Published online Jun 7, 2016. doi: 10.3748/wjg.v22.i21.5088
Peer-review started: February 2, 2016
First decision: March 7, 2016
Revised: March 22, 2016
Accepted: April 7, 2016
Article in press: April 7, 2016
Published online: June 7, 2016
Processing time: 118 Days and 7.8 Hours
AIM: To investigate whether an elevated preoperative neutrophil-to-lymphocyte ratio (NLR) can predict poor survival in patients with hepatocellular carcinoma (HCC).
METHODS: We retrospectively reviewed 526 patients with HCC who underwent surgery between 2004 and 2011.
RESULTS: Preoperative NLR ≥ 2.81 was an independent predictor of poor disease-free survival (DFS, P < 0.001) and overall survival (OS, P = 0.044). Compared with patients who showed a preoperative NLR < 2.81 and postoperative increase, patients who showed preoperative NLR ≥ 2.81 and postoperative decrease had worse survival (DFS, P < 0.001; OS, P < 0.001). Among patients with preoperative NLR ≥ 2.81, survival was significantly higher among those showing a postoperative decrease in NLR than among those showing an increase (DFS, P < 0.001; OS, P < 0.001). When elevated, alpha-fetoprotein (AFP) provided no prognostic information, and so preoperative NLR ≥ 2.81 may be a good complementary indicator of poor OS whenever AFP levels are low or high.
CONCLUSION: Preoperative NLR ≥ 2.81 may be an indicator of poor DFS and OS in patients with HCC undergoing surgery. Preoperative NLR ≥ 2.81 may be a good complementary indicator of poor OS when elevated AFP levels provide no prognostic information.
Core tip: We retrospectively analyzed a relatively large cohort of patients and used propensity score matching to balance out biases related to patient selection. Our results suggest that preoperative neutrophil-to-lymphocyte ratio (NLR) is a significant predictor of poor overall and disease-free survival. We further suggest that postoperative decrease in NLR is associated with poor survival, although only in patients with high preoperative NLR. Finally, we show that preoperative NLR ≥ 2.81 may be a good complementary indicator of poor overall survival when elevated alpha-fetoprotein levels provide no prognostic information.
- Citation: Yang HJ, Guo Z, Yang YT, Jiang JH, Qi YP, Li JJ, Li LQ, Xiang BD. Blood neutrophil-lymphocyte ratio predicts survival after hepatectomy for hepatocellular carcinoma: A propensity score-based analysis. World J Gastroenterol 2016; 22(21): 5088-5095
- URL: https://www.wjgnet.com/1007-9327/full/v22/i21/5088.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i21.5088
Hepatocellular carcinoma (HCC) is a devastating malignancy that is the third most frequent cause of cancer-associated mortality worldwide. Hepatectomy and transplantation are considered curative treatments for HCC, but long-term survival is far from satisfactory due to the high frequency of tumor recurrence[1,2].
The prognosis of HCC patients who undergo resection varies, as it is dependent on such factors as tumor size, tumor number, vascular invasion, and tumor capsule, but these factors can only be assessed after surgery and so cannot be used for preoperative patient selection. One potential preoperative prognostic indicator is the neutrophil-to-lymphocyte ratio (NLR). This indicator of systemic inflammation is easy and inexpensive to determine[3-7], and elevated pretreatment NLR has been associated with poor outcome in numerous malignancies, including colon cancer[4], gastric cancer[8], HCC[3], and breast cancer[6].
Although studies have suggested that an elevated pretreatment NLR may correlate with a poor outcome in patients with HCC[9-11], other studies failed to detect such a correlation[12-14]. To gain a clearer picture of the influence of preoperative NLR on survival and recurrence after surgery for HCC, we carried out a retrospective study on propensity score-matched patients.
NLR often changes after hepatic resection in HCC patients, perhaps reflecting the shifting balance between inflammatory activity and immune activity. This raises the question of whether a postoperative change in NLR also serves as a predictor of prognosis after surgery. A study of 189 patients with early stage HCC suggested that a postoperative increase in NLR was associated with poorer overall survival and disease-free survival[13], but this has yet to be confirmed in larger samples.
The present study retrospectively analyzed a relatively large sample of Chinese patients with HCC in order to assess the usefulness of both preoperative NLR and postoperative change in NLR as prognostic indicators.
This research was approved by the Ethics Committee of the Tumor Hospital of Guangxi Medical University. Written informed consent was obtained from participating patients.
