Published online Jun 21, 2016. doi: 10.3748/wjg.v22.i23.5342
Peer-review started: December 4, 2015
First decision: December 21, 2015
Revised: April 6, 2016
Accepted: April 20, 2016
Article in press: April 20, 2016
Published online: June 21, 2016
Processing time: 193 Days and 11.3 Hours
AIM: To develop a potent and safe gene therapy for esophageal cancer.
METHODS: An expression vector carrying fusion suicide gene (yCDglyTK) and shRNA against vascular endothelial growth factor (VEGF) was constructed and delivered into EC9706 esophageal cancer cells by calcium phosphate nanoparticles (CPNP). To achieve tumor selectivity, expression of the fusion suicide gene was driven by a tumor-specific human telomerase reverse transcriptase (hTERT) promoter. The biologic properties and therapeutic efficiency of the vector, in the presence of prodrug 5-fluorocytosine (5-FC), were evaluated in vitro and in vivo.
RESULTS: Both in vitro and in vivo testing showed that the expression vector was efficiently introduced by CPNP into tumor cells, leading to cellular expression of yCDglyTK and decreased VEGF level. With exposure to 5-FC, it exhibited strong anti-tumor effects against esophageal cancer. Combination of VEGF shRNA with the fusion suicide gene demonstrated strong anti-tumor activity.
CONCLUSION: The shVEGF-hTERT-yCDglyTK/5-FC system provided a novel approach for esophageal cancer-targeted gene therapy.
Core tip: Esophageal cancer is a highly aggressive neoplasm with poor prognosis and low survival rates. In this study, an expression vector carrying a fusion suicide gene (yCDglyTK) and shRNA against vascular endothelial growth factor (VEGF) was constructed and delivered into EC9706 esophageal cancer cells by calcium phosphate nanoparticles (CPNP). To achieve tumor selectivity, the expression of the fusion suicide gene was driven by a tumor-specific human telomerase reverse transcriptase promoter. Our results showed that the novel expression vector was efficiently introduced into EC9706 cells by CPNP, leading to cellular expression of yCDglyTK and decreased VEGF level. With exposure to 5-fluorocytosine, it exhibited strong anti-tumor effects against esophageal cancer both in vitro and in vivo. Combination of VEGF shRNA with the fusion suicide gene demonstrated strong anti-cancer effects. Our study provides a novel approach for esophageal cancer-targeted gene therapy.
- Citation: Liu T, Wu HJ, Liang Y, Liang XJ, Huang HC, Zhao YZ, Liao QC, Chen YQ, Leng AM, Yuan WJ, Zhang GY, Peng J, Chen YH. Tumor-specific expression of shVEGF and suicide gene as a novel strategy for esophageal cancer therapy. World J Gastroenterol 2016; 22(23): 5342-5352
- URL: https://www.wjgnet.com/1007-9327/full/v22/i23/5342.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i23.5342
Esophageal cancer is one of the most prevalent cancers in the world. It has high incidence and mortality rates, especially in Africa, East Asia, and North America[1]. Histologically, the most common type of esophageal cancer is esophageal squamous cell cancer (ESCC), which accounts for more than 90% of all cases[2]. Despite advances in diagnosis and treatment of ESCC during recent years, postoperative survival rates have not improved in the last decade. The 5-year overall survival rate is still less than 20%[3,4]. Novel and effective treatments are urgently needed for esophageal cancer.
Gene therapy has been recognized as a promising strategy for cancer treatment[5]. Among various gene therapy approaches, suicide gene therapy holds great promise since suicide gene expression can be manipulated to a particular tissue[6]. It transfers prodrug-activating enzyme genes into cancer cells. These genes encode enzymes that can catalyze non-toxic prodrugs into cytotoxic agents to confer drug sensitivity to certain types of cells[7]. Cytosine deaminase (CD) and herpes simplex type I thymidine kinase are the two most intensively studied suicide genes. Previous studies showed that a fusion gene (CDglyTK) of yeast cytosine deaminase (yCD) and thymidine kinase (TK) was more effective than either single suicide gene[8]. In addition, suicide gene expressed in localized areas of tumor can produce high local concentration of cytotoxic agent [e.g., 5-fluorouracil (5-FU)], thus killing the neighboring tumor cells[9]. Expression of yCDglyTK, together with the treatment of prodrug 5-fluorocytosine (5-FC) exhibited targeted therapeutic effects[10-12].
A major limitation for the clinical use of suicide gene therapy for cancer is its proneness to cause side effects due to a lack of tumor specificity. The tissue-specific expression of suicide genes in tumor cells could be achieved by taking advantage of certain tumor-specific transcription regulatory elements, such as promoters and enhancers[13]. A number of tumor-specific promoters were employed in previous studies[14,15], and among them, the human telomerase reverse transcriptase (hTERT) promoter demonstrated the greatest tumor specificity[10]. The hTERT promoter is inactive in normal somatic cells[16,17] and may enable tumor-selective pharmaceutical effects of therapeutic genes.
