Published online Mar 21, 2016. doi: 10.3748/wjg.v22.i11.3234
Peer-review started: May 31, 2015
First decision: June 19, 2015
Revised: September 27, 2015
Accepted: December 8, 2015
Article in press: December 8, 2015
Published online: March 21, 2016
Processing time: 290 Days and 16.9 Hours
AIM: To evaluate short-term outcomes following intraoperative biliary lavage for hepatolithiasis.
METHODS: A total of 932 patients who were admitted to the West China Medical Center of Sichuan University between January 2010 and January 2014 and underwent bile duct exploration and lithotomy were retrospectively included in our study. The patients were divided into the lavage group and the control group. Related pre-, intra-, and postoperative factors were recorded, analyzed, and compared between the two groups in order to verify the effects of biliary lavage on the short-term outcome of patients with hepatolithiasis.
RESULTS: Amongst the patients who were included, 678 patients with hepatolithiasis were included in the lavage group, and the other 254 patients were enrolled in the control group. Data analyses revealed that preoperative baseline and related intraoperative variables were not significantly different. However, patients who underwent intraoperative biliary lavage had prolonged postoperative hospital stays (6.67 d vs 7.82 d, P = 0.024), higher hospitalization fees (RMB 28437.1 vs RMB 32264.2, P = 0.043), higher positive rates of bacterial cultures from blood (13.3% vs 25.8%, P = 0.001) and bile (23.6% vs 40.7%, P = 0.001) samples, and increased usage of advanced antibiotics (26.3% vs 38.2%, P = 0.001). In addition, in the lavage group, more patients had fever (> 37.5 °C, 81.4% vs 91.1%, P = 0.001) and hyperthermia (> 38.5°C,39.7% vs 54.9%, P = 0.001), and higher white blood cell counts within 7 d after the operation compared to the control group.
CONCLUSION: Intraoperative biliary lavage might increase the risk of postoperative infection, while not significantly increasing gallstone removal rate.
Core tip: Hepatolithiasis remains a prevalent disease in Asia-Pacific regions, and the most important treatment procedures are hepatectomy and intraoperative choledochofiberscopy combined with basket stone extraction Intraoperative biliary lavage is also a commonly used, simple, and effective procedure for the extraction of intrahepatic stones. However, few investigators have noted the potential risks associated with this method. In this study, we collected and analyzed the data, and the aim of this retrospective study was to evaluate the risks and short-term patient outcomes following intraoperative biliary lavage.
- Citation: Jiang O, Zhou RX, Yang K, Cai CX, Liu Y, Cheng NS. Negative short-term impact of intraoperative biliary lavage in patients with hepatolithiasis. World J Gastroenterol 2016; 22(11): 3234-3241
- URL: https://www.wjgnet.com/1007-9327/full/v22/i11/3234.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i11.3234
Despite its rarity in Western countries, hepatolithiasis remains a prevalent disease in Asia-Pacific regions[1-3]. As an important component of primary stones, intrahepatic stones associated with hepatolithiasis are frequently pigment or mixed stones[4]. Intrahepatic stones can cause recurring episodes of localized proliferative cholangitis, possibly leading to ductal wall thickening and biliary stricture or obstruction. This can also be accompanied by irregular distal dilatation, which might influence biliary fluid dynamics. In addition, the role of infections should also be considered[2,3,5]. Taken together, the proliferation of the stones may result in a vicious cycle, eventually leading to hepatic abscesses, atrophy, cirrhosis, or even cholangiocarcinoma[4-6].
The current treatment for hepatolithiasis includes gallstone extraction, removal of affected segments, elimination of strictures, and prevention of recurrence[3,4,7-10]. In conventional bile duct exploration and lithotomy, the most important treatment procedures are hepatectomy and intraoperative choledochofiberscopy combined with basket stone extraction[8-10]. In addition, some other passages to access biliary are commonly adopted, such as, combined endoscopic retrograde cholangiopancreatography (ERCP) with endoscopic sphincterotomy (EST), percutaneous transhepatic cholangioscopy (PTCS), and hepatocutaneous jejunostomy[2,11-13]. Similarly, biliary lavage is also a commonly used method for stone extraction. Many surgeons or endoscopists perform biliary lavage at a certain pressure in order to remove minuscule or uncaptured stones and to loosen entrapped stones for easier extraction. In contrast, choledochofiberscopy combined with basket stone extraction enables the removal of only one stone at a time; hence, biliary lavage is a more convenient extraction method, and this has led to its widespread application.
Intraoperative biliary lavage is a commonly used, simple, and effective procedure for the extraction of intrahepatic stones. However, few investigators have noted the potential risks associated with this method. Since Jan 2010, our team has noticed and suspected that intraoperative biliary lavage may increase postoperative infective complications. We avoided employing intraoperative lavage since then, while the other surgeons have continued to perform this procedure. We collected and analyzed the data, and the aim of this retrospective study was to evaluate the risks and short-term patient outcomes following intraoperative biliary lavage.