All patients who underwent hepatic resection for primary HCC as initial treatment at the Affiliated Tumor Hospital of Guangxi Medical University between May 2004 and September 2011 were considered for inclusion in the study. Patients were diagnosed with primary HCC when two types of imaging technique showed features typical of HCC, or when one imaging technique gave positive findings and the alpha fetoprotein (AFP) level was > 400 ng/mL. Diagnosis of HCC was confirmed by histopathological examination.
Patients were excluded from the study if they underwent transarterial chemoembolization (TACE), radiofrequency ablation (RFA), percutaneous ethanol injection, or other anti-tumor therapies before hepatic resection. Patients were also excluded if they suffered from preoperative fever.
Baseline clinical characteristics and laboratory results were recovered from the hospital database.
NLR was calculated by dividing the neutrophil count by the lymphocyte count. Preoperative NLR was determined within 7 d of surgery, and postoperative NLR was determined at the first follow-up visit in the outpatient department a month after surgery. Postoperative change in NLR was calculated by dividing postoperative NLR by preoperative NLR. The resulting numerical changes were transformed into a binary outcome of postoperative increase in NLR (when the ratio was ≥ 1) or postoperative decrease in NLR (when the ratio was < 1). For certain analyses, patients were divided into groups with low or high preoperative NLR using a cutoff value of 2.81, as reported in the literature[9,11].
All patients were followed up 1 mo after liver resection, every subsequent 3 mo during the first postoperative year, and every 6 mo thereafter until 60 mo after surgery or until death. At each follow-up visit, routine blood tests, serum AFP assay, ultrasound and computed tomography (CT), or magnetic resonance imaging (MRI) were performed.
Outcomes were overall survival (OS) and disease-free survival (DFS). DFS was defined as the interval from hepatectomy to imaging-based discovery of tumor relapse. OS was defined as 60 mo for those who survived more than 60 mo and DFS was defined as 60 mo if tumor relapse did not occur within 60 mo.
Since patients were assigned to groups based on a preoperative NLR cut-off rather than randomization, propensity score analysis was used to balance out patient differences related to patient selection for hepatic resection. Propensity scores for all patients were estimated using a logistic regression model, which included all covariates that might have affected patient assignment to a high or low preoperative NLR group, as well as patient survival (Table 1). One-to-one nearest-neighbor matching was performed between high and low preoperative NLR using a 0.1 caliper width[14]. The resulting score-matched pairs were used in subsequent analyses as indicated.
| Variable | Before propensity matching | After propensity matching | ||||
| NLR < 2.81 | NLR≥2.81 | P value | NLR < 2.81 | NLR≥2.81 | P value | |
| n = 401 | n = 125 | n = 111 | n = 111 | |||
| Gender, M/F | 358/43 | 107/18 | 0.262 | 94/17 | 97/14 | 0.561 |
| Age (yr) | 46.8 ± 10.9 | 47.9 ± 11.5 | 0.309 | 46.1 ± 11.6 | 47.2 ± 11.2 | 0.487 |
| HbsAg | ||||||
| Negative | 50 | 25 | 0.041 | 18 | 19 | 0.857 |
| Positive | 351 | 100 | 93 | 92 | ||
| Liver cirrhosis | ||||||
| Yes | 338 | 94 | 0.023 | 85 | 86 | 0.873 |
| No | 63 | 31 | 26 | 25 | ||
| AFP (ng/mL) | ||||||
| < 400 | 268 | 70 | 0.032 | 67 | 67 | 1.000 |
| ≥ 400 | 133 | 55 | 44 | 44 | ||
| Edmonson grade | ||||||
| I-II | 238 | 84 | 0.166 | 71 | 71 | 1.000 |
| III-IV | 163 | 41 | 40 | 40 | ||
| Surgical margin (cm) | ||||||
| < 1 | 195 | 51 | 0.126 | 48 | 50 | 0.787 |
| ≥ 1 | 206 | 74 | 63 | 61 | ||
| BCLC stage | ||||||
| 0 or A | 180 | 69 | 0.044 | 53 | 57 | 0.591 |
| B or C | 221 | 56 | 58 | 54 | ||
| Child-Pugh class | ||||||
| A | 385 | 117 | 0.260 | 105 | 105 | 1.000 |
| B | 16 | 8 | 6 | 6 | ||
| Tumor number | ||||||
| Single | 293 | 86 | 0.363 | 75 | 79 | 0.560 |
| Multiple | 108 | 39 | 36 | 32 | ||
| Tumor size (cm) | 6 (4-8) | 8 (5.5-12) | < 0.001 | 7.5 (6-11) | 8 (5-12) | 0.769 |
| Tumor capsule | ||||||
| Complete | 181 | 40 | 0.010 | 33 | 39 | 0.390 |
| Incomplete | 220 | 85 | 78 | 72 | ||
| Vascular invasion | ||||||
| Absent | 354 | 98 | 0.008 | 91 | 91 | 1.000 |
| Present | 47 | 27 | 20 | 20 | ||
| Albumin (g/L) | 40.1 ± 4.4 | 39.8 ± 4.1 | 0.399 | 40.3 ± 5.1 | 39.9 ± 4.2 | 0.583 |
| Platelet count (109/L) | 180.6 ± 76.6 | 202.1 ± 84.7 | 0.008 | 206.5 ± 82.6 | 200.91 ± 85.73 | 0.624 |
| AST (U/L) | 41 (36-60) | 49 (37-67.5) | 0.371 | 47 (31-70) | 49 (37-70) | 0.138 |
| ALT (U/L) | 40 (29-58) | 42 (27.5-54) | 0.393 | 37 (26-59) | 42 (33-55) | 0.400 |
| Total bilirubin (μmol/L) | 12 (9-16.8) | 14 (9.9-19.5) | 0.010 | 12.3 (9.2-17.4) | 14 (9.9-19.4) | 0.051 |
Statistical analysis was performed using SPSS 19.0 (IBM, United States). Intergroup differences in categorical data were assessed for significance using χ2 test, while intergroup differences in continuous data were assessed using the Mann-Whitney U test or t test. OS and DFS were analyzed using the Kaplan-Meier approach, and differences were assessed for significance using the log-rank test. Independent prognostic factors were identified using the Cox proportional hazards model. P < 0.05 served as the threshold of significance.
Between May 2004 and September 2011, 858 patients underwent hepatectomy for HCC at the Affiliated Tumor Hospital of Guangxi Medical University. Of these, 332 (38.7%) were excluded from our study because they (1) received initial HCC treatment at other centers (n = 288, 33.5%); (2) had already undergone RFA, TACE, percutaneous ethanol injection, or another pre-resection procedure (n = 24, 2.8%); or (3) suffered from preoperative fever (n = 20, 2.3%).
Ultimately, 526 patients (61.5%) were enrolled in the study, of whom 452 (85.9%) received curative hepatectomy. The remaining 74 patients (14.1%) received hepatectomy that was considered palliative because they had macroscopic vessel invasion[15].
Of the 526 patients, 125 (23.8%) had NLR levels higher than the cut-off value and were included in the high NLR group, while the remaining 401 (76.2%) were included in the low NLR group. The two groups were balanced in terms of gender, age, Edmondson grade, surgical margin, Child-Pugh class, and tumor number, as well as levels of albumin, aspartate aminotransferase (AST), and alanine aminotransferase (ALT) (all P > 0.05; Table 1). However, the two groups were unbalanced in terms of the presence of hepatitis B surface antigen (HbsAg), liver cirrhosis, tumor capsule, and vascular invasion; levels of AFP, platelets, and total bilirubin; Barcelona Clinic Liver Cancer (BCLC) stage; and tumor size (all P < 0.05; Table 1). Propensity score matching was used to generate 111 pairs of patients from the two groups, who showed no significant differences (Table 1).
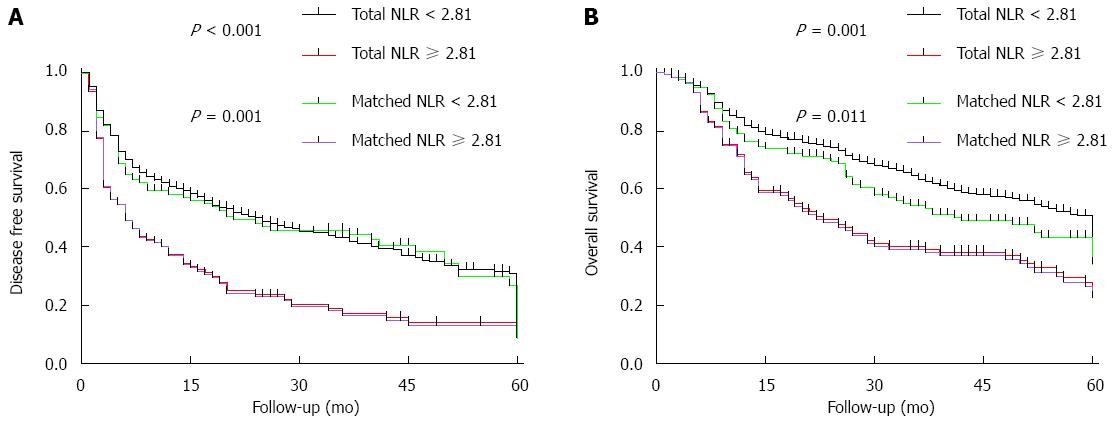
Among all patients in the study, DFS was 55.4% at 1 year, 37.3% at 3 years, and 19.6% at 5 years. The corresponding OS rates were 78.2%, 57.9%, and 35.6%, respectively. Among propensity-matched pairs of patients, DFS was significantly higher in the low-NLR group than in the high-NLR group at 1, 3, and 5 years (Figure 1A). Similar results were obtained for OS (Figure 1B).
Among all patients in the study, univariate analysis identified several factors significantly associated with poor DFS: AFP ≥ 400 ng/mL, Edmondson grade III-IV, surgical margin < 1 cm, multiple tumors, tumor size ≥ 5 cm, incomplete tumor capsule, vascular invasion, preoperative NLR ≥ 2.81, AST ≥ 80 U/L, and BCLC stage B or C. With the exception of AFP level, all of the aforementioned factors were also found to be significantly associated with poor OS.
Multivariate analysis (Table 2) identified the following independent predictors of poor DFS: AFP ≥ 400 ng/mL, multiple tumors, tumor size ≥ 5 cm, vascular invasion, and preoperative NLR ≥ 2.81. Excluding AFP level, all of these factors were also found to be independent predictors of poor OS.
| Factor | HR | 95%CI | P value |
| Disease-free survival | |||
| AFP > 400 ng/mL | 1.493 | 1.062-2.100 | < 0.001 |
| Multiple tumors | 1.766 | 1.385-2.252 | < 0.001 |
| Tumor size ≥ 5 cm | 1.313 | 1.018-1.693 | 0.036 |
| Vascular invasion | 2.656 | 1.962-3.594 | < 0.001 |
| NLR ≥ 2.81 | 1.610 | 1.250-2.075 | < 0.001 |
| Overall survival outcome | |||
| Multiple tumors | 1.649 | 1.257-2.16 | < 0.001 |
| Tumor size ≥ 5 cm | 1.912 | 1.407-2.59 | < 0.001 |
| Incomplete tumor capsule | 1.480 | 1.139-1.92 | 0.003 |
| Vascular invasion | 2.239 | 1.496-3.350 | < 0.001 |
| NLR ≥ 2.81 | 1.333 | 1.007-1.76 | 0.044 |
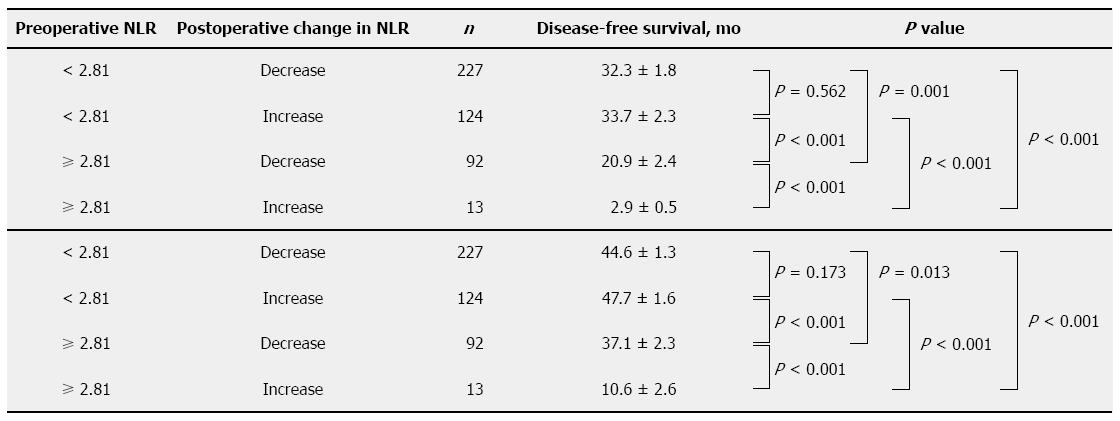
In the complete cohort of 526 patients, postoperative NLR data were available for 456 (86.7%). These fell into the following four subgroups (Figure 2): 227 patients (49.8%) who had a preoperative NLR < 2.81 and showed a postoperative decrease in NLR; 124 (27.2%) who had a preoperative NLR < 2.81 and showed a postoperative increase in NLR; 92 (20.2%) with preoperative NLR ≥ 2.81 and a postoperative decrease in NLR; and 13 (2.9%) with NLR ≥ 2.81 and a postoperative increase in NLR.
Compared with the patients who show preoperative NLR < 2.81 and postoperative increase, the patients who show preoperative NLR ≥ 2.81 and postoperative decrease have worse survival (DFS, P < 0.001; OS, P < 0.001; Figure 2). Among patients with preoperative NLR ≥ 2.81, survival was significantly higher for those showing a postoperative decrease in NLR than for those showing a postoperative increase (DFS, P < 0.001; OS, P < 0.001; Figure 2).
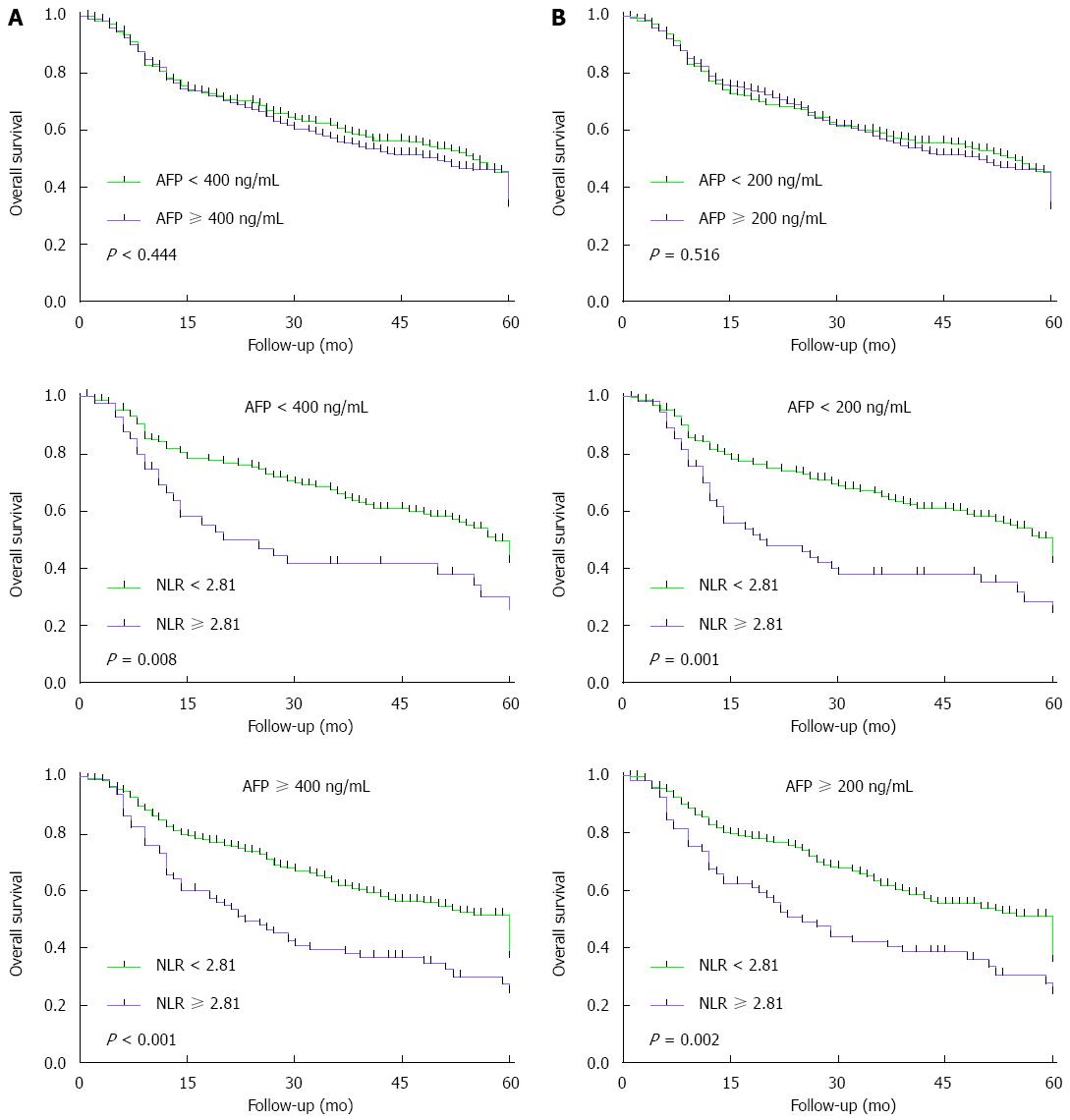
Since univariate analysis identified preoperative AFP ≥ 400 ng/mL as a predictor of poor DFS but not OS, we wanted to examine whether the prognostic value of preoperative NLR varied with AFP level. Analysis of patient subgroups with AFP levels of 200, 400 ng/mL showed that, when elevated, AFP levels provide no prognostic information and that preoperative NLR ≥ 2.81 may be a complementary indicator of poor OS whenever alpha-fetoprotein (AFP) levels are low or high (Figure 3).
The present retrospective study with a relatively large cohort of Chinese HCC patients suggests that elevated preoperative NLR is associated with poor OS and DFS, and that a postoperative increase in NLR is associated with poor survival. This result may not only assist surgeons in predicting HCC patient survival before and after surgery, but also act to remind the surgeon to perform timely adjuvant treatment to improve the prognosis of patients with preoperative NLR ≥ 2.81.
In our cohort, elevated serum AFP levels were not significant predictors of poor OS after resection: OS did not vary significantly with preoperative AFP levels of 200, 400 ng/mL. AFP remains controversial as a predictor of HCC patient survival after resection; while some studies have associated elevated serum AFP levels with poor prognosis[11,16-18], others have failed to find such an association[10,13,15,19]. Our results suggest that, when elevated AFP levels provide no prognostic information, preoperative NLR ≥ 2.81 may be a complementary indicator of poor OS whenever AFP levels are low or high.
Why elevated NLR and postoperative NLR increase should predict poor survival remains unclear, but some studies have proposed explanations. One such explanation is that many patients with elevated NLR have lymphocytopenia, which may contribute to a weak lymphocyte-mediated immune response to tumors[20]. This lymphocyte-mediated response normally aids in the elimination of abnormal cells and in the production of cytokines that inhibit tumor proliferation, invasion, and metastasis[21]. Another possible explanation is that elevated NLR reflects a stronger neutrophil response and higher numbers of peripheral neutrophils, leading to higher secretion of pro-angiogenic factors such as interleukin-8[22], vascular endothelial growth factor (VEGF)[23,24], and matrix metalloproteinase (MMP)[25,26], which may contribute to tumor growth and therefore to poor prognosis. Studies have indicated that a postoperative NLR increase may reflect that the body has not recovered from tumor control after surgery[27], potentially leading to worse survival.
The findings of the present study should be interpreted with caution in light of several limitations. First, this study is retrospective and based on patients at a single institution. Indeed, more than 85% of our cohort was chronically infected with hepatitis B virus, which is not the case in other parts of the world. Secondly, the cut-off value of NLR was obtained from published papers[9,11]. Thirdly, owing to the distribution of HCC patients, the number of patients who showed preoperative NLR ≥ 2.81 and postoperative increase was more than 10 times less than others, which may have led to variance in the results.
In conclusion, our results suggest that both preoperative NLR and postoperative change in NLR are predictors of OS and DFS in HCC patients undergoing hepatic surgery. Elevated NLR may be a complementary, or even an alternative, biomarker of survival when elevated AFP levels prove uninformative.
The authors thank Armando Chapin Rodríguez, PhD, for his language editing, which substantially improved the quality of the manuscript.
An elevated preoperative neutrophil-to-lymphocyte ratio (NLR) may predict poor survival in patients with hepatocellular carcinoma (HCC), but this requires confirmation.
The prognosis of HCC patients who undergo resection depends on factors such as tumor size and number, vascular invasion, and tumor capsule, but these factors can only be assessed after surgery and so cannot be used for preoperative patient selection. One potential preoperative prognostic indicator is the neutrophil-to-lymphocyte ratio. This indicator of systemic inflammation is easy and inexpensive to determine.
The authors retrospectively analyzed a relatively large cohort of patients and used propensity score matching to balance out biases related to patient selection in order to investigate the impact of preoperative NLR and postoperative NLR on survival. This study will provide more evidence for NLR after curative resection of HCC in the future.
Preoperative NLR ≥ 2.81 may be an indicator of poor DFS and OS in patients with HCC undergoing surgery. Preoperative NLR ≥ 2.81 may be a good complementary indicator of poor OS when elevated AFP levels provide no prognostic information.
NLR was calculated by dividing the neutrophil count by the lymphocyte count. Postoperative change in NLR was calculated by dividing postoperative NLR by preoperative NLR. The resulting numerical changes were transformed into a binary outcome of postoperative increase in NLR (when the ratio was ≥ 1) or postoperative decrease in NLR (when the ratio was < 1).
This is an interesting manuscript that shows the benefit of using an easily available tool for prognosis after resection for HCC.
| 1. | Ercolani G, Grazi GL, Ravaioli M, Del Gaudio M, Gardini A, Cescon M, Varotti G, Cetta F, Cavallari A. Liver resection for hepatocellular carcinoma on cirrhosis: univariate and multivariate analysis of risk factors for intrahepatic recurrence. Ann Surg. 2003;237:536-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 263] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 2. | Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3249] [Cited by in RCA: 3630] [Article Influence: 259.3] [Reference Citation Analysis (12)] |
| 3. | Sukato DC, Tohme S, Chalhoub D, Han K, Zajko A, Amesur N, Orons P, Marsh JW, Geller DA, Tsung A. The Prognostic Role of Neutrophil-to-Lymphocyte Ratio in Patients with Unresectable Hepatocellular Carcinoma Treated with Radioembolization. J Vasc Interv Radiol. 2015;26:816-24.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 4. | Galizia G, Lieto E, Zamboli A, De Vita F, Castellano P, Romano C, Auricchio A, Cardella F, De Stefano L, Orditura M. Neutrophil to lymphocyte ratio is a strong predictor of tumor recurrence in early colon cancers: A propensity score-matched analysis. Surgery. 2015;158:112-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 69] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 5. | Stotz M, Gerger A, Eisner F, Szkandera J, Loibner H, Ress AL, Kornprat P, AlZoughbi W, Seggewies FS, Lackner C. Increased neutrophil-lymphocyte ratio is a poor prognostic factor in patients with primary operable and inoperable pancreatic cancer. Br J Cancer. 2013;109:416-421. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 315] [Cited by in RCA: 407] [Article Influence: 31.3] [Reference Citation Analysis (1)] |
| 6. | Azab B, Bhatt VR, Phookan J, Murukutla S, Kohn N, Terjanian T, Widmann WD. Usefulness of the neutrophil-to-lymphocyte ratio in predicting short- and long-term mortality in breast cancer patients. Ann Surg Oncol. 2012;19:217-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 418] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 7. | Sharaiha RZ, Halazun KJ, Mirza F, Port JL, Lee PC, Neugut AI, Altorki NK, Abrams JA. Elevated preoperative neutrophil: lymphocyte ratio as a predictor of postoperative disease recurrence in esophageal cancer. Ann Surg Oncol. 2011;18:3362-3369. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 246] [Cited by in RCA: 282] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 8. | Shimada H, Takiguchi N, Kainuma O, Soda H, Ikeda A, Cho A, Miyazaki A, Gunji H, Yamamoto H, Nagata M. High preoperative neutrophil-lymphocyte ratio predicts poor survival in patients with gastric cancer. Gastric Cancer. 2010;13:170-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 300] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 9. | Okamura Y, Ashida R, Ito T, Sugiura T, Mori K, Uesaka K. Preoperative neutrophil to lymphocyte ratio and prognostic nutritional index predict overall survival after hepatectomy for hepatocellular carcinoma. World J Surg. 2015;39:1501-1509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 106] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 10. | Liao W, Zhang J, Zhu Q, Qin L, Yao W, Lei B, Shi W, Yuan S, Tahir SA, Jin J. Preoperative Neutrophil-to-Lymphocyte Ratio as a New Prognostic Marker in Hepatocellular Carcinoma after Curative Resection. Transl Oncol. 2014;7:248-255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 11. | Mano Y, Shirabe K, Yamashita Y, Harimoto N, Tsujita E, Takeishi K, Aishima S, Ikegami T, Yoshizumi T, Yamanaka T. Preoperative neutrophil-to-lymphocyte ratio is a predictor of survival after hepatectomy for hepatocellular carcinoma: a retrospective analysis. Ann Surg. 2013;258:301-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 277] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 12. | Huang J, Xu L, Luo Y, He F, Zhang Y, Chen M. The inflammation-based scores to predict prognosis of patients with hepatocellular carcinoma after hepatectomy. Med Oncol. 2014;31:883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 13. | Peng W, Li C, Wen TF, Yan LN, Li B, Wang WT, Yang JY, Xu MQ. Neutrophil to lymphocyte ratio changes predict small hepatocellular carcinoma survival. J Surg Res. 2014;192:402-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 69] [Article Influence: 5.8] [Reference Citation Analysis (1)] |
| 14. | Sullivan KM, Groeschl RT, Turaga KK, Tsai S, Christians KK, White SB, Rilling WS, Pilgrim CH, Gamblin TC. Neutrophil-to-lymphocyte ratio as a predictor of outcomes for patients with hepatocellular carcinoma: a Western perspective. J Surg Oncol. 2014;109:95-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 15. | Uemura M, Sasaki Y, Yamada T, Gotoh K, Eguchi H, Yano M, Ohigashi H, Ishikawa O, Imaoka S. Serum antibody titers against hepatitis C virus and postoperative intrahepatic recurrence of hepatocellular carcinoma. Ann Surg Oncol. 2014;21:1719-1725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Jiang JH, Guo Z, Lu HF, Wang XB, Yang HJ, Yang FQ, Bao SY, Zhong JH, Li LQ, Yang RR. Adjuvant transarterial chemoembolization after curative resection of hepatocellular carcinoma: propensity score analysis. World J Gastroenterol. 2015;21:4627-4634. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 43] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 17. | Guo Z, Zhong JH, Jiang JH, Zhang J, Xiang BD, Li LQ. Comparison of survival of patients with BCLC stage A hepatocellular carcinoma after hepatic resection or transarterial chemoembolization: a propensity score-based analysis. Ann Surg Oncol. 2014;21:3069-3076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 18. | Zhong JH, Ke Y, Gong WF, Xiang BD, Ma L, Ye XP, Peng T, Xie GS, Li LQ. Hepatic resection associated with good survival for selected patients with intermediate and advanced-stage hepatocellular carcinoma. Ann Surg. 2014;260:329-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 387] [Article Influence: 32.3] [Reference Citation Analysis (1)] |
| 19. | Fu SJ, Shen SL, Li SQ, Hua YP, Hu WJ, Liang LJ, Peng BG. Prognostic value of preoperative peripheral neutrophil-to-lymphocyte ratio in patients with HBV-associated hepatocellular carcinoma after radical hepatectomy. Med Oncol. 2013;30:721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 20. | Chew V, Tow C, Teo M, Wong HL, Chan J, Gehring A, Loh M, Bolze A, Quek R, Lee VK. Inflammatory tumour microenvironment is associated with superior survival in hepatocellular carcinoma patients. J Hepatol. 2010;52:370-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 206] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 21. | Ding PR, An X, Zhang RX, Fang YJ, Li LR, Chen G, Wu XJ, Lu ZH, Lin JZ, Kong LH. Elevated preoperative neutrophil to lymphocyte ratio predicts risk of recurrence following curative resection for stage IIA colon cancer. Int J Colorectal Dis. 2010;25:1427-1433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 167] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 22. | Zurek OW, Pallister KB, Voyich JM. Staphylococcus aureus Inhibits Neutrophil-derived IL-8 to Promote Cell Death. J Infect Dis. 2015;212:934-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 23. | Phan VT, Wu X, Cheng JH, Sheng RX, Chung AS, Zhuang G, Tran C, Song Q, Kowanetz M, Sambrone A. Oncogenic RAS pathway activation promotes resistance to anti-VEGF therapy through G-CSF-induced neutrophil recruitment. Proc Natl Acad Sci USA. 2013;110:6079-6084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 94] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 24. | Ohki Y, Heissig B, Sato Y, Akiyama H, Zhu Z, Hicklin DJ, Shimada K, Ogawa H, Daida H, Hattori K. Granulocyte colony-stimulating factor promotes neovascularization by releasing vascular endothelial growth factor from neutrophils. FASEB J. 2005;19:2005-2007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 184] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 25. | Odabasi M, Yesil A, Ozkara S, Paker N, Ozkan S, Eris C, Yildiz MK, Abuoglu HH, Gunay E, Tekeşin K. Role of human neutrophil gelatinase associated lipocalin (NGAL) and Matrix Metalloproteinase-9 (MMP-9) overexpression in neoplastic colon polyps. Int J Clin Exp Med. 2014;7:2804-2811. [PubMed] |
| 26. | Candido S, Abrams SL, Steelman LS, Lertpiriyapong K, Fitzgerald TL, Martelli AM, Cocco L, Montalto G, Cervello M, Polesel J. Roles of NGAL and MMP-9 in the tumor microenvironment and sensitivity to targeted therapy. Biochim Biophys Acta. 2016;1863:438-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 77] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 27. | Kishi Y, Kopetz S, Chun YS, Palavecino M, Abdalla EK, Vauthey JN. Blood neutrophil-to-lymphocyte ratio predicts survival in patients with colorectal liver metastases treated with systemic chemotherapy. Ann Surg Oncol. 2009;16:614-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 272] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Kapoor S, Morales-Gonzalez J S- Editor: Qi Y L- Editor: Rutherford A E- Editor: Ma S