Angiogenesis plays a vital role in the process of growth and metastasis of solid tumors[18]. Vascular endothelial growth factor (VEGF) has been found to be an important angiogenesis factor in cancer development[19]. It exerts various biological effects on the growth and spread of tumors, including induction of proteinases, cell mitogenesis, cell migration, increase of vascular permeability, and survival maintenance of newly formed blood vessels. Overexpression of VEGF has been found in about 60% of esophageal carcinoma cases[20,21]. Downregulating VEGF may be a potential targeted treatment strategy for esophageal cancer[22].
Another key consideration for developing a successful gene therapy system is improving transfection efficiency while minimizing toxicity and enhancing stability[23,24]. A number of viral vectors and non-viral plasmids have been developed for gene therapy. However, viral vectors have some serious drawbacks, such as triggering immune response[25], severe hepatic inflammation[26], random chromosomal integration[27], and cytotoxicity to host cells[28]. Non-viral vectors are considered more promising as gene delivery vehicles because they are safe, easy to synthesize, cost-effective, and have a low degree of immunogenicity[29]. In our previous work, we developed the calcium phosphate nanoparticle (CPNP) as a novel non-viral tool for efficient gene delivery[11,12,30]. CPNP-delivered suicide genes mediated tumor specific cytotoxicity in gastric cancer and colon cancer[11,12].
In this study, tumor-specific shVEGF-yCDglyTK expression cassette was delivered using CPNP into human esophageal cancer cells and xenograft esophageal carcinoma. The therapeutic efficacy of this novel gene therapy system was evaluated, and the results suggest it was specific and effective for esophageal carcinoma.
Human esophageal squamous cell cancer cell line EC9706 and normal human lung fibroblast cell line HLF were obtained from the Central Laboratory of Xiangya Hospital, Central South University. A human cervical adenocarcinoma cell line HeLa was acquired from the Cancer Research Institute, Central South University. Cells were cultured in Roswell Park Memorial Institute 1640 medium (Hyclone, Logan, UT, United States) supplemented with 10% heat-inactivated fetal bovine serum and maintained in a humidified atmosphere of 5% CO2 and 95% air at 37 °C.
Human hTERT promoter was amplified by polymerase chain reaction (PCR) from HeLa genomic DNA by using the following primers: forward primer 5′- GCGACGCGTGATTCGCGGGCACAGACG-3′and reverse primer 5′- AAACTCGAGCCACGTGCGCAGCAGGAC-3′. The product was cloned into the Mlu I and Xho I sites of the pGL3-Basic vector. pcDNA3.1(-)-CV-yCDglyTK[11] and pGenesil-shVEGF[31] were constructed in previous studies. pcDNA3.1(-) hTERT-yCDglyTK was made by replacing the carcinoembryonic antigen (CEA) promoter with the hTERT promoter. An expression cassette pcDNA3.1(-)-shVEGF-hTERT-yCDglyTK, which expressed shVEGF and yCDglyTK, was constructed as shown in Supplementary Figure 1. All constructs were confirmed by sequencing.
EC9706 and HLF cells were seeded in 24-well plates with 1 × 105 cells per well. Three plasmids, hTERT-pGL3 Basic, pGL3-Basic, and pGL3-control, were transfected when the cell monolayer reached 80%-85% confluence. The activities of luciferase and beta-galactosidase (β-gal) were detected 48 h post transfection. The luciferase activity was normalized against β-gal activity and expressed as relative luciferase activity.
CPNP was produced according to previous studies[11,12,30]. The characteristics of CPNP have been described previously[11]. Two micrograms of pEGFP-N1 DNA were mixed with 20 μg of CPNP to generate the CPNP-pEGFP-N1 complexes. EC9706 cells were seeded in 12-well plates at a density of 2 × 105 cells per well. When the cell monolayer reached 80%-85% confluence, the CPNP-pEGFP-N1 complexes were added to the cells. The pEGFP-N1 DNA-liposome complexes (10 μg:2 μg) were used as a positive control. Transient transfection efficiency after 48 h was determined by examining GFP expression using flow cytometry and fluorescence microscopy.
EC9706 cells were seeded in 6-well plates at a density of 2 × 105 cells each well. When the cell reached 70%-80% of confluence, pcDNA3.1(-) null, pGenesil-shVEGF, pcDNA3.1(-)-hTERT-yCDglyTK, and pcDNA3.1(-)-hTERT -shVEGF-yCDglyTK were mixed with CPNP (2 μg of DNA: 20 μg of CPNP), respectively[30]. At 48 h after transfection, G418 was added to cell culture medium, the final concentration of which was 600 μg/mL. The G418-resistant colonies were picked 16 d later and kept in 96-well plates. These cells were maintained in selective medium containing 200 μg/mL of G418.
Total RNA from parental EC9706 cells and stable transfected cells was prepared by using TRIzol reagent (Invitrogen, Carlsbad, CA, United States) according to the manufacturer’s protocol. Reverse transcription (RT)-PCR of yCDglyTK and VEGF were carried out with AMV reverse transcription kit (Promega, Madison, WI, United States). Primers for yCDglyTK were: forward primer 5’-GGGAGATTAGAGGGCAAAGTGT-3’, reverse primer5’-ACGGCGTCGGTCACGGCATAA-3’. The yCDglyTK PCR product was 707 bp. A VEGF PCR product of 112 bp was produced by forward primer 5’-TCTTCAAGCCATCCTGTGTG-3’and reverse primer 5’-ATCCGCATAATCTGCATGGT-3’. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the internal control, and a PCR product of 101 bp was produced by forward primer 5’-CCTGTTCGACAGTCA GCCG-3’; reverse primer 5’-CGACCAAATCCGTTGACTCC-3’. The amplified fragments were separated in 2% agarose gels for visualization.