All patients with hepatolithiasis who were admitted to the West China Hospital of Sichuan University between January 2010 and January 2014 were retrospectively included in the study. This study was approved by the review board of West China Hospital.
Patients were assigned to either the lavage group or the control group based on whether or not they had received intraoperative biliary lavage. Patients who had undergone emergency surgery or who had presented with acute preoperative suppurative cholangitis or other infections with evidence of fever (axillary temperature > 37.5 °C) were excluded.
In our hospital, EST was the primary treatment choice for extrahepatic bile stones, while all intrahepatic bile stone patients were resolved by surgical procedure. PTCS was implemented recently, and patients who underwent PTCS were not included in this study. All surgical procedures were performed by a total of six surgeons. All the surgeons were engaged in hepatobiliary surgery for more than 10 years, therefore, there were no significant gaps in surgical technique or management among them.
Medical histories were obtained from all patients; in addition, all patients underwent physical examinations followed by preoperative liver and kidney function tests, routine blood tests, electrocardiography, chest radiography, and other tests. B-mode ultrasound and magnetic resonance cholangiopancreatography or computed tomography as well as intraoperative choledochofiberscopy were performed to assess comprehensively the location of the gallstones and to determine pathological changes in the liver and biliary strictures.
Intraoperative biliary lavage involved inserting an 8-14F catheter into the intrahepatic bile duct neighboring the stones, attaching a 20 mL hollow needle to the catheter, and rinsing the intrahepatic bile duct by rapid expulsion of physiological saline in about 2 s. The stones were discharged with the rapid outflow stream. The pressure of lavage was not detected directly, while it was much higher than the normal pressure of the bile duct (8-10 cm H2O).
Thirty minutes before abdominal incision, 2 g of first-generation or second-generation cephalosporin was routinely administered intravenously. Cephalosporin administration was also repeated every 3 h during the operating time or when blood loss exceeded 1000 mL. All patients were under general anesthesia, and the surgery approach, including open or laparoscope, was decided by the chief surgeon according to the condition of each patient. We opened and explored the common bile duct while biliary endoscopy and lithotomy were routinely performed through CBD. Patients in the lavage group received one or repeated intraoperative biliary lavage, while the control group patients did not. We would remove all stones intraoperatively through the common bile duct unless the lithotomy procedure led to hemobilia. Bile samples were collected after opening the bile duct for bacterial culture. For patients with unilateral hepatolithiasis or with accompanying liver atrophy, liver resection was also performed during surgery unless the patients refused. After completing the lithotomy, T-tube and peritoneal drainage catheter placement was performed routinely before closing the abdominal wall. Six weeks after surgery, lithotomy was performed repeatedly by T tube sinus with choledochoscope for those patients with incomplete stone removal.
Cephalosporin administration did not exceed 72 h following surgery, unless the patient had an infection or was clearly at risk of infection. Axillary temperature was measured 2-4 times daily after the operation, and the highest daily temperature was recorded and analyzed. If the patient’s axillary temperature exceeded 38.5 °C, one or more biological samples, including peripheral venous blood, bile, sputum, and peritoneal fluid, were collected for bacterial culture. Antibiotic administration was then adjusted based on the results of the culture. If antibiotics were ineffective, the medication was adjusted further based on the results of the bacterial culture. Routine blood tests and liver function tests were performed on day 1, 3 and 7 after the operation, and bile samples for bacterial culture were collected from the T-tube on postoperative days 1-3. Other surgery-related complications were also recorded and analyzed.
Related preoperative, intraoperative, and postoperative factors were recorded, analyzed, and compared between the lavage and control groups; the effects of biliary lavage on postoperative short-term patient outcomes were then analyzed and compared.
SPSS version 19.0 (SPSS Inc., Chicago, IL, United States) was used for all analyses. Results are presented as mean ± SE of the mean. The t or U tests were applied to continuous variables, while binary categorical variables were assessed using the χ2 test. Two-sided P value less than 0.05 was considered statistically significant.
