INTRODUCTION
Since 1995, regulatory T (Treg) cells as a particular lineage of CD4+T cells have been found to play a central role in the effective control of self-tolerance and maintenance of immune homeostasis[1,2]. Insufficient quantity or dysfunction of Treg cells invariably leads to inflammatory diseases, autoimmune diseases or lymphoproliferative syndrome including inflammatory bowel disease (IBD), rheumatoid arthritis and systemic lupus erythematosus in humans and animals[3-6]. Many previous studies have demonstrated that the suppressive function of Treg cells limits convincingly favorable host effector responses and restrains inflammatory responses in diverse anatomical locations as mucosal barriers against chronic inflammations and tumors[7-10]. Distinct Treg subsets coexist in the intestinal mucosa and mesenteric lymph nodes. Some studies have demonstrated that the suppressive function of Treg cells is partly implemented via modulation of dendritic cells (DCs)[11-13]. Usually, the transfer of Treg cells leads to the development of colitis via accumulation of T cells and DCs[14]. However, adequate Tregs or transfusion of Treg cells maintains mucosal tolerance to prevent and cure experimental colitis by directly inhibiting the expression of costimulatory molecules (such as CD40, CD154, CD134 and CD134L) of DCs or the migration of DCs to the MLNs, or by reducing DC activation[15,16]. These findings indicate that the interactions of DCs and Treg cells are closely related to the pathogenesis of IBD, and probably become a target for treatment of IBD.
Curcumin (Cur), a major active component of the rhizome of Curcuma longa (turmeric), has been used widely to treat cardiovascular disease, diabetes mellitus and IBD for several centuries in China[17,18]. Cur is known for its low toxicity and a wide range of reported pharmacological effects, which include antioxidant, anti-inflammatory, antiplatelet, hypoglycemic, cholesterol-lowering, anti-bacterial, wound-healing and anti-fungal effects[19-21]. Several studies have demonstrated that Cur can effectively attenuate inflammation in humans and animals with colitis[19-21]. Although anti-inflammatory actions of Cur in IBD may be associated with the inhibition of nuclear factor κB (NF-κB) pathway, such as p38 mitogen-activated protein kinases, and the reduction of pro-inflammatory cytokine response[22,23], its exact mechanisms remain unclear. In the present study, we explored the pathway by which Cur regulates function of Treg cells by observing the expression of costimulatory molecules of DCs in an animal model of colitis.
MATERIALS AND METHODS
Animals
Male C57BL/6 mice (9-12-wk-old) were purchased from the Animal Center of Peking University Health Science Center (Animal certificate number: SCXK 2012-0001). They were housed at 23 ± 2 °C with a humidity of 50% ± 5% in a 12 h light/dark cycle and provided with standard diet and water ad libitum. Mice were randomly divided into four groups: normal, TNBS, TNBS + Cur, and TNBS + mesalazine (Mes) groups. Each group contained ten mice. The experimental protocol (JZ2015-016) was approved by Institutional Animal Care and Use Committee of Jiangxi University of Traditional Chinese Medicine.
Induction of colitis
Colitis was induced with 2, 4, 6-trinitrobenzene sulfonic acid (TNBS; batch number: p2297, Sigma, United States) as described by previous studies[24,25]. Except for mice in the normal group, mice in other groups were mildly anesthetized with pentobarbital sodium (40 mg/kg) via intraperitoneal injection before 100 mg/kg TNBS dissolved in 0.3 mL of 50% ethanol was instilled into the colon approximately 2 cm to the anus. Equal volume of physiological saline was given in the normal group.
Treatment protocol
Twenty-four hours after colitis was induced, the mice in the TNBS+Cur and TNBS+Mes groups were, respectively, administered with Cur (200 mg/kg) (purity > 95% by HPLC, batch number: GR-133-140421, GANGRUN Biotechnology, Nanjing, China) and Mes (300 mg/kg) (batch number: 130407, Sunflower Pharma Jiamusi, China) intragastrically for 7 d. The mice in the normal and TNBS groups received equal volume of physiological saline every day until the end of the experiment. All the mice were sacrificed on the 8th day.