For Western blot assay, 30 μg protein from each sample was loaded for 10% sodium dodecyl sulfate gel electrophoresis and transferred to polyvinylidene fluoride membrane. The membranes were blocked with 5% (w/v) non-fat dry milk in Tris-buffered saline with tween (TBST) buffer at room temperature for 1 h. The membranes were then incubated overnight at 4 °C with anti-CD antibody (QED Bioscience Inc., San Diego, CA, United States). After washing three times with TBST buffer, blots were incubated for 2 h at room temperature with horseradish peroxidase-conjugated rabbit anti-mouse secondary antibody (Sigma, St. Louis, MO, United States). Signals were detected by enhanced chemiluminescence with Western blot detection system (Amersham, Piscataway, NJ, United States). GAPDH was used as a control to ensure equal protein loading.
Parental and stable EC9706 cells were seeded at a density of 5000 cells per well in 96-well plates and incubated at 37 °C for 24 h. 5-FC was added into the culture medium, and the final concentration was 200 μg/mL. Methylthiazole tetrazolium (MTT) assays were conducted after incubation for 24, 48, 72, and 96 h, respectively. At the end of the incubation period, 20 μL of MTT stock solution (5 mg/mL, Sigma) was added per well, and the supernatant was carefully removed 4 h later. The formazan crystals were dissolved in dimethyl sulfoxide (DMSO, Promega). Optical density (OD) was determined by using a multi-well plate reader by measuring absorbance at 570 nm with a 690 nm reference wavelength. The background absorbance of medium was subtracted. All samples were assayed in triplicate, and the mean value for each experiment was analyzed. Cell growth curves were plotted with culture time on the horizontal axis and OD570 values on the vertical axis.
Nuclear morphology changes of apoptotic cells were detected by staining with Hoechst 33258. Parental EC9706 cells and stable cells transfected with empty vector, shVEGF, yCDglyTK, and shVEGF-yCDglyTK were cultured in complete medium supplemented with 5-FC (200 μg/mL). After 48 h, cells were washed three times with phosphate buffered saline (PBS) and stained with 5 μg/mL Hoechst 33258 for 30 min in the dark. Stained nuclei were visualized using a fluorescence microscope with a wavelength of excitation at 355 to 366 nm.
Flow cytometry was performed as previously described[11]. EC9706 cells stably expressing shVEGF, yCDglyTK, and shVEGF-yCDglyTK and untransfected EC9706 cells were seeded in 10 cm dishes, respectively. 5-FC was added to the culture medium at a concentration of 200 μg/mL when the cells reached 90%-95% confluence. Cells were pelleted after 48 h, washed with PBS, and then resuspended in staining buffer (HEPES supplemented with 2.5 mmol/L CaCl2). Flow cytometry was employed for detecting cell apoptosis after addition of fluorescein isothiocyanate-labeled annexin V and incubation for 15 min at 4 °C.
The animal use protocol was reviewed and approved by the Institutional Animal Care and Use Committee of Central South University. Twenty-five female BALB/c nude mice (4-6 wk old, 18-20 g) were acquired from Slac Laboratory Animal of Shanghai Co. Ltd, China. A suspension of 5 × 106 EC9706 cells was inoculated subcutaneously into the right flank of nude mice. After 10 d, when transplanted tumors reached a volume of 100-200 mm3, the mice were randomized into five groups, named A, B, C, D, and E, with five mice in each group. Group A was the non-treating control group; Group B received intratumoral injection of the CPNPs/pcDNA3.1(-)null complex; Group C received intratumoral injection of the CPNPs/pGenesil-shVEGF complex; Group D received intratumoral injection of the CPNPs/pcDNA3.1(-)-hTERT-yCDglyTK complex; and Group E received intratumoral injection of the CPNPs/pcDNA3.1(-)shVEGF-hTERT -yCDglyTK complex.
The intratumoral injections of CPNP/DNA complexes were carried out every other day and repeated three times in total. One day after the first intratumoral injection, mice in groups B, D, and E received daily intraperitoneal injection of 5-FC (500 mg/kg) for 14 consecutive days. The body weights of mice were recorded twice every week, and symptoms of side effects including change in behavior and food withdrawal were closely monitored. The longest (L) and shortest (W) perpendicular tumor diameters were measured every 3 d with calipers. To estimate the tumor volume; the following formula: V = (1/2)W2× L was used to calculate the three-dimensional volume of the xenograft. The growth curve was drawn according to the tumor volume. Animals were euthanized if the tumor diameter reached 1.5 cm or weight loss was more than 20% during the experiment. All surviving mice were euthanized by CO2 inhalation and cervical dislocation at the end of the experiment.
The esophagus cancer xenograft tissue was cut at 4-μm thickness. The sections were incubated overnight at 4 °C with primary polyclonal antibody (anti-CD). The anti-rabbit antibody (Abgent, San Diego, CA, United States) was used as secondary antibody and incubated with sections for 30 min at 37 °C. Color development was performed with the streptavidin-peroxidase system (Sigma-Aldrich). The chromogen was 3,3-diaminobenzidine tetrahydrochloride. Nuclei were lightly counterstained with hematoxylin. Microvessel counts (MVC) were analyzed to evaluate the degree of angiogenesis. Sections were immunostained with anti-CD34 antibody and examined at 200 × magnification. At least three microscopic images were collected and further analyzed with Axion Vision Rel 4.6 software to obtain MVC.
In order to develop a more effective treatment for esophageal cancer, a novel expression plasmid, which could express a shRNA for VEGF and a fusion suicide gene yCDglyTK (Supplementary Figure 1), was constructed. The expression of shVEGF was under the control of the U6 promoter, a pol III promoter; the expression of yCDglyTK was controlled by the tumor-specific hTERT promoter.
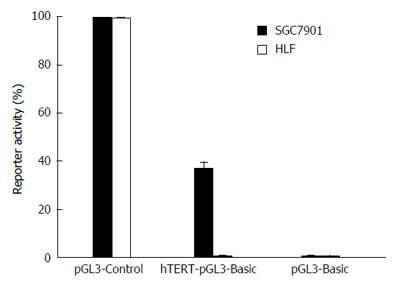
Activities of the hTERT promoter were determined by luciferase assay in EC9706 esophageal cancer cells and HLF normal lung fibroblast cells. The pGL3-Control vector containing SV40 promoter and enhancer sequences was used as a positive control. Luciferase activity driven by the hTERT promoter was much higher in EC9706 cells than in the HLF cells (Figure 1). The results indicated that tumor specificity driven by the hTERT promoter could be achieved in EC9706 esophageal carcinoma cells.
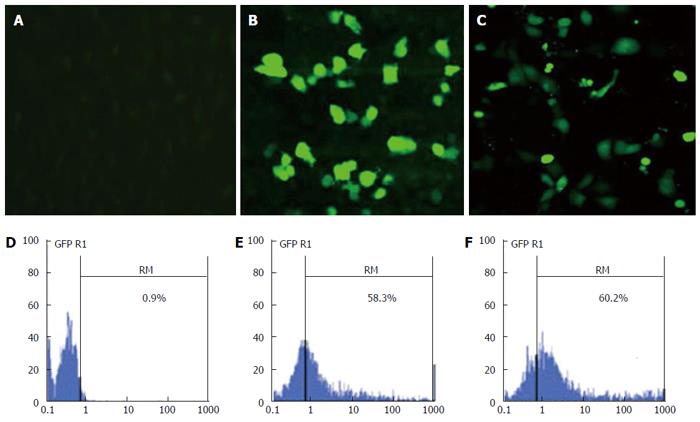
EC9706 cells were transiently transfected with CPNP/green fluorescent protein (GFP) complex to evaluate the transfection efficiency of CPNP. EC9706 cells were assessed 48 h after transfection for GFP expression by using fluorescence microscopy and flow cytometry analysis. As shown in Figure 2, the proportion of GFP expressing cells transfected with CPNP was 58.3%, similar to that in liposome-medicated transfection group (60.2%). These data suggested that CPNP is an efficient strategy to deliver DNA into EC9706 esophageal cancer cells.
MTT assay was carried out to test the toxicity. Compared to the control group, liposome inhibited the cell growth by about 21%, while CPNP-DNA group had little effect on the cell growth (Supplementary Figure 1).
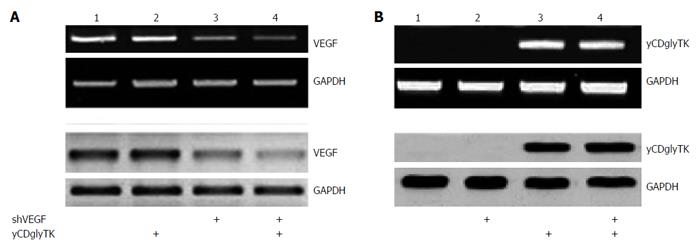
RT-PCR and western blot were used to determine the expression of VEGF and yCDglyTK. Compared with cells transfected with pcDNA3.1(-), EC9706 cells transfected with pcDNA3.1(-)-shVEGF-hTERT-yCDglyTK showed significantly decreased expression of VEGF at both the mRNA and protein levels. No significant changes of VEGF expression were observed in EC9706 cells transfected with pGenesil-shVEGF and pcDNA3.1(-)-shVEGF- hTERT-yCDglyTK (Figure 3A). yCDglyTK expression was not present in cells transfected with pcDNA3.1(-). Its expression was substantially increased at both the mRNA and protein level by introduction of pcDNA3.1(-) -shVEGF-hTERT-yCDglyTK into EC9706 cells (Figure 3B).
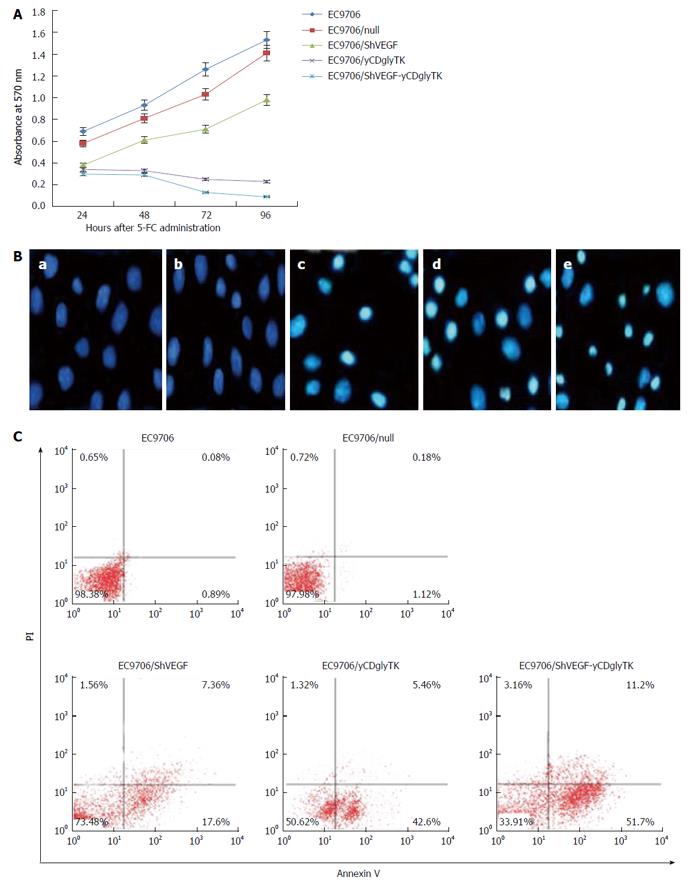
MTT assay was performed to assess cytotoxicity of the shVEGF-yCDglyTK/prodrug system in EC9706 cells. Parental and stable EC9706 cells were treated with 200 μg/mL of 5-FC. Cells were harvested at 24, 48, 72, and 96 h after 5-FC administration and subjected to MTT assay. As shown in Figure 4A, 96 h after 5-FC treatment, cells growth in the shVEGF group, yCDglyTK group, and shVEGF-yCDglyTK group was inhibited by 36%, 85%, and 94%, respectively.
Forty eight hours after 5-FC exposure, cell apoptosis were determined by Hoechst staining and flow cytometry, respectively. Hoechst staining showed that 5-FC induced a significant increase in apoptosis in EC9706 cells stably modified with the pcDNA3.1(-)-shVEGF-hTERT-yCDglyTK (Figure 4B). In contrast, apoptotic cells were barely found in parental EC9706 cells and cells carrying empty vector. Consistent results were found by the flow cytometry analysis (Figure 4C). Apoptotic population (early and late apoptotic) in EC9706 cells expressing shVEGF, yCDglyTK, and shVEGF together with yCDglyTK were 24.96%, 48.06%, and 62.9%, respectively. The percentage of apoptotic cells in untransfected EC9706 cells and EC9706 null cells transfected with an empty vector was only 0.97% and 1.3%, respectively. These results showed that both shVEGF and yCDglyTK expression could induce apoptosis and that combining them had the strongest effect on apoptosis induction.
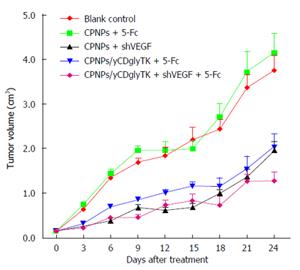
The anti-tumor activity of the shVEGF-yCDglyTK/5-FC system was investigated with EC9706 xenograft models. Tumors receiving CPNPs/null injection grew at a similar rate to that of the blank control group. On day 24, when compared with blank control group, CPNPs/yCDglyTK + 5-FC group and CPNPs + shVEGF group showed about 46% and 48% tumor growth inhibition. When combining CPNPs/yCDglyTK + 5-FC and shVEGF, the tumor growth was inhibited by 66% at day 24. These results demonstrated the potential benefit of the shVEGF-yCDglyTK/5-FC system for treating esophageal cancer (Figure 5).
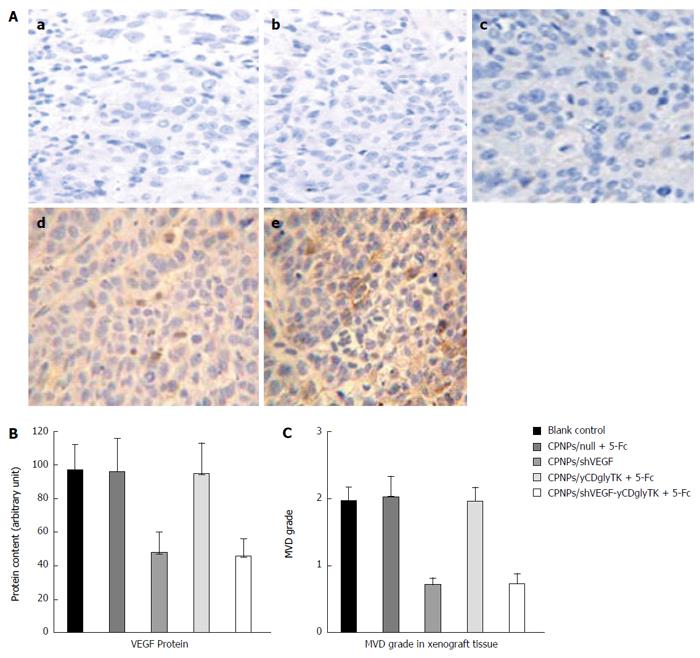
The expression and distribution of yCDglyTK and VEGF in the tumor xenograft were visualized by using immunohistochemistry staining. As shown in Figure 6A, tumor tissue expression of yCDglyTK was detected in mice injected with CPNPs/yCDglyTK or CPNPs/shVEGF-yCDglyTK. Meanwhile, it was not present in other groups, including non-treatment, sham control, and CPNPs/shVEGF groups. VEGF expression was quantified and represented by integrated optical density (IOD) values (Figure 6B). VEGF levels were significantly decreased in tumor tissues receiving injection of CPNPs/shVEGF or CPNPs/shVEGF-yCDglyTK, when compared with those of other groups (P < 0.01). No significant difference in VEGF expression was found between these two groups (P > 0.05). Since VEGF is an important angiogenesis factor, neovascularization was assessed by quantification of MVD. CPNPs/shVEGF and CPNPs/shVEGF-yCDglyTK groups showed lower MVD as compared to other groups (Figure 6C). The results suggested that the intratumoral injection of CPNPs/shVEGF-yCDglyTK could effectively inhibit neovascularization by down-regulating VEGF expression.
Esophageal carcinoma is a common cause of death globally. Traditional remedies for esophageal cancer, such as surgery, chemotherapy, and radiotherapy, all have drawbacks. For example, surgery is applicable only if the tumor is diagnosed at an early stage. Chemotherapy and radiotherapy have serious side effects as a result of lacking tumor specificity. Therefore, novel methods are required for the treatment of esophageal cancer. Gene therapy, especially suicide gene therapy, has been studied extensively for cancer treatment[32]. For suicide gene therapy, the therapeutic transgenes can convert a non-toxic pro-drug, which easily penetrates the tumor cell membrane, into a cytotoxic drug[33]. However, present suicide gene therapies have limited success due to lack of tumor specificity and an effective gene delivery tool.
One key point for successful gene therapy is the development of a safe and effective gene delivery system. Viral vectors are the most widely investigated delivering system because of their high transfection efficiency. However, viral vectors have some serious drawbacks, such as triggering immune response, severe hepatic inflammation, and random chromosomal integration[25-27]. Among non-viral vectors, cationic lipids could cause toxic effects when repeatedly used and induce potent anti-inflammatory activity in vivo; calcium phosphate precipitation had low transfection efficiency. The use of nanoparticles has become one of the most promising vectors because of their high transfection efficiency and low toxicity. In previous studies, we developed a new gene delivery system using either CPNP or calcium carbonate nanoparticles (CCNP)[11,12,30,31,34]. In this study, CPNP successfully delivered the suicide gene into EC9706 esophageal cells, with low toxicity.
Inserting a tumor-specific promoter upstream of suicide genes has been proven to be a successful strategy to achieve targeted expression of suicide genes in tumor tissues. Previously, we successfully used CEA promoter for targeting gastric cancer and colon cancer[11,12]. In this study, the specificity against esophageal cancer cells was achieved using the hTERT promoter.
VEGF stimulates tumor cell proliferation and angiogenesis in tumor tissue, and overexpression of VEGF has been found in the majority of human cancers[35,36]. Silencing VEGF with RNA interference (RNAi) could inhibit tumor growth and metastasis[37,38]. In this study, a novel fusion gene vector carrying a suicide gene (yCDglyTK) and a VEGF shRNA was developed. This expression vector was transfected into EC9706 esophageal cells by CPNP. Increased expression of yCDglyTK and decreased expression of VEGF were confirmed at both the mRNA and protein level in EC9706 cells.
In vitro antitumor activity of this novel system in the presence of prodrug was tested by MTT assay, Hoechst staining, and flow cytometry. The antitumor effect of the CPNP/shVEGF-yCDglyTK/5-FC system was further evaluated in vivo by using EC9706 cell xenograft model. Both yCDglyTK/5-FC and shVEGF successfully inhibited the tumor growth, and the combination of the two showed the strongest anti-tumor activity. Subsequent immunohistochemistry staining confirmed the expression of yCDglyTK, the knockdown of VEGF, and decreased neovascularization in the cancer tissue.
In summary, the CPNP/shVEGF-hTERT-yCDglyTK/5-FC system developed in this study may overcome several challenges for cancer gene therapy. The use of CPNP increased delivery efficiency, and insertion of hTERT promoter improved tumor specificity. VEGF-targeted shRNA further enhanced the anti-tumor effect. This combination of gene therapies exerted more potent anti-esophageal cancer activity in vitro and in vivo. The present study provides a novel and promising strategy for esophageal cancer treatment. We will try to optimize this approach to make it more feasible in the future clinical trials.
Esophageal cancer is one of the most prevalent cancers in the world. It has high incidence and mortality rates. Novel and effective treatment options are urgently needed for esophageal cancer.
In recent years, gene therapy, especially suicide gene therapy, has been recognized as a promising strategy for cancer treatment.
In this study, tumor-specific shVEGF-yCDglyTK expression cassette was delivered using calcium phosphate nanoparticle (CPNP) into human esophageal cancer cells. The therapeutic efficacy of this novel gene therapy system was evaluated in vitro and in vivo, and results showed that it was an effective strategy for esophageal carcinoma treatment.
The present study provides a novel and promising strategy for esophageal cancer treatment.
CPNP is a novel non-viral tool for efficient gene delivery.
This study investigated a novel technique for targeted gene therapy in an esophageal cancer model. The authors report transfection rates and altered gene expression profiles. The study is well conducted and well described. The techniques are appropriate and the experiments are clearly written. The study topic is interesting and relevant.
| 1. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23762] [Cited by in RCA: 25604] [Article Influence: 1706.9] [Reference Citation Analysis (10)] |
| 2. | Tew WP, Kelsen DP, Ilson DH. Targeted therapies for esophageal cancer. Oncologist. 2005;10:590-601. [PubMed] |
| 3. | Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med. 2003;349:2241-2252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2115] [Cited by in RCA: 2232] [Article Influence: 97.0] [Reference Citation Analysis (0)] |
| 4. | Morita M, Yoshida R, Ikeda K, Egashira A, Oki E, Sadanaga N, Kakeji Y, Yamanaka T, Maehara Y. Advances in esophageal cancer surgery in Japan: an analysis of 1000 consecutive patients treated at a single institute. Surgery. 2008;143:499-508. [PubMed] |
| 5. | Alexandrova R. Experimental strategies in gene therapy of cancer. J BUON. 2009;14 Suppl 1:S23-S32. [PubMed] |
| 6. | Yazawa K, Fisher WE, Brunicardi FC. Current progress in suicide gene therapy for cancer. World J Surg. 2002;26:783-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 59] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 7. | Denny WA. Prodrug strategies in cancer therapy. Eur J Med Chem. 2001;36:577-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 210] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 8. | Zhao Y, Li Z, Sheng W, Miao J, Yang J. Adenovirus-mediated ING4/IL-24 double tumor suppressor gene co-transfer enhances antitumor activity in human breast cancer cells. Oncol Rep. 2012;28:1315-1324. [PubMed] |
| 9. | Freeman SM, Ramesh R, Shastri M, Munshi A, Jensen AK, Marrogi AJ. The role of cytokines in mediating the bystander effect using HSV-TK xenogeneic cells. Cancer Lett. 1995;92:167-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 60] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | Liu TWC, He XW, Zhang GW, Leng AM, Liu H, Ye L, Zhang GY. In Vitro and In Vivo Tissue SpecificCytotoxicity of Gastric Cancer Cells Resulting from CD: UPRT/5-FC Gene Therapy System Driven by hTERT Promoter. Advanced Science Letters. 2011;4:1-6. |
| 11. | Liu T, Zhang G, Chen YH, Chen Y, Liu X, Peng J, Xu MH, Yuan JW. Tissue specific expression of suicide genes delivered by nanoparticles inhibits gastric carcinoma growth. Cancer Biol Ther. 2006;5:1683-1690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Zhang G, Liu T, Chen YH, Chen Y, Xu M, Peng J, Yu S, Yuan J, Zhang X. Tissue specific cytotoxicity of colon cancer cells mediated by nanoparticle-delivered suicide gene in vitro and in vivo. Clin Cancer Res. 2009;15:201-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Nagata T, Nakamori M, Iwahashi M, Yamaue H. Overexpression of pyrimidine nucleoside phosphorylase enhances the sensitivity to 5’-deoxy-5-fluorouridine in tumour cells in vitro and in vivo. Eur J Cancer. 2002;38:712-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Majumdar AS, Hughes DE, Lichtsteiner SP, Wang Z, Lebkowski JS, Vasserot AP. The telomerase reverse transcriptase promoter drives efficacious tumor suicide gene therapy while preventing hepatotoxicity encountered with constitutive promoters. Gene Ther. 2001;8:568-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 81] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 15. | Ebara S, Shimura S, Nasu Y, Kaku H, Kumon H, Yang G, Wang J, Timme TL, Aguilar-Cordova E, Thompson TC. Gene therapy for prostate cancer: toxicological profile of four HSV-tk transducing adenoviral vectors regulated by different promoters. Prostate Cancer Prostatic Dis. 2002;5:316-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Neumann AA, Reddel RR. Telomere maintenance and cancer -- look, no telomerase. Nat Rev Cancer. 2002;2:879-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 94] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 17. | Gu J, Kagawa S, Takakura M, Kyo S, Inoue M, Roth JA, Fang B. Tumor-specific transgene expression from the human telomerase reverse transcriptase promoter enables targeting of the therapeutic effects of the Bax gene to cancers. Cancer Res. 2000;60:5359-5364. [PubMed] |
| 18. | Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4848] [Cited by in RCA: 4822] [Article Influence: 160.7] [Reference Citation Analysis (0)] |
| 19. | Veikkola T, Karkkainen M, Claesson-Welsh L, Alitalo K. Regulation of angiogenesis via vascular endothelial growth factor receptors. Cancer Res. 2000;60:203-212. [PubMed] |
| 20. | Kitadai Y, Haruma K, Tokutomi T, Tanaka S, Sumii K, Carvalho M, Kuwabara M, Yoshida K, Hirai T, Kajiyama G. Significance of vessel count and vascular endothelial growth factor in human esophageal carcinomas. Clin Cancer Res. 1998;4:2195-2200. [PubMed] |
| 21. | Shih CH, Ozawa S, Ando N, Ueda M, Kitajima M. Vascular endothelial growth factor expression predicts outcome and lymph node metastasis in squamous cell carcinoma of the esophagus. Clin Cancer Res. 2000;6:1161-1168. [PubMed] |
| 22. | Gorski DH, Beckett MA, Jaskowiak NT, Calvin DP, Mauceri HJ, Salloum RM, Seetharam S, Koons A, Hari DM, Kufe DW. Blockage of the vascular endothelial growth factor stress response increases the antitumor effects of ionizing radiation. Cancer Res. 1999;59:3374-3378. [PubMed] |
| 23. | Thomas M, Klibanov AM. Non-viral gene therapy: polycation-mediated DNA delivery. Appl Microbiol Biotechnol. 2003;62:27-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 356] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 24. | Felgner PL. Nonviral strategies for gene therapy. Sci Am. 1997;276:102-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 177] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 25. | Yang Y, Nunes FA, Berencsi K, Furth EE, Gönczöl E, Wilson JM. Cellular immunity to viral antigens limits E1-deleted adenoviruses for gene therapy. Proc Natl Acad Sci USA. 1994;91:4407-4411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1179] [Cited by in RCA: 1164] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 26. | Engelhardt JF, Ye X, Doranz B, Wilson JM. Ablation of E2A in recombinant adenoviruses improves transgene persistence and decreases inflammatory response in mouse liver. Proc Natl Acad Sci USA. 1994;91:6196-6200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 455] [Cited by in RCA: 435] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 27. | Tenenbaum L, Lehtonen E, Monahan PE. Evaluation of risks related to the use of adeno-associated virus-based vectors. Curr Gene Ther. 2003;3:545-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 95] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 28. | Han S, Mahato RI, Sung YK, Kim SW. Development of biomaterials for gene therapy. Mol Ther. 2000;2:302-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 291] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 29. | Li S, Huang L. Nonviral gene therapy: promises and challenges. Gene Ther. 2000;7:31-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 456] [Cited by in RCA: 415] [Article Influence: 16.0] [Reference Citation Analysis (10)] |
| 30. | Liu T, Tang A, Zhang G, Chen Y, Zhang J, Peng S, Cai Z. Calcium phosphate nanoparticles as a novel nonviral vector for efficient transfection of DNA in cancer gene therapy. Cancer Biother Radiopharm. 2005;20:141-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 52] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 31. | Liu T, Ye L, He Y, Chen X, Peng J, Zhang X, Yi H, Peng F, Leng A. Combination gene therapy using VEGF-shRNA and fusion suicide gene yCDglyTK inhibits gastric carcinoma growth. Exp Mol Pathol. 2011;91:745-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 32. | Zarogoulidis P, Darwiche K, Sakkas A, Yarmus L, Huang H, Li Q, Freitag L, Zarogoulidis K, Malecki M. Suicide Gene Therapy for Cancer - Current Strategies. J Genet Syndr Gene Ther. 2013;4. [PubMed] |
| 33. | Kim KY, Kim SU, Leung PC, Jeung EB, Choi KC. Influence of the prodrugs 5-fluorocytosine and CPT-11 on ovarian cancer cells using genetically engineered stem cells: tumor-tropic potential and inhibition of ovarian cancer cell growth. Cancer Sci. 2010;101:955-962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 34. | He XW, Liu T, Chen YX, Cheng DJ, Li XR, Xiao Y, Feng YL. Calcium carbonate nanoparticle delivering vascular endothelial growth factor-C siRNA effectively inhibits lymphangiogenesis and growth of gastric cancer in vivo. Cancer Gene Ther. 2008;15:193-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 83] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 35. | Ellis LM, Takahashi Y, Fenoglio CJ, Cleary KR, Bucana CD, Evans DB. Vessel counts and vascular endothelial growth factor expression in pancreatic adenocarcinoma. Eur J Cancer. 1998;34:337-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 122] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 36. | Koide N, Nishio A, Kono T, Yazawa K, Igarashi J, Watanabe H, Nimura Y, Hanazaki K, Adachi W, Amano J. Histochemical study of vascular endothelial growth factor in squamous cell carcinoma of the esophagus. Hepatogastroenterology. 1999;46:952-958. [PubMed] |
| 37. | Li M, Ye C, Feng C, Riedel F, Liu X, Zeng Q, Grandis JR. Enhanced antiangiogenic therapy of squamous cell carcinoma by combined endostatin and epidermal growth factor receptor-antisense therapy. Clin Cancer Res. 2002;8:3570-3578. [PubMed] |
| 38. | Arora A, Scholar EM. Role of tyrosine kinase inhibitors in cancer therapy. J Pharmacol Exp Ther. 2005;315:971-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 777] [Cited by in RCA: 706] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Deans C, Shimoyama S S- Editor: Yu J L- Editor: Filipodia E- Editor: Zhang DN