Published online Jun 21, 2016. doi: 10.3748/wjg.v22.i23.5400
Peer-review started: August 18, 2015
First decision: November 26, 2015
Revised: January 12, 2016
Accepted: January 30, 2016
Article in press: January 30, 2016
Published online: June 21, 2016
Processing time: 299 Days and 21.9 Hours
AIM: To analyze the efficacy of last line sorafenib treatment in colorectal cancer patients.
METHODS: All patients receiving chemotherapy for colorectal cancer in the outpatient clinic of the University of Mainz since 2006 were retrospectively analyzed for last line sorafenib exposure. Charts of identified patients were analyzed for clinic-pathological parameters, like data on gender, age, date of initial diagnosis, UICC stage, number and kind of the pre-therapies, therapy start and end of sorafenib, sorafenib mediated treatment cessation, side effects, response rates, time to progression and overall survival.
RESULTS: Ten patients with a median of 3.0 prior chemotherapy lines had received a last line sorafenib therapy either alone (10%) or in combination with 5-fluorouracil derivates (90%). All patients suffered from colorectal cancer stage UICC 4 and were routinely seen in 2-wk intervals in the oncology outpatient clinic. Median duration of treatment was 142.0 d. At 8 wk 80% of patients showed stable disease but we did not observe any remissions. Median time to progression was 140.5 d (4.7 mo), while median overall survival reached 176.5 d. One patient ceased treatment due to side effects. Reason for treatment stop was bleeding complication in one case and non-specified sorafenib intolerance in another case. Due to the retrospective approach we did not further quantify side effects.
CONCLUSION: This retrospective analysis encourages further investigation of sorafenib in colorectal cancer last line therapy.
Core tip: In this restrospective analysis we demonstrate that sorafenib monotherapy or in combination with 5-fluorouracil derivates seems to be feasible. Eighty percent of the patients showed stable disease with a median time to progression of 140.5 d and acceptable toxicity profile. In our eyes, the reported overall as well as progression free survival under sorafenib treatment are of clinical and financial interest.
- Citation: Martchenko K, Schmidtmann I, Thomaidis T, Thole V, Galle PR, Becker M, Möhler M, Wehler TC, Schimanski CC. Last line therapy with sorafenib in colorectal cancer: A retrospective analysis. World J Gastroenterol 2016; 22(23): 5400-5405
- URL: https://www.wjgnet.com/1007-9327/full/v22/i23/5400.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i23.5400
Colorectal cancer ranges among the most frequent malignancies in Western countries[1,2]. Annually, more than 1.2 million patients are diagnosed with colorectal cancer resulting in more than 600000 death each year (1 Sora). Survival is delineated by local recurrence and tumor dissemination[3]. Noteworthy, 50% of patients develop metastases in the due course of the disease (2 Sora).
Due to improved therapeutic strategies, the overall survival in metastatic stage IV colorectal cancer has increased from eight months to more than two years during the last decade. Chemotherapeutics, such as platinum derivates (oxaliplatin) or topoisomerase II inhibitors (irinotecan) as well as the introduction of biologicals targeting tumor neo-vascularisation (anti-VEGF: bevacizumab and aflibercept) or growth-signaling (anti-EGFR: cetuximab and panitumumab) have significantly augmented response rates and prognosic parameters[4-10].
Those new strategies have resulted in an unexpected dilemma: Numerous patients in good condition have experienced progression following treatment with all available agents. This therapeutic gap has recently been targeted by the multi-tyrosine kinase inhibitor regorafenib[11]. Last line treatment with regorafenib resulted in a progression free survival of 1.9 mo (vs 1.7 mo in the placebo arm) and an overall survival of 6.4 mo (vs 5.0 mo in the placebo arm). Receptor tyrosine kinases (RTKs) are transmembrane-receptors containing extracellular ligand-binding domains connected to intracellular catalytic domains[12]. The growth factors VEGF/PDGF/EGF and their receptors VEGFR1-3, PDGFRα/β and EGFR are critical in the process of (lymphatic) neo-angiogenesis and dissemination in human cancer[13-17].