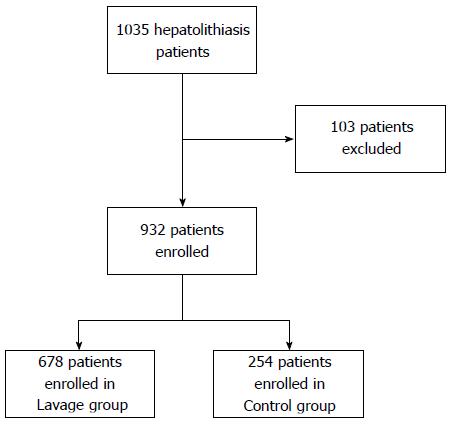
Between January 2010 and January 2014, a total of 1035 patients with hepatolithiasis were included in the study. After applying the exclusion criteria, 932 patients were included in the study, including 261 men and 662 women (Figure 1). As our hospital is a regional center for intensive care, the patients admitted to our hospital typically had serious conditions. Among the patients included in our study, 44% had histories of single or multiple bile duct exploration or lithotomy, and 10% of these patients had received partial liver resection. In 298 patients, the stones were located on both the right and left sides of the liver and the extrahepatic bile duct. In 134 patients, the stones were located bilaterally in the liver only. In 275 patients, the stones were located either on the left or right side of the liver, as well as in the extrahepatic bile duct. For the remaining 225 patients, the stones were located either on the left or right side of the liver only.
Two patients in our study died: one due to severe abdominal infection and the other due to hemorrhagic shock.
In all, 678 patients were included in the lavage group and 254 patients in the control group. There were no statistically significant differences between the two groups with regard to age, gender, comorbid diseases, gallstone location, past history of bile duct exploration, liver resection, and cholangioenterostomy, and laboratory serological test results (Table 1).
| Control group(n = 254) | Lavage group(n = 678) | P value | |
| Age (yr) | 55.96 ± 13.48 | 50.81 ± 10.91 | 0.223 |
| Gender (M:F) | 81:173 | 180:498 | 0.119 |
| Pulmonary disease | 45 (17.7) | 154 (22.7) | 0.106 |
| Hypertension | 25 (9.8) | 63 (9.3) | 0.803 |
| Diabetes mellitus | 11 (4.3) | 27 (3.9) | 0.853 |
| Past history of biliary tract surgery | 119 (46.9) | 287 (42.3) | 0.235 |
| Past history of cholangioenterostomy | 11 (4.3) | 15 (2.2) | 0.115 |
| Past history of liver resection | 23 (9.1) | 72 (10.6) | 0.713 |
| Stone location | 0.892 | ||
| Right | 159 (62.5) | 395 (58.3) | |
| Left | 215 (84.6) | 577 (85.1) | |
| Extra | 158 (62.2) | 406 (59.8) | |
| Total bilirubin | 15.8 ± 10.42 | 18.3 ± 24.33 | 0.110 |
| ALB (g/L) | 40.31 ± 3.45 | 39.45 ± 6.92 | 0.197 |
| WBC (× 109/L) | 5.61 ± 2.02 | 6.05 ± 4.7 | 0.462 |
| AST (U/L) | 39.6 ± 45.12 | 53.51 ± 61.38 | 0.155 |
| ALT (U/L) | 38.76 ± 54.37 | 64.61 ± 76.79 | 0.306 |
The intraoperative variables of the two groups are outlined in Table 2. Blood loss, presence of liver atrophy and biliary stricture, addition of liver resection, cholangioenterostomy during surgery, and other related variables were not significantly different between the two groups, while the operation time of the control group was slightly longer than the lavage group.
| Control group(n = 254) | Lavage group(n = 678) | P value | |
| Operation time (min) | 124.2 ± 48.1 | 100.1 ± 51.9 | 0.036 |
| Surgical approach | 0.128 | ||
| Open | 233 (91.4) | 641 (94.5) | |
| Laparoscopy | 21 (8.6) | 37 (5.5) | |
| Blood loss (mL) | 235.3 ± 358.2 | 241.6 ± 256.9 | 0.918 |
| Combined liver resection | 137 (53.9) | 355 (52.4) | 0.713 |
| Cirrhosis | 71 (27.9) | 229 (33.8) | 0.098 |
| Liver atrophy | 97 (38.2) | 282 (41.6) | 0.369 |
| Biliary stricture | 23 (9.1) | 92 (13.6) | 0.113 |
| Biliary distal dilatation | 118 (46.4) | 294 (43.4) | 0.416 |
| Cholangioenterostomy | 8 (3.1) | 15 (2.2) | 0.491 |
| Complete stone remove | 207 (81.5) | 523 (77.1) | 0.249 |
| Bile bacterial culture positive | 15 (5.9) | 38 (5.6) | 0.874 |
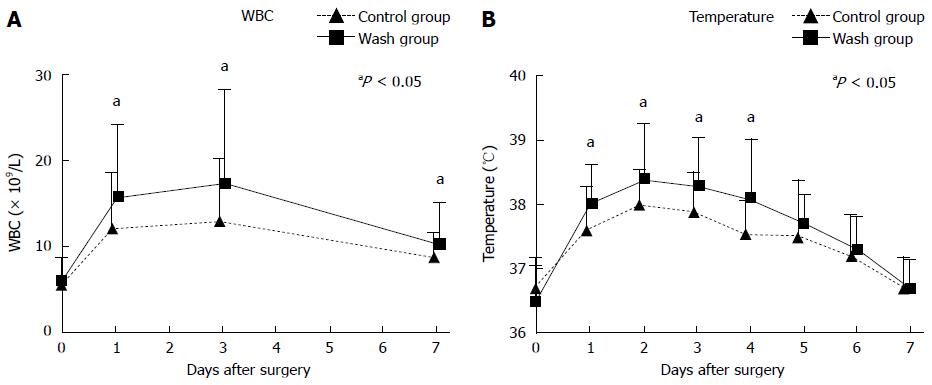
