Published online Jul 14, 2016. doi: 10.3748/wjg.v22.i26.5879
Peer-review started: March 24, 2016
First decision: May 12, 2016
Revised: May 13, 2016
Accepted: May 21, 2016
Article in press: May 23, 2016
Published online: July 14, 2016
Processing time: 104 Days and 16.8 Hours
HER2 is overexpressed in approximately 10%-20% of gastric and gastroesophageal junction carcinomas. In these types of cancer, accurate assessment of HER2 status is mandatory, for selecting patients who may benefit from targeted therapies with anti-HER2 drugs such as Trastuzumab. This manuscript focuses on HER2 in gastric carcinogenesis, on optimal evaluation of HER2 and on the possible causes which may contribute to inaccurate HER2 evaluation. Similarly to breast cancer HER2 evaluation, standardization of HER2 testing in gastric cancer is necessary in diagnostic practice. The three principle aspects which require consideration are: (1) the choice of sample with regards to cancer morphology - intestinal vs diffuse areas; (2) the choice of scoring criteria - use of HER2 scoring criteria specific for gastric cancer; and (3) the choice of HER2 evaluation methods - use of an algorithm in which both immunohistochemistry and in situ hybridization play a role. Problematic issues include: (1) pre-analytic variables with particular emphasis on fixation; (2) recommended methodology for HER2 assessment (immunohistochemistry vs in situ hybridization); (3) HER2 heterogeneity both within the primary tumor and between primary tumor and metastases; (4) reliability of biopsies in HER 2 evaluation; and (5) quantity of sample (FFPE blocks from surgical specimens or endoscopic biopsies) necessary for an adequate assessment.
Core tip: Accurate assessment of HER2 status is mandatory in gastric/gastroesophageal cancer, for selecting patients who may benefit from targeted therapies with anti-HER2 drugs. The three principle aspects of HER2 evaluation which require consideration are: (1) choice of sample with regards to cancer morphology; (2) choice of scoring criteria; and (3) choice of HER2 evaluation methods. Problematic issues include: (1) pre-analytic variables; (2) recommended methodology for HER2 assessment; (3) HER2 heterogeneity both within the primary tumor and between primary tumor and metastases; (4) reliability of biopsies in HER 2 evaluation; and (5) quantity of sample necessary for adequate assessment.
- Citation: Grillo F, Fassan M, Sarocchi F, Fiocca R, Mastracci L. HER2 heterogeneity in gastric/gastroesophageal cancers: From benchside to practice. World J Gastroenterol 2016; 22(26): 5879-5887
- URL: https://www.wjgnet.com/1007-9327/full/v22/i26/5879.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i26.5879
Much has changed in gastric cancer (GC) treatment in the last decade as advances are being made with regards to new, tailored and integrated therapeutic approaches. Nonetheless, prognosis for GC patients still remains dismal as diagnosis is often late and, at least in Western countries, only about half of patients undergo curative resection. Even though worldwide incidence of distal GC has been slowly decreasing, it remains one of the most common causes of cancer-related deaths, with approximately 950000 new cases/year[1] and an estimated number of deaths close to 720000. GC incidence is closely related to geographic distribution and this is mainly due to varied lifestyle characteristics, such as diet and smoking habits, as well as Helicobacter pylori infection[2]. Gastroesophageal junction carcinoma (GEJC) is showing, on the other hand, a rapid rise in incidence in Western countries with a strong predilection for white males[3].
Despite advances in cytotoxic therapies as well as various multimodality treatments, both in the neoadjuvant and adjuvant settings, survival for patients with metastatic disease remains poor, with overall survival rates of 5%-20% at 5 years[4,5]. A relatively recent, randomized phase III trial [Trastuzumab for Gastric Cancer (ToGA)][6] showed improved response rate, median progression-free survival, and overall survival when the monoclonal antibody against HER2, Trastuzumab, was added to the first-line fluoropyrimidine/platinum based treatment in HER2 positive GC/GEJC. Trastuzumab and chemotherapy have since become the new standard of treatment for patients with advanced, HER2 positive, GC/GEJC. Further trials for HER2 positive cases are ongoing, using combination therapies (e.g., Trastuzumab and Bevacizumab[7] and new molecules, such as Lapatinib[8]).
While the predictive role of HER2 has been widely proven, its validity as a prognostic factor in GC/GEJC is still debated, even though more recent reports favor its negative impact on prognosis[9-12]. Contrasting results in different studies may be partly explained by: (1) different study populations with regards to ethnicity and cancer histotype; (2) use of different assays for HER2 evaluation; (3) use of variable criteria for HER2 status evaluation (older reports used HER2 breast scoring criteria); and (4) tumor heterogeneity and its impact on the type of sample tested .
The HER2 proto-oncogene, located on chromosome 17q21[13], encodes for a transmembrane tyrosine-kinase receptor, involved in cell proliferation and survival. Although HER2 gene amplification, with consequent HER2 protein overexpression, were identified in GC soon after their description in breast carcinoma[14], clinical interest in HER2 remained focused on breast cancer for many years. Following the enthusiasm of ToGA trial results, HER2 has become object of great interest even though its role in gastro-esophageal carcinogenesis is still largely unexplored.
Both distal esophagus (adenocarcinoma in Barrett’s esophagus) and gastric (intestinal-type adenocarcinoma) carcinogenesis rely on a multistep process in which a major role is played by longstanding inflammation with replacement of native mucosa by metaplastic epithelium. In this setting, intestinal metaplasia (IM) represents the “carcinogenic field” in which neoplasia (intra-epithelial neoplasia and invasive adenocarcinoma) can develop[15-17]. Few studies have focused on HER-2 status in pre-neoplastic and/or pre-invasive esophageal lesions[18-20], and these demonstrated that the rate of HER2 overexpression/amplification increases along the carcinogenetic cascade, from low grade dysplasia (LGD) to adenocarcinoma, while Barrett’s esophagus metaplastic epithelium is invariably negative. These findings suggest a possible role of HER2 in the dysplasia-adenocarcinoma sequence of the esophagus. Contrasting results were published in a larger series by Hu et al[21] including 116 adenocarcinomas, 18 LGD, 15 high grade dysplasia (HGD), 34 Barrett’s Esophagus (BE) as well as 81 cases of non-intestinal columnar cell metaplasia and 86 cases of esophageal squamous epithelium. HER2 amplification was detected in only one case of HGD and in 21 (18%) adenocarcinomas while all other categories were completely negative.