Inhibition of RTKs with sorafenib has been successful in renal and hepatocellular cancer[18,19]. Two phase I studies revealed a disease stabilization in pretreated colorectal cancer patients receiving sorafenib in combination with either irinotecan or oxaliplatin[20,21]. Therefore, the impact of combinational therapies (sorafenib + chemotherapy) remains controversial.
However due to a lack of treatment options in the augmenting number of colorectal cancer patients pretreated with all available chemotherapeutic and biological options, we identified 10 patients which had received off-label sorafenib within a risk sharing program of Bayer Healthcare. The current publication reports on the results of those patients in a retrospective approach.
We retrospectively analyzed all medical records of colorectal cancer patients which received any treatment in the outpatient clinic of the university if Mainz between January 1, 2007 and December 31, 2011 in order to identify patients that had received sorafenib as last line treatment. We then retrospectively collected and analyzed data from the medical records. In particular we collected data on gender, age, date of initial diagnosis, UICC stage, number and kind of the pre-therapies, therapy start and end of sorafenib, sorafenib mediated treatment cessation, progression free survival (PFS), overall survival (OS) and relative risk.
We identified 10 patients, which had received off-lable sorafenib after entering a risk sharing program of Bayer Healthcare. All patients were routinely seen in 2-wk intervals in the oncology outpatient clinic. At visits following assessments were done: general condition of the patients, blood counts, side effects. Staging analyses (CT scan abdomen and thorax) were done every 8 wk. All patients (100%) suffered from colorectal cancer stage UICC 4. Eighty percent of patients were male, while 20% were of female gender. Average patient age was 65 years. One hundred percent of patients were in good condition as indicated by an ECOG of 0-1. Patients had received an average of 3 prior chemotherapy regimens prior to treatment with sorafenib (Tables 1 and 2).
| Patient characteristics | n (%) |
| Total number | 10.0 |
| Average age (yr) | 65.4 |
| Gender | |
| Female | 2 (20) |
| Male | 8 (80) |
| Location | |
| Colon | 8 (80) |
| Rectum | 2 (20) |
| UICC | |
| 1 + 2 + 3 | 0 (0) |
| 4 | 10 (100) |
| Metastases | |
| Liver | 6 (60) |
| Lung | 8 (80) |
| Lymph nodes | 5 (50) |
| Prior ctx regimen | |
| 3 | 7 (70) |
| 4 | 2 (20) |
| 5 | 0 (0) |
| 6 | 1 (10) |
| Patient | Age | Gender | Primary | UICC | Liver metastases | Lunge metastases | Number of pre-therapies |
| 1 | 68 | M | Sigma | IV | 1 | 1 | 3 |
| 2 | 52 | M | Colon desc. | IV | 0 | 1 | 3 |
| 3 | 51 | M | Sigma | IV | 1 | 1 | 3 |
| 4 | 81 | M | Rectum | IV | 1 | 1 | 3 |
| 5 | 59 | F | Colon desc. | IV | 1 | 1 | 3 |
| 6 | 74 | M | Sigma | IV | 0 | 1 | 3 |
| 7 | 74 | M | Rectum | IV | 1 | 0 | 4 |
| 8 | 65 | F | Sigma | IV | 1 | 1 | 6 |
| 9 | 69 | M | Sigma | IV | 0 | 1 | 3 |
| 10 | 61 | M | Colon desc. | IV | 0 | 0 | 4 |
One patient (10%) had received a sorafenib monotherapy, while 9 patients (90%) had had a combination of sorafenib with different 5-fluorouracil (5-FU) derivates. 5-FU derivates applied were intravenous 5-FU (n = 2; folinic acid 400 mg/qm d1, 5-FU 400 mg/q.m. bolus d1, 2400 mg/q.m. d1 and d2), capecitabine (n = 4; dose 2000 mg/q.m. d1-d14) or Tegafur-Uracil (n = 3; 300 mg/q.m. and Calciumfolinat 90 mg/d d1-d28). All patients were initially administered a reduced dose of sorafenib of 400 mg/d (200 mg b.i.d.). The dose was adjusted to 800 mg/d (400 mg b.i.d.) after 1 to 2 wk. Treatment duration was 142 d in median. Maximal treatment duration was 176 d. Eighty percent of patients (8/10) received treatment until progression, while 20% (2/10) ceased treatment due to side effects after average treatment duration of 56 d. Reason for treatment stop was bleeding complication in one case and non-specified sorafenib intolerance in another case. Due to the retrospective approach we did not further quantify side effects (Table 3).
