COMMENTARY ON HOT TOPICS
The epithelial monolayer on the luminal border of the intestinal wall has certain physiological functions, such as diffusion and absorption of small molecules, or acts as a physical, chemical and immunological barrier defence against luminal microbiota. Due to the high cellular turnover (2-3 d) the continuous replacement of shedding epithelial cells is required from a local stem cell pool even in the healthy intestine. Stem cells are located at the crypt base, and their progenies migrate towards the luminal surface where they undergo terminal differentiation[1-3].
During the years, different methods have been developed to study the destiny, renewal, and differentiation potential of epithelial stem cells. The first functional demonstration of stemness in case of epithelial cells was performed using methods established originally for culturing epidermal keratinocytes under conditions where they were able to maintain and propagate for hundreds of colonies without losing stemness[4,5]. Proliferative capacity assays and ultrastructural analyses of the intestinal crypt led to opening the hypothesis that crypt base columnar cells refer to intestinal stem cells[6]. Consequentially, assignment of stemness favored cells at the +4 position, given their mode of chromosome segregation and higher resistance to cell death induced by deoxyribonucleotide-acid (DNA) damage[7,8]. Lineage tracings of 0→+4 crypt basal cells with Lgr5-CreER (leucine-rich repeat-containing G-protein coupled receptor 5), Bmi1 (B cell-specific Moloney murine leukemia virus integration site 1), mTERT (mouse telomerase reverse transcriptase), and Hopx-CreER (homeodomain-only protein homeobox) indicated that all crypt columnar basal cells display interconvertible multipotent intestinal stem cell characteristics[9-13]. This fact is further illustrated by diphteria toxin-targeted ablation of Lgr5+ cells, which does not influence intestinal homeostasis[14]. Based on these results, the intestine is characterized by spatially separated interconvertible stem cells existing in quiescent and/or activated states.
Initially, it has been proposed that all Lgr5+ intestinal stem cells are cycling rapidly[9]. Recent data of Buczacki et al[15] revealed that in case of physiological circumstances approx. 20% of Lgr5+ intestinal cells not only cycle less frequently, but differentiate into Paneth and neuroendocrine cells, as well do not contribute to intestinal homeostasis. However, this population of cells retain the ability to reacquire stem cell function, and also can be recruited to serve as a functional clonogenic stem population under conditions of regeneration.
DETECTION OF IMMORTAL DNA STRAND SEGREGATION
The Cairn’s theory (i.e., the immortal DNA strand hypothesis) describes a mechanism for adult stem cells to minimize genomic mutations[16]. According to this hypothesis, in adult stem cells DNA division is asymmetric, and stem cells retain a distinct template set of DNA strands over successive generations. Adult stem cells by non-random DNA division could transmit mutations arising from DNA replication errors onto terminally differentiating daughter cells, thus they may reduce the rate of accumulated mutations frequently leading to genetic disorders, like cancer. Further analysis of this hypothesis may yield insights into areas, such as tissue regeneration, cancer development or the process of aging.
One of the used methods to detect immortal DNA strand segregation is the label-retention assay[17,18]. In this approach, symmetrically cycling cells are labeled by a repeated or continuous supply of tritiated thymidine or bromodeoxyuridine (BrdU) for one generation time, producing cells with hemi-labeled DNA. Further, asymmetric cell kinetics is induced, and simultaneously the tritiated thymidine or BrdU is removed. Then the cells continue cycling for a long chase period (usually of five generation time) during which the label is lost from all the cycling, transit amplifying cells, therefore only quiescent or slowly cycling cells (i.e., stem cells) remain to be labeled. These cells are termed label-retaining cells (LRCs).
The other technique, the label-release assay, does not require the manipulation of cell division kinetics. In this technique non-immortal DNA strands are labeled and observed, and the captured event is the release of the labeled non-immortal DNA strands from the stem cell turning into the next cell cycle[19].
EPITHELIAL STEM CELL MARKERS AND REGULATORS
Stem cells exhibit both the self renewal and the ability to give rise of differentiated progenies. Stem cells can be classified as pluripotent embryonic or as multi(uni)potent adult stem cells. In tissues with high cell turnover rates, such as the intestine adult stem cells are cycling asymmetrically[20]. Currently two types of stem cells are defined in intestinal crypts, the cycling crypt base columnar cells and the quiescent (+4) cells[21]. So far, several candidate markers and/or regulators of rapidly cycling and quiescent epithelial stem cells have already been identified.
CDX2
Caudal related homeobox protein 2 (CDX2) is a nuclear transcription factor with an important function in early differentiation and maintenance of the intestinal epithelial phenotype[22], and controlling the expression of a number of downstream genes, some of which contribute to inflammation[23]. Further, CDX2 was shown to inhibit in vitro cell growth and migration as well as dissemination of colon tumor cells in vivo[24]. CDX2 could also be useful for detection of crypt epithelial stem cells[22]. The induction of CDX2 in intestinal epithelium can lead to expression of Math1 (a basic helix-loop-helix transcription factor), which plays an important role in differentiation of stem cells into goblet cells[25].
