomy for gastric cancer. Gastric Cancer. 2011;14:279-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 70. | Nomura E, Lee SW, Tokuhara T, Nitta T, Kawai M, Uchiyama K. Functional outcomes according to the size of the gastric remnant and the type of reconstruction following distal gastrectomy for gastric cancer: an investigation including total gastrectomy. Jpn J Clin Oncol. 2013;43:1195-1202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 71. | Nomura E, Lee SW, Tokuhara T, Kawai M, Uchiyama K. Functional outcomes according to the size of the gastric remnant and type of reconstruction following open and laparoscopic proximal gastrectomy for gastric cancer. Hepatogastroenterology. 2012;59:1677-1681. [PubMed] |
| 72. | Miwa K, Miyashita T, Kinami S, Nakagawa H, Ohba H, Takai Y, Fujimura T. Gastric carcinoma following function-preserving limited gastrectomy for early gastric cancer (in Japanese with English abstract). Stom Int. 2005;40:1647-1654. |
| 73. | Miwa K, Miyazaki I, Sahara H, Fujimura T, Yonemura Y, Noguchi M, Falla R. Rationale for extensive lymphadenectomy in early gastric carcinoma. Br J Cancer. 1995;72:1518-1524. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Manuscript source: Invited manuscript
P- Reviewer: Komatsu S, Lianos GD, Yang MH S- Editor: Qi Y L- Editor: A E- Editor: Wang CH