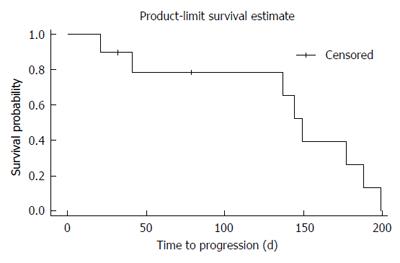
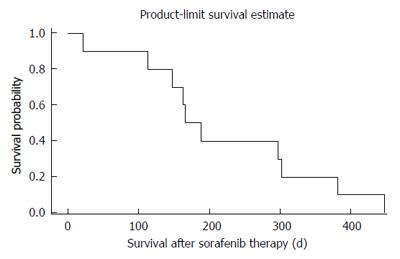
At the first staging at treatment week 6, 80% of patients (8/10) revealed stable disease (SD) as compared to progressive disease (PD) in 20% (2/10; one of both patients had died after 21 d of treatment due to clinical progress). No partial or complete responses were observed. At week 12 only six patients were evaluable. Of those 50% (3/6) revealed stable disease and 50% (3/6) progressive disease. Median PFS was 140 d, median OS was 176 d (Figures 1 and 2).
The most frequent sorafenib-related adverse events of grade 3 or higher were fatigue (30%), anemia (20%), emesis (10%), mucositis (10%), pain (10%), leucocytopenia (10%) and thrombocytopenia (10%; Table 4). Relevant sorafenib-related adverse events grade 1 or 2 were fatigue (70%), diarrhea (40%), anemia (40%), mucositis (30%), hand foot syndrome (30%) and thrombocytopenia (30%) among others (Table 4).
| Patient | I°-II° | III°-IV° | Total |
| Fatigue | 70% (7/10) | 30% (3/10) | 100% |
| Nausea | 20% (2/10) | 0% (0/10) | 20% |
| Emesis | 20% (2/10) | 10% (1/10) | 30% |
| Diarrhea | 40% (4/10) | 0% (0/10) | 40% |
| Mukositis | 30% (3/10) | 10% (1/10) | 40% |
| Rash | 20% (2/10) | 0% (0/10) | 20% |
| HFS | 30% (3/10) | 0% (0/10) | 30% |
| PNP | 20% (2/10) | 0% (0/10) | 20% |
| Pain | 20% (2/10) | 10% (1/10) | 30% |
| Weight loss | 10% (1/10) | 0% (0/10) | 10% |
| Infection | 20% (2/10) | 0% (0/10) | 20% |
| Anemia | 40% (4/10) | 20% (2/10) | 60% |
| Thrombopenia | 30% (3/10) | 10% (1/10) | 40% |
| Leucopenia | 20% (2/10) | 10% (1/10) | 30% |
As to our best knowledge, this is the first retrospective study investigating last line sorafenib in colorectal cancer patients. Prospective studies have not been performed or published, so far.