Musashi-1
Musashi-1, an RNA (ribonucleic acid)-binding protein, possesses a regulatory function of the asymmetric cell division in ectodermal precursor cells[26], and exhibits importance in regulating the maintenance and differentiation of stem cells even in the intestine[27,28]. On the other hand, Musashi-1+ cells might represent circulating smooth muscle cell precursors, as well[29].
Lgr5
Lgr5, an orphan receptor, has been identified as a downstream target of the Wnt-pathway[9]. In situ hybridization and lineage marking using an inducible Lgr5-Cre knockin and Rosa26-lacZ reporter demonstrated that Lgr5 is a marker for small and large bowel epithelial stem cells or long-lived multipotent progenitor cells[9,30]. Lgr5 might also be involved in cancer stemness[31]. Interestingly, knockout of the Lgr5 gene has no detectable effect on intestinal development, however, there are closely related receptors which may replace its function[32].
Bmi1
Bmi1, originally described as a member of a nuclear protein complex in sarcoma and leukemic cells[33,34], has been found to be expressed in distinct cells located near the bottom of crypts in the small intestine, predominantly in the +4 position[10]. It has been recently reported that Bmi1+ cells found in the proximal small intestine can be clonally expanded, and repopulate Lgr5+ stem cells after conditional Lgr5 cell deletion[10,14].
Wip1
Wip1 (wildtype p53-induced phosphatase; PPM1D) was originally described as a human phosphatase that is induced in response to ionizing radiation in a p53-dependent manner in Burkitt lymphoma cells[35]. Wip1, as a negative regulator of the nuclear factor (NF)-κB p65 subunit regulates the homeostasis of intestinal stem cells, and co-localizes with LRCs in a supra-Paneth cell position[36,37]. The loss of Wip1 gene can lower the threshold of p53-dependent apoptosis, so it prevents the conversion of adult stem cells into tumor-initiating ones[36].
TERT
TERT is a ribonucleoprotein complex that maintains the telomeric ends of chromosomes, which could help to prevent cellular senescence being relevant for the self-renewal of adult stem cells[38]. TERT is implicated in the direct regulation of epidermal stem cell proliferation and mobilization[39,40]. The epithelial expression of TERT together with other growth factor receptors has been found to be increased in the crypt-base affected by chronic inflammation, which may lead to the pathologic survival and proliferation of epithelial cells carrying genetic defects[41]. In mice, TERT expression has been found to be present in slowly cycling intestinal stem cells[11].
Bone morphogenetic protein type I receptor alpha
Bone morphogenetic protein type I receptor alpha (BMPR1α), a receptor of BMP-signaling pathway has been shown to be a negative regulator of intestinal stem cell proliferation[42]. BMPR1α is highly expressed on LRCs, and the intestine-specific knockout of BMPR1α resulted in polyposis, probably due to an increase in intestinal stem cell self-renewal[43].
Phosphatase and tensin homolog
Phosphatase and tensin homolog (PTEN), a known tumor suppressor has been found to limit the number of intestinal stem cells[43]. Its inactive form is co-localized with BrdU+ LRCs[44]. It has been proposed that BMP-signaling leads to the increase of PTEN activity, which as a negative regulator of phosphoinositol-3-kinase/Akt suppresses Wnt signaling, and thus controls stem cell proliferation[43].
Ephrin ligands
The interplay of ephrin receptors and ephrin ligands (Eph) regulates cell migration and boundary formation during development and tumorigenesis[45]. EphB2 and B3 were shown to be strongly expressed at the bottom of the crypts, even at cell positions 4-6, i.e., the putative intestinal stem cell location[46]. Moreover, gene expression analysis of laser microdissected crypt epithelial cells identified EphA6 as a potential stem cell marker[47].
HOPX
HOPX, an unusual homeodomain protein, was originally described as a key regulator of cardiac development[48]. In the intestine, LRCs in the +4 position can be marked by HOPX[12]. HOPX-expressing cells give rise to columnar base cells and all mature intestinal epithelial lineages. These cells can convert to Lgr5+ population maintaining clonogenic growth[12]. These findings support the presence of a bidirectional lineage relationship between active and quiescent stem cells in their niches.
Leucine-rich repeats and immunoglobulin-like domains protein 1
Leucine-rich repeats and immunoglobulin-like domains protein 1 (Lrig1), a negative-feedback regulator of the epidermal growth factor receptor family, was proposed to maintain epidermal stem cells in a quiescent, nondividing stage[49]. In situ hybridisation has revealed that Lrig1 is highly expressed in the stem cell niche of the small intestine and colon, and it regulates proliferation within the niche by inhibiting EGFR-signaling[50].
Interestingly, a recent report has demonstrated that several quiescent stem cell markers (i.e., Bmi1, TERT, Hopx or Lrig1) are highly expressed by the Lgr5 population[21]. The previously listed putative biomarkers may provide useful cell surface/cytoplasmic/nuclear markers to study the intestinal stem cells, but remain to be carefully examined and functionally validated. On the whole, the exact nature of quiescent stem cells is still questionable and needs to be clarified, since so far candidate markers rather than the LRCs themselves have been investigated.