Even less information is available for gastric carcinogenesis. HER2 positivity was demonstrated in 12.6% of gastric HGDs in comparison to 20.2% of invasive carcinomas by analyzing both the pre-invasive and invasive component of cancer in the same patient[22].
New insights were provided by a comprehensive and exhaustive analysis on HER2 status in the multi-step process of esophageal (non-intestinal columnar metaplasia, IM, LGD, HGD and adenocarcinoma) and gastric (antral IM, LGD, HGD and intestinal-type adenocarcinoma) carcinogenesis[23]. In detail, HER2 amplification was seen in 2/25 LGD, 5/25 HGD and 7/25 adenocarcinomas of the esophagus and in 1/25 LGD, 4/25 HGD and 8/25 adenocarcinomas of the stomach while native esophageal and gastric mucosa and metaplastic lesions were invariably negative. The progressive increase of HER2 amplification rate from LGD to HGD to adenocarcinoma, provides evidence of the possible early involvement of HER2 in esophageal and gastric carcinogenesis. Recent studies on the role of microRNAs (miRNAs) further reinforce the hypothesis that HER2 dysregulation is an early event in these carcinogenetic cascades[24] in a minority of GC/GEJCs.
National and International guidelines regarding HER2 testing have tried to standardize, as much as possible, HER2 testing in diagnostic practice. The three principle aspects which require consideration are: (1) the choice of sample with regards to cancer morphology; (2) the choice of scoring criteria; and (3) the choice of HER2 evaluation methods.
HER2 positive GC/GEJC are more frequently of intestinal type or mixed while diffuse type, including signet ring cell tumors, are generally HER2 negative[25]. In mixed-type carcinomas, samples with a prevalence of intestinal-type areas should be selected when performing HER2 evaluation. Gastro-esophageal carcinomas tend to be more often HER2 positive (33%) compared to GC (21%) according to the ToGA trial and its post hoc exploratory analysis[6,26]. These findings may be related as GEJCs are more often of intestinal-type when compared to GC[27,28]. HER2 expression in unusual histologic subtypes is still controversial as published reports vary greatly, even with opposite findings[29-31].
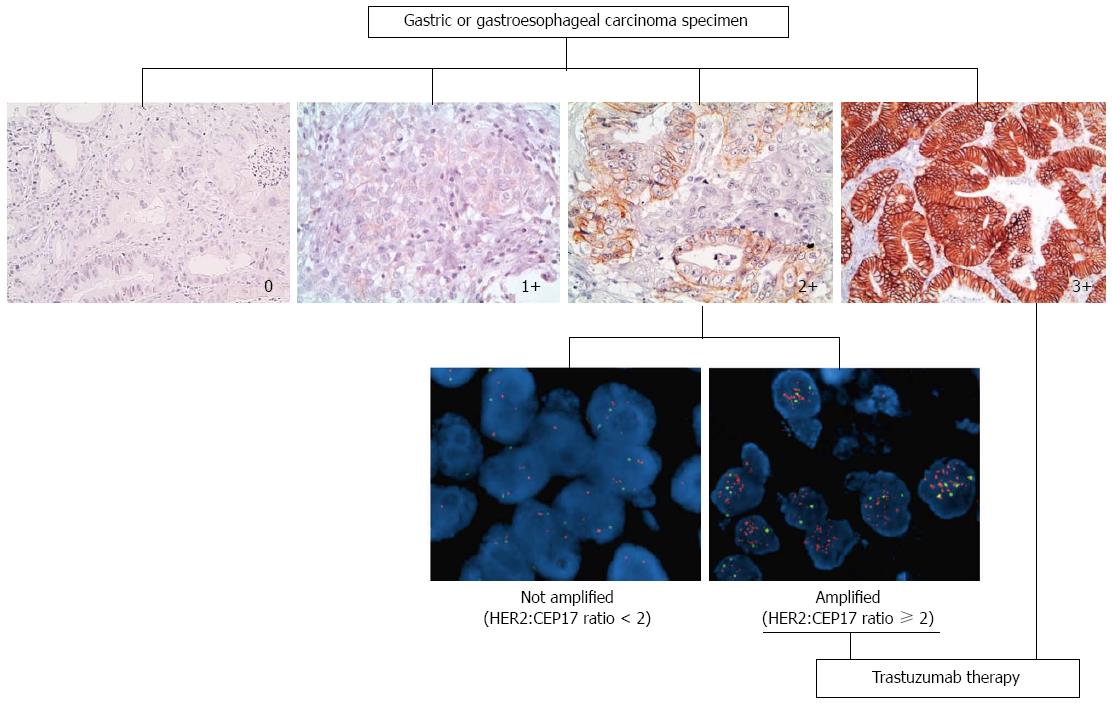
Differences in HER2 staining between breast carcinoma and gastric adenocarcinoma led to a modified Immunohistochemistry (IHC) scoring system for gastric cancer (used in the ToGA trial), which was validated on 168 GC specimens with 93.5% concordance with FISH[32]. Differently to breast cancer, GC HER2 staining does not always show complete membranous staining and so baso-lateral or lateral staining was also considered positive, as this reflects the physiological prevalence of growth factor receptors at these sites. The new scoring system also took into account the more heterogeneous staining pattern in GC and distinguished between surgical and biopsy samples. A 10% cut off was established when evaluation was performed on surgical samples whereas a single cluster of at least 5 positive cells was sufficient in endoscopic biopsies[33]. Staining intensity was however maintained similar to breast cancer in three categories i.e., faint, moderate or intense. Such modified criteria proved to be effective in predicting response to treatment[6] within the ToGA trial.
Early studies reported concordance rates between IHC and in situ hybridization (ISH) to be lower in GC/GEJC than breast cancer, thus suggesting that additional mechanisms, other than gene amplification, may be at the basis of protein overexpression[34,35]. In contrast, more recent studies reported high concordance rates between IHC protein overexpression and ISH amplification with 87%-98% concordance rates[26,29,33], when IHC score 2+, equivocal cases are excluded. For these reasons a simple algorithm has been recommended by the European Medicines Agency (EMA)[36] (http://www.ema.europa.eu/docs/) which states IHC as the initial testing method and ISH only for equivocal score 2+ cases. See Figure 1.
HER2 assessment relies on optimal tissue handling[37,38], abundant tissue for evaluation and optimal scoring criteria. Problematic issues include pre-analytic variables, the identification of the best tissue samples on which to perform HER2 testing, heterogeneity within the primary tumor and heterogeneity between primary tumor and metastases.
Standardized tissue handling, fixation regimens and immunohistochemistry techniques are mandatory for successful and reliable HER2 assessment. Clinicians should be aware that the time from biopsy/surgery to fixation (so called cold ischemia) must be minimized (especially for biopsies which dehydrate quickly) and that this may affect HER2[39,40]. Fixation should be exclusively based on the use of 10% neutral-buffered formalin and fixation time in formalin should be a minimum of 8 h and a maximum of 48 h; prolonged fixation may also lead to unreliable HER2 results[41,42]. HER2 testing must be performed in quality assured laboratories with validated and standardized immunohistochemical testing kits and on freshly cut sections as precut sections tend to lose their antigenicity[43]. This last point is especially important when centralizing tissue in multicenter trials.
The ToGA trial identified approximately 22% of patients who showed HER2 gene amplification at fluorescent in situ hybridization (FISH) but no or faint protein staining at IHC (0 or 1+). These patients did not benefit from treatment with Trastuzumab. Conversely, the highest survival advantage was seen in patients whose cancers were HER2 score 3+ at IHC and FISH amplified or HER2 score 2+ and FISH amplified. Differently to breast cancer, for which ISH testing may be the first approach, HER2 testing in GC should be performed by IHC as the first approach (although in the United States, the FDA approved test is indifferently by IHC or ISH). This does not mean that ISH testing is less important. Indeed, a relatively recent prospective study showed that the level of HER2 gene amplification predicts response and overall survival in HER2 positive gastric cancer treated with Trastuzumab[44]. In close to 70 patients with advanced GC treated with Trastuzumab, the HER2/CEP17 ratio was used to predict response to treatment (optimal HER2/CEP17 ratio threshold of 4.7). ISH testing therefore, may also become useful in stratifying response rates.
Concordance studies between FISH, chromogenic (CISH) or silver based in situ hybridization (SISH) showed concordance rates of 91%-100%, making all these methodologies reliable for HER2 amplification testing[45,46]. Bright field ISH techniques (CISH and SISH), may become the preferred assay in the future, as these methods enable parallel evaluation of the microscopic morphology (i.e., to choose areas with intestinal morphology) as well as alignment between ISH and IHC slides.
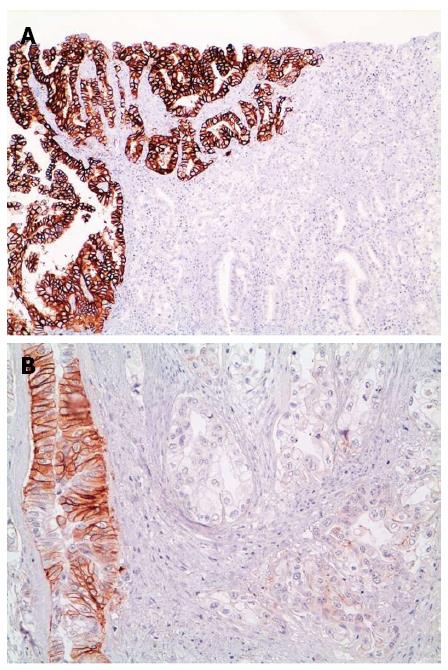
Although initial studies reported that HER2 amplification was highly homogeneous[47,48], tumor heterogeneity (see Figure 2) has now been shown to be extremely relevant in GC/GEJC[49,50]. A wide range of percentages of cases showing heterogeneity appears in the Literature, from a minimum of 5% to a maximum of 69%[22,26,32,50-52]. A possible reason for such discrepancy is that a universally accepted definition of heterogeneity is missing and, indeed, different studies applied widely different definitions. For example, Hofmann et al[32] defined heterogeneity as < 10% of tumor cells staining positive or only focal staining of tumor, Van Cutsem et al[26], identified a cut off of < 30% and Anh et al[52] defined it as a staining pattern between 10%-90% of tumor cells, leading to marked differences in heterogeneity percentages (4.8% vs 50.3% vs 68.6% respectively).
In the post hoc analysis of the HER2 screening data in the ToGA trial[26], a comprehensive (IHC scores 1+, 2+, 3+) HER2 staining heterogeneity of about 50% was detailed and was shown to be at its greatest in the lower IHC categories (IHC score 1+ and score 2+). If assessment of heterogeneity was limited only to IHC 3+ cases, as in many other publications, percentage of heterogeneity dropped to 30%.
Another explanation for variable heterogeneity is HER2 status evaluation on tissue microarray (TMA) samples vs whole slides[53-55], thus leading to its possible underestimation. The TMA technique performs well on homogeneously expressed proteins, however heterogeneous expression may not be correctly picked up on, even if multiple cores are used[37].
The mechanisms leading to HER2 expression heterogeneity are still largely unknown but possibilities include neoplastic clones in which HER2 is amplified/overexpressed in an otherwise HER2 negative tumor or silencing of HER2 expression in an area of a tumor with homogeneous HER2 amplification.
Some experts question the validity of having a minimum area of > 10% cut off value in a clearly heterogeneous expression pattern. An expert panel[37] recommends that cases with < 10% IHC strongly stained tumor cells should also be subjected to ISH testing to reduce false-negative results, and that if amplification is detected, the case should be considered HER2 positive. This proposal has been picked up on by some international guidelines (e.g., Belgium Guidelines for HER2 testing[56]). Furthermore, other authors suggest that a 10% cut off is subject to significant inter-observer variability[57] and that there is a risk of misinterpretation of the staining results leading to denial of treatment. Similarly to the modifications adopted for breast cancer HER2 testing, for which minimum area cut offs were changed from 30% to 10%[58,59], a change in the GC HER2 staining analysis protocol may be considered in the future.
Multi-block analysis[60-64] has been shown to increase sensitivity and accuracy. False negative rates for one block analysis compared to multiblock analysis are between 7% and 10%. This is especially important if one considers that these patients, who would benefit from Trastuzumab therapy, would have been denied this chance. Laboratories should adopt a decisional workflow chart to maximize HER2 positive case discovery, taking into account costs and workload for pathologists. If one block is chosen, this must at least contain the largest amount of differentiated, intestinal type tumor, which is more likely to express HER2.
With the exception of patients who have recurrent disease after surgical resection, HER2 status in inoperable patients is based on endoscopic biopsy evaluation. An important question, which stems from HER2 status heterogeneity, is whether small endoscopic biopsies are reliable for HER2 assessment. The HER2 scoring system for GC/GEJC has, in part, taken into account evaluation on biopsy tissue and, indeed, a IHC 3+ group of 5 neoplastic cells is considered sufficient to define the biopsy as HER-positive[32,33]. A recent study by our group, analyzed[61] a cohort of 103 matched biopsy and surgical specimens for HER2 with IHC and FISH and concordance between the two types of specimens was the main aim of the study. Eighty-nine percent of biopsies were predictive of HER2 status in surgical samples with a concordance rate of 80%, showing a high predictive value of IHC biopsy material. Most of the discordant cases were IHC HER2 negative at biopsy but showed IHC positivity and gene amplification on the surgical specimen. A probable explanation for false negative HER2 status on biopsy is heterogeneity[52,65] whereas HER2 positivity on biopsy and not on surgical resections may be due to prolonged cold ischemia and/or over or under-fixation in larger specimens[66]. Other studies have found variable concordance rates between biopsy and paired surgical resections ranging from 45.5% to 94%[10,22,50,52,65,67-69] questioning the reliability of HER2 status on biopsy material. From a practical point of view, one possible option is to consider ISH analysis for both IHC score 2+ and 1+ biopsy cases, while a second approach is in the repeat assessment[10] of endoscopic biopsies, especially if IHC 1+ or 2+ at initial biopsy as suggested by the GASTHER 1 study. A rescue rate of 8.7% has been shown and, indeed, cases with IHC expression of IHC 1+ or 2+ at initial biopsy are 3.1 times more likely to show HER2 positivity on repeat biopsy than those with IHC 0.
A major problem in HER2 assessment on endoscopic biopsies is the definition of the minimum set of biopsies which the endoscopist must submit for evaluation. National Comprehensive Cancer Network guidelines recommend more than 6 samples to be taken, but this is not evidence-based. Two recent publications[52,70] have focused on this topic. Gullo et al[70] applied virtual biopsies on digitally scanned whole slides of resected GC/GEJCs identifying five superficial samples to be the optimal biopsy set. Anh et al[52] on the other hand used a large series of paired biopsy/resection specimens and identified 4 biopsy samples of cancer as the optimal number. In a real life situation not all samples submitted by the endoscopist prove to contain assessable neoplastic cells at histologic examination. Furthermore, endoscopists should preferentially take biopsies in the lateral parts of the tumor as this area has been shown to be more frequently HER2 positive[71] while the central part of the tumor should be avoided when macroscopically ulcerated.
Discordance in HER2 status between primary and metastatic sites has been found in breast cancer[72] but variable data are available for GC/GEJC[47,73].
Most studies have reported a high concordance between primary and metastases in HER2 status with a discordance rate which varies between 1% to 14%[47,64,73-77]. Both positive (negative in primary tumor and positive in metastasis)[64] and negative (positive in primary tumor and negative in metastasis)[75] conversion have been described. Possible explanations for discrepancies are genetic drift or clonal selection of HER2, during neoplastic progression, or as a consequence of intratumor heterogeneity of HER2. The second hypothesis is probably more likely as heterogeneous HER2 status is often found in the primary tumor[64] and may not be identified if tissue is limited. An inherent problem in many of these studies[47,64,75,78] is the evaluation of HER2 status on TMA which may underestimate heterogeneity and overestimate discrepancies between primary and metastatic sites.
The previously mentioned GASTHER 1 study[10] has shown that repeat HER2 assessment in recurrent sites may be recommended in patients with advanced GC/GEJC whose initial evaluation was HER2 negative (5.7% patients were HER2 positive on biopsy of metastases) and that these patients show similar treatment benefits with Trastuzumab as patients identified as HER2 positive at initial evaluation. In particular, liver as site of metastasis was 5.88 times more likely to show HER2 positivity on repeat biopsy than those who had HER2 reassessment in other metastatic sites. An evaluation of cost and potential harm of repeat biopsies is necessary, however this approach may become important in selected patients.
Despite the fact that the hitherto published data are not always consistent, the following considerations can be made: (1) HER2 assessment in GC/GEJC cancer is reliable once the pre-analytical variables and technical procedures are standardized; (2) endoscopic biopsies can provide reliable HER2 status assessment when a sufficient number of samples are available; (3) IHC and ISH assessment are both reliable, but confirmation by ISH is mandatory in cases of equivocal IHC; (4) tumor heterogeneity is a major problem (but not insurmountable) which must be taken into account when selecting samples; and (5) there is relative consistency between HER2 status in the primary tumor and in distant metastases.
The guidelines and recommendations published so far provide a good basis on which to base technical procedures and diagnostic criteria.
| 1. | Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-E386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20108] [Cited by in RCA: 20718] [Article Influence: 1883.5] [Reference Citation Analysis (23)] |
| 2. | Forman D, Burley VJ. Gastric cancer: global pattern of the disease and an overview of environmental risk factors. Best Pract Res Clin Gastroenterol. 2006;20:633-649. [PubMed] |
| 3. | Edgren G, Adami HO, Weiderpass E, Nyrén O. A global assessment of the oesophageal adenocarcinoma epidemic. Gut. 2013;62:1406-1414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 269] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 4. | Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24:2137-2150. [PubMed] |
| 5. | Green D, Ponce de Leon S, Leon-Rodriguez E, Sosa-Sanchez R. Adenocarcinoma of the stomach: univariate and multivariate analysis of factors associated with survival. Am J Clin Oncol. 2002;25:84-89. [PubMed] |
| 6. | Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5823] [Cited by in RCA: 5527] [Article Influence: 345.4] [Reference Citation Analysis (3)] |
| 7. | De Vita F, Di Martino N, Fabozzi A, Laterza MM, Ventriglia J, Savastano B, Petrillo A, Gambardella V, Sforza V, Marano L. Clinical management of advanced gastric cancer: the role of new molecular drugs. World J Gastroenterol. 2014;20:14537-14558. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 35] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 8. | Hecht JR, Bang YJ, Qin SK, Chung HC, Xu JM, Park JO, Jeziorski K, Shparyk Y, Hoff PM, Sobrero A. Lapatinib in Combination With Capecitabine Plus Oxaliplatin in Human Epidermal Growth Factor Receptor 2-Positive Advanced or Metastatic Gastric, Esophageal, or Gastroesophageal Adenocarcinoma: TRIO-013/LOGiC--A Randomized Phase III Trial. J Clin Oncol. 2016;34:443-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 466] [Cited by in RCA: 494] [Article Influence: 49.4] [Reference Citation Analysis (0)] |
| 9. | Gravalos C, Jimeno A. HER2 in gastric cancer: a new prognostic factor and a novel therapeutic target. Ann Oncol. 2008;19:1523-1529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 737] [Cited by in RCA: 902] [Article Influence: 50.1] [Reference Citation Analysis (2)] |
| 10. | Park SR, Park YS, Ryu MH, Ryoo BY, Woo CG, Jung HY, Lee JH, Lee GH, Kang YK. Extra-gain of HER2-positive cases through HER2 reassessment in primary and metastatic sites in advanced gastric cancer with initially HER2-negative primary tumours: Results of GASTric cancer HER2 reassessment study 1 (GASTHER1). Eur J Cancer. 2016;53:42-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 85] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 11. | Begnami MD, Fukuda E, Fregnani JH, Nonogaki S, Montagnini AL, da Costa WL, Soares FA. Prognostic implications of altered human epidermal growth factor receptors (HERs) in gastric carcinomas: HER2 and HER3 are predictors of poor outcome. J Clin Oncol. 2011;29:3030-3036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 217] [Article Influence: 14.5] [Reference Citation Analysis (1)] |
| 12. | Yoon HH, Shi Q, Sukov WR, Wiktor AE, Khan M, Sattler CA, Grothey A, Wu TT, Diasio RB, Jenkins RB. Association of HER2/ErbB2 expression and gene amplification with pathologic features and prognosis in esophageal adenocarcinomas. Clin Cancer Res. 2012;18:546-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 115] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 13. | Normanno N, Bianco C, Strizzi L, Mancino M, Maiello MR, De Luca A, Caponigro F, Salomon DS. The ErbB receptors and their ligands in cancer: an overview. Curr Drug Targets. 2005;6:243-257. [PubMed] |
| 14. | Yokota J, Yamamoto T, Toyoshima K, Terada M, Sugimura T, Battifora H, Cline MJ. Amplification of c-erbB-2 oncogene in human adenocarcinomas in vivo. Lancet. 1986;1:765-767. [PubMed] |
| 15. | Cassaro M, Rugge M, Tieppo C, Giacomelli L, Velo D, Nitti D, Farinati F. Indefinite for non-invasive neoplasia lesions in gastric intestinal metaplasia: the immunophenotype. J Clin Pathol. 2007;60:615-621. [PubMed] |
| 16. | Correa P, Piazuelo MB, Wilson KT. Pathology of gastric intestinal metaplasia: clinical implications. Am J Gastroenterol. 2010;105:493-498. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 290] [Cited by in RCA: 295] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 17. | Saraggi D, Fassan M, Bornschein J, Farinati F, Realdon S, Valeri N, Rugge M. From Barrett metaplasia to esophageal adenocarcinoma: the molecular background. Histol Histopathol. 2016;31:25-32. [PubMed] |
| 18. | Villanacci V, Rossi E, Grisanti S, Bassotti G, Ferrari VD, Missale G, Minelli L, Cengia P, Marini G, Cestari R. Targeted therapy with trastuzumab in dysplasia and adenocarcinoma arising in Barrett’s esophagus: a translational approach. Minerva Gastroenterol Dietol. 2008;54:347-353. [PubMed] |
| 19. | Rossi E, Grisanti S, Villanacci V, Della Casa D, Cengia P, Missale G, Minelli L, Buglione M, Cestari R, Bassotti G. HER-2 overexpression/amplification in Barrett’s oesophagus predicts early transition from dysplasia to adenocarcinoma: a clinico-pathologic study. J Cell Mol Med. 2009;13:3826-3833. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 20. | Rossi E, Villanacci V, Bassotti G, Donato F, Festa A, Cengia G, Grisanti S, Cestari R. TOPOIIalpha and HER-2/neu overexpression/amplification in Barrett’s oesophagus, dysplasia and adenocarcinoma. Histopathology. 2010;57:81-89. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 21. | Hu Y, Bandla S, Godfrey TE, Tan D, Luketich JD, Pennathur A, Qiu X, Hicks DG, Peters JH, Zhou Z. HER2 amplification, overexpression and score criteria in esophageal adenocarcinoma. Mod Pathol. 2011;24:899-907. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 22. | Lee S, de Boer WB, Fermoyle S, Platten M, Kumarasinghe MP. Human epidermal growth factor receptor 2 testing in gastric carcinoma: issues related to heterogeneity in biopsies and resections. Histopathology. 2011;59:832-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 109] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 23. | Fassan M, Mastracci L, Grillo F, Zagonel V, Bruno S, Battaglia G, Pitto F, Nitti D, Celiento T, Zaninotto G. Early HER2 dysregulation in gastric and oesophageal carcinogenesis. Histopathology. 2012;61:769-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 24. | Fassan M, Pizzi M, Realdon S, Balistreri M, Guzzardo V, Zagonel V, Castoro C, Mastracci L, Farinati F, Nitti D. The HER2-miR125a5p/miR125b loop in gastric and esophageal carcinogenesis. Hum Pathol. 2013;44:1804-1810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 25. | Barros-Silva JD, Leitão D, Afonso L, Vieira J, Dinis-Ribeiro M, Fragoso M, Bento MJ, Santos L, Ferreira P, Rêgo S. Association of ERBB2 gene status with histopathological parameters and disease-specific survival in gastric carcinoma patients. Br J Cancer. 2009;100:487-493. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 135] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 26. | Van Cutsem E, Bang YJ, Feng-Yi F, Xu JM, Lee KW, Jiao SC, Chong JL, López-Sanchez RI, Price T, Gladkov O. HER2 screening data from ToGA: targeting HER2 in gastric and gastroesophageal junction cancer. Gastric Cancer. 2015;18:476-484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 452] [Cited by in RCA: 456] [Article Influence: 41.5] [Reference Citation Analysis (1)] |
| 27. | Shah MA, Khanin R, Tang L, Janjigian YY, Klimstra DS, Gerdes H, Kelsen DP. Molecular classification of gastric cancer: a new paradigm. Clin Cancer Res. 2011;17:2693-2701. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 243] [Cited by in RCA: 246] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 28. | Rüschoff J. Adenocarcinoma of the GEJ: gastric or oesophageal cancer? Recent Results Cancer Res. 2012;196:107-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 29. | Yano T, Doi T, Ohtsu A, Boku N, Hashizume K, Nakanishi M, Ochiai A. Comparison of HER2 gene amplification assessed by fluorescence in situ hybridization and HER2 protein expression assessed by immunohistochemistry in gastric cancer. Oncol Rep. 2006;15:65-71. [PubMed] |
| 30. | Roh JH, Srivastava A, Lauwers GY, An J, Jang KT, Park CK, Sohn TS, Kim S, Kim KM. Micropapillary carcinoma of stomach: a clinicopathologic and immunohistochemical study of 11 cases. Am J Surg Pathol. 2010;34:1139-1146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 31. | Giuffrè G, Ieni A, Barresi V, Caruso RA, Tuccari G. HER2 status in unusual histological variants of gastric adenocarcinomas. J Clin Pathol. 2012;65:237-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 32. | Hofmann M, Stoss O, Shi D, Büttner R, van de Vijver M, Kim W, Ochiai A, Rüschoff J, Henkel T. Assessment of a HER2 scoring system for gastric cancer: results from a validation study. Histopathology. 2008;52:797-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 777] [Cited by in RCA: 868] [Article Influence: 48.2] [Reference Citation Analysis (0)] |
| 33. | Rüschoff J, Dietel M, Baretton G, Arbogast S, Walch A, Monges G, Chenard MP, Penault-Llorca F, Nagelmeier I, Schlake W. HER2 diagnostics in gastric cancer-guideline validation and development of standardized immunohistochemical testing. Virchows Arch. 2010;457:299-307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 342] [Cited by in RCA: 372] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 34. | Kameda T, Yasui W, Yoshida K, Tsujino T, Nakayama H, Ito M, Ito H, Tahara E. Expression of ERBB2 in human gastric carcinomas: relationship between p185ERBB2 expression and the gene amplification. Cancer Res. 1990;50:8002-8009. [PubMed] |
| 35. | Lemoine NR, Jain S, Silvestre F, Lopes C, Hughes CM, McLelland E, Gullick WJ, Filipe MI. Amplification and overexpression of the EGF receptor and c-erbB-2 proto-oncogenes in human stomach cancer. Br J Cancer. 1991;64:79-83. [PubMed] |
| 36. | European Medicines Agency. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/00278/WC500074922. |
| 37. | Rüschoff J, Hanna W, Bilous M, Hofmann M, Osamura RY, Penault-Llorca F, van de Vijver M, Viale G. HER2 testing in gastric cancer: a practical approach. Mod Pathol. 2012;25:637-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 379] [Cited by in RCA: 450] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 38. | Sauter G, Lee J, Bartlett JM, Slamon DJ, Press MF. Guidelines for human epidermal growth factor receptor 2 testing: biologic and methodologic considerations. J Clin Oncol. 2009;27:1323-1333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 389] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 39. | Khoury T, Sait S, Hwang H, Chandrasekhar R, Wilding G, Tan D, Kulkarni S. Delay to formalin fixation effect on breast biomarkers. Mod Pathol. 2009;22:1457-1467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 164] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 40. | Portier BP, Wang Z, Downs-Kelly E, Rowe JJ, Patil D, Lanigan C, Budd GT, Hicks DG, Rimm DL, Tubbs RR. Delay to formalin fixation ‘cold ischemia time’: effect on ERBB2 detection by in-situ hybridization and immunohistochemistry. Mod Pathol. 2013;26:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 41. | Moatamed NA, Nanjangud G, Pucci R, Lowe A, Shintaku IP, Shapourifar-Tehrani S, Rao N, Lu DY, Apple SK. Effect of ischemic time, fixation time, and fixative type on HER2/neu immunohistochemical and fluorescence in situ hybridization results in breast cancer. Am J Clin Pathol. 2011;136:754-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 57] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 42. | Yamashita-Kashima Y, Shu S, Yorozu K, Hashizume K, Moriya Y, Fujimoto-Ouchi K, Harada N. Importance of formalin fixing conditions for HER2 testing in gastric cancer: immunohistochemical staining and fluorescence in situ hybridization. Gastric Cancer. 2014;17:638-647. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 43. | Grillo F, Pigozzi S, Ceriolo P, Calamaro P, Fiocca R, Mastracci L. Factors affecting immunoreactivity in long-term storage of formalin-fixed paraffin-embedded tissue sections. Histochem Cell Biol. 2015;144:93-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 84] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 44. | Gomez-Martin C, Plaza JC, Pazo-Cid R, Salud A, Pons F, Fonseca P, Leon A, Alsina M, Visa L, Rivera F. Level of HER2 gene amplification predicts response and overall survival in HER2-positive advanced gastric cancer treated with trastuzumab. J Clin Oncol. 2013;31:4445-4452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 163] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 45. | Hanna WM, Kwok K. Chromogenic in-situ hybridization: a viable alternative to fluorescence in-situ hybridization in the HER2 testing algorithm. Mod Pathol. 2006;19:481-487. [PubMed] |
| 46. | Dietel M, Ellis IO, Höfler H, Kreipe H, Moch H, Dankof A, Kölble K, Kristiansen G. Comparison of automated silver enhanced in situ hybridisation (SISH) and fluorescence ISH (FISH) for the validation of HER2 gene status in breast carcinoma according to the guidelines of the American Society of Clinical Oncology and the College of American Pathologists. Virchows Arch. 2007;451:19-25. [PubMed] |
| 47. | Marx AH, Tharun L, Muth J, Dancau AM, Simon R, Yekebas E, Kaifi JT, Mirlacher M, Brümmendorf TH, Bokemeyer C. HER-2 amplification is highly homogenous in gastric cancer. Hum Pathol. 2009;40:769-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 170] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 48. | Bilous M, Osamura RY, Rüschoff J, van de Vijver M, Hanna W, Penault-Llorca F, Roche P. HER-2 amplification is highly homogenous in gastric cancer. Hum Pathol. 2010;41:304-305; author reply 305-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 49. | Grabsch H, Sivakumar S, Gray S, Gabbert HE, Müller W. HER2 expression in gastric cancer: Rare, heterogeneous and of no prognostic value - conclusions from 924 cases of two independent series. Cell Oncol. 2010;32:57-65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 131] [Reference Citation Analysis (0)] |
| 50. | Lee HE, Park KU, Yoo SB, Nam SK, Park do J, Kim HH, Lee HS. Clinical significance of intratumoral HER2 heterogeneity in gastric cancer. Eur J Cancer. 2013;49:1448-1457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 138] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 51. | Wang T, Hsieh ET, Henry P, Hanna W, Streutker CJ, Grin A. Matched biopsy and resection specimens of gastric and gastroesophageal adenocarcinoma show high concordance in HER2 status. Hum Pathol. 2014;45:970-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 52. | Ahn S, Ahn S, Van Vrancken M, Lee M, Ha SY, Lee H, Min BH, Lee JH, Kim JJ, Choi S. Ideal number of biopsy tumor fragments for predicting HER2 status in gastric carcinoma resection specimens. Oncotarget. 2015;6:38372-38380. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 53. | Warneke VS, Behrens HM, Böger C, Becker T, Lordick F, Ebert MP, Röcken C. Her2/neu testing in gastric cancer: evaluating the risk of sampling errors. Ann Oncol. 2013;24:725-733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 104] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 54. | Kim KC, Koh YW, Chang HM, Kim TH, Yook JH, Kim BS, Jang SJ, Park YS. Evaluation of HER2 protein expression in gastric carcinomas: comparative analysis of 1,414 cases of whole-tissue sections and 595 cases of tissue microarrays. Ann Surg Oncol. 2011;18:2833-2840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 130] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 55. | Stahl P, Seeschaaf C, Lebok P, Kutup A, Bockhorn M, Izbicki JR, Bokemeyer C, Simon R, Sauter G, Marx AH. Heterogeneity of amplification of HER2, EGFR, CCND1 and MYC in gastric cancer. BMC Gastroenterol. 2015;15:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 107] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 56. | Jouret-Mourin A, Hoorens A, De Hertogh G, Vanderveken J, Demetter P, Van Cutsem E. Analysis of HER2 expression and gene amplification in adenocarcinoma of the stomach and the gastro-oesophageal junction: rationale for the Belgian way of working. Acta Gastroenterol Belg. 2012;75:9-13. [PubMed] |
| 57. | Behrens HM, Warneke VS, Böger C, Garbrecht N, Jüttner E, Klapper W, Mathiak M, Oschlies I, Rudolph U, Stuhlmann-Laeisz C. Reproducibility of Her2/neu scoring in gastric cancer and assessment of the 10% cut-off rule. Cancer Med. 2015;4:235-244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 58. | Moeder CB, Giltnane JM, Harigopal M, Molinaro A, Robinson A, Gelmon K, Huntsman D, Camp RL, Rimm DL. Quantitative justification of the change from 10% to 30% for human epidermal growth factor receptor 2 scoring in the American Society of Clinical Oncology/College of American Pathologists guidelines: tumor heterogeneity in breast cancer and its implications for tissue microarray based assessment of outcome. J Clin Oncol. 2007;25:5418-5425. [PubMed] |
| 59. | Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, Allred DC, Bartlett JM, Bilous M, Fitzgibbons P. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31:3997-4013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2910] [Cited by in RCA: 3135] [Article Influence: 241.2] [Reference Citation Analysis (0)] |
| 60. | Asioli S, Maletta F, Verdun di Cantogno L, Satolli MA, Schena M, Pecchioni C, Botta C, Chiusa L, Molinaro L, Conti L. Approaching heterogeneity of human epidermal growth factor receptor 2 in surgical specimens of gastric cancer. Hum Pathol. 2012;43:2070-2079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 61. | Grillo F, Fassan M, Ceccaroli C, Giacometti C, Curto M, Zagonel V, Ceppa P, Nitti D, Castoro C, Fiocca R. The Reliability of Endoscopic Biopsies in Assessing HER2 Status in Gastric and Gastroesophageal Junction Cancer: A Study Comparing Biopsies with Surgical Samples. Transl Oncol. 2013;6:10-16. [PubMed] |
| 62. | Ge X, Wang H, Zeng H, Jin X, Sujie A, Xu C, Liu Y, Huang J, Ji Y, Tan Y. Clinical significance of assessing Her2/neu expression in gastric cancer with dual tumor tissue paraffin blocks. Hum Pathol. 2015;46:850-857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 63. | Grillo F, Fassan M, Fiocca R, Mastracci L. Heterogeneous Her2/Neu expression in gastric and gastroesophageal cancer. Hum Pathol. 2016;48:173-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 64. | Kim MA, Lee HJ, Yang HK, Bang YJ, Kim WH. Heterogeneous amplification of ERBB2 in primary lesions is responsible for the discordant ERBB2 status of primary and metastatic lesions in gastric carcinoma. Histopathology. 2011;59:822-831. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 115] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 65. | Yang J, Luo H, Li Y, Li J, Cai Z, Su X, Dai D, Du W, Chen T, Chen M. Intratumoral heterogeneity determines discordant results of diagnostic tests for human epidermal growth factor receptor (HER) 2 in gastric cancer specimens. Cell Biochem Biophys. 2012;62:221-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 88] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 66. | Kumarasinghe AP, de Boer B, Bateman AC, Kumarasinghe MP. DNA mismatch repair enzyme immunohistochemistry in colorectal cancer: a comparison of biopsy and resection material. Pathology. 2010;42:414-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 67. | Watson S, Validire P, Cervera P, Zorkani N, Scriva A, Lemay F, Tournigand C, Perniceni T, Garcia ML, Bennamoun M. Combined HER2 analysis of biopsies and surgical specimens to optimize detection of trastuzumab-eligible patients in eso-gastric adenocarcinoma: a GERCOR study. Ann Oncol. 2013;24:3035-3039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 68. | Yoshida H, Yamamoto N, Taniguchi H, Oda I, Katai H, Kushima R, Tsuda H. Comparison of HER2 status between surgically resected specimens and matched biopsy specimens of gastric intestinal-type adenocarcinoma. Virchows Arch. 2014;465:145-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 69. | Yan B, Yau EX, Choo SN, Ong CW, Yong KJ, Pang B, Salto-Tellez M. Dual-colour HER2/chromosome 17 chromogenic in situ hybridisation assay enables accurate assessment of HER2 genomic status in gastric cancer and has potential utility in HER2 testing of biopsy samples. J Clin Pathol. 2011;64:880-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 70. | Gullo I, Grillo F, Molinaro L, Fassan M, De Silvestri A, Tinelli C, Rugge M, Fiocca R, Mastracci L. Minimum biopsy set for HER2 evaluation in gastric and gastro-esophageal junction cancer. Endosc Int Open. 2015;3:E165-E170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 71. | Tominaga N, Gotoda T, Hara M, Hale MD, Tsuchiya T, Matsubayashi J, Kono S, Kusano C, Itoi T, Fujimoto K. Five biopsy specimens from the proximal part of the tumor reliably determine HER2 protein expression status in gastric cancer. Gastric Cancer. 2016;19:553-560. [PubMed] |
| 72. | Santinelli A, Pisa E, Stramazzotti D, Fabris G. HER-2 status discrepancy between primary breast cancer and metastatic sites. Impact on target therapy. Int J Cancer. 2008;122:999-1004. [PubMed] |
| 73. | Fassan M, Ludwig K, Pizzi M, Castoro C, Guzzardo V, Balistreri M, Zaninotto G, Ruol A, Giacomelli L, Ancona E. Human epithelial growth factor receptor 2 (HER2) status in primary and metastatic esophagogastric junction adenocarcinomas. Hum Pathol. 2012;43:1206-1212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 74. | Bozzetti C, Negri FV, Lagrasta CA, Crafa P, Bassano C, Tamagnini I, Gardini G, Nizzoli R, Leonardi F, Gasparro D. Comparison of HER2 status in primary and paired metastatic sites of gastric carcinoma. Br J Cancer. 2011;104:1372-1376. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 122] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 75. | Cho EY, Park K, Do I, Cho J, Kim J, Lee J, Kim S, Kim KM, Sohn TS, Kang WK. Heterogeneity of ERBB2 in gastric carcinomas: a study of tissue microarray and matched primary and metastatic carcinomas. Mod Pathol. 2013;26:677-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 80] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 76. | Fusco N, Rocco EG, Del Conte C, Pellegrini C, Bulfamante G, Di Nuovo F, Romagnoli S, Bosari S. HER2 in gastric cancer: a digital image analysis in pre-neoplastic, primary and metastatic lesions. Mod Pathol. 2013;26:816-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 77. | Cappellesso R, Fassan M, Hanspeter E, Bornschein J, d’Amore ES, Cuorvo LV, Mazzoleni G, Barbareschi M, Pizzi M, Guzzardo V. HER2 status in gastroesophageal cancer: a tissue microarray study of 1040 cases. Hum Pathol. 2015;46:665-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 78. | Reichelt U, Duesedau P, Tsourlakis MCh, Quaas A, Link BC, Schurr PG, Kaifi JT, Gros SJ, Yekebas EF, Marx A, Simon R, Izbicki JR, Sauter G. Frequent homogeneous HER-2 amplification in primary and metastatic adenocarcinoma of the esophagus. Mod Pathol. 2007;20:120-129. [PubMed] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Manuscript source: Invited manuscript
P- Reviewer: Arsenijevic T, Di Lauro L, Huang CM S- Editor: Ma YJ L- Editor: A E- Editor: Zhang DN