Due to augmented therapeutic options, the overall survival in metastatic stage IV colorectal cancer has increased from eight months to more than two years during the last decade[4-7]. Those new strategies have resulted in an unexpected dilemma: Numerous patients in good condition have experienced progression following treatment with all available agents. This therapeutic gap has recently been targeted by the multi-tyrosine kinase inhibitor regorafenib[11]. Last line treatment with regorafenib resulted in a progression free survival of 1.9 mo (vs 1.7 mo in the placebo arm) and an overall survival of 6.4 mo (vs 5.0 mo in the placebo arm). In the light of the regorafenib approval in 2013 we decided to analyze the efficacy of sorafenib in a retrospective approach. Diverse colorectal cancer patients in good condition had previously received sorafenib in a last line approach over the recent years prior to admission of regorafenib.
Our retrospective data must be handled with care due to their limitation by the retrospective approach and the small number of patients. All patients were UICC stage IV, in good condition and had received an average of 3 prior chemotherapy regimens. However, the majority of patients were of male gender, thus not representing the normal distribution of colorectal cancer among genders.
Tolerability of last line sorafenib was generally good: 30% of patients developed a grade 3/4 fatigue, 20% a grade 3/4 anemia and respectively 10% a grade 3/4 emesis, mucositis, leucocytopenia or thrombocytopenia. Thus grade 3/4 fatigue, anemia and thrombocytopenia seems to occur more often than under regorafenib (10%, 3% and 4%, respectively)[11]. However, due to the low number of cases this data is at best descriptive.
We were surprised to find a median PFS of 4.7 mo (140 d) among our patients as compared to 1.9 mo under regorafenib treatment as reported by Axel Grothey and coauthors. In addition our retrospective analysis revealed an overall survival of 5.9 mo (176 d) and thus is in line with the reported overall survival of last line regorafenib (6.4 mo vs 5.0 mo in the placebo arm). As to be expected we did not observe any remissions but found an interesting effect in disease stabilization (80% at week 8). Noteworthy, the majority of patients (90%) had received a combination of sorafenib with 5-FU (or its pro-drugs) and only one patient had ceased treatment due to side effects.
However, the combination of sorafenib with 5-FU might not explain the observed effects. As reported by our own group, we previously studied the combination of sorafenib with 5-FU in vitro as well as in vivo in xenograft models[22]. We demonstrated that a sorafenib-monotherapy (5 mg/kg; approximately 400 mg in an 80 kg patient) was equally effective as a combination therapy of both sorafenib and 5-FU. Thus, a combination therapy did not result in any additive effects, but might add adverse events. However, these data became available only after the off-label treatment of colorectal cancer patients.
In our eyes, the reported overall as well as progression free survival under sorafenib treatment are of clinical and financial interest, as treatment costs of regorafenib sum up to €5573 per 28-d-cycle as compared to €2611 for its predecessor sorafenib. Therefore a trial focusing on non-inferiority for sorafenib vs regorafenib might be feasible.
Improved therapeutic strategies have resulted in an unexpected dilemma: Numerous patients in good condition have experienced progression following treatment with all available agents. This therapeutic gap has recently been targeted by the multi-tyrosine kinase inhibitor regorafenib. Last line treatment with regorafenib resulted in a progression free survival of 1.9 mo (vs 1.7 mo in the placebo arm) and an overall survival of 6.4 mo (vs 5.0 mo in the placebo arm). Receptor tyrosine kinases (RTKs) are transmembrane-receptors containing extracellular ligand-binding domains connected to intracellular catalytic domains. The growth factors VEGF/PDGF/EGF and their receptors VEGFR1-3, PDGFRα/β and EGFR are critical in the process of (lymphatic) neo-angiogenesis and dissemination in human cancer. Inhibition of RTKs with sorafenib has been successful in renal and hepatocellular cancer. Two phase I studies revealed a disease stabilization in pretreated colorectal cancer patients receiving sorafenib in combination with either irinotecan or oxaliplatin. Therefore, the impact of combinational therapies (sorafenib + chemotherapy) remains controversial.
Due to novel therapeutic approaches patients with stage IV colorectal cancer show an improvement in overall survival. In this palliative setting it remains a major goal to reduce therapy induced toxicity and still preserve improvement of progression free survival or overall survival.
Ten patients with a median of 3.0 prior chemotherapy lines had received a last line sorafenib therapy either alone (10%) or in combination with 5-fluorouracil derivates (90%). All patients suffered from colorectal cancer stage UICC 4 and were routinely seen in 2-wk intervals in the oncology outpatient clinic. Median duration of treatment was 142.0 d. At 8 wk 80% of patients showed stable disease. Therefore sorafenib could be a very efficient and cost effective therapeutic last line approach to compared to regorafenib.
The authors evaluate a cost effective last line therapeutic approach for colorectal cancer patient with a favorable toxicity profile.
Receptor tyrosine kinases (RTKs), are transmembrane-receptors containing extracellular ligand-binding domains connected to intracellular catalytic domains. The growth factors VEGF/PDGF/EGF and their receptors VEGFR1-3, PDGFRα/β and EGFR are critical in the process of (lymphatic) neo-angiogenesis and dissemination in human cancer. Sorafenib and regorafenib are inhibitors for these RTKs.
The retrospective study in order to analyze the efficacy of sorafenib treatment in colorectal cancer present a small number of patients, but the results are valuable.
| 1. | Greenlee RT, Murray T, Bolden S, Wingo PA. Cancer statistics, 2000. CA Cancer J Clin. 2000;50:7-33. [PubMed] |
| 2. | Weir HK, Thun MJ, Hankey BF, Ries LA, Howe HL, Wingo PA, Jemal A, Ward E, Anderson RN, Edwards BK. Annual report to the nation on the status of cancer, 1975-2000, featuring the uses of surveillance data for cancer prevention and control. J Natl Cancer Inst. 2003;95:1276-1299. [PubMed] |
| 3. | August DA, Ottow RT, Sugarbaker PH. Clinical perspective of human colorectal cancer metastasis. Cancer Metastasis Rev. 1984;3:303-324. [PubMed] |
| 4. | Douillard JY, Siena S, Cassidy J, Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham D, Jassem J. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol. 2010;28:4697-4705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1296] [Cited by in RCA: 1413] [Article Influence: 88.3] [Reference Citation Analysis (0)] |
| 5. | Douillard JY, Siena S, Cassidy J, Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham D, Jassem J. Final results from PRIME: randomized phase III study of panitumumab with FOLFOX4 for first-line treatment of metastatic colorectal cancer. Ann Oncol. 2014;25:1346-1355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 439] [Article Influence: 36.6] [Reference Citation Analysis (7)] |
| 6. | Sobrero A, Ackland S, Clarke S, Perez-Carrión R, Chiara S, Gapski J, Mainwaring P, Langer B, Young S. Phase IV study of bevacizumab in combination with infusional fluorouracil, leucovorin and irinotecan (FOLFIRI) in first-line metastatic colorectal cancer. Oncology. 2009;77:113-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 92] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 7. | Welch S, Spithoff K, Rumble RB, Maroun J. Bevacizumab combined with chemotherapy for patients with advanced colorectal cancer: a systematic review. Ann Oncol. 2010;21:1152-1162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 138] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 8. | Nielsen DL, Palshof JA, Larsen FO, Jensen BV, Pfeiffer P. A systematic review of salvage therapy to patients with metastatic colorectal cancer previously treated with fluorouracil, oxaliplatin and irinotecan +/- targeted therapy. Cancer Treat Rev. 2014;40:701-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 9. | Akhtar R, Chandel S, Sarotra P, Medhi B. Current status of pharmacological treatment of colorectal cancer. World J Gastrointest Oncol. 2014;6:177-183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 10. | Kirstein MM, Lange A, Prenzler A, Manns MP, Kubicka S, Vogel A. Targeted therapies in metastatic colorectal cancer: a systematic review and assessment of currently available data. Oncologist. 2014;19:1156-1168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 84] [Article Influence: 7.0] [Reference Citation Analysis (1)] |
| 11. | Grothey A, Van Cutsem E, Sobrero A, Siena S, Falcone A, Ychou M, Humblet Y, Bouché O, Mineur L, Barone C. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:303-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2194] [Cited by in RCA: 2213] [Article Influence: 170.2] [Reference Citation Analysis (0)] |
| 12. | Li E, Hristova K. Role of receptor tyrosine kinase transmembrane domains in cell signaling and human pathologies. Biochemistry. 2006;45:6241-6251. [PubMed] |
| 13. | Möbius C, Stein HJ, Becker I, Feith M, Theisen J, Gais P, Jütting U, Siewert JR. The ‘angiogenic switch’ in the progression from Barrett’s metaplasia to esophageal adenocarcinoma. Eur J Surg Oncol. 2003;29:890-894. [PubMed] |
| 14. | Liu XE, Sun XD, Wu JM. Expression and significance of VEGF-C and FLT-4 in gastric cancer. World J Gastroenterol. 2004;10:352-355. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 39] [Cited by in RCA: 41] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Yonemura Y, Endo Y, Tabata K, Kawamura T, Yun HY, Bandou E, Sasaki T, Miura M. Role of VEGF-C and VEGF-D in lymphangiogenesis in gastric cancer. Int J Clin Oncol. 2005;10:318-327. [PubMed] |
| 16. | Takahashi Y, Cleary KR, Mai M, Kitadai Y, Bucana CD, Ellis LM. Significance of vessel count and vascular endothelial growth factor and its receptor (KDR) in intestinal-type gastric cancer. Clin Cancer Res. 1996;2:1679-1684. [PubMed] |
| 17. | Wang KL, Wu TT, Choi IS, Wang H, Resetkova E, Correa AM, Hofstetter WL, Swisher SG, Ajani JA, Rashid A. Expression of epidermal growth factor receptor in esophageal and esophagogastric junction adenocarcinomas: association with poor outcome. Cancer. 2007;109:658-667. [PubMed] |
| 18. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9016] [Cited by in RCA: 10531] [Article Influence: 585.1] [Reference Citation Analysis (9)] |
| 19. | Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Staehler M, Negrier S, Chevreau C, Desai AA, Rolland F. Sorafenib for treatment of renal cell carcinoma: Final efficacy and safety results of the phase III treatment approaches in renal cancer global evaluation trial. J Clin Oncol. 2009;27:3312-3318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 833] [Cited by in RCA: 857] [Article Influence: 50.4] [Reference Citation Analysis (0)] |
| 20. | Mross K, Steinbild S, Baas F, Gmehling D, Radtke M, Voliotis D, Brendel E, Christensen O, Unger C. Results from an in vitro and a clinical/pharmacological phase I study with the combination irinotecan and sorafenib. Eur J Cancer. 2007;43:55-63. [PubMed] |
| 21. | Kupsch P, Henning BF, Passarge K, Richly H, Wiesemann K, Hilger RA, Scheulen ME, Christensen O, Brendel E, Schwartz B. Results of a phase I trial of sorafenib (BAY 43-9006) in combination with oxaliplatin in patients with refractory solid tumors, including colorectal cancer. Clin Colorectal Cancer. 2005;5:188-196. [PubMed] |
| 22. | Wehler TC, Hamdi S, Maderer A, Graf C, Gockel I, Schmidtmann I, Hainz M, Berger MR, Theobald M, Galle PR. Single-agent therapy with sorafenib or 5-FU is equally effective in human colorectal cancer xenograft--no benefit of combination therapy. Int J Colorectal Dis. 2013;28:385-398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Stanilova SA S- Editor: Yu J L- Editor: Cant MR E- Editor: Ma S