INJURY-ASSOCIATED REACQUIRING OF STEMNESS
It is widely accepted that adult intestinal stem cells are able to acquire different progeny fates. However, several recent studies have demonstrated that committed epithelial cells could have the capacity to reverse their destinies[15,51]. Furthermore, the continuous debate regarding lineage and hierarchy in the intestinal epithelium is still existing. The possibility to reacquire stem cell function and the argument for functional plasticity have been strengthened by the recent results of Buczacki et al[15].
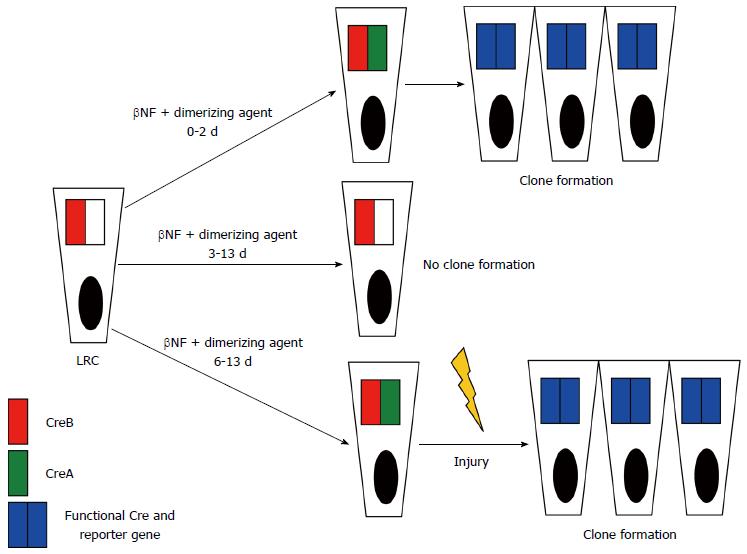
Instead of focusing on different putative stem cell markers, for directly studying quiescence the authors[15] identified cells retaining nuclear-localized fluorescent histone 2B-yellow fluorescent protein (H2B-YFP) fusion protein during a chase period following a pulse of induced expression. After the successful identification of YFP-LRCs, their transcriptional profiling proved that most of them belong to a subpopulation of Lgr5-expressing cells. Next, it has been demonstrated that YFP-LRCs appear to exist mainly as a Paneth cell precursor population, however, they are also capable of enteroendocrine differentiation. During the most fascinating and unique step of the study only LRCs in the intestinal epithelium were marked by a smart lineage tracing strategy. They fused a fragment of Cre recombinase (CreA) to H2B under the control of a β-naphthoflavone (βNF)-inducible promoter expressed by intestinal epithelial cells. After a βNF pulse-chase analysis only LRCs retained H2B-CreA. Administration of an intravenous dimerization agent reunited the H2B-CreA fragment with its ubiquitously expressed counterpart CreB triggering lineage tracing in LRCs via recombination. By varying the interval between βNF induction and Cre dimerization further combined with intestinal injury the authors found that during homeostasis, LRCs were Lgr5+ and destined to become Paneth and neuroendocrine cells. After epithelial injury, however, they demonstrated that LRCs are capable of clonogenic growth by recall to the self-renewing pool of stem cells (Figure 1). In a mammalian system this is the first model of in vivo lineage tracing based on label retention.
Figure 1 Schematic illustration of conditions for clone formation in the commented study.
Dimerizing agent is competent for clone formation from 0 to 2 d post-βNF-induction (top line) but not between 3 and 13 d (middle line). Between 6-13 d both dimerizing agent and injury (hydroxyurea) are required for clone formation (bottom line). LRC: Label-retaining cell; βNF: β-naphthoflavone; Cre: Recombinase.
The results of Buczacki et al[15] seem to resolve the supposed paradox that quiescence markers are expressed within a population of Lgr5+ cells thought to be rapidly cycling ones[21]. According to their results approx. 20% of Lgr5+ cells are largely quiescent and continue to express Lgr5 before maturation to Paneth cell.
On the other hand, it has been recently shown that suppression of apoptosis (even caused by chemotherapeutic agents) may decrease the number of lineage tracing clones from Lgr5+ cells, whereas lineage tracing from other cells with stem characteristics (e.g., Bmi1-expression) may be increased[52]. Based on these findings one can speculate that in a stem cell population apoptosis favors the proliferation of another stem cell population. Furthermore, environmental or genetic factors possibly can differentially influence progeny production by a given stem cell pool.
The debate about the origin, identity, and location of crypt epithelial stem cells is still continued by this new study of Buczacki et al[15]. Their findings highlight the hierarchical and spatial organization of intestinal epithelial homeostasis and the important plasticity of progenitors during tissue regeneration. The cellular plasticity and lineage reversibility may represent an adaptