Published online Jul 7, 2016. doi: 10.3748/wjg.v22.i25.5655
Peer-review started: March 24, 2016
First decision: May 12, 2016
Revised: May 27, 2016
Accepted: June 15, 2016
Article in press: June 15, 2016
Published online: July 7, 2016
Processing time: 102 Days and 18.4 Hours
The incidence of inflammatory bowel diseases (IBD) - Crohn’s disease (CD) and ulcerative colitis (UC) - has been increasing on a global scale, and progressively, more gastroenterologists will be included in the diagnosis and treatment of IBD. Although IBD primarily affects the intestinal tract, extraintestinal manifestations of the disease are often apparent, including in the oral cavity, especially in CD. Specific oral manifestations in patients with CD are as follows: indurate mucosal tags, cobblestoning and mucogingivitis, deep linear ulcerations and lip swelling with vertical fissures. The most common non-specific manifestations, such as aphthous stomatitis and angular cheilitis, occur in both diseases, while pyostomatitis vegetans is more pronounced in patients with UC. Non-specific lesions in the oral cavity can also be the result of malnutrition and drugs. Malnutrition, followed by anemia and mineral and vitamin deficiency, affects the oral cavity and teeth. Furthermore, all of the drug classes that are applied to the treatment of inflammatory bowel diseases can lead to alterations in the oral cavity due to the direct toxic effects of the drugs on oral tissues, as well as indirect immunosuppressive effects with a risk of developing opportunistic infections or bone marrow suppression. There is a higher occurrence of malignant diseases in patients with IBD, which is related to the disease itself and to the IBD-related therapy with a possible oral pathology. Treatment of oral lesions includes treatment of the alterations in the oral cavity according to the etiology together with treatment of the primary intestinal disease, which requires adequate knowledge and a strong cooperation between gastroenterologists and specialists in oral medicine.
Core tip: Inflammatory bowel diseases (IBD), Crohn’s disease (CD) and ulcerative colitis affect the intestinal tract, but can also present with extraintestinal manifestations and complications. In CD, disease-specific lesions with granulomatous changes can occur in the oral cavity. However, non-specific lesions are more common in IBD and are mostly caused by malnutrition and medications. All of the drug classes that are applied in the treatment of IBD can lead to lesions in the oral cavity. This paper offers an overview of the oral pathology with a detailed description of the complications related to malnutrition and IBD therapy.
- Citation: Muhvić-Urek M, Tomac-Stojmenović M, Mijandrušić-Sinčić B. Oral pathology in inflammatory bowel disease. World J Gastroenterol 2016; 22(25): 5655-5667
- URL: https://www.wjgnet.com/1007-9327/full/v22/i25/5655.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i25.5655
Crohn’s disease (CD) and ulcerative colitis (UC) belong to the group of chronic inflammatory bowel diseases (IBD). Although the etiology of these diseases has not been completely ascertained, it is well known that the factors contributing to disease pathogenesis include environmental aspects, intestinal microflora, genetic predisposition and pathological immune responses[1-3]. North America and North-Western Europe exhibit the highest incidence and prevalence of the disease, but an increase in the number of patients has been observed worldwide, indicating its emergence as a global disease[4,5]. It appears that the global increase in the disease is related to a “Western” lifestyle and diet, which shows the strong impact of the environment on the occurrence of the disease[1,3,6].
In addition to affecting the intestinal tract, the disease can manifest with extraintestinal symptoms in almost every organ system and significantly influence the quality of life and the functional state of the patient. There is a distinction between extraintestinal manifestations (EIM) and extraintestinal complications, although they are sometimes difficult to distinguish. EIMs occur in 6% to 47% of patients[7-10] with different rates of occurrence in relation to the primary disease. Peripheral arthropathy type I, aphthous stomatitis, erythema nodosum and episcleritis are usually related to an active disease. Ankylosing spondylitis and peripheral arthropathy type II have their own course of disease, independent of the activity of the bowel disease. Primary sclerosing cholangitis, uveitis and pyoderma gangrenosum have a variable course and can be associated with, but do not have to be related to, the activity of the bowel disease[10]. Patients with perianal CD, patients with colonic disease and smokers are at an increased risk of developing EIMs[9,10]. Furthermore, patients can develop several EIMs at the same time, and the occurrence of one EIM increases the risk of developing another EIM[10].
The pathogenesis of EIMs is still not fully identified. It appears that the inflamed intestinal mucosa can trigger immunological responses by sharing common epitopes (e.g., intestinal bacteria and synovia). Bacteria that can translocate because of greater permeability of the intestinal mucosa trigger an acquired immune response that does not distinguish between a bacterial epitope and a joint or skin epitope[10]. In patients with extraintestinal disease manifestations, there is also a strong genetic predisposition; the connection between the EIMs and the major histocompatibility complex loci has been demonstrated, and there is a concordance for EIMs in 84% of siblings[8,9].
Extraintestinal complications of inflammatory bowel diseases frequently occur due to malnutrition, chronic inflammation and side effects of drugs[2,8-10].
Oral lesions are common in patients with IBD and epidemiology data vary over a wide range of 5%-50% due to contradictory studies[11,12]. The goal of our paper is to present the oral pathology of IBD, whether it includes extraintestinal manifestations of the disease or its complications.
Oral manifestations of CD include specific and non-specific lesions, while in patients with UC, only non-specific lesions in the oral cavity are observed. Characteristics of specific lesions include the presence of non-caseous granulomas, which occur only in CD patients. CD manifestations in the oral cavity can precede the intestinal manifestations, occur at the same time as the intestinal manifestations or occur after the occurrence of the intestinal manifestations. Oral lesions can be of significance when diagnosing CD because of a characteristic non-caseous granulomatous inflammation[12-17]. This condition can be easily confirmed by a histopathological examination of accessible lesions in the oral cavity. Turchi et al[18] described a rare case of tonsillar granuloma as a manifestation of CD. Oral manifestations of the disease are more common in males[15,19,20] and children[12,19,21,22]. They are also more common in patients with CD than in patients with UC[23,24], and their prevalence in CD patients ranges from 20%-50%[11], while in UC the prevalence is 8%[25]. However, some studies have not demonstrated a statistically significant difference[26]. Furthermore, data from the literature are contradictory; in some studies, it has been stated that the changes in the oral cavity are not related to the CD activity index[27] or to the localization of intestinal manifestations[23,24,28,29], although there are papers that do link and attribute the changes in the oral cavity to inflammatory responses following the exacerbation of CD[26,30].
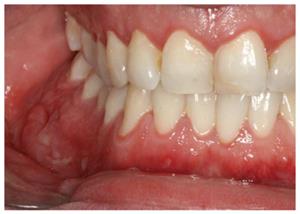
Specific oral lesions in patients with CD include a cobblestone appearance of the oral mucosa; deep linear ulcerations; mucosal tags; swelling of the lips, cheeks and face; lip and tongue fissures; and mucogingivitis[15,19,21,22,25,31]. The mucosa of the oral cavity is hyperplastic and resembles a “cobblestone”, which marks the nodular, granulomatous swelling of the oral mucosa (Figure 1). In addition, there are also indurated polypoid fringe-like lesions of the vestibule and the retromolar region. Mucosal tags and deep ulcerations with hyperplastic edges, firm or boggy to palpation, are mostly present in the labial and buccal mucosa and in retromolar regions. Attached gingiva and alveolar mucosa become swollen, granulated and hyperplastic with or without ulcerations[19,32]. Edema of the face, of one or both lips and of the buccal mucosa may also occur. This condition is unpleasant for patients because it can lead to facial deformation[15,19,22,25]. Non-caseous granulomatous inflammation[33] can be histologically detected in such lesions. The lips are the most commonly affected, and they are usually painless, tender and firm to palpation[34]. Numerous patients with swollen lips also develop painful vertical fissures where many microorganisms can be isolated[35].
Furthermore, patients with CD often experience autoimmune changes of the minor salivary glands and dry mouth[23,36]. Mills et al[36] reported a case of patient with CD in which characteristic granulomatous lesions caused rupture of the excretory salivary duct leading to the formation of a cutaneous salivary duct fistula. Chronic inflammatory processes near the parotid duct resulted in partial to total duct obstruction and caused dilated ducts and cyst formation, which can lead to the formation of cutaneous fistula. All of these changes can cause reduction in saliva and dry mouth[23].
Non-specific oral lesions associated with CD and UC include aphthous stomatitis, angular cheilitis, pyostomatitis vegetans, glossitis and lichen planus[13,17,19,23,31,37,38]. Non-specific lesions, present in both CD and UC patients, are more common than specific lesions, which makes the differential diagnosis very difficult[13]. Non-specific lesions occur due to chronic inflammation, malnutrition and as a side effect of drugs. The occurrence of halitosis[23], dental erosion, dental caries[20,30,39,40], candidiasis, odynophagia and dysphagia[23,26] is more common in patients with IBD than in the general population. Furthermore, patients with UC exhibit diffuse pustules and non-specific gingivitis in the oral cavity[19].
Aphthous stomatitis, which occurs in both CD and UC patients, presents as shallow, round ulcers surrounded by an erythematous “halo” with a central fibrin membrane[19,31]. Aphthous stomatitis does not differ from the stomatitis that occurs in the general population. If aphthous ulcerations are present, the presence of inflammatory bowel disease must be suspected, although intestinal symptoms may not yet be present[28,29]. Because oral lesions are more common in children and can precede the development of inflammatory bowel disease, cooperation between a specialist in oral medicine and a gastroenterologist is crucial to detect the disease as early as possible[12,13].
Angular cheilitis is clinically manifested as erythema with or without painful fissures and sores at the corners of the mouth. It can occur due to anemia or as a manifestation of a fungal or bacterial infection[25,41-43].
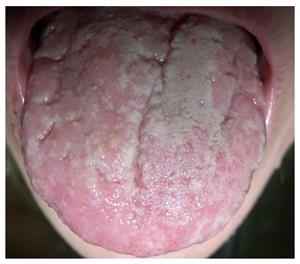
Pyostomatitis vegetans is a rare benign chronic inflammatory mucocutaneous disorder characterized by pustules of an unknown etiology. It is related to inflammatory bowel disease, occurs more often in combination with UC (although it can occur in CD) and is more common in male patients. It represents a specific inflammatory marker of UC[19,33]. Only about fifty cases of this disorder have been described to-date[37,38,44-50]. Macroscopically, the disease manifests as small exophytic lesions with an erythematous perimeter and a creamy white or yellow surface. They are covered with vulnerable membranes and their cracking results in small superficial erosions or ulcers. The confluence of those lesions results in the characteristic morphology sign of a “snail track”[33]. The alterations occur in the upper and lower frontal vestibule, on the tongue (Figure 2) and gingiva, as well as on the soft and hard palate[33]. Microscopically, there are no granulomas in the lesions.
Although IBD complications lead to non-specific lesions in the oral cavity, the consequences of two of the most common complications, malnutrition and medications administered for the treatment of inflammatory bowel disease, must be emphasized. Table 1 summarizes the oral manifestations and complications of IBD.
| Oral tissue | Manifestations | Etiology |
| Lips | Lips swelling with or without fissures[12,15,16,21,22,34,36,156] | Crohn's disease |
| Angular cheilitis[15,22,31] | Fungal and bacterial infections, nutritional deficiency | |
| Erythema multiforme, Stevens-Johnson syndrome[143,144] | Infliximab; adalimumab | |
| Tongue | Fissuring[23] | Crohn's disease |
| Cobblestone plaques[17] | Crohn's disease | |
| Pyostomatitis vegetans[17,37,38,45] | Ulcerative colitis (more common) and Crohn's disease | |
| Aphthous stomatitis[11,31] | Nutritional deficiency; decreased heat shock protein 27 expression | |
| Taste disturbance[17,23,147,155] | Related to disease activity and nutritional habits; sulfasalazine; metronidazole | |
| Candida infections[78,131,132,141] | Corticosteroids; thiopurines; cyclosporin A; infliximab | |
| Erythema multiforme, Stevens-Johnson syndrome[143,144] | Infliximab; adalimumab | |
| Oral mucosa | Buccal edema[15,34,48] | Crohn's disease |
| (labial/buccal/ | Cobblestoning[12,14,15,21,24,34] | Crohn's disease |
| palatal/vestibular) | Deep linear ulceration[15,16,21-24,34,38] | Crohn's disease |
| Mucosal tags[12,16,21,29,34] | Crohn's disease | |
| Buccal sulcus ulcerations[20,34] | Crohn's disease | |
| Palatal ulcerations[20,44] | Crohn's disease | |
| Pyostomatitis vegetans[33,37,45-47,49,50] | Ulcerative colitis (more common) and Crohn's disease | |
| Aphthous stomatitis[11,22,29,31,45] | Nutritional deficiency; decreased heat shock protein 27 expression | |
| Lichen planus/lichenoid reaction[29,85,140,142] | Sulfasalazine; mesalazine; infliximab; | |
| certolizumab pegol | ||
| Erythema multiforme, Stevens-Johnson syndrome[143,144] | Infliximab; adalimumab | |
| Periodontal tissue | Mucogingivitis[12,21,22,32,34] | Crohn's disease |
| Cobblestoning[29,32] | Crohn's disease | |
| Pyostomatitis vegetans/pustular ulcerations[17,32,33,37,44,46,50] | Ulcerative colitis (more common) and Crohn's disease | |
| Nonspecific gingivitis[13,19,147] | Cause not clearly specified | |
| Periodontal diseases/periodontitis[20,156] | Cause not clearly specified | |
| Alveolar bone | Periapical lesions[30] | Related to disease activity |
| Alveolar bone loss[156] | Cause not clearly specified | |
| Teeth | Caries[20,30,39,40] | Related to disease activity and malabsorption |
| Hypoplasia of enamel[40] | Related to disease activity and malabsorption | |
| Salivary glands | Hyposalivation/dry mouth[23,36] | Granulomatous inflammation |
| Autoimmune changes in minor salivary glands | ||
| Salivary duct fistula[36] | Crohn's disease |
Malnutrition is present in 23% of outpatients and 85% of hospitalized patients suffering from IBD[51]. It is caused by a reduced food intake, reduced resorption of nutrients, gastrointestinal losses, increased metabolic needs and as a side effect of drugs[51,52]. As a result of a nutrient deficiency, patients develop anemia due to lower iron, vitamin B12 and folate levels. In addition to anemia, a deficiency of electrolytes, trace elements and vitamins is also quite common[52,53]. Anemia is a common complication of inflammatory bowel disease, which can be manifested in oral pathology. Suffering from anemia can strongly influence the patient’s quality of life. Bleeding and reduced iron reabsorption (due to inflammatory changes in the duodenum and upper jejunum) lead to a microcytic, hypochromic anemia, while the presence of proinflammatory cytokines causes chronic anemia with a high hyperferritinemia. The deficiency of B12 and folates leads to macrocytic anemia. The deficiency of B12 occurs most often in Crohn’s disease due to reabsorption deficiency in the terminal ileum, while the deficiency of folates can be caused by reduced reabsorption, inadequate dietary intake and as a side effect of methotrexate and sulfasalazine[7,54].
Anemia caused by an iron deficiency is manifested as paleness of the oral mucosa[55], generalized oral mucosal atrophy, pricking[56,57], atrophic glossitis with tongue pain[57] and angular cheilitis[43,55]. The deficiency of vitamin B12 is manifested in the oral cavity as a painful atrophy of the oral mucosa and the tongue, recurrent aphthous ulcerations[58,59], angular cheilitis, oral candidiasis, diffuse erythematous stomatitis and pale yellowish mucosa, especially on the palate[60]. Patients can also complain of altered taste, a burning sensation in the mouth and dysphagia[43]. If anemia is caused by a folate deficiency, the manifestations in the oral cavity are the same as in anemia caused by vitamin B12 deficiency but without neurological symptoms[43]. In more severe cases, ulcerative stomatitis and pharyngitis[43] are also detected.
Today, it is well known that vitamin D not only plays an important role in the mineralization of bones and teeth but also contributes to numerous metabolic processes and has a protective role in immune-mediated diseases as well as allergies. A vitamin D deficiency, in addition to disorders in the metabolism of calcium and phosphate in the oral cavity, is accompanied by the development of bone hypomineralization and an increased risk of fractures[61]. Malabsorption of calcium, vitamin K and other nutrients, treatment with corticosteroids, inflammatory cytokines in IBD and hypogonadism caused by IBD are additional factors that contribute to the decreased bone mineral density[62]. A vitamin D deficiency is also associated with the increased prevalence of periodontal diseases (gingivitis and periodontitis), dental caries and tooth loss[63-65]. Vitamin D also exerts an immunomodulatory effect, and its deficiency increases the risk of infection, malignancy and autoimmune disease[66] with possible oral manifestations. Calcium is a mineral that plays an important role in tooth development and mineralization. Experimental studies have shown that a calcium deficiency causes a disorder affecting the mineralization of dentin and enamel[40,67]. A decreased mineralization of bones and teeth is expected in children, who have developed IBD with a deficiency of calcium and vitamin D. However, according to the literature, there are no studies addressing this issue.
Vitamin A and vitamin C deficiencies are also described in IBD patients. A vitamin A deficiency is manifested in the oral cavity as angular cheilitis, atrophy and dryness of oral mucosa. The lips are described as “retreating” because the mucosa contracts towards the oral cavity[68]. A vitamin C deficiency is manifested in the oral cavity as generalized gingival swelling and spontaneous bleeding, ulcerations, tooth mobility, increased severity of periodontal infections and bone loss[55,69]. Spontaneous bleeding of the mucosa can be observed as well[70]. The development of bones and teeth is disrupted in children because both dentin and osteoid depend on vitamin C.
A zinc deficiency is quite common in CD patients. It is manifested in the oral cavity with erosions, ulcers and fissures, a crusting and scaling rash on the lips[29,71],burning mouth syndrome[56] and altered taste[72].
Treatment of inflammatory bowel diseases includes 5-aminosalicylic acid (5-ASA) derivatives, corticosteroids, immunomodulators, calcineurin inhibitors, biological therapy and antibiotics. The selection of treatment depends on the site of the disease, the disease activity and the course or behavior of the disease[73,74]. Currently, the personalized approach to therapy is advocated, and “risk matrices” for assessing the risk of developing severe forms of the disease have been developed[75,76]. The therapy applied to treating inflammatory bowel diseases can lead to alterations in the oral cavity due to the direct toxic effect of the drug on the oral tissue, the indirect immunosuppressive effect which increases the risk for opportunistic infections or bone marrow suppression. The immunomodulators commonly used in IBD treatment include the following: corticosteroids (a total dose equivalent to ≥ 20 mg of prednisolone for ≥ 2 wk), thiopurines, methotrexate, anti-tumor necrosis factor alpha (anti-TNF) agents and other biologics, which increase the risk of opportunistic infections[77]. The incidence of infection is higher if the patients simultaneously receive several immunosuppressive drugs, are malnourished, suffer from other associated diseases or have a prior history of serious infections[77-80]. The risk of infection also increases with age[77-79]. Potential myelotoxicity of the drugs, including the development of leucopenia and agranulocytosis, also increases the risk of developing opportunistic and serious infections[79-81]. In addition to the risk of developing gastrointestinal tumors, patients with inflammatory bowel diseases are at risk of developing hematological malignancies. Compared to the general population, patients suffering from CD are at risk of developing lymphoma, especially non-Hodgkin lymphoma, while patients with UC are more likely to develop leukemia[82]. Early disease onset, male gender and age > 65 are risk factors for developing hematological malignancies. With regard to malignancies related to IBD therapy, patients receiving thiopurines have an increased risk of developing cancer. The risk of developing lymphoma is also increased but can be reversed by thiopurine withdrawal. There is no evidence of an increased risk of developing cancer or lymphoma in patients who received monotherapy with anti TNF drugs[82].
Thus far, only one study addressing the risks of developing malignancies in the oral cavity of patients with IBD has been published[83]. Katsanos et al[83] have established that patients with IBD are at a higher risk of developing tumors in the oral cavity, especially on the tongue. The authors have also concluded that female IBD patients are at a higher risk than male patients. However, this study did not include a risk analysis based on the type of treatment.
Sulfasalazine has been used to treat UC for 50 years[84]. It is a prodrug made of sulfapyridine and an active substance of 5-aminosalicylic acid (5-ASA). Sulfapyridine, or more precisely the sulfur component, is considered to be responsible for numerous side effects and allergic reactions caused by this drug. In the oral cavity, sulfasalazine causes an oral lichen planus/oral lichenoid reaction[85-87]. The occurrence of these lesions in the oral cavities of patients with IBD is rare, and only a few cases have been described[85]. Some patients receiving sulfasalazine may complain of a metallic taste in the mouth[72]. Patients suffering from inflammatory bowel diseases who are receiving sulfasalazine can also develop oral complications caused by myelotoxicity and hepatotoxicity due to the sulfasalazine treatment, presented through signs of aplastic anemia, bleeding (ecchymosis and petechiae) and oral infections. Side effects of 5-ASA derivatives (non-sulfa-containing drugs) are less numerous[88] but have been described in the literature[89]. Mesalazine can cause hematological side effects, such as leucopenia, thrombocytopenia and aplastic anemia[89], resulting in alterations in the oral cavity. Alstead et al[85] presented a case of a patient who developed oral lichen as a reaction to sulfasalazine, which was replaced with mesalazine, but the lesions did not withdraw. After withdrawing the mesalazine, the oral lesions withdrew, and the authors concluded that the lesions were related to 5-ASA.
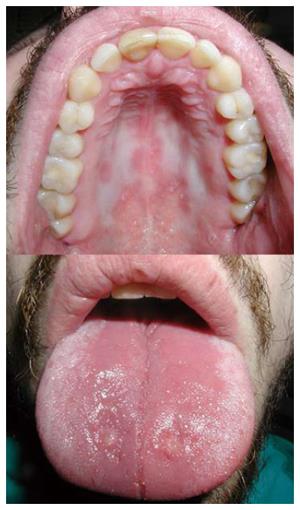
Despite their efficiency, corticosteroids were declared unsuitable for long-term use due to a high percentage of side effects (reported in 50% of patients)[90]. Early adverse effects in the orofacial region, which occur as a result of a supra-physiological dose, include acne, moon face, petechiae and ecchymosis due to blood vessel vulnerability[90-92]. The long-term use of corticosteroids increases the risk of opportunistic infection[78,79]. The risk of infection also depends on the dose[78]. The use of these drugs is related to the occurrence of candidiasis in the oral cavity, pharynx and esophagus[78]. Pseudomembranous candidiasis and chronic atrophic candidiasis are the most prevalent forms of oral candidiasis in such patients (Figure 3)[92,93]. Patients receiving corticosteroids and thiopurines simultaneously have an increased risk of developing opportunistic infections than patients who receive corticosteroids only[78]. Tourner et al[78] observed a 10% prevalence of Candida albicans infections in patients with IBD receiving corticosteroid therapy only. However, the clinical site of these infections was not specified. Numerous case reports and clinical studies on primary varicella zoster virus and herpes zoster virus infections[78,79,81,94] in patients with IBD were published, but checking the literature, no papers on these infections occurring in the oral cavity and orofacial region have been published. Herpes simplex virus (HSV) infections in such patients are also common, whether they are of primary or recurrent character[78,95-98]. Tourner et al[78] have described an 18% prevalence of HSV infections on the face, in the esophagus or on the extremities of patients with IBD.
In children with IBD, especially with CD, the disease itself[99] and the use of corticosteroids[100] can affect growth. There are no studies analyzing bone development in the orofacial region of children with IBD, especially in children who have received long-term corticosteroid therapy. Long-term corticosteroid use can also result in osteoporosis, affecting the patients’ jawbones and increasing the risk of periodontal diseases and fractures. Experimental and clinical studies have shown that the long-term use of corticosteroids leads to the occurrence of calcifications in the dental pulp and pulp obliteration[101-103].
Azathioprine (AZA) and 6-mercaptopurine (6-MP) are thiopurine drugs that have been used in the treatment of IBD for the past 50 years, primarily for the maintenance of disease remission[90]. Unfortunately, in addition to the beneficial treatment effects in some patients with IBD, these drugs can also cause complications and lead to adverse effects. AZA has been shown to cause taste disturbances in the form of ageusia/hypogeusia and dysgeusia[72]. Other side effects of thiopurines include opportunistic infections, myelotoxicity and hepatotoxicity as well as a risk of developing malignant lymphomas, which can also lead to alterations in the oral cavity. The use of AZA/6-MP increases the risk of opportunistic infections for patients taking steroids from approximately 2-3-fold to approximately 15-fold[78]. Atypical clinical features and longer disease duration are characteristics of these infections. Cases of recurrent HSV infections with large and irregular ulcerations occurring in any region of the oral mucosa and lasting for weeks or months have been described. The lesions can also occur on non-keratinized mucosa (a region not usually affected) and are not easily distinguished from aphthous lesions[104]. Due to a potential myelotoxic effect of thiopurines, such as the development of leucopenia and neutropenia[105,106], the risk of developing opportunistic infections is increased.
It is well known that the use of thiopurines increases the risk of developing lymphoma and cancer[82,107,108]. However, clinical studies on the prevalence of malignant diseases in the oral cavity of patients with IBD receiving thiopurines are lacking. Only one clinical study was published on this subject. Pasternak et al[109] established that patients with IBD who are receiving thiopurines are at no greater risk of developing lip, oral cavity or pharynx cancer. Dojcinov et al[110] were the first to present the cases of two rheumatoid arthritis (RA) patients with an azathioprine-related lymphoproliferative disorder (LPD) in the oral cavity.
Methotrexate is a stomatotoxic drug, which causes oral ulcers, ulcerative stomatitis and mucositis[87,111-113]. The occurrence of lesions in the oral cavity is associated with a folic acid deficiency and toxic effects of the drug[112]. The effects of the drug in the oral cavity depend on the administered dose. Lower doses cause ulcers and stomatitis, while higher doses, administered to treat malignant diseases, cause mucositis[114]. Several clinical studies and cases have described oral ulcers, stomatitis and mucositis in patients treated with methotrexate due to RA, psoriasis and malignancies[113,115,116]. Furthermore, methotrexate can cause agusea/hypogeusia in the oral cavity[72]. However, there are no published papers on the occurrence of oral ulcers, stomatitis and taste disturbances in patients with IBD treated with methotrexate.
Bone marrow suppression (in the form of leucopenia, thrombocytopenia or pancytopenia) is also described in patients receiving methotrexate (more often in patients treated with high doses and less often in patients treated with low doses)[117,118]. Oral alterations can develop as a consequence of bone marrow suppression. The only study on oral infections in patients receiving low doses of methotrexate that we found was published by Pedrazas et al[119]. The authors described a significantly higher prevalence of oral candidiasis (10.7%) and oral ulcers (60.7%) in patients with RA receiving methotrexate than in patients who were not treated with methotrexate.
Several clinical cases of methotrexate-related Epstein-Barr virus associated lymphoproliferative disorders in the oral cavity in patients with RA and Still’s disease[110,111,120-126] have been published. Clinical LPD is manifested in the oral cavity as swelling or painful ulcers of irregular edges on the gingiva, tongue, floor of the mouth and buccal mucosa[110,111,120-126]. In some cases, the bone[111,122,125] was also affected. The common characteristic of these disorders is that the majority of lesions regress completely following the withdrawal of methotrexate[123]. According to the literature, there are no descriptions of these disorders in the oral cavity caused by methotrexate in patients with IBD.
Gingival hyperplasia (or gingivae overgrowth) is common in patients receiving cyclosporine (CsA). The severity of gingival hyperplasia depends on the duration of CsA therapy, but its occurrence is also influenced by bacterial plaque, local irritants and possibly the simultaneous use of other drugs that cause gingival hyperplasia (e.g., nifedipine)[127,128]. Gingival hyperplasia can interfere with oral functions and speech and can cause delayed and/or ectopic dentition and difficulties in maintaining oral hygiene, increasing the risk of caries, infection and periodontal disease[129].
Furthermore, in patients receiving CsA, filiform papillae hypertrophy on the tongue[130], opportunistic infections (candidiasis)[131,132], squamous-cell carcinomas on the lip[133], non-Hodgkin lymphomas[134,135] and lymphoproliferative disorders[110,136,137] have been described in the oral cavity. The side effects of tacrolimus are less common compared to cyclosporine[138], and currently there is no evidence of complications associated with tacrolimus treatment in the oral cavity.
In addition to exerting a revolutionary effect in treating inflammatory bowel diseases, TNF alpha inhibitors have numerous side effects. These drugs can cause oral lichen/lichenoid reactions and opportunistic infections in the oral cavity[87,139-142]. Asarch et al[139] reported two cases of oral lichenoid reaction in patients with psoriasis receiving infliximab and adalimumab. Moss et al[140] described a case of a patient with CD receiving infliximab that resulted in an oral lichenoid reaction. Cases of erythema multiforme and Stevens-Johnson syndrome in the oral cavity and on the skin in patients with CD receiving infliximab and adalimumab[143,144] were also reported. Oral opportunistic infections (primarily candidiasis) can be expected in patients with IBD treated with anti-TNF drugs but so far only one case report has been published[141]. There are no clinical studies on the prevalence of opportunistic oral infections in these patients. One of the most often described side effects of vedolizumab (a gut selective anti-integrin) is pharyngitis[145,146].
The most common side effect of metronidazole is a metallic taste in the mouth[147]. In addition to the metallic taste, there are no other described side effects of these antibiotics in the oral cavity.
The goal of treating oral lesions in patients with IBD is to reduce pain, accelerate the healing of lesions and prevent secondary infections. The treatment depends on the etiology, severity of the clinical presentation and the symptoms of the oral lesions. Treatment options include topical or/and systemic medications. Although the first European evidence-based consensus on extraintestinal manifestations in inflammatory bowel disease has been recently published[148], there are still no statements on the treatment of oral manifestations and complications of IBD.
Treatment of specific oral lesions in Crohn’s disease and pyostomatitis vegetans (which occur in both diseases) is always aimed at treating and controlling the underlying disease, and lesions usually respond well to IBD treatment[37-39,41-43,46,50]. In addition to systemic therapy, topical agents can be used; therefore, a multidisciplinary approach is essential. Topical treatments include steroids (topical or intralesional injections), topical tacrolimus, 5-ASA mouthwashes, topical anesthetics for pain relief, non-steroidal anti-inflammatory pastes and antiseptic mouthwash for preventing secondary infections[11,14,19,33,34,149].
In IBD patients, the treatment of aphthous stomatitis includes nutrition supplements and topical or systemic medication therapy. The choice of therapy depends on the severity of symptoms and the type and numbers of aphthous lesions. Choices for therapy include steroids (topical, intralesional or systemic) as a first line of treatment, topical anesthetics, antiseptic mouthwash or non-steroidal anti-inflammatory pastes[150]. Furthermore, non-medical treatments, such as ozone therapy and low-level laser therapy, can be used for pain relief and to accelerate lesion healing[151,152]. When the aphthous lesions are numerous and very painful, systemic steroids, immunosuppressive agents and thalidomide are indicated[11,150].
Angular cheilitis and glossitis are frequently caused by anemia and malnutrition; in cases with specific deficiencies, the replacement of iron, B12, folate and zinc is necessary[52-54]. Angular cheilitis can also be caused by fungi (Candida spp.) and bacteria (Staphylococcus aureus or β-hemolytic streptococci). In the case of fungal etiology, treatment options include topical antifungals (e.g., nystatin, miconazole, ketoconazole or clotrimazole). When the infection is caused by Staphylococcus aureus, topical treatments include combinations of mupirocin or fusidic acid and 1% hydrocortisone cream[153].
Most often, oral infections occur as a consequence of immunosuppressive drug therapy, and candidiasis is the most commonly occurring infection. Oral candidiasis does not require the interruption of therapy[77]. Topical therapy options include nystatin, amphotericin B, miconazole, fluconazole, ketoconazole or clotrimazole. In some cases, systemic therapy with fluconazole, itraconazole or ketoconazole is necessary[154].
HSV infection is not a contraindication for immunosuppressive therapy. In recurrent oral HSV infections, oral antiviral therapy should be considered[77].
When oral lichen/oral lichenoid reactions or taste disturbances are present, the interruption and replacement of medication can be considered.
The occurrence of erythema multiforme or Stevens-Johnson syndrome requires the prompt interruption of biologic drugs[143,144]. The patients with erythema multiforme can be treated as out-patients with systemic and topical steroids[143]. Stevens-Johnson syndrome requires treatment in a hospital setting[144].
Oral lesions in patients with inflammatory bowel disease can be extraintestinal manifestations of the disease or can occur as complications of the disease and treatment. They occur more often in CD patients than in UC patients, although pyostomatitis vegetans is more common in UC patients. One or more oral lesions can simultaneously appear in the oral cavity. The severity of their clinical presentation can range from mild and painless to extensive and painful. The lesions can compromise oral functions. Cooperation between specialists in oral medicine and gastroenterologists is essential for the successful diagnosis of IBD (in cases when oral pathology precedes intestinal manifestations), as well as in the diagnosis and treatment of oral lesions in such patients.
| 1. | Sartor RB. Mechanisms of disease: pathogenesis of Crohn’s disease and ulcerative colitis. Nat Clin Pract Gastroenterol Hepatol. 2006;3:390-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1158] [Cited by in RCA: 1306] [Article Influence: 65.3] [Reference Citation Analysis (2)] |
| 2. | Danese S, Fiocchi C. Etiopathogenesis of inflammatory bowel diseases. World J Gastroenterol. 2006;12:4807-4812. [PubMed] |
| 3. | Leone V, Chang EB, Devkota S. Diet, microbes, and host genetics: the perfect storm in inflammatory bowel diseases. J Gastroenterol. 2013;48:315-321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 114] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 4. | Burisch J, Pedersen N, Čuković-Čavka S, Brinar M, Kaimakliotis I, Duricova D, Shonová O, Vind I, Avnstrøm S, Thorsgaard N. East-West gradient in the incidence of inflammatory bowel disease in Europe: the ECCO-EpiCom inception cohort. Gut. 2014;63:588-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 294] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 5. | Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema HW. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46-54.e42; quiz e30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3789] [Cited by in RCA: 3593] [Article Influence: 256.6] [Reference Citation Analysis (6)] |
| 6. | Sincić BM, Vucelić B, Persić M, Brncić N, Erzen DJ, Radaković B, Mićović V, Stimac D. Incidence of inflammatory bowel disease in Primorsko-goranska County, Croatia, 2000-2004: A prospective population-based study. Scand J Gastroenterol. 2006;41:437-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 70] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 7. | Danese S, Semeraro S, Papa A, Roberto I, Scaldaferri F, Fedeli G, Gasbarrini G, Gasbarrini A. Extraintestinal manifestations in inflammatory bowel disease. World J Gastroenterol. 2005;11:7227-7236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 223] [Cited by in RCA: 229] [Article Influence: 11.5] [Reference Citation Analysis (2)] |
| 8. | Rothfuss KS, Stange EF, Herrlinger KR. Extraintestinal manifestations and complications in inflammatory bowel diseases. World J Gastroenterol. 2006;12:4819-4831. [PubMed] |
| 9. | Ardizzone S, Puttini PS, Cassinotti A, Porro GB. Extraintestinal manifestations of inflammatory bowel disease. Dig Liver Dis. 2008;40 Suppl 2:S253-S259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 140] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 10. | Vavricka SR, Schoepfer A, Scharl M, Lakatos PL, Navarini A, Rogler G. Extraintestinal Manifestations of Inflammatory Bowel Disease. Inflamm Bowel Dis. 2015;21:1982-1992. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 546] [Cited by in RCA: 500] [Article Influence: 45.5] [Reference Citation Analysis (0)] |
| 11. | Katsanos KH, Torres J, Roda G, Brygo A, Delaporte E, Colombel JF. Review article: non-malignant oral manifestations in inflammatory bowel diseases. Aliment Pharmacol Ther. 2015;42:40-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 12. | Pittock S, Drumm B, Fleming P, McDermott M, Imrie C, Flint S, Bourke B. The oral cavity in Crohn’s disease. J Pediatr. 2001;138:767-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 79] [Article Influence: 3.2] [Reference Citation Analysis (1)] |
| 13. | Litsas G. Crohn’s disease of the mouth: report of a case. Eur J Paediatr Dent. 2011;12:198-200. [PubMed] |
| 14. | Urek MM, Sinčić BM, Braut A. Oral manifestations of Crohn’s disease: a case report. Sanamed. 2015;10:205-208. |
| 15. | Dupuy A, Cosnes J, Revuz J, Delchier JC, Gendre JP, Cosnes A. Oral Crohn disease: clinical characteristics and long-term follow-up of 9 cases. Arch Dermatol. 1999;135:439-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 63] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Ghandour K, Issa M. Oral Crohn’s disease with late intestinal manifestations. Oral Surg Oral Med Oral Pathol. 1991;72:565-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 1.0] [Reference Citation Analysis (2)] |
| 17. | Ficarra G, Cicchi P, Amorosi A, Piluso S. Oral Crohn’s disease and pyostomatitis vegetans. An unusual association. Oral Surg Oral Med Oral Pathol. 1993;75:220-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 1.4] [Reference Citation Analysis (1)] |
| 18. | Turchi RM, Soriano H, Rodgers GL. Tb or not TB: Crohn’s disease presenting with tonsillar granulomas. Otolaryngol Head Neck Surg. 2006;134:528-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Lankarani KB, Sivandzadeh GR, Hassanpour S. Oral manifestation in inflammatory bowel disease: a review. World J Gastroenterol. 2013;19:8571-8579. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 146] [Cited by in RCA: 123] [Article Influence: 9.5] [Reference Citation Analysis (1)] |
| 20. | Zbar AP, Ben-Horin S, Beer-Gabel M, Eliakim R. Oral Crohn’s disease: is it a separable disease from orofacial granulomatosis? A review. J Crohns Colitis. 2012;6:135-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 21. | Mays JW, Sarmadi M, Moutsopoulos NM. Oral manifestations of systemic autoimmune and inflammatory diseases: diagnosis and clinical management. J Evid Based Dent Pract. 2012;12:265-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 79] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 22. | Galbraith SS, Drolet BA, Kugathasan S, Paller AS, Esterly NB. Asymptomatic inflammatory bowel disease presenting with mucocutaneous findings. Pediatrics. 2005;116:e439-e444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 41] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Katz J, Shenkman A, Stavropoulos F, Melzer E. Oral signs and symptoms in relation to disease activity and site of involvement in patients with inflammatory bowel disease. Oral Dis. 2003;9:34-40. [PubMed] |
| 24. | Asquith P, Thompson RA, Cooke WT. Oral manifestations of Crohn’s disease. Gut. 1975;16:249-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 80] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 25. | Siegel MA, Solomon LV, Majia LM. Diseases of Gastrointestinal Tract. Burket’s Oral Medicine. Shelton, Connecticut: People’s Medical Publishing House 2015; 389-410. |
| 26. | Laranjeira N, Fonseca J, Meira T, Freitas J, Valido S, Leitão J. Oral mucosa lesions and oral symptoms in inflammatory bowel disease patients. Arq Gastroenterol. 2015;52:105-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 27. | Best WR, Becktel JM, Singleton JW, Kern F. Development of a Crohn’s disease activity index. National Cooperative Crohn’s Disease Study. Gastroenterology. 1976;70:439-444. [PubMed] |
| 28. | Veloso FT, Carvalho J, Magro F. Immune-related systemic manifestations of inflammatory bowel disease. A prospective study of 792 patients. J Clin Gastroenterol. 1996;23:29-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 245] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 29. | Plauth M, Jenss H, Meyle J. Oral manifestations of Crohn’s disease. An analysis of 79 cases. J Clin Gastroenterol. 1991;13:29-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 138] [Article Influence: 3.9] [Reference Citation Analysis (1)] |
| 30. | Halme L, Meurman JH, Laine P, von Smitten K, Syrjänen S, Lindqvist C, Strand-Pettinen I. Oral findings in patients with active or inactive Crohn’s disease. Oral Surg Oral Med Oral Pathol. 1993;76:175-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 54] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 31. | Trost LB, McDonnell JK. Important cutaneous manifestations of inflammatory bowel disease. Postgrad Med J. 2005;81:580-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 95] [Article Influence: 4.8] [Reference Citation Analysis (1)] |
| 32. | Ojha J, Cohen DM, Islam NM, Stewart CM, Katz J, Bhattacharyya I. Gingival involvement in Crohn disease. J Am Dent Assoc. 2007;138:1574-181; quiz 1574-181;. [PubMed] |
| 33. | Field EA, Allan RB. Review article: oral ulceration--aetiopathogenesis, clinical diagnosis and management in the gastrointestinal clinic. Aliment Pharmacol Ther. 2003;18:949-962. [PubMed] |
| 34. | Fatahzadeh M, Schwartz RA, Kapila R, Rochford C. Orofacial Crohn’s disease: an oral enigma. Acta Dermatovenerol Croat. 2009;17:289-300. [PubMed] |
| 35. | Gibson J, Wray D, Bagg J. Oral staphylococcal mucositis: A new clinical entity in orofacial granulomatosis and Crohn’s disease. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;89:171-176. [PubMed] |
| 36. | Mills CC, Amin M, Manisali M. Salivary duct fistula and recurrent buccal space infection: a complication of Crohn’s disease. J Oral Maxillofac Surg. 2003;61:1485-1487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 37. | Bens G, Laharie D, Beylot-Barry M, Vergier B, Noblesse I, Beylot C, Amouretti M. Successful treatment with infliximab and methotrexate of pyostomatitis vegetans associated with Crohn’s disease. Br J Dermatol. 2003;149:181-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (1)] |
| 38. | Mijandrusić-Sincić B, Licul V, Gorup L, Brncić N, Glazar I, Lucin K. Pyostomatitis vegetans associated with inflammatory bowel disease--report of two cases. Coll Antropol. 2010;34 Suppl 2:279-282. [PubMed] |
| 39. | Grössner-Schreiber B, Fetter T, Hedderich J, Kocher T, Schreiber S, Jepsen S. Prevalence of dental caries and periodontal disease in patients with inflammatory bowel disease: a case-control study. J Clin Periodontol. 2006;33:478-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 83] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 40. | Ansaldi N, Morabito A, Balocco P, Galleano E. [Dental changes in children with malabsorption]. Minerva Pediatr. 1989;41:581-585. [PubMed] |
| 41. | Appleton SS. Candidiasis: pathogenesis, clinical characteristics, and treatment. J Calif Dent Assoc. 2000;28:942-948. [PubMed] |
| 42. | Rogers RS, Bekic M. Diseases of the lips. Semin Cutan Med Surg. 1997;16:328-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 37] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 43. | Huber MA, Sankar V. Hematologic diseases. Burket’s Oral Medicine. Shelton, Connecticut: People’s Medical Publishing House 2015; 463-488. |
| 44. | Chaudhry SI, Philpot NS, Odell EW, Challacombe SJ, Shirlaw PJ. Pyostomatitis vegetans associated with asymptomatic ulcerative colitis: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;87:327-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 45. | Ruiz-Roca JA, Berini-Aytés L, Gay-Escoda C. Pyostomatitis vegetans. Report of two cases and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;99:447-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 35] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 46. | Ayangco L, Rogers RS, Sheridan PJ. Pyostomatitis vegetans as an early sign of reactivation of Crohn’s disease: a case report. J Periodontol. 2002;73:1512-1516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (1)] |
| 47. | Chan SW, Scully C, Prime SS, Eveson J. Pyostomatitis vegetans: oral manifestation of ulcerative colitis. Oral Surg Oral Med Oral Pathol. 1991;72:689-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 48. | Markiewicz M, Suresh L, Margarone J, Aguirre A, Brass C. Pyostomatitis vegetans: A clinical marker of silent ulcerative colitis. J Oral Maxillofac Surg. 2007;65:346-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (1)] |
| 49. | Wu YH, Chang JY, Chen HM, Wang YP. Pyostomatitis vegetans: An oral manifestation of inflammatory bowel disease. J Formos Med Assoc. 2015;114:672-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 50. | Femiano F, Lanza A, Buonaiuto C, Perillo L, Dell’Ermo A, Cirillo N. Pyostomatitis vegetans: a review of the literature. Med Oral Patol Oral Cir Bucal. 2009;14:E114-E117. [PubMed] |
| 51. | Triantafillidis JK, Vagianos C, Papalois AE. The role of enteral nutrition in patients with inflammatory bowel disease: current aspects. Biomed Res Int. 2015;2015:197167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 52. | Hartman C, Eliakim R, Shamir R. Nutritional status and nutritional therapy in inflammatory bowel diseases. World J Gastroenterol. 2009;15:2570-2578. [PubMed] |
| 53. | Halmos EP, Gibson PR. Dietary management of IBD--insights and advice. Nat Rev Gastroenterol Hepatol. 2015;12:133-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 54. | Dignass AU, Gasche C, Bettenworth D, Birgegård G, Danese S, Gisbert JP, Gomollon F, Iqbal T, Katsanos K, Koutroubakis I. European consensus on the diagnosis and management of iron deficiency and anaemia in inflammatory bowel diseases. J Crohns Colitis. 2015;9:211-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 449] [Cited by in RCA: 440] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 55. | Mulliken RA, Casner MJ. Oral manifestations of systemic disease. Emerg Med Clin North Am. 2000;18:565-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 56. | Sun A, Wu KM, Wang YP, Lin HP, Chen HM, Chiang CP. Burning mouth syndrome: a review and update. J Oral Pathol Med. 2013;42:649-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 88] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 57. | Wu YC, Wang YP, Chang JY, Cheng SJ, Chen HM, Sun A. Oral manifestations and blood profile in patients with iron deficiency anemia. J Formos Med Assoc. 2014;113:83-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 107] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 58. | Piskin S, Sayan C, Durukan N, Senol M. Serum iron, ferritin, folic acid, and vitamin B12 levels in recurrent aphthous stomatitis. J Eur Acad Dermatol Venereol. 2002;16:66-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 55] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 59. | Adeyemo TA, Adeyemo WL, Adediran A, Akinbami AJ, Akanmu AS. Orofacial manifestations of hematological disorders: anemia and hemostatic disorders. Indian J Dent Res. 2011;22:454-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 60. | Derossi SS, Raghavendra S. Anemia. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;95:131-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 61. | Turner AG, Anderson PH, Morris HA. Vitamin D and bone health. Scand J Clin Lab Invest Suppl. 2012;243:65-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 62. | Vestergaard P. Prevalence and pathogenesis of osteoporosis in patients with inflammatory bowel disease. Minerva Med. 2004;95:469-480. [PubMed] |
| 63. | Amano Y, Komiyama K, Makishima M. Vitamin D and periodontal disease. J Oral Sci. 2009;51:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 64. | Zhan Y, Samietz S, Holtfreter B, Hannemann A, Meisel P, Nauck M, Völzke H, Wallaschofski H, Dietrich T, Kocher T. Prospective Study of Serum 25-hydroxy Vitamin D and Tooth Loss. J Dent Res. 2014;93:639-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 65. | Jimenez M, Giovannucci E, Krall Kaye E, Joshipura KJ, Dietrich T. Predicted vitamin D status and incidence of tooth loss and periodontitis. Public Health Nutr. 2014;17:844-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 66. | Holick MF, Chen TC. Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr. 2008;87:1080S-1086S. [PubMed] |
| 67. | Nanci A, Mocetti P, Sakamoto Y, Kunikata M, Lozupone E, Bonucci E. Morphological and immunocytochemical analyses on the effects of diet-induced hypocalcemia on enamel maturation in the rat incisor. J Histochem Cytochem. 2000;48:1043-1058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 68. | Schlosser BJ, Pirigyi M, Mirowski GW. Oral manifestations of hematologic and nutritional diseases. Otolaryngol Clin North Am. 2011;44:183-203, vii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 69. | Rubinoff AB, Latner PA, Pasut LA. Vitamin C and oral health. J Can Dent Assoc. 1989;55:705-707. [PubMed] |
| 70. | Fontana M. Vitamin C (ascorbic acid): clinical implications for oral health--a literature review. Compendium. 1994;15:916, 918, 920 passim; quiz 930. [PubMed] |
| 71. | Kambe T, Fukue K, Ishida R, Miyazaki S. Overview of Inherited Zinc Deficiency in Infants and Children. J Nutr Sci Vitaminol (Tokyo). 2015;61 Suppl:S44-S46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 72. | Spielman AI. Chemosensory function and dysfunction. Crit Rev Oral Biol Med. 1998;9:267-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 58] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 73. | Van Assche G, Dignass A, Panes J, Beaugerie L, Karagiannis J, Allez M, Ochsenkühn T, Orchard T, Rogler G, Louis E. The second European evidence-based Consensus on the diagnosis and management of Crohn’s disease: Definitions and diagnosis. J Crohns Colitis. 2010;4:7-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 818] [Cited by in RCA: 798] [Article Influence: 49.9] [Reference Citation Analysis (0)] |
| 74. | Dignass A, Lindsay JO, Sturm A, Windsor A, Colombel JF, Allez M, D’Haens G, D’Hoore A, Mantzaris G, Novacek G. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 2: current management. J Crohns Colitis. 2012;6:991-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 728] [Cited by in RCA: 708] [Article Influence: 50.6] [Reference Citation Analysis (0)] |
| 75. | Solberg IC, Cvancarova M, Vatn MH, Moum B. Risk matrix for prediction of advanced disease in a population-based study of patients with Crohn’s Disease (the IBSEN Study). Inflamm Bowel Dis. 2014;20:60-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 76. | Solberg IC, Høivik ML, Cvancarova M, Moum B. Risk matrix model for prediction of colectomy in a population-based study of ulcerative colitis patients (the IBSEN study). Scand J Gastroenterol. 2015;50:1456-1462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 77. | Rahier JF, Magro F, Abreu C, Armuzzi A, Ben-Horin S, Chowers Y, Cottone M, de Ridder L, Doherty G, Ehehalt R. Second European evidence-based consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J Crohns Colitis. 2014;8:443-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 694] [Cited by in RCA: 759] [Article Influence: 63.3] [Reference Citation Analysis (1)] |
| 78. | Toruner M, Loftus EV, Harmsen WS, Zinsmeister AR, Orenstein R, Sandborn WJ, Colombel JF, Egan LJ. Risk factors for opportunistic infections in patients with inflammatory bowel disease. Gastroenterology. 2008;134:929-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 719] [Cited by in RCA: 762] [Article Influence: 42.3] [Reference Citation Analysis (1)] |
| 79. | Naganuma M, Kunisaki R, Yoshimura N, Takeuchi Y, Watanabe M. A prospective analysis of the incidence of and risk factors for opportunistic infections in patients with inflammatory bowel disease. J Gastroenterol. 2013;48:595-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 74] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 80. | Dave M, Purohit T, Razonable R, Loftus EV. Opportunistic infections due to inflammatory bowel disease therapy. Inflamm Bowel Dis. 2014;20:196-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 102] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 81. | Long MD, Martin C, Sandler RS, Kappelman MD. Increased risk of herpes zoster among 108 604 patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2013;37:420-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 160] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 82. | Annese V, Beaugerie L, Egan L, Biancone L, Bolling C, Brandts C, Dierickx D, Dummer R, Fiorino G, Gornet JM. European Evidence-based Consensus: Inflammatory Bowel Disease and Malignancies. J Crohns Colitis. 2015;9:945-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 336] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 83. | Katsanos KH, Roda G, McBride RB, Cohen B, Colombel JF. Increased Risk of Oral Cancer in Patients With Inflammatory Bowel Diseases. Clin Gastroenterol Hepatol. 2016;14:413-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 84. | Hanauer SB. Review article: aminosalicylates in inflammatory bowel disease. Aliment Pharmacol Ther. 2004;20 Suppl 4:60-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 85. | Alstead EM, Wilson AG, Farthing MJ. Lichen planus and mesalazine. J Clin Gastroenterol. 1991;13:335-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 86. | Ghosh S, Jain VK, Chaudhuri S, Mathur SK. Sulfasalazine induced lichen planus in a patient of rheumatoid arthritis. Indian J Dermatol Venereol Leprol. 2013;79:541-544. [PubMed] |
| 87. | Yuan A, Woo SB. Adverse drug events in the oral cavity. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;119:35-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 83] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 88. | Jick H, Myers MW, Dean AD. The risk of sulfasalazine- and mesalazine-associated blood disorders. Pharmacotherapy. 1995;15:176-181. [PubMed] |
| 89. | Farrell RJ, Peppercorn MA, Fine SN, Michetti P. Mesalamine-associated thrombocytopenia. Am J Gastroenterol. 1999;94:2304-2306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 90. | Carter MJ, Lobo AJ, Travis SP. Guidelines for the management of inflammatory bowel disease in adults. Gut. 2004;53 Suppl 5:V1-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 746] [Cited by in RCA: 776] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 91. | Sandborn WJ. Steroid-dependent Crohn’s disease. Can J Gastroenterol. 2000;14 Suppl C:17C-22C. [PubMed] |
| 92. | Akintoye SO, Collins MT, Ship JA. Diabetes mellitus and endocrine diseases. Burket’s oral medicine: Diagnosis & treatment 11th ed. Hamilton: Bc Decker Inc 2008; 509-536. |
| 93. | Sharon V, Fazel N. Oral candidiasis and angular cheilitis. Dermatol Ther. 2010;23:230-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 94. | Cullen G, Baden RP, Cheifetz AS. Varicella zoster virus infection in inflammatory bowel disease. Inflamm Bowel Dis. 2012;18:2392-2403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 78] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 95. | Santos-Antunes J, Abreu C, Magro F, Coelho R, Vilas-Boas F, Andrade P, Lopes S, Macedo G. Disseminated cutaneous herpes simplex infection in a patient with Crohn’s disease under azathioprine and steroids: First case report and literature review. J Crohns Colitis. 2014;8:326-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 96. | Shlien RD, Meyers S, Lee JA, Dische R, Janowitz HD. Fulminant herpes simplex hepatitis in a patient with ulcerative colitis. Gut. 1988;29:257-261. [PubMed] |
| 97. | Seksik P, Gozlan J, Guitton C, Galula G, Maury E, Offenstadt G. Fatal herpetic hepatitis in adult following short corticotherapy: a case report. Intensive Care Med. 1999;25:415-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 98. | Alimohamadi SM, Malekzadeh R, Mirmadjless SH, Mohamadnejad M, Zamani F. Herpes simplex virus encephalistis during immunosuppressive treatment of ulcerative colitis. MedGenMed. 2004;6:7. [PubMed] |
| 99. | Gasparetto M, Guariso G. Crohn’s disease and growth deficiency in children and adolescents. World J Gastroenterol. 2014;20:13219-13233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 77] [Cited by in RCA: 83] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 100. | Ezri J, Marques-Vidal P, Nydegger A. Impact of disease and treatments on growth and puberty of pediatric patients with inflammatory bowel disease. Digestion. 2012;85:308-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 65] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 101. | Symons AL, Henry AC, Chang S, Daley TJ, Harbrow DJ, Joseph BK. The effect of glucocorticosteroid treatment on dentine formation in the Lewis rat, a histological study. Growth Factors. 2000;18:157-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 102. | Näsström K. Dentin formation after corticosteroid treatment. A clinical study and an experimental study on rats. Swed Dent J Suppl. 1996;115:1-45. [PubMed] |
| 103. | van Hogezand RA, Hamdy NA. Skeletal morbidity in inflammatory bowel disease. Scand J Gastroenterol Suppl. 2006;59-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 104. | Atkinson JC, Moutsopoulos N, Pillemer SR, Imanguli MM. Transplantation Medicine. Glick M, Burket’s oral medicine. Shelton, Connecticut: People’s Medical publishing House, beaugertroelkuoogata 2015; 463-488. |
| 105. | Sandborn WJ. Azathioprine: state of the art in inflammatory bowel disease. Scand J Gastroenterol Suppl. 1998;225:92-99. [PubMed] |
| 106. | Cunliffe RN, Scott BB. Review article: monitoring for drug side-effects in inflammatory bowel disease. Aliment Pharmacol Ther. 2002;16:647-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 56] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 107. | Subramaniam K, D’Rozario J, Pavli P. Lymphoma and other lymphoproliferative disorders in inflammatory bowel disease: a review. J Gastroenterol Hepatol. 2013;28:24-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 108. | Beaugerie L, Brousse N, Bouvier AM, Colombel JF, Lémann M, Cosnes J, Hébuterne X, Cortot A, Bouhnik Y, Gendre JP. Lymphoproliferative disorders in patients receiving thiopurines for inflammatory bowel disease: a prospective observational cohort study. Lancet. 2009;374:1617-1625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 774] [Cited by in RCA: 823] [Article Influence: 48.4] [Reference Citation Analysis (0)] |
| 109. | Pasternak B, Svanström H, Schmiegelow K, Jess T, Hviid A. Use of azathioprine and the risk of cancer in inflammatory bowel disease. Am J Epidemiol. 2013;177:1296-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 189] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 110. | Dojcinov SD, Venkataraman G, Raffeld M, Pittaluga S, Jaffe ES. EBV positive mucocutaneous ulcer--a study of 26 cases associated with various sources of immunosuppression. Am J Surg Pathol. 2010;34:405-417. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 483] [Cited by in RCA: 409] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 111. | Kalantzis A, Marshman Z, Falconer DT, Morgan PR, Odell EW. Oral effects of low-dose methotrexate treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;100:52-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 73] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 112. | Deeming GM, Collingwood J, Pemberton MN. Methotrexate and oral ulceration. Br Dent J. 2005;198:83-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 113. | Troeltzsch M, von Blohn G, Kriegelstein S, Woodlock T, Gassling V, Berndt R, Troeltzsch M. Oral mucositis in patients receiving low-dose methotrexate therapy for rheumatoid arthritis: report of 2 cases and literature review. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;115:e28-e33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 114. | Stein RB, Hanauer SB. Comparative tolerability of treatments for inflammatory bowel disease. Drug Saf. 2000;23:429-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 123] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 115. | Kremer JM, Phelps CT. Long-term prospective study of the use of methotrexate in the treatment of rheumatoid arthritis. Update after a mean of 90 months. Arthritis Rheum. 1992;35:138-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 172] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 116. | Ince A, Yazici Y, Hamuryudan V, Yazici H. The frequency and clinical characteristics of methotrexate (MTX) oral toxicity in rheumatoid arthritis (RA): a masked and controlled study. Clin Rheumatol. 1996;15:491-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 117. | Sosin M, Handa S. Low dose methotrexate and bone marrow suppression. BMJ. 2003;326:266-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 118. | Lang B, Riegel W, Peters T, Peter HH. Low dose methotrexate therapy for rheumatoid arthritis complicated by pancytopenia and Pneumocystis carinii pneumonia. J Rheumatol. 1991;18:1257-1259. [PubMed] |
| 119. | Pedrazas CH, Azevedo MN, Torres SR. Oral events related to low-dose methotrexate in rheumatoid arthritis patients. Braz Oral Res. 2010;24:368-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 120. | Kikuchi K, Miyazaki Y, Tanaka A, Shigematu H, Kojima M, Sakashita H, Kusama K. Methotrexate-related Epstein-Barr Virus (EBV)-associated lymphoproliferative disorder--so-called “Hodgkin-like lesion”--of the oral cavity in a patient with rheumatoid arthritis. Head Neck Pathol. 2010;4:305-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 121. | Kojima M, Itoh H, Hirabayashi K, Igarashi S, Tamaki Y, Murayama K, Ogura H, Saitoh R, Kashiwabara K, Takimoto J. Methtrexate-associated lymphoproliferative disorders. A clinicopathological study of 13 Japanese cases. Pathol Res Pract. 2006;202:679-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 47] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 122. | Tanaka A, Shigematsu H, Kojima M, Sakashita H, Kusama K. Methotrexate-associated lymphoproliferative disorder arising in a patient with adult Still’s disease. J Oral Maxillofac Surg. 2008;66:1492-1495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 123. | Uneda S, Sonoki T, Nakamura Y, Matsuoka H, Nakakuma H. Rapid vanishing of tumors by withdrawal of methotrexate in Epstein-Barr virus-related B cell lymphoproliferative disorder. Intern Med. 2008;47:1445-1446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 124. | Ishida M, Hodohara K, Yoshii M, Okuno H, Horinouchi A, Nakanishi R, Harada A, Iwai M, Yoshida K, Kagotani A. Methotrexate-related Epstein-Barr virus-associated lymphoproliferative disorder occurring in the gingiva of a patient with rheumatoid arthritis. Int J Clin Exp Pathol. 2013;6:2237-2241. [PubMed] |
| 125. | Acero J, Navarro-Cuellar C, Menarguez J, Herencia H, Navarro-Vila C. Naso-maxillary non-Hodgkin lymphoma associated with methotrexate treatment in a patient with rheumatoid arthritis. J Oral Maxillofac Surg. 2006;64:708-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 126. | Pastor-Nieto MA, Kilmurray LG, López-Chumillas A, O’Valle F, García-Del Moral R, Puig AM, Bautista P. [Methotrexate-associated lymphoproliferative disorder presenting as oral ulcers in a patient with rheumatoid arthritis]. Actas Dermosifiliogr. 2009;100:142-146. [PubMed] |
| 127. | O’Valle F, Mesa F, Aneiros J, Gómez-Morales M, Lucena MA, Ramírez C, Revelles F, Moreno E, Navarro N, Caballero T. Gingival overgrowth induced by nifedipine and cyclosporin A. Clinical and morphometric study with image analysis. J Clin Periodontol. 1995;22:591-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 128. | Ciavarella D, Guiglia R, Campisi G, Di Cosola M, Di Liberto C, Sabatucci A, Escudero N, Bascones A, Lo Muzio L. Update on gingival overgrowth by cyclosporine A in renal transplants. Med Oral Patol Oral Cir Bucal. 2007;12:E19-E25. [PubMed] |
| 129. | Hassell TM, Hefti AF. Drug-induced gingival overgrowth: old problem, new problem. Crit Rev Oral Biol Med. 1991;2:103-137. [PubMed] |
| 130. | Rateitschak-Plüss EM, Hefti A, Lörtscher R, Thiel G. Initial observation that cyclosporin-A induces gingival enlargement in man. J Clin Periodontol. 1983;10:237-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 182] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 131. | King GN, Healy CM, Glover MT, Kwan JT, Williams DM, Leigh IM, Thornhill MH. Prevalence and risk factors associated with leukoplakia, hairy leukoplakia, erythematous candidiasis, and gingival hyperplasia in renal transplant recipients. Oral Surg Oral Med Oral Pathol. 1994;78:718-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 58] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 132. | Olczak-Kowalczyk D, Pawłowska J, Garczewska B, Smirska E, Grenda R, Syczewska M, Kowalczyk W. Oral candidiasis in immunosuppressed children and young adults after liver or kidney transplantation. Pediatr Dent. 2010;32:189-194. [PubMed] |
| 133. | Seymour RA, Thomason JM, Nolan A. Oral lesions in organ transplant patients. J Oral Pathol Med. 1997;26:297-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 44] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 134. | Kuo PC, Dafoe DC, Alfrey EJ, Sibley RK, Scandling JD. Posttransplant lymphoproliferative disorders and Epstein-Barr virus prophylaxis. Transplantation. 1995;59:135-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 27] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 135. | Maxymiw WG, Wood RE, Lee L. Primary, multi-focal, non-Hodgkin’s lymphoma of the jaws presenting as periodontal disease in a renal transplant patient. Int J Oral Maxillofac Surg. 1991;20:69-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 136. | Cole-Hawkins H, Fyfe E, Price C, Pring M. Posttransplant lymphoproliferative disorder presenting as a nonhealing extraction socket: a case report and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;113:e12-e18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 137. | León JE, Takahama Júnior A, Vassallo J, Soares FA, de Almeida OP, Lopes MA. EBV-associated polymorphic posttransplant lymphoproliferative disorder presenting as gingival ulcers. Int J Surg Pathol. 2011;19:241-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 138. | Ogata H, Matsui T, Nakamura M, Iida M, Takazoe M, Suzuki Y, Hibi T. A randomised dose finding study of oral tacrolimus (FK506) therapy in refractory ulcerative colitis. Gut. 2006;55:1255-1262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 335] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 139. | Asarch A, Gottlieb AB, Lee J, Masterpol KS, Scheinman PL, Stadecker MJ, Massarotti EM, Bush ML. Lichen planus-like eruptions: an emerging side effect of tumor necrosis factor-alpha antagonists. J Am Acad Dermatol. 2009;61:104-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 126] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 140. | Moss AC, Treister NS, Marsee DK, Cheifetz AS. Clinical challenges and images in GI. Oral lichenoid reaction in a patient with Crohn’s disease receiving infliximab. Gastroenterology. 2007;132:488, 829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 141. | Kaur N, Mahl TC. Pneumocystis carinii pneumonia with oral candidiasis after infliximab therapy for Crohn’s disease. Dig Dis Sci. 2004;49:1458-1460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 142. | Mocciaro F, Orlando A, Renna S, Rizzuto MR, Cottone M. Oral lichen planus after certolizumab pegol treatment in a patient with Crohn’s disease. J Crohns Colitis. 2011;5:173-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 143. | Edwards D, Boritz E, Cowen EW, Brown RS. Erythema multiforme major following treatment with infliximab. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;115:e36-e40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 144. | Salama M, Lawrance IC. Stevens-Johnson syndrome complicating adalimumab therapy in Crohn’s disease. World J Gastroenterol. 2009;15:4449-4452. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 145. | Sandborn WJ, Feagan BG, Rutgeerts P, Hanauer S, Colombel JF, Sands BE, Lukas M, Fedorak RN, Lee S, Bressler B. Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2013;369:711-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1416] [Cited by in RCA: 1630] [Article Influence: 125.4] [Reference Citation Analysis (1)] |
| 146. | Van Kemseke C, Louis E, Reenaers C. [Vedolizumab (Entyvio®) for the treatment of inflammatory bowel diseases]. Rev Med Liege. 2015;70:575-582. [PubMed] |
| 147. | Frankel DH, Mostofi RS, Lorincz AL. Oral Crohn’s disease: report of two cases in brothers with metallic dysgeusia and a review of the literature. J Am Acad Dermatol. 1985;12:260-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 22] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 148. | Harbord M, Annese V, Vavricka SR, Allez M, Barreiro-de Acosta M, Boberg KM, Burisch J, De Vos M, De Vries AM, Dick AD. The First European Evidence-based Consensus on Extra-intestinal Manifestations in Inflammatory Bowel Disease. J Crohns Colitis. 2016;10:239-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 619] [Cited by in RCA: 569] [Article Influence: 56.9] [Reference Citation Analysis (9)] |
| 149. | Casson DH, Eltumi M, Tomlin S, Walker-Smith JA, Murch SH. Topical tacrolimus may be effective in the treatment of oral and perineal Crohn’s disease. Gut. 2000;47:436-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 96] [Article Influence: 3.7] [Reference Citation Analysis (1)] |
| 150. | Jurge S, Kuffer R, Scully C, Porter SR. Mucosal disease series. Number VI. Recurrent aphthous stomatitis. Oral Dis. 2006;12:1-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 236] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 151. | Dharmavaram AT, Reddy RS, Nallakunta R. “Ozone” - the new NEMESIS of canker sore. J Clin Diagn Res. 2015;9:ZC01-ZC04. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 152. | Albrektson M, Hedström L, Bergh H. Recurrent aphthous stomatitis and pain management with low-level laser therapy: a randomized controlled trial. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;117:590-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 153. | Lamey PJ, Lewis MA. Oral medicine in practice: angular cheilitis. Br Dent J. 1989;167:15-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 154. | Garcia-Cuesta C, Sarrion-Pérez MG, Bagán JV. Current treatment of oral candidiasis: A literature review. J Clin Exp Dent. 2014;6:e576-e582. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 113] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 155. | Zopf Y, Rabe C, Kollmann S, Hahn EG, Thürauf N, Schwab D. Alterations of taste perception in Crohn’s disease and their dependency on disease activity and nutritional behavior. J Clin Gastroenterol. 2009;43:617-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 156. | Sigusch BW. Periodontitis as manifestation of Crohn’s disease in primary dentition: a case report. J Dent Child (Chic). 2004;71:193-196. [PubMed] |
Manuscript Source: Invited manuscript
Specialty Type: Gastroenterology and Hepatology
Country of Origin: Croatia
Peer-Review Report Classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Ahluwalia NK, Freeman HJ, Johnson MW S- Editor: Ma YJ L- Editor: A E- Editor: Wang CH