Published online Jun 7, 2016. doi: 10.3748/wjg.v22.i21.5096
Peer-review started: January 5, 2016
First decision: February 18, 2016
Revised: February 25, 2016
Accepted: March 18, 2016
Article in press: March 18, 2016
Published online: June 7, 2016
Processing time: 146 Days and 13.1 Hours
AIM: To assess significance of serum adipokines to determine the histological severity of non-alcoholic fatty liver disease.
METHODS: Patients with persistent elevation in serum aminotransferase levels and well-defined characteristics of fatty liver at ultrasound were enrolled. Individuals with a history of alcohol consumption, hepatotoxic medication, viral hepatitis or known liver disease were excluded. Liver biopsy was performed to confirm non-alcoholic liver disease (NAFLD). The degrees of liver steatosis, lobular inflammation and fibrosis were determined based on the non-alcoholic fatty liver activity score (NAS) by a single expert pathologist. Patients with a NAS of five or higher were considered to have steatohepatitis. Those with a NAS of two or lower were defined as simple fatty liver. Binary logistic regression was used to determine the independent association of adipokines with histological findings. Receiver operating characteristic (ROC) analysis was employed to determine cut-off values of serum adipokines to discriminate the grades of liver steatosis, lobular inflammation and fibrosis.
RESULTS: Fifty-four participants aged 37.02 ± 9.82 were enrolled in the study. Higher serum levels of visfatin, IL-8, TNF-α levels were associated independently with steatosis grade of more than 33% [β = 1.08 (95%CI: 1.03-1.14), 1.04 (95%CI: 1.008-1.07), 1.04 (95%CI: 1.004-1.08), P < 0.05]. Elevated serum IL-6 and IL-8 levels were associated independently with advanced lobular inflammation [β = 1.4 (95%CI: 1.09-1.8), 1.07 (95%CI: 1.003-1.15), P < 0.05]. Similarly, higher TNF-α, resistin, and hepcidin levels were associated independently with advanced fibrosis stage [β = 1.06 (95%CI: 1.002-1.12), 19.86 (95%CI: 2.79-141.19), 560.72 (95%CI: 5.98-5255.33), P < 0.05]. Serum IL-8 and TNF-α values were associated independently with the NAS score, considering a NAS score of 5 as the reference value [β = 1.05 (95%CI: 1.01-1.1), 1.13 (95%CI: 1.04-1.22), P < 0.05].
CONCLUSION: Certain adipokines may determine the severity of NAFLD histology accurately.
Core tip: Considering the drawbacks of current assays, it seemed reasonable to find appropriate serum biomarkers to define the extent of liver damage in non-alcoholic liver disease (NAFLD). We investigated several key adipokines together with metabolic profiles and liver function tests, providing an advantage over previous studies. We concluded that serum visfatin, IL-8, TNF-α levels were associated with liver steatosis degree; serum IL-6 and IL-8 concentrations correlated with lobular inflammation grade; and TNF-α, resistin, and hepcidin levels correlated with fibrosis stage. The study suggested that certain adipokines might have better accuracy than currently used serum biomarkers to determine NAFLD histology.
- Citation: Jamali R, Razavizade M, Arj A, Aarabi MH. Serum adipokines might predict liver histology findings in non-alcoholic fatty liver disease. World J Gastroenterol 2016; 22(21): 5096-5103
- URL: https://www.wjgnet.com/1007-9327/full/v22/i21/5096.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i21.5096
Non-alcoholic liver disease (NAFLD) is a health concern worldwide. The burden of the disease is increasing because of the epidemic of obesity and the development of insulin resistance (IR) syndrome[1]. Liver function tests, metabolic profiles, liver ultrasound and clinical data are used routinely to detect the disease. Considering the limitations of these assays, liver biopsy is still the gold standard method to diagnose NAFLD[2]. However, concerns about the possible complications and invasiveness of the method have limited its application by physicians. It seems reasonable to identify appropriate serum biomarkers to diagnose and define the extent of liver damage in NAFLD. In this regard, interest in the roles of adipokines that are secreted from visceral adipose tissue (VAT) has been increasing.
NAFLD comprises a wide spectrum of liver cell injury that is induced by insulin resistance. Primarily, the accumulation of fat occurs in hepatocytes (simple fatty liver) as a consequence of hepatic insulin resistance. A growing body of evidence supports the view that adipokines modulate these metabolic processes by regulating insulin mediated glucose metabolism, fatty acid utilization and lipid accumulation of visceral tissues. At the later stages of disease, inflammatory phenomena arise that might progress to steatohepatitis and, ultimately, cirrhosis. It has been suggested that the development of steatohepatitis is a consequence of the balance between pro and anti-inflammatory effects of adipokines.
There is a paucity of literature regarding the serum threshold values and efficacy of adipokines in the diagnosis and follow-up of fatty liver patients. In this research, we evaluated certain important adipokines that were reported to be associated with NAFLD, in a cohort of biopsy-proven NAFLD patients[3-9].
The aims of this study were: (1) to evaluate the association of histological findings (steatosis, lobular inflammation and fibrosis) and serum biomarkers (including adipokines, inflammatory cytokines, liver function tests and metabolic profiles); and (2) to determine cut-off values of serum biomarkers to identify the grades of steatosis, lobular inflammation and fibrosis.
This study was conducted in the outpatient gastroenterology clinic of Shahid Beheshti general hospital, from September 2012 to September 2014. Initially, patients with persistent elevated serum aminotransferase levels and well-defined characteristics of a fatty liver via abdominal ultrasound (Hitachi EUB 405 apparatus equipped with a convex 3.5 MHz probe) were included (Phase 1)[1,10]. The upper normal limit of the serum aminotransferases level was considered as 40 units per liter[11]. Individuals with a history of alcohol consumption, hepatotoxic medication, viral hepatitis and known liver disease were excluded from the study (Phase 2)[1,12]. Liver biopsy was performed on the remaining patients from phase 2 to confirm diagnosis of NAFLD for final enrolment (Phase 3).
The study was conducted according to ethical standards for human experimentation (Helsinki Declaration). The ethics committee of the hospital approved the study protocol (No: 8861). The purpose of study was explained to the participants. They were enrolled in the study upon providing written informed consent.
The sample size was n = 54, considering the mean prevalence of NAFLD (P = 28%, α = 0.05, z = 1.96, and d = 0.12), according to a previous study[10].
Fasting serum samples were obtained to assess the level of adiponectin, visfatin, resistin, hepcidin, IL-6, IL-8 and tumor necrosis factor (TNF)-α by enzyme-linked immunosorbent assay (ELISA), according to the manufacturer’s instructions. The following kits were used in this study: Human adiponectin and visfatin ELISA kits (Production numbers: AG-45A-0001 and AG-45A-0006 respectively; ADIPOGEN Inc., South Korea), resistin (human resistin ELISA kit, Biovendor, Czech Republic), hepcidin (Lot: RN- 24429; DEMEDITEC GmbH, Kiel-Wellsee, Germany), IL-6 (Lot: 233737; Bendered Systems GmbH, Vienna, Austria), IL-8 (Lot: ab46032; IL-8 human ELISA kit, Abcam, United States), and TNF-α (Lot: ab46087; TNF-α human ELISA kit, Abcam, United States). Fasting blood glucose, insulin, lipid profiles and liver function tests were performed as previously described[1,10-13].
Percutaneous liver biopsy was performed using a true cut needle (G14). A sample larger than 10 mm or with at least five portal tracts was considered acceptable for histological evaluation. Hematoxylin and Eosin (HE) and Masson’s Trichrome stainings were performed to evaluate necroinflammation and fibrosis, respectively. To avoid inter-observer disagreement, a single expert pathologist who was blinded to the patient data interpreted samples. The degree of liver steatosis, lobular inflammation and fibrosis was defined based on the “non-alcoholic fatty liver activity score (NAS)”[14]. Patients with a NAS of five or higher were considered to have NASH. Those with a NAS of two or lower were defined as simple fatty liver[14].
Continuous variables were reported as the mean ± SD and categorical variables were shown as counts (percent). The Kolmogorov-Smirnov test was used to assess the distribution of serum adipokines. A χ2 or t-test was applied to assess differences among groups, where appropriate. Binary logistic regression analysis using the standard model was applied to evaluate the association of independent variables (including serum adipokines and clinical data) and liver histology findings.
Hepatic steatosis severity was categorized into four degrees according to the NAS. The first two degrees (0-1) represented no and mild liver steatosis, and the next degrees (2-3) indicated moderate to severe liver steatosis. To define the risk of lower liver steatosis versus a more advanced degrees of steatosis, we considered the patients with steatosis grades of less than 33% as the “mild group”. Meanwhile, those with higher degrees (2-3) were merged to form the “moderate to severe group”.
The lobular inflammation range was graded from 0 to 3 by the NAS. To estimate the risk of lower lobular inflammation against more advanced grades, we labeled the individuals with lobular inflammation of less than two foci per HPF (grade 1) as the “mild group”. At the same time, those with higher lobular inflammation grades (2-3) were combined to form the “moderate to severe group”.
Hepatic fibrosis content was categorized into 5 stages based on NAS. The former two stages (0-1) demonstrated none/mild fibrosis and the latter stages stand for more advanced fibrosis (2-4). In order to determine the probability of lower fibrosis versus more advanced fibrosis, we labeled the subjects with perisinusoidal or periportal (stage 1) as the “mild group”. Those with higher fibrosis stages (2-4) were mixed to form the “moderate to severe group”.
For the regression model, liver steatosis, lobular inflammation, fibrosis stage, and NAS were employed as dependent variables; Steatosis grade of less than 33%, lobular inflammation of less than two foci per high powered field (HPF), fibrosis stage of one (perisinusoidal or periportal), and a NAS of five or higher were set as the reference groups, respectively. Standardized correlation coefficient (OR) with the 95%CI was calculated. Serum adipokines that were independently associated with the histological findings were selected for receiver operating characteristic (ROC) analysis. ROC analysis explored the serum adipokines’ cut-off values and their sensitivities and specificities to discriminate higher grades of liver steatosis, lobular inflammation and fibrosis. Values with the highest sum of the sensitivity and specificity were reported as the best cut-off values. All statistical analyses were performed by SPSS, version 17 (SPSS, Chicago, United States). The probability of a difference between groups was considered statistically significant if the two-sided P value was less than 0.05.
Seventy participants presumed to have NAFLD were evaluated from September 2012 to September 2014 (phase 1). Reasons for leaving certain patients out of the study were patient refusal to participate in the study (n = 8), normalization of ALT during the lead-in phase (n = 6), autoimmune hepatitis (n = 1) and viral hepatitis (n = 1) (phase 2). Finally, fifty-four patients with biopsy proven NAFLD were included in the study (phase 3). The clinico-demographic and laboratory data of the participants are presented in Table 1.
| Variable | Total | Simple fatty liver | Non-alcoholic steatohepatitis |
| n = 54 | n = 2 | n = 28 | |
| Age (yr) | 37.02 ± 9.82 | 27.00 ± 2.82 | 35.00 ± 8.47 |
| Male gender, n (%) | 35 (64.8) | 2 (100) | 17 (60.7) |
| Waist circumference (cm) | 102.13 ± 2.69 | 101.00 ± 42.24 | 101.57 ± 2.71 |
| Body mass index (kg/m2) | 30.55 ± 3.97 | 28.09 ± 7.77 | 29.92 ± 3.79 |
| Diabetes mellitus present, n (%) | 12 (22.2) | 0 (100) | 11 (39.3) |
| Metabolic syndrome present, n (%) | 36 (66.7) | 1 (50) | 21 (75) |
| Adiponectin (mg/L) | 8.14 ± 2.91 | 8.20 ± 2.67 | 7.00 ± 0.28 |
| Visfatin (ng/mL) | 19.96 ± 17.5 | 5.40 ± 0.84 | 18.34 ± 16.18 |
| Resistin (mg/mL) | 2.51 ± 1.08 | 1.70 ± 1.83 | 2.10 ± 1.04 |
| Hepcidin (ng/mL) | 64.0 ± 0.62 | 48.50 ± 0.38 | 75.00 ± 0.49 |
| Tumor necrosis factor-alpha (pg/mL) | 2.68 ± 19.32 | 0.96 ± 9.05 | 3.68 ± 20.93 |
| Interleukin 6 (pg/mL) | 7.59 ± 5.75 | 4.70 ± 0.24 | 7.41 ± 4.78 |
| Interleukin 8 (pg/mL) | 27.41 ± 24.99 | 13.60 ± 13.01 | 38.60 ± 28.21 |
| Alanine aminotransferase (U/L) | 65.91 ± 36.11 | 37.00 ± 0.00 | 82.10 ± 39.25 |
| Aspartate aminotransferase (U/L) | 42.18 ± 20.48 | 25.00 ± 4.24 | 49.67 ± 24.14 |
| Alkaline phosphatase (U/L) | 181.50 ± 76.14 | 144.21 ± 42.41 | 180.23 ± 45.22 |
| Gamma glutamyl transpeptidase (U/L) | 54.12 ± 62.55 | 44.40 ± 30.54 | 55.40 ± 33.26 |
| Fasting blood sugar (mg/dL) | 98.41 ± 14.12 | 98.25 ± 16.31 | 103.0 ± 0.00 |
| Insulin (mU/L) | 15.16 ± 13.42 | 10.68 ± 3.65 | 17.40 ± 18.00 |
| Triglyceride (mg/dL) | 150.09 ± 70.18 | 60.20 ± 9.70 | 167.57 ± 77.19 |
| Total cholesterol (mg/dL) | 177.55 ± 34.17 | 165.85 ± 40.65 | 183.77 ± 37.76 |
| Low density lipoprotein cholesterol (mg/dL) | 100.89 ± 29.03 | 98.75 ± 35.42 | 103.15 ± 32.19 |
| High density lipoprotein cholesterol (mg/dL) | 48.51 ± 9.06 | 55.05 ± 7.14 | 48.66 ± 9.67 |
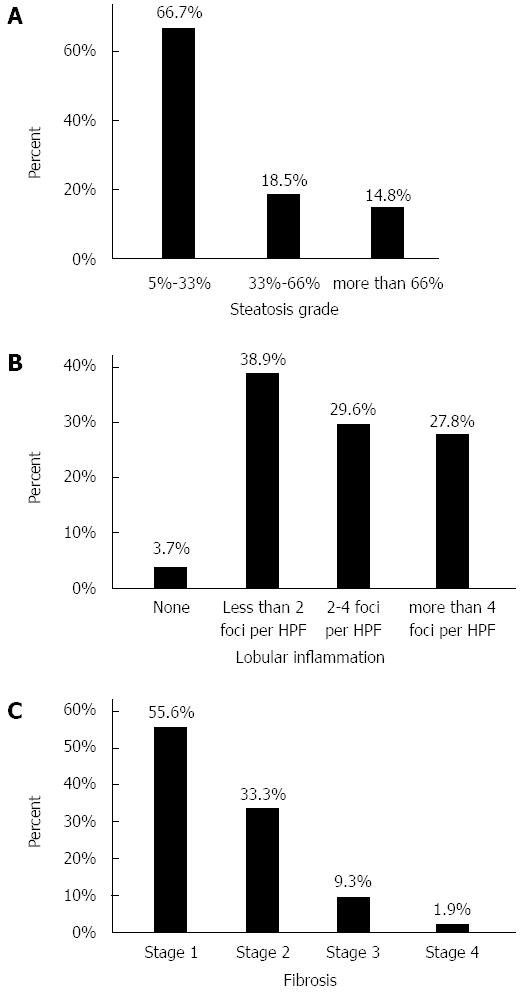
Participants showed NAS of 4.87 ± 1.71. The frequency of histological findings in the study population is depicted in Figure 1.
Binary logistic regression showed a positive association between serum visfatin, IL-8 and TNF-α level and the grades of steatosis. Similarly, serum IL-6 and IL-8 levels were independently associated with the degrees of lobular inflammation. Serum TNF-α, resistin and hepcidin levels were independently associated with perisinusoidal fibrosis stage. Serum IL-8 and TNF-α values were positively associated with NAS (Table 2).
| Adipokine | OR | 95%CI | P value |
| Steatosis degree | |||
| Visfatin | 1.08 | 1.030-1.14 | 0.001 |
| TNF-α | 1.04 | 1.004-1.08 | 0.030 |
| Interleukin 8 | 1.04 | 1.006-1.07 | 0.020 |
| Lobular inflammation grade | |||
| Interleukin 6 | 1.4 | 1.090-1.80 | 0.008 |
| Interleukin 8 | 1.07 | 1.003-1.15 | 0.040 |
| Fibrosis stage | |||
| Resistin | 19.86 | 2.790-141.19 | 0.003 |
| Hepcidin | 560.72 | 5.980-5255.33 | 0.006 |
| TNF-α | 1.06 | 1.002-1.12 | 0.040 |
| NAS | |||
| Interleukin 8 | 1.05 | 1.01-1.10 | 0.040 |
| TNF-α | 1.13 | 1.04-1.22 | 0.004 |
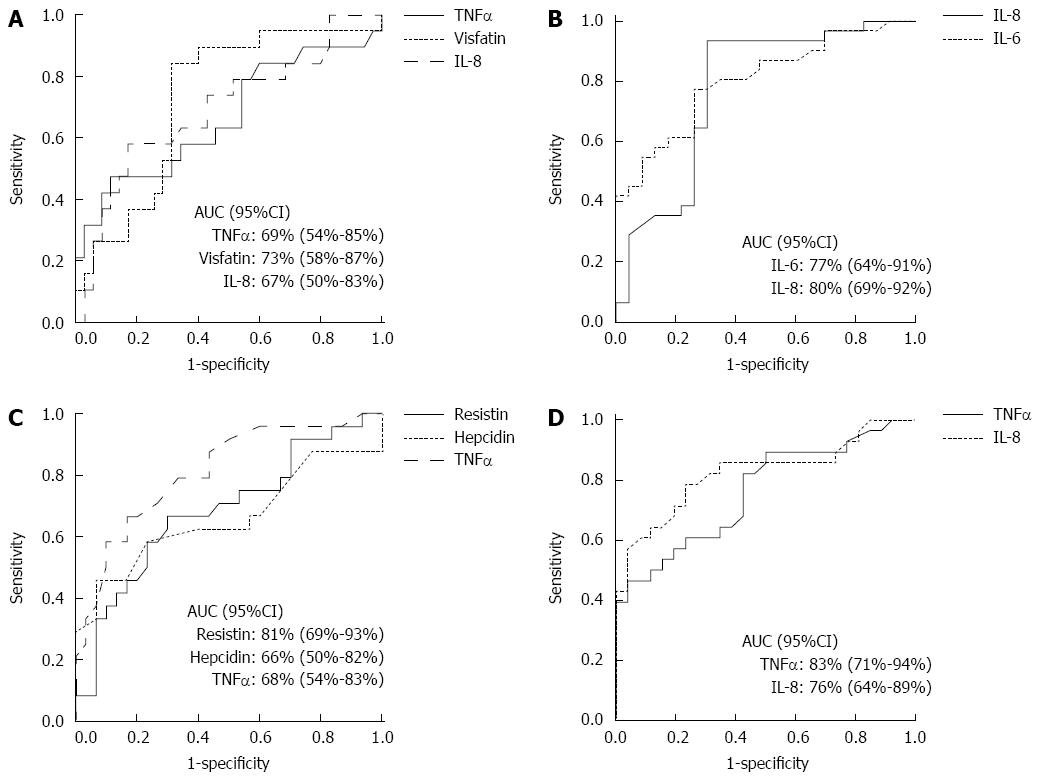
The ROC curves with calculated AUC (± 95%CI) to determine the best cut-off values of serum adipokines to differentiate histological groups are shown in Figure 2. The sensitivities and specificities of the cut-off values of biomarkers to identify histological groups appear in Table 3.
| Adipokine | Serum concentration | Sensitivity (%) | Specificity (%) |
| TNF-α (pg/mL) | 2.13 | 74 | 58 |
| Visfatin (ng/mL) | 13.00 | 84 | 69 |
| Interleukin 8 (pg/mL) | 24.25 | 58 | 66 |
| Cut-off values of serum adipokine levels to differentiate lobular inflammation grade of less than 2 foci per high power field from more advanced grades of inflammation. | |||
| Interleukin 6 (pg/mL) | 3.70 | 94 | 70 |
| Interleukin 8 (pg/mL) | 13.00 | 77 | 74 |
| Cut-off values of serum adipokine levels to differentiate perisinusoidal or periportal fibrosis from more advanced stages of fibrosis. | |||
| Resistin (mg/mL) | 1.65 | 79 | 66 |
| Hepcidin (ng/mL) | 45.00 | 63 | 60 |
| TNF-α (pg/mL) | 2.44 | 67 | 70 |
| Cut-off values of serum adipokine levels to differentiate steatohepatitis from simple fatty liver group based on non-alcoholic fatty liver disease activity score (NAS). | |||
| Interleukin 8 (pg/mL) | 9.80 | 82 | 54 |
| TNF-α (pg/mL) | 2.44 | 71 | 81 |
| Cut-off values of serum adipokine levels to differentiate steatosis degree of less than 33% from more advanced degrees of steatosis. | |||
This study concluded that serum visfatin, IL-8 and TNF-α levels were independently associated with liver steatosis degree; serum IL-6 and IL-8 concentrations were independently associated with lobular inflammation grade; and TNF-α, resistin and hepcidin levels were independently associated with fibrosis stage in a cohort of biopsy-proven NAFLD patients. Moreover, the best cut-off values for the above-mentioned serum adipokines were calculated to identify the liver histological findings.
The associations between certain adipokines with NAFLD were evaluated in previous reports[3-9]. We investigated several key adipokines, together with metabolic profiles and LFT, which provided an advantage over the previous studies. To improve the accuracy of the study, the cases were recruited from a cohort of biopsy-proven NAFLD patients. We used NAS for to determine the severity of the liver histology. Notably, NAS is a valid scoring system for NAFLD that differentiates the spectrum of disease with an acceptable reliability and validity[14].
Visfatin is a new adipokine with proinflammatory and metabolic properties. It is increased in IR syndrome. The expression of visfatin in VAT facilitates the maturation of preadipocyte cells to differentiated adipocytes (Paracrine effect)[3]. This fact might explain the correlation between serum visfatin levels and hepatic steatosis degree in our study. Visfatin is also associated with body fat mass in alcoholic fatty liver disease[15]. Previous studies have reported a correlation between visfatin and fibrosis stage, but not with steatosis or lobular inflammation grade in NAFLD[16]. Meanwhile, an increase in serum visfatin was shown to be associated with portal inflammation[17].
TNF-α is a pro-inflammatory cytokine and is associated with hepatic IR in NAFLD[4]. It mediates the early stage of NAFLD by fat accumulation in hepatocytes. In addition, it facilitates disease progression to a more advanced stage[18]. The relationship between serum TNF-α and liver steatosis and fibrosis in our research is in line with previous observations.
IL-8 is also a pro-inflammatory cytokine that activate monocytes and attracts polymorphonuclear leukocytes to the site of inflammation[19]. It is increased in obese individuals with IR. In accordance with the literature, our results showed that serum IL-8 was associated with steatosis degree and lobular inflammation[5].
IL-6 is a liver and adipose tissue-derived proinflammatory cytokine that is implicated in hepatic and skeletal muscle IR. IL-6 is thought to act as a second hit in the pathophysiology of NAFLD, causing the progression of simple fatty liver to NASH[19]. The correlation of IL-6 with lobular inflammation grade in our study is comparable to the findings by other groups[6,20,21].
With regard to hepcidin, the circulatory level was strongly associated with fibrosis stage in our study. Nevertheless, a previous found no correlation between hepcidin and histological findings[7]. Body iron stores in NAFLD regulate hepcidin levels[22]. Therefore, it seems reasonable to adjust for patients iron storage when evaluating hepcidin levels in NAFLD patients.
Resistin is an adipokine that is considered an indicator of IR in obesity[23]. However, the pathophysiological role of resistin in NAFLD is not clear. In this study, we observed that serum resistin levels were related with fibrosis stage. On the other hand, advanced liver fibrosis was associated with reduced resistin concentration in chronic hepatitis C patients with normal body weight, glucose and lipid profiles[24]. Previous studies demonstrated a correlation between high serum resistin levels and the presence of steatosis and necroinflammation in NAFLD[8,25]. Meanwhile, another study demonstrated an association of low serum resistin levels with excessive fat accumulation in the liver[26].
Adiponectin is a well-known adipokine that regulates hepatic IR[27]. It was suggested that adiponectin might be related to steatosis grade and the severity of NAFLD; however, its definitive role remains to be addressed[28]. A decrease in serum adiponectin is the primary event in children with NAFLD before the rise of inflammatory cytokines and the development of overt diabetes[9,29]. One previous study showed that adiponectin could predict patients with higher necroinflammatory grade and fibrosis stage from those with milder histological findings[30]. Another study showed that adiponectin is related to hepatic fat content and not to necroinflammatory activity and fibrosis stage[31]. Meanwhile, our study showed no correlation between adiponectin and liver histology. This study, despite its advantages, suffers from several drawbacks: first, the study was performed in a single institution; therefore, the findings need to be generalized with caution. Second, our study was cross-sectional, which limited the interpretation of causal associations.
There is currently no defined “normal range” for serum adipokines. Moreover, adipokine levels might fluctuate over time according to the metabolic environment. These concerns might explain the differences in the results of the above-mentioned studies with our results. Further well-controlled prospective studies to determine the association of VAT-derived proteins (including proinflammatory cytokines and polypeptide hormones) and liver histological findings are recommended.
The associations of some important adipokines, together with the currently used serum biomarkers, with the liver histological findings were evaluated. Certain adipokines were independently associated with the liver histological findings. Finally, the best cut-off values of these serum adipokines were determined to detect the severity of liver steatosis, lobular inflammation and fibrosis.
In conclusion, this study suggested that certain adipokines might determine accurately the severity of NAFLD based on histological findings.
We are grateful to Dr. Hassan Ehteram at the Kashan University of Medical Sciences for reviewing the liver histology samples, and to Dr. Ayat Ahmadi at the Tehran University of Medical Sciences (TUMS) for reviewing the statistical analysis. The authors would like to expand their gratitude to Drs. Vafa Rahimi Movaghar and Arsia Jamali at TUMS for their critical review of the manuscript.
Non-alcoholic liver disease (NAFLD) is a health concern worldwide. The burden of disease is increasing because of an epidemic of obesity. Considering the limitations of current modalities, finding an appropriate serum biomarker to diagnose and assess the severity of liver damage in NAFLD is crucial.
The roles of adipokines in the pathogenesis of NAFLD have received research interest recently. Nevertheless, there is a paucity of studies that used serum levels of adipokines in the diagnosis and follow up of NAFLD patients.
The associations between certain adipokines with NAFLD were evaluated in previous reports. The authors investigated several key adipokines together with metabolic profiles and LFT, providing an advantage over the previous studies. To improve the accuracy of the study, the cases were selected from a cohort of biopsy-proven NAFLD patients. To assess the severity of NAFLD based on histology, we applied NAS, a valid scoring system for NAFLD that categorizes the spectrum of disease with acceptable reliability and validity.
This study suggested that certain adipokines might determine accurately the presence and severity of NAFLD.
This manuscript evaluated the association between histological grade of NAFLD and serum biomarkers, and suggested cut-off values of the biomarkers for NASH. The authors used a direct method to define NASH, and measured various biomarkers related to adiposity and inflammation. Finally, the authors showed successfully that the indices were related to each component of NASH.
| 1. | Jamali R, Khonsari M, Merat S, Khoshnia M, Jafari E, Bahram Kalhori A, Abolghasemi H, Amini S, Maghsoudlu M, Deyhim MR. Persistent alanine aminotransferase elevation among the general Iranian population: prevalence and causes. World J Gastroenterol. 2008;14:2867-2871. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 60] [Cited by in RCA: 71] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 2. | Jamali R. Non-Alcoholic Fatty Liver Disease: Diagnosis and Evaluation of Disease Severity. Thrita. 2013;2:43-51. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 3. | Aller R, de Luis DA, Izaola O, Sagrado MG, Conde R, Velasco MC, Alvarez T, Pacheco D, González JM. Influence of visfatin on histopathological changes of non-alcoholic fatty liver disease. Dig Dis Sci. 2009;54:1772-1777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 4. | Chu CJ, Lu RH, Wang SS, Chang FY, Wu SL, Lu CL, Chun BC, Chang CY, Wu MY, Lee SD. Risk factors associated with non-alcoholic fatty liver disease in Chinese patients and the role of tumor necrosis factor-alpha. Hepatogastroenterology. 2007;54:2099-2102. [PubMed] |
| 5. | Chu CJ, Lu RH, Wang SS, Chang FY, Lin SY, Yang CY, Lin HC, Chang CY, Wu MY, Lee SD. Plasma levels of interleukin-6 and interleukin-8 in Chinese patients with non-alcoholic fatty liver disease. Hepatogastroenterology. 2007;54:2045-2048. [PubMed] |
| 6. | Grigorescu M, Crisan D, Radu C, Grigorescu MD, Sparchez Z, Serban A. A novel pathophysiological-based panel of biomarkers for the diagnosis of nonalcoholic steatohepatitis. J Physiol Pharmacol. 2012;63:347-353. [PubMed] |
| 7. | Senates E, Yilmaz Y, Colak Y, Ozturk O, Altunoz ME, Kurt R, Ozkara S, Aksaray S, Tuncer I, Ovunc AO. Serum levels of hepcidin in patients with biopsy-proven nonalcoholic fatty liver disease. Metab Syndr Relat Disord. 2011;9:287-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (1)] |
| 8. | Pagano C, Soardo G, Pilon C, Milocco C, Basan L, Milan G, Donnini D, Faggian D, Mussap M, Plebani M. Increased serum resistin in nonalcoholic fatty liver disease is related to liver disease severity and not to insulin resistance. J Clin Endocrinol Metab. 2006;91:1081-1086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 139] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 9. | Louthan MV, Barve S, McClain CJ, Joshi-Barve S. Decreased serum adiponectin: an early event in pediatric nonalcoholic fatty liver disease. J Pediatr. 2005;147:835-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 62] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 10. | Razavizade M, Jamali R, Arj A, Talari H. Serum parameters predict the severity of ultrasonographic findings in non-alcoholic fatty liver disease. Hepatobiliary Pancreat Dis Int. 2012;11:513-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Jamali R, Pourshams A, Amini S, Deyhim MR, Rezvan H, Malekzadeh R. The upper normal limit of serum alanine aminotransferase in Golestan Province, northeast Iran. Arch Iran Med. 2008;11:602-607. [PubMed] |
| 12. | Jamali R, Mofid A, Vahedi H, Farzaneh R, Dowlatshahi S. The effect of helicobacter pylori eradication on liver fat content in subjects with non-alcoholic Fatty liver disease: a randomized open-label clinical trial. Hepat Mon. 2013;13:e14679. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 13. | Razavizade M, Jamali R, Arj A, Matini SM, Moraveji A, Taherkhani E. The effect of pioglitazone and metformin on liver function tests, insulin resistance, and liver fat content in nonalcoholic Fatty liver disease: a randomized double blinded clinical trial. Hepat Mon. 2013;13:e9270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 14. | Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6807] [Cited by in RCA: 8559] [Article Influence: 407.6] [Reference Citation Analysis (9)] |
| 15. | Kalafateli M, Triantos C, Tsochatzis E, Michalaki M, Koutroumpakis E, Thomopoulos K, Kyriazopoulou V, Jelastopulu E, Burroughs A, Lambropoulou-Karatza C. Adipokines levels are associated with the severity of liver disease in patients with alcoholic cirrhosis. World J Gastroenterol. 2015;21:3020-3029. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 36] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 16. | Kukla M, Ciupińska-Kajor M, Kajor M, Wyleżoł M, Żwirska-Korczala K, Hartleb M, Berdowska A, Mazur W. Liver visfatin expression in morbidly obese patients with nonalcoholic fatty liver disease undergoing bariatric surgery. Pol J Pathol. 2010;61:147-153. [PubMed] |
| 17. | Gaddipati R, Sasikala M, Padaki N, Mukherjee RM, Sekaran A, Jayaraj-Mansard M, Rabella P, Rao-Guduru V, Reddy-Duvvuru N. Visceral adipose tissue visfatin in nonalcoholic fatty liver disease. Ann Hepatol. 2010;9:266-270. [PubMed] |
| 18. | Manco M, Marcellini M, Giannone G, Nobili V. Correlation of serum TNF-alpha levels and histologic liver injury scores in pediatric nonalcoholic fatty liver disease. Am J Clin Pathol. 2007;127:954-960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 140] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 19. | Tarantino G, Savastano S, Colao A. Hepatic steatosis, low-grade chronic inflammation and hormone/growth factor/adipokine imbalance. World J Gastroenterol. 2010;16:4773-4783. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 158] [Cited by in RCA: 172] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 20. | Lemoine M, Ratziu V, Kim M, Maachi M, Wendum D, Paye F, Bastard JP, Poupon R, Housset C, Capeau J. Serum adipokine levels predictive of liver injury in non-alcoholic fatty liver disease. Liver Int. 2009;29:1431-1438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 106] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 21. | Wieckowska A, Papouchado BG, Li Z, Lopez R, Zein NN, Feldstein AE. Increased hepatic and circulating interleukin-6 levels in human nonalcoholic steatohepatitis. Am J Gastroenterol. 2008;103:1372-1379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 395] [Cited by in RCA: 450] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 22. | Nelson JE, Brunt EM, Kowdley KV. Lower serum hepcidin and greater parenchymal iron in nonalcoholic fatty liver disease patients with C282Y HFE mutations. Hepatology. 2012;56:1730-1740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 23. | Liu F, Fan HQ, Qiu J, Wang B, Zhang M, Gu N, Zhang CM, Fei L, Pan XQ, Guo M. A paradox: insulin inhibits expression and secretion of resistin which induces insulin resistance. World J Gastroenterol. 2008;14:95-100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 24. | Wójcik K, Jabłonowska E, Omulecka A, Piekarska A. Insulin resistance, adipokine profile and hepatic expression of SOCS-3 gene in chronic hepatitis C. World J Gastroenterol. 2014;20:10449-10456. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | Aller R, de Luis DA, Fernandez L, Calle F, Velayos B, Olcoz JL, Izaola O, Sagrado MG, Conde R, Gonzalez JM. Influence of insulin resistance and adipokines in the grade of steatosis of nonalcoholic fatty liver disease. Dig Dis Sci. 2008;53:1088-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 26. | Perseghin G, Lattuada G, De Cobelli F, Ntali G, Esposito A, Burska A, Belloni E, Canu T, Ragogna F, Scifo P. Serum resistin and hepatic fat content in nondiabetic individuals. J Clin Endocrinol Metab. 2006;91:5122-5125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 36] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 27. | Buechler C, Wanninger J, Neumeier M. Adiponectin, a key adipokine in obesity related liver diseases. World J Gastroenterol. 2011;17:2801-2811. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 110] [Reference Citation Analysis (0)] |
| 28. | Finelli C, Tarantino G. What is the role of adiponectin in obesity related non-alcoholic fatty liver disease? World J Gastroenterol. 2013;19:802-812. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 144] [Cited by in RCA: 148] [Article Influence: 11.4] [Reference Citation Analysis (4)] |
| 29. | Musso G, Gambino R, Durazzo M, Biroli G, Carello M, Fagà E, Pacini G, De Michieli F, Rabbione L, Premoli A. Adipokines in NASH: postprandial lipid metabolism as a link between adiponectin and liver disease. Hepatology. 2005;42:1175-1183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 210] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 30. | Musso G, Gambino R, Biroli G, Carello M, Fagà E, Pacini G, De Michieli F, Cassader M, Durazzo M, Rizzetto M. Hypoadiponectinemia predicts the severity of hepatic fibrosis and pancreatic Beta-cell dysfunction in nondiabetic nonobese patients with nonalcoholic steatohepatitis. Am J Gastroenterol. 2005;100:2438-2446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 148] [Article Influence: 7.0] [Reference Citation Analysis (1)] |
| 31. | Bugianesi E, Pagotto U, Manini R, Vanni E, Gastaldelli A, de Iasio R, Gentilcore E, Natale S, Cassader M, Rizzetto M. Plasma adiponectin in nonalcoholic fatty liver is related to hepatic insulin resistance and hepatic fat content, not to liver disease severity. J Clin Endocrinol Metab. 2005;90:3498-3504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 299] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Kim YS S- Editor: Qi Y L- Editor: Stewart G E- Editor: Ma S