Table 3 shows a comparison of postoperative variables between the two groups. Compared to the control group, the lavage group had a longer average length of hospital stay, increased hospitalization fees, and increased risk of postoperative fever and hyperthermia, as well as an increased probability of positive blood and bile bacterial cultures. The number of patients who required stronger antibiotic was also higher in the lavage group. The peak axillary temperature on day 1 to day 4 after surgery was also significantly higher in the lavage group compared to the control group. In addition, white blood cell counts during the first 7 d after surgery were significantly higher in the lavage group than that in the control group (Figure 2). However, the incidence of other surgery-related complications, such as bile leakage and lung infections, was not significantly different between the two groups. Furthermore, although the probability of abdominal infection was higher in the lavage group compared to the control group, this difference was not statistically significant. Moreover, there was no significant difference between the two groups with regard to stone clearance rate, which is an important prognostic factor for hepatolithiasis; this was likely because of the use of choledochofiberscopy intraoperatively and postoperatively.
| Control group(n = 254) | Lavage group(n = 678) | P value | |
| Death | 1 | 1 | 0.471 |
| Cost | RMB 28437.1 ± 8530.5 | RMB 32264.2 ± 11893.2 | 0.043 |
| Postoperative hospital stay (d) | 6.67 ± 3.78 | 7.82 ± 4.34 | 0.024 |
| Fever (> 37.5 °C) | 207 (81.4) | 618 (91.2) | 0.001 |
| High fever (> 38.5 °C) | 101 (39.8) | 372 (54.9) | 0.001 |
| Antibiotic up-regulation | 67 (26.3) | 259 (38.2) | 0.001 |
| Blood bacterial culture positive | 34 (13.4) | 175 (25.8) | 0.001 |
| Bile bacterial culture positive | 60 (23.6) | 276 (40.1) | 0.001 |
| Cholangitis | 51 (20.1) | 238 (35.1) | 0.001 |
| Pulmonary infection | 5 (1.9) | 22 (3.2) | 0.383 |
| Abdominal infection | 12 (4.7) | 59 (8.7) | 0.051 |
| Bile leakage | 8 (3.1) | 33 (4.8) | 0.287 |
| Complications | |||
| Grade I | 69 (27.1) | 174 (25.7) | 0.250 |
| Grade II | 87 (34.2) | 305 (45.0) | |
| Grade III | 7 (2.8) | 16 (2.3) | |
| Grade IV | 2 (0.8) | 3 (0.4) |
The results of postoperative bacterial culture using blood and bile samples revealed that 545 colonies of bacteria were cultivated from 438 patients. There were 172 colonies of Escherichia coli (E. coli), which had the highest count, followed by 141 colonies of Klebsiella pneumoniae, 94 colonies of Enterobacter cloacae, 75 colonies of Pseudomonas aeruginosa, 43 colonies of Enterococcus faecalis, and 87 colonies of other bacteria. In terms of the total bacterial culture count, the lavage group had significantly higher counts for both blood and bile cultures compared to the control group. However, with blood cultures, there were no statistically significant differences in the counts of Pseudomonas aeruginosa, Enterobacter cloacae, and Enterococcus faecalis between the two groups. Similarly, with bile cultures, Enterococcus faecalis counts were not significantly different between the two groups (Table 4).
| Blood | Bile | |||||
| Control group(n = 254) | Lavage group(n = 678) | P value | Control group(n = 254) | Lavage group(n = 678) | P value | |
| Escherichia coli | 11 (4.3) | 57 (8.4) | 0.034 | 19 (7.4) | 85 (12.5) | 0.035 |
| Klebsiella pneumoniae | 9 (3.5) | 48 (7.1) | 0.046 | 15 (5.9) | 69 (27.2) | 0.041 |
| Pseudomonas aeruginosa | 8 (3.1) | 18 (2.7) | 0.659 | 7 (2.8) | 42 (6.2) | 0.046 |
| Enterobacter cloacae | 5 (2.0) | 22 (3.2) | 0.383 | 11 (4.3) | 56 (8.3) | 0.045 |
| Enterococcus faecalis | 2 (0.8) | 13 (1.9) | 0.379 | 7 (2.8) | 21 (3.1) | 0.265 |
| Others | 5 (2.0) | 34 (5.0) | 0.043 | 8 (3.1) | 40 (5.9) | 0.098 |
| Total | 40 (15.7) | 192 (28.3) | 0.001 | 67 (26.4) | 313 (46.2) | 0.001 |
Higher level antibiotics were more frequently required in the lavage group than the control group, and this difference was statistically significant. There were significant differences between the two groups in the antibiotic usage of imipenem, moxifloxacin, piperacillin-tazobactam, and cefoperazone-sulbactam. However, there were no significant differences in the antibiotic use of ceftriaxone, levofloxacin, and ciprofloxacin between the two groups (Table 5).