Histological evaluation
The whole colon (n = 10) was separated rapidly, and its length was measured. After clearing its contents, the colon was weighed and then divided into two parts. The weight index of the colon was calculated as colonic weight/body weight × 100%. The colonic segments were fixed in 4% paraformaldehyde solution, cut into sections, and stained with hematoxylin and eosin (HE). According to the previous study reported by Nicole and his colleagues[26], the criterion of the histological damage score (n = 10) was implemented to evaluate colonic injury. Histological score was calculated based on inflammatory cell infiltration and tissue damage. Scores for infiltration are as follows: 0: no infiltration; 1: increased number of inflammatory cells in the lamina propria; 2: inflammatory cells extending into the submucosa; and 3: transmural inflammatory cell infiltration. Scores of tissue damage are as follows: 0: no mucosal damage; 1: discrete epithelial lesions; 2: erosions or focal ulcerations; and 3: severe mucosal damage with extensive ulceration extending into the bowel wall.
Isolation of lymphocyte from gut-associated lymphoid tissue
Gut-associated lymphoid tissue (GALT) was separated and collected from the whole small intestine to the terminal rectum. GALT was triturated in 3% fetal calf serum (FCS)/PBS solution on ice cake, and filtrated via 300 section stainless steel cell cribble. The cell suspensions were centrifuged at 1500 rpm/min at 4 °C for 5 min and suspended in 3% FCS/PBS solution at 1 × 106-1 × 107/mL.
Assay of CD4+CD25+Foxp3+ T cells by flow cytometry
The cell suspensions (n = 8) were incubated for 30 min with V450 -anti-mouse CD4+Ab (0.125 μg/100 μL, BD Bioscience) and PerCP/Cy5.5 anti-mouse CD25+Ab (0.25 μg/100 μL, Biolegend) at 37 °C in the dark. Cells were centrifuged at 1500 rpm/min and 4 °C for 5 min, fixed in Fix/Perm Buffer (eBioscience, San Diego, CA) for at least 1 h at 37 °C, and then incubated with APC -anti-mouse Foxp3+Ab (0.5 μg/100 μL; eBioscience) for 30 min at 37 °C in the dark. Cells labeled with PE rat IgG2a were used as the isotype negative control. Fluorescence-activated cell sorting analysis was performed on a FACSCalibur (BD Biosciences).
Measurement of costimulatory molecules by flow cytometry
Data acquisition was performed using a flow cytometer (FACSCalibur, BD-Pharmingen, San Diego, CA, United States) by collecting a minimum of 10000 events per sample. The frequency of positive cells was analyzed using the program Cell Quest in two regions. The lymphocyte region was determined using granularity (SSC) and size (FSC) plot. DCs were identified as a MHC+ [CD205+, CD54 (ICAM-1)+, TLR4+, CD252 (OX40 L)+, CD256 (RANK)+ and CD254 (RANK L)+] population, and within this gate the CD11c+ population was assessed. The following mAbs were used: APC/Cy7 anti-mouse CD11c, PerCP/Cy5.5 anti-mouse CD205, FITC anti-mouse CD54, PerCP-Cy™5.5 anti-mouse I-A/I-E (MHC-II), PE-cyanine7 anti-mouse TLR4, APC anti-mouse CD252, PE anti-mouse CD256 and PE rat anti-mouse CD254 (eBioscience, San Diego, CA). Limits for the quadrant markers were always set based on negative populations and isotype controls.
Enzyme-linked immunosorbent assay
The colonic mucosa was separated from all mice to prepare tissue homogenate. After centrifugation at 5000 rpm/min for 10 min, the supernatant was collected and used for testing the levels of cytokines by enzyme-linked immunosorbent assay (ELISA). The levels of TNF-α, IL-2, IL-6, IL-12 p40, IL-17 and IL-21 (n = 8) were determined using commercial ELISA kits according to the manufacturer’s instructions (eBioscience, San Diego, CA).
Statistical analysis
Data are expressed as mean ± SE. The statistical significance was evaluated by one-way analysis of variance, and statistical analyses were performed with Prism 4.0 (GraphPad Software, La Jolla, CA). P values less than 0.05 were considered statistically significant.
RESULTS
Cur effectively alleviates inflammatory injury of the colonic mucosa in colitis mice
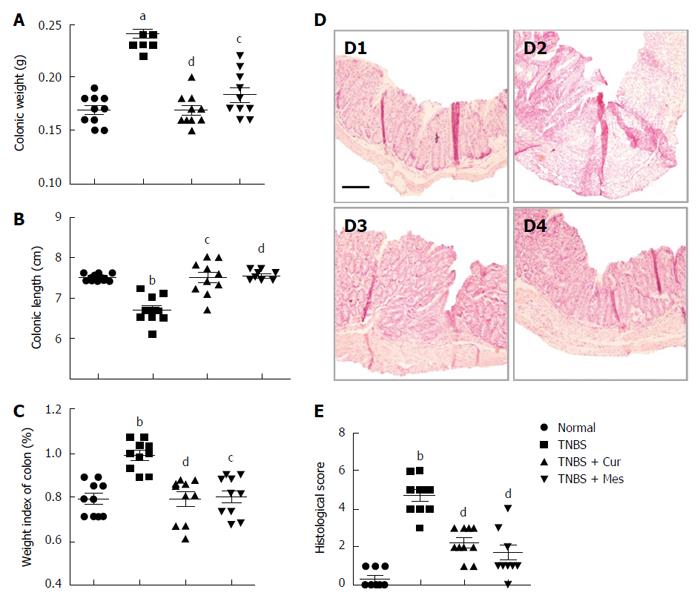
Administration of TNBS led to a severe illness that was characterized by damaged colonic mucosa, increased colonic weight and so on. As shown in Figure 1, compared with mice in the TNBS group, the colonic weight (Figure 1A) and weight index of the colon (Figure 1C) were significantly decreased in the TNBS + Cur and TNBS + Mes groups, while the colonic length (Figure 1B) was lengthened. Pathological observation found that mucosal architecture was damaged, the colon wall was thicken, ulcers formed, and extensive inflammatory cells infiltrated in the colonic mucosa of colitis mice, while its histological score was increased (Figure 1D-2 and E). However, the extent of damaged colonic mucosa was alleviated in the TNBS + Cur and TNBS + Mes groups (Figure 1D-3 and D-4) as revealed by decreased histological scores (Figure 1E). These results suggest that Cur alleviates inflammatory injury of the colonic mucosa effectively.
Figure 1 Histological evaluation.
A: Colonic weight; B: Colonic length; C: Weight index of the colon; D: Typical histological images stained with HE. D1, D2, D3 and D4, respectively, represent the normal, TNBS, TNBS + Cur and TNBS + Mes groups. Bar = 200 μm; E: Histological scores. Data are presented as mean ± SE (n = 10). aP < 0.05 and bP < 0.01 vs normal group; cP < 0.05 and dP < 0.01 vs TNBS group.
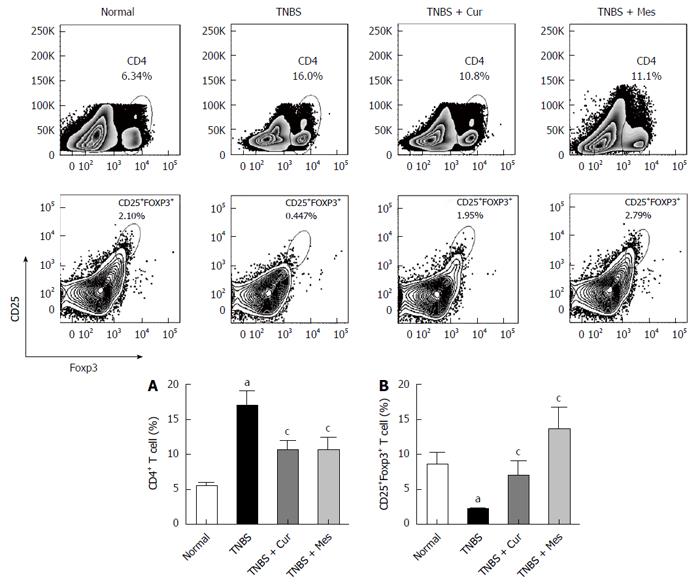
Cur improves levels of Treg cells in GALT
As shown in Figure 2, the total number of CD4+ T cells in GALT decreased in the normal, TNBS + Cur, and TNBS + Mes groups compared with the TNBS group. However, the number of CD4+CD25+Foxp3 + T cells (Treg cells), which is a marker of Treg cells, increased in the three groups compared with the TNBS group.
Figure 2 Typical graphs and levels of CD4+ and CD25+Foxp3+ T cells.
Lymphocytes were isolated from GALT of normal mice or mice with TNBS-induced colitis, and analyzed by flow cytometry. A: Typical graphs and levels (MFI) of CD4+ T cells; B: Typical graphs and levels (MFI) of CD25+Foxp3+ T cells. Data are expressed as mean ± SE (n = 8). aP < 0.05 vs normal group; cP < 0.05 vs TNBS group.
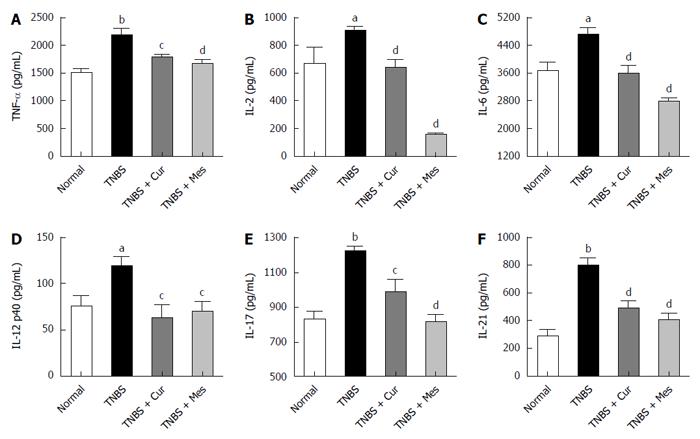
Cur inhibits secretion of related cytokines in the colonic mucosa of mice with colitis
Significant increases in the secretions of TNF-α, IL-2, IL-6, IL-12 p40, IL-17, and IL-21, as assessed by ELISA in Figure 3, were observed in the TNBS group compared to the normal group. Notably, the increased expression of TNF-α, IL-2, IL-6, IL-12 p40, IL-17, and IL-21 was remarkably reduced in the TNBS + Cur group after treatment with Cur compared to the TNBS group.
Figure 3 Concentrations of cytokines in the colonic mucosa.
The tissue supernatant was prepared from the colonic mucosa in all mice. The levels of cytokines were analyzed by ELISA. A: Concentrations of TNF-α in the colonic mucosa from different groups; B: Concentrations of IL-2 in the colonic mucosa from different groups; C: Concentrations of IL-6 in the colonic mucosa from different groups; D: Concentrations of IL-12 p40 in the colonic mucosa from different groups; E: Concentrations of IL-17 in the colonic mucosa from different groups; F: Concentrations of IL-21 in the colonic mucosa from different groups. Data are expressed as mean ± SE (n = 8). aP < 0.05 and bP < 0.01 vs normal group; cP < 0.05 and dP < 0.01 vs TNBS group.
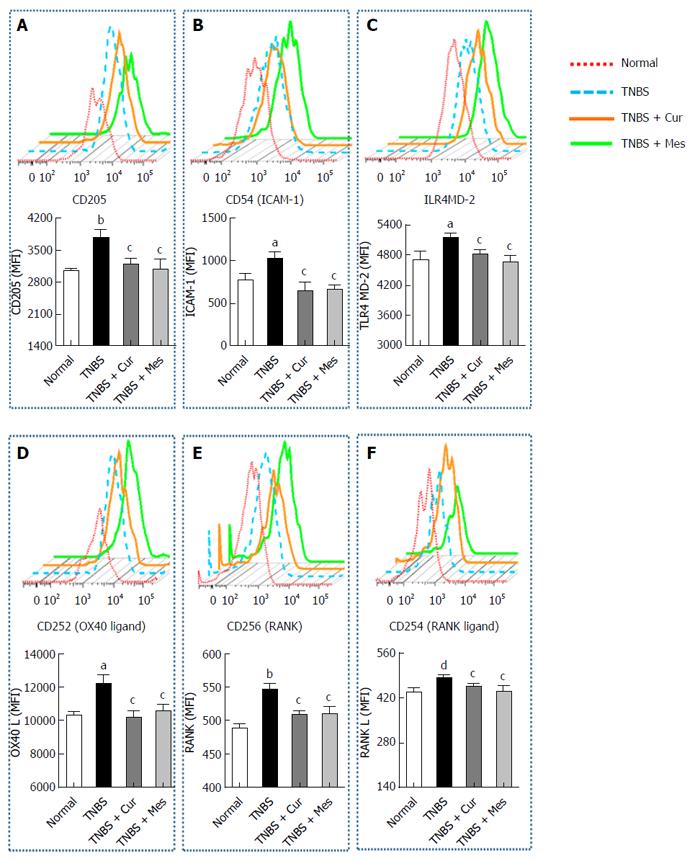
Cur reduces expression of costimulatory molecules in GALT
Figure 3 shows the expression of CD205 (Figure 4A), CD54 (ICAM-1) (Figure 4B), TLR4 (Figure 4C), CD252 (OX40 L) (Figure 4D), CD256 (RANK) (Figure 4E) and CD254 (RANK L) (Figure 4F) in GALT. The expression of these costimulatory molecules was increased in the TNBS group compared with the normal group. Interestingly, after 7 d treatment the increased levels of costimulatory molecules were coincidently inhibited or down-regulated in the TNBS + Cur and TNBS + Mes groups compared with the TNBS group.
Figure 4 Typical curves and levels of costimulatory molecules in dendritic cells.
Lymphocytes were isolated from GALT of normal mice or mice with TNBS-induced colitis, and analyzed by flow cytometry. A: Typical pseudocolor and level (MFI) of CD205; B: Typical pseudocolor and level (MFI) of CD54(ICAM-1); C:Typical pseudocolor and level (MFI) of TLR4; D: Typical pseudocolor and level (MFI) of CD252 (OX40 L); E: Typical pseudocolor and level (MFI) of CD256 (RNAK); F: Typical pseudocolor and level (MFI) of CD254 (RNAK L). Data are expressed as mean ± SE (n = 8). aP < 0.05 and bP < 0.01 vs normal group; cP < 0.05 and dP < 0.01 vs TNBS group.
DISCUSSION
Many studies have indicated that IBD is usually characterized by inflammatory injury of the colonic mucosa and disorder immune responses in inflamed mucosa, along with the dominance of IL-17-producing cells and deficiency of Treg cells[27-29]. In the present study, the results showed that Cur repaired colonic structure, decreased colonic weigh and histological injury score, recovered colonic length, indicating that Cur effectively restored damaged colonic mucosa in mice with TNBS-induced colitis. The overexpression of IL-17 and decreased Treg cells in the development of TNBS-induced colitis in our experiments were in agreement with those previous studies. After treatment with Cur, the level of IL-17 decreased, and Treg cells increased, indicating that the protective effect of Cur against TNBS-induced colitis is closely associated with decreased IL-17 expression and recovered levels of Treg cells. Also, we found that the production of related cytokines (as TNF-α, IL-2, IL-6, IL-12 p40 and IL-21) and expression of costimulatory molecules were suppressed by treatment with Cur. It is known that these inhibitory cytokines are secreted by DCs. Therefore, the effect of Cur in experimental colitis is closely with the suppressive function of Treg cells and activation of DCs.
Treg cells, also known as CD4+CD25+Foxp3+T cells, are involved in the maintenance of peripheral tolerance and control of the immune response by initiating suppressive effects on activated immune cells[30,31]. Treg-mediated suppression can be regulated primarily by the four broad categories of mechanisms including suppression by inhibitory cytokines (IL-10, IL-35 and TGF-β), cytolysis, metabolic disruption, or modulation of DC maturation or function[11-13]. One of mechanisms of Treg-mediated suppression is realized by anti-inflammatory cytokines (IL-10, IL-35) and TGF-β, which are in balance with pro-inflammatory cytokines such as TNF-α, IL-1, IL-6, IL-12, and IL-21. These pro-inflammatory cytokines are inhibited by anti-inflammatory cytokines, and as a result, Th17 cells are promoted to secrete IL-17 and eventually induce inflammatory damage. IL-17 is overwhelmingly produced by Th17 cells[12,32], and is expressed extensively in the mucosa and serum of IBD patients and TNBS-induced colitis mice[33]. This suggests that the balance of Treg cells and Th17 cells was possibly broken in GALT of mice with colitis.
Foxp3, a forkhead/winged helix transcription factor, can maintain the generation, function, and stabilization of Treg cells[12]. Hence, the level of Treg cells is usually evaluated by the expression of Foxp3 in the nucleus of Treg cells. High expression of Foxp 3 is essential for effector cytokines of T helper (Th) 1, Th2, and Th17 lineages[34,35]. For example, IL-2 can maintain the stable Foxp3 expression in Treg cells via STAT5 phosphorylation[36,37]. Moreover, IL-6 may cause Treg cells to transfer into Th17 cells and secrete abundant IL-17 and IL-21. IL-21 combined with IL-23 induces signal transducer and activator of transcription-3 expression, promotes retinoid-related orphan receptor-γt activation, and finally improves secretion of IL-17[38-40]. In our study, down-regulation of Treg cells led to an imbalance of Treg and Th17 cells and also an imbalance of Th1 and Th2 cells, and thus induced strong expression of pro-inflammatory cytokines that resulted in inflammatory damages in the colonic mucosa.
As the uppermost part, DCs can modulate Treg-mediated suppressive function via expression of costimulatory molecules[41,42]. DCs are critical for regulation of intestinal immunity and mucosal immune tolerance to commensal microorganisms, which is one of the pivotal etiologies of IBD[41]. Maturation and migration of DCs in lymph nodes are “danger” signals that induce inflammatory injuries in the intestinal mucosa[42,43]. Mature DCs with expression of costimulatory molecules can regulate the balance between Th1 and Th2 cells, and the balance of Treg and Th17 cells. Misra and colleagues have demonstrated that low expression of costimulatory molecules of DCs can induce immature and limited antigen presentation of DCs, which will ineffectively stimulate T cell responses and then induce an increase in the number of Treg cells[44]. Certainly, Treg cells can secrete inhibitory cytokines to repress activation of MHC-II and costimulatory molecules of DCs via negative feedback to suppress the antigen presentation of DCs[45,46]. Furthermore, DCs can induce the differentiation of Th17 cells to regulate the balance of Treg cells and Th17 cells by secreting IL-6 or IL-12 p40 and producing more pro-inflammatory cytokines (such as TNF-α, IL-6, and IL-15) that mediate inflammatory injury[47,48]. Meanwhile, costimulatory molecules of DCs, which include TNF/TNF receptor protein families (CD40/CD40L, OX40/OX40L, TNFR/TNF and so on) and immune globulin superfamily (ICAM-1/LAF-1, CD28/CTLA4/B7, etc.), participate in the polarization of Th1 and Th2 cell responses. As an example, receptor activator of NF-κB (RANK)/receptor activator of NF-κB ligand (RANKL) signal is an important costimulatory factor that activates DCs and prolongs their lifespan. This is achieved by activating Bcl-XL gene and cooperating with other costimulatory molecules (ICAM-1, TLR4, OX40 L, etc.) and some cytokines (IL-6 and IL-12) to activate NF-κB and induce the secretion of pro-inflammatory cytokines (TNF-α, IL-6, IL-17, and IL-21)[49]. The ICAM-1/LFA-1 signal and B7-1 molecule (B7/CD28 signal) can activate DCs to induce Th1 cell response via secretion of IL-12. However, B7-2 molecule and OX40/OX40L signal promote the polarization of Th2 cells. These costimulatory molecules of DCs were highly expressed in human and animal colitis[50,51]. Thus, DCs are closely related to the development of IBD, suppression of Treg cells and balance of Treg/Th17 cells. In the present study, Cur noticeably inhibited the expression of costimulatory molecules of DCs that ineffectively stimulated T cell response to increase the suppression or number of Treg cells, and maintain Treg-mediated suppression. Also, Cur down-regulated the secretion of cytokines including IL-6 and IL-12 p40 by inhibiting DCs to prevent Treg cells from transferring into Th17 cells, inhibit the production of pro-inflammatory cytokines (TNF-α, IL-2, and IL-6) and decrease the destructive effects of IL-17 and IL-21. The mechanisms by which Cur maintains Treg-mediated suppression were only partly revealed in the present study. Therefore, further study is needed to determine whether cytolysis of Treg cells and metabolic disruption of Treg cells are also mechanisms through which Cur maintains Treg-mediated suppression.
In conclusion, Cur potentially modulates activation of DCs to enhance the suppressive functions of Treg cells and promote the recovery of damaged colonic mucosa in IBD.
COMMENTS
Background
Regulatory T cells (Treg) play a crucial role in the maintenance of self tolerance and the prevention of inflammatory bowel disease. Treg-mediated suppression can be implemented primarily by the four broad categories of mechanisms including suppression by inhibitory cytokine, cytolysis, metabolic disruption, or via modulation of dendritic cells (DCs) maturation or function. In these four facts, the activation of DCs is thought as the main role to maintain the suppression of Treg cells.
Research frontiers
The mature DCs with an expression of costimulatory molecules can regulate the balance between balance of Treg and Th17 cells. The previous had demonstrated that higher level expression of costimulatory molecules of DCs can lead to a decreased numbers of Treg cells to induce IBD.
Innovations and breakthroughs
The present study is firstly shown that curcumin (Cur) potentially modulated activation of DCs to enhance the suppressive functions of Treg cells and restore damaged colonic mucosa of inflammatory bowel disease (IBD).
Applications
It is known that Cur effectively treated experimental colitis by many pathway including the inhibition of nuclear factor κB pathway and reduction of pro-inflammatory cytokine response. However the level and pathway are ambiguous that Cur can or not regulate Treg cell. In the present study, our results had hinted that Cur can height the suppressive function of Treg cells via inhibiting the activation of DCs. The results are favorable to explore the mechanism of Cur treated chronic colitis.
Terminology
These costimulatory molecules of DCs, which include TNF/TNF receptor protein families (CD40/CD40L, OX40/OX40L, TNFR/TNF and so on) and immune globulin superfamily (ICAM-1/LAF-1, CD28/CTLA4/B7, etc.), are marker of activation of DCs, and decreased to inhibit the suppressive function of Treg cells in many previous documents .
Peer-review
This is a well-designed and well-presented study for examining the anti-inflammatory potential of curcumine in TNBS colitis of mice. The authors found that curcumine modulates the action of dendritic cells to enhance suppressive Treg functions, leading to an accelerated mucosal healing.