| Control group(n = 254) | Lavage group(n = 678) | P value | |
| Imipenem | 4 (1.6) | 29 (4.3) | 0.047 |
| Ceftriaxone | 8 (3.1) | 21 (3.1) | 1.000 |
| Moxifloxacin | 11 (4.3) | 57 (8.4) | 0.034 |
| Piperacillin-tazobactam | 15 (5.9) | 69 (10.2) | 0.041 |
| Cefoperazone-sulbactam | 18 (7.1) | 80 (11.8) | 0.041 |
| Levofloxacin | 4 (1.6) | 10 (14.7) | 0.261 |
| Ciprofloxacin | 5 (2.0) | 7 (10.3) | 1.000 |
| Others | 7 (2.8) | 18 (26.5) | 0.439 |
| Total | 72 (28.3) | 291 (42.9) | 0.005 |
In this study, we found that performing intraoperative biliary lavage during biliary lithotomy may negatively affect patients with hepatolithiasis, and it may not increase the clearance rate of gallstones. As hepatolithiasis can lead to recurring localized chronic cholangitis, which can cause ductal wall thickening and biliary stricture[2,3,5], it creates large obstacles against stone removal in the drainage segments of the bile ducts. Furthermore, miniscule and soft stones are not easily removed by choledochofiberscope-guided basket stone extraction; therefore, surgeons commonly apply biliary lavage at a certain pressure to expel the stones. Therefore, unfortunately, according to our investigations, this commonly used method of stone extraction might significantly increase the risk of postoperative infection, fever, and hyperthermia in patients, thereby prolonging the patients’ average length of hospital stay and increasing hospitalization fees. Moreover, the technological advancements and application of choledochofiberscopy and shock wave lithotripsy, which may prolong the operation time as well as increase the use of liver resection, are sufficiently able to maintain a high clearance rate of intrahepatic bile duct stones[2], and therefore, the relative effectiveness of biliary lavage for stone removal may be decreasing.
The causes of hepatolithiasis are complicated and related to various health conditions. In China, a large proportion of patients have a history of roundworm infection[14], while several studies have shown that bacteria play an important role in the occurrence of hepatolithiasis[15-17]. Normal bile should be sterile; however, hepatolithiasis will occur because of the involvement of bacteria. For example, E. coli can secrete β-glucuronidase, which breaks down conjugated bilirubin found in bile into free bilirubin; the free bilirubin will then combine with calcium to form pigment stones, the main type of gallstone found in hepatolithiasis[18]. Another study has shown that there were remnants of bacterial DNA in a proportion of intrahepatic bile duct stones[19]. Therefore, a few scholars believe that chronic bacterial infection may be present even in patients with hepatolithiasis who do not experience acute attacks and that the bacteria still retain a certain degree of activity[15,17]. Bile duct exploration and lithotomy for hepatolithiasis can easily lead to the occurrence of acute postoperative biliary infection or even sepsis. This might be because patients with hepatolithiasis also present with accompanying biliary strictures and chronic proliferative cholangitis. Firstly, the gallstones might have sharper edges, and the use of bougienage, choledochofiberscopy, and other procedures during surgery might damage the biliary mucosa. Subsequently, bacteria that were already involved in chronic biliary infection may then enter the blood stream and cause sepsis. In addition, in theory, pressurized lavage may cause bacteria to break free from the gallstones, which will exacerbate or increase the risk of retrograde flow of bacteria into the blood. Besides, a portion of the hepatolithiasis patients had liver cirrhosis, which can impair immune system[20]. Our study has shown that acute biliary infection and concurrent blood infection occur frequently in patients with hepatolithiasis, and intraoperative biliary lavage can exacerbate the occurrence and severity of acute infections.
In the absence of intraoperative biliary lavage, patients with hepatolithiasis are already prone to fever and hyperthermia after bile duct exploration and lithotomy, with occurrence rates of 81.4% and 39.7%, respectively. However, the application of biliary lavage increased these occurrence rates to 91.1% and 54.9%, respectively. Our results also demonstrated that bacterial cultures from bile and blood samples of patients with postoperative infection had lower positive rates than those without postoperative infection (blood: 13.3% and 25.8%, respectively; bile: 23.6% and 40.7%, respectively). This might be due to the use of prophylactic antibiotics. Bacterial cultures from patients with postoperative infection contained mostly gram-negative bacteria, of which E. coli, K. pneumoniae, Pseudomonas aeruginosa, and Enterobacter cloacae were the most common types; of note, these bacteria are normal intestinal bacteria. Currently, studies have shown that causative pathogens of biliary infections come mostly from the intestines[11-13], which is consistent with our results.
Since hepatolithiasis is usually accompanied by bacterial infection, the general consensus for treatment is that the intraoperative use of prophylactic antibiotics is beneficial; however, the most effective type of antibiotic for prophylaxis has not been established[21,22]. At present, it is believed that first- and second-generation cephalosporins should be used during abdominal surgery as prophylactic antibiotics for surgical site infection[23]. In this study, we used first- and second-generation cephalosporins as prophylactic antibiotics for all cases. However, most patients with hepatolithiasis have concurrent gram-negative bacterial infections, and the antibacterial activity of first- and second-generation cephalosporins is quite poor against these bacteria. Furthermore, most of the patients who were recruited for this study had previously undergone multiple biliary operations and had histories of multiple acute cholangitis. Thus, different types of antibiotics were administered to patients, and non-standard antibiotics were used in certain cases. Accordingly, there may be several types of bacteria present with resistance to various drugs. Therefore, for these reasons, there was a higher proportion of patients in our study who had fever, cholangitis, and sepsis. In addition, these proportions were even higher in the lavage group. With regard to the treatment of biliary infections, as some patients developed concurrent blood infection, antibiotic selection was based on drug sensitivity, while also taking into account drug concentrations in the blood and bile[24,25]. It is currently believed that piperacillin-tazobactam, cefoperazone-sulbactam, ceftriaxone, and levofloxacin, which have high bile concentrations, are the better choices, and those with additional β-lactamase inhibitor activity might have even better efficacies[26-30]. The results of our study have shown that the use of cefoperazone-sulbactam, piperacillin-tazobactam, and moxifloxacin within our medical center is in line with the abovementioned drug selection principle, while the lack of difference between the two groups in terms of drug usage for ceftriaxone, levofloxacin, and ciprofloxacin is likely due to surgeons’ preferences.
In this study, we investigated and clarified the postoperative short-term effects of biliary lavage in a large group of patients with hepatolithiasis. However, there were certain limitations to our study. First, our study was a retrospective analysis; thus, data collection, statistical analyses, and other aspects of the study might be biased, which could have influenced the results. Second, intraoperative biliary lavage involves manual control of the syringe by the surgeon, and the water pressure is not set automatically by machines; therefore, each surgeon might use different levels of water pressure for each operation, and the pressure gradient might theoretically be positively correlated to the retrograde flow of bacteria into the blood. In reality, the use of choledochofiberscopy during surgery also requires a certain level of water pressure in order to maintain a clear field of vision. Our study showed that intraoperative pressurized biliary lavage correlated with postoperative occurrence of bacteremia and sepsis. However, we were not able to analyze water pressure as one of the factors, and, therefore, we were not able to determine what level of water pressure may cause the abovementioned effects. In order to address these issues, our research group has registered a prospective randomized controlled trial on the World Health Organization website (currently under ethical review) in order to further clarify and comprehensively evaluate the effects of different levels of lavage pressure gradients and the addition of antibiotic intervention on patients with hepatolithiasis.
In conclusion, intraoperative biliary lavage is a commonly used method for stone extraction. Although it does not increase the occurrence of severe and fatal complications, our study shows that it might increase the postoperative risk of infection-related complications in patients with hepatolithiasis. These complications will lead to prolonged hospital stays and increased hospitalization fees, while not significantly increasing the rate of gallstone removal. Therefore, we suggest that this procedure should be used with caution.
Hepatolithiasis remains a prevalent disease in China. As hepatolithiasis can lead to recurring localized chronic cholangitis, which can cause ductal wall thickening and biliary stricture, it creates large obstacles against stone removal in the drainage segments of the bile ducts. Furthermore, miniscule and soft stones are not easily removed by choledochofiberscope-guided basket stone extraction; therefore, surgeons commonly apply biliary lavage at a certain pressure to expel the stones. However, few investigators have noted the potential risk of biliary lavage.
Intraoperative biliary lavage is commonly used to clear the intrahepatic stone, however, there are very few English studies available in PubMed concerning the risk of this procedure. This study focused on the short-term impact of biliary lavage in hepatolithiasis patients.
Intraoperative biliary lavage is commonly used as one of the traditional methods to remove intrahepatic biliary stones. However, very few experts have noticed the risk associated with this procedure. In this study, the authors research the short-term impact of intraoperative biliary lavage. The results suggest that intraoperative biliary lavage may increase the rate of postoperative infection and prolong hospital stay.
The data in this study suggested intraoperative biliary lavage may negatively impact hepatolithiasis patients in the short-term, however, this study was retrospectively designed, and further prospective randomized controlled trials are necessary to confirm our conclusions.
Intraoperative biliary lavage is inserting an 8-14F catheter into the intrahepatic bile duct neighboring the stones, attaching a 20 mL hollow needle to the catheter, and rinsing the intrahepatic bile duct by rapid expulsion of physiological saline in about 2 s. Escherichia coli, Klebsiella pneumonia, Pseudomonas aeruginosa, Enterobacter cloacae, and Enterococcus faecalis are various kinds of bacteria. Imipenem, ceftriaxone, moxifloxacin, piperacillin-tazobactam, cefoperazone-sulbactam, levofloxacin, and ciprofloxacin are different kinds of antibiotics.
This manuscript addresses the necessity and safety of intraoperative biliary lavage for hepatolithiasis. This paper is interesting and provides information to answer an important clinical question when dealing with recurrent pyogenic cholangitis, i.e., sepsis.
| 1. | Zhang GW, Lin JH, Qian JP, Zhou J. Identification of risk factors for intraoperative hemobilia and its correlation with early postoperative complications in patients with hepatolithiasis. Am J Surg. 2015;209:260-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 2. | Tsuyuguchi T, Miyakawa K, Sugiyama H, Sakai Y, Nishikawa T, Sakamoto D, Nakamura M, Yasui S, Mikata R, Yokosuka O. Ten-year long-term results after non-surgical management of hepatolithiasis, including cases with choledochoenterostomy. J Hepatobiliary Pancreat Sci. 2014;21:795-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 3. | Suzuki Y, Mori T, Yokoyama M, Nakazato T, Abe N, Nakanuma Y, Tsubouchi H, Sugiyama M. Hepatolithiasis: analysis of Japanese nationwide surveys over a period of 40 years. J Hepatobiliary Pancreat Sci. 2014;21:617-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (2)] |
| 4. | Kim HJ, Kim JS, Suh SJ, Lee BJ, Park JJ, Lee HS, Kim CD, Bak YT. Cholangiocarcinoma Risk as Long-term Outcome After Hepatic Resection in the Hepatolithiasis Patients. World J Surg. 2015;39:1537-1542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (1)] |
| 5. | Zhang GW, Lin JH, Qian JP, Zhou J. Identification of prognostic factors and the impact of palliative resection on survival of patients with stage IV hepatolithiasis-associated intrahepatic cholangiocarcinoma. J Surg Oncol. 2014;109:494-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (1)] |
| 6. | Liu FB, Yu XJ, Wang GB, Zhao YJ, Xie K, Huang F, Cheng JM, Wu XR, Liang CJ, Geng XP. Preliminary study of a new pathological evolution-based clinical hepatolithiasis classification. World J Gastroenterol. 2015;21:2169-2177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Ma WJ, Zhou Y, Shrestha A, Mao H, Li FY, Cheng NS, Zhang W, Xu RH, Zhang YQ, Jiang T. Applying chemical bile duct embolization to achieve chemical hepatectomy in hepatolithiasis: a further experimental study. J Surg Res. 2014;187:113-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Li SQ, Liang LJ, Peng BG, Hua YP, Lv MD, Fu SJ, Chen D. Outcomes of liver resection for intrahepatic stones: a comparative study of unilateral versus bilateral disease. Ann Surg. 2012;255:946-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 9. | Fang CH, Liu J, Fan YF, Yang J, Xiang N, Zeng N. Outcomes of hepatectomy for hepatolithiasis based on 3-dimensional reconstruction technique. J Am Coll Surg. 2013;217:280-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 10. | Tian J, Li JW, Chen J, Fan YD, Bie P, Wang SG, Zheng SG. Laparoscopic hepatectomy with bile duct exploration for the treatment of hepatolithiasis: an experience of 116 cases. Dig Liver Dis. 2013;45:493-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Tan J, Tan Y, Chen F, Zhu Y, Leng J, Dong J. Endoscopic or laparoscopic approach for hepatolithiasis in the era of endoscopy in China. Surg Endosc. 2015;29:154-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 12. | Cheon YK, Cho YD, Moon JH, Lee JS, Shim CS. Evaluation of long-term results and recurrent factors after operative and nonoperative treatment for hepatolithiasis. Surgery. 2009;146:843-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 117] [Article Influence: 6.9] [Reference Citation Analysis (3)] |
| 13. | Parlak E, Disibeyaz S, Oztas E, Cicek B, Ulas M, Ozogul Y, Ozdemir E, Olcer T, Sasmaz N, Sahin B. Endoscopic treatment of biliary disorders in patients with Roux-en-Y hepaticojejunostomy via a permanent access loop. Endoscopy. 2011;43:73-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Heimes JK, Waller S, Olyee M, Schmitt TM. Hepatolithiasis after Hepaticojejunostomy: Ascaris lumbricoides in the biliary tract. Surg Infect (Larchmt). 2013;14:470-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 15. | Yu JK, Pan H, Huang SM, Huang NL, Yao CC, Hsiao KM, Wu CW. Calcium content of different compositions of gallstones and pathogenesis of calcium carbonate gallstones. Asian J Surg. 2013;36:26-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Weerakoon HT, Ranasinghe S, Navaratne A, Sivakanesan R, Galketiya KB, Rosairo S. Serum lipid concentrations in patients with cholesterol and pigment gallstones. BMC Res Notes. 2014;7:548. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Katz SC, Ryan K, Ahmed N, Plitas G, Chaudhry UI, Kingham TP, Naheed S, Nguyen C, Somasundar P, Espat NJ. Obstructive jaundice expands intrahepatic regulatory T cells, which impair liver T lymphocyte function but modulate liver cholestasis and fibrosis. J Immunol. 2011;187:1150-1156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 65] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 18. | Tsui WM, Lam PW, Lee WK, Chan YK. Primary hepatolithiasis, recurrent pyogenic cholangitis, and oriental cholangiohepatitis: a tale of 3 countries. Adv Anat Pathol. 2011;18:318-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 19. | Wu T, Zhang Z, Liu B, Hou D, Liang Y, Zhang J, Shi P. Gut microbiota dysbiosis and bacterial community assembly associated with cholesterol gallstones in large-scale study. BMC Genomics. 2013;14:669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 167] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 20. | Acalovschi M. Gallstones in patients with liver cirrhosis: incidence, etiology, clinical and therapeutical aspects. World J Gastroenterol. 2014;20:7277-7285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 61] [Cited by in RCA: 74] [Article Influence: 6.2] [Reference Citation Analysis (1)] |
| 21. | Kobayashi S, Gotohda N, Nakagohri T, Takahashi S, Konishi M, Kinoshita T. Risk factors of surgical site infection after hepatectomy for liver cancers. World J Surg. 2009;33:312-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 50] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 22. | Kondo K, Chijiiwa K, Ohuchida J, Kai M, Fujii Y, Otani K, Hiyoshi M, Nagano M, Imamura N. Selection of prophylactic antibiotics according to the microorganisms isolated from surgical site infections (SSIs) in a previous series of surgeries reduces SSI incidence after pancreaticoduodenectomy. J Hepatobiliary Pancreat Sci. 2013;20:286-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 23. | Bergman S, Deban M, Martelli V, Monette M, Sourial N, Hamadani F, Teasdale D, Holcroft C, Zakrzewski H, Fraser S. Association between quality of care and complications after abdominal surgery. Surgery. 2014;156:632-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Westphal JF, Brogard JM. Biliary tract infections: a guide to drug treatment. Drugs. 1999;57:81-91. [PubMed] |
| 25. | Mukaiya M, Hirata K, Katsuramaki T, Kihara C, Kimura Y, Yamaguchi K, Furuhata T, Hata F. Isolated bacteria and susceptibilities to antimicrobial agents in biliary infections. Hepatogastroenterology. 2005;52:686-690. [PubMed] |
| 26. | Weickert U, Wiesend F, Subkowski T, Eickhoff A, Reiss G. Optimizing biliary stent patency by coating with hydrophobin alone or hydrophobin and antibiotics or heparin: an in vitro proof of principle study. Adv Med Sci. 2011;56:138-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (1)] |
| 27. | Ortega M, Marco F, Soriano A, Almela M, Martínez JA, López J, Pitart C, Mensa J. Epidemiology and prognostic determinants of bacteraemic biliary tract infection. J Antimicrob Chemother. 2012;67:1508-1513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 28. | Darkahi B, Sandblom G, Liljeholm H, Videhult P, Melhus Å, Rasmussen IC. Biliary microflora in patients undergoing cholecystectomy. Surg Infect (Larchmt). 2014;15:262-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 29. | Hwang JJ, Lee DH, Yoon H, Shin CM, Park YS, Kim N. Efficacy of moxifloxacin-based sequential and hybrid therapy for first-line Helicobacter pylori eradication. World J Gastroenterol. 2015;21:10234-10241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 30. | Ober MC, Hoppe-Tichy T, Köninger J, Schunter O, Sonntag HG, Weigand MA, Encke J, Gutt C, Swoboda S. Tissue penetration of moxifloxacin into human gallbladder wall in patients with biliary tract infections. J Antimicrob Chemother. 2009;64:1091-1095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Co M, Tsutsumi K S- Editor: Yu J L- Editor: Filipodia E- Editor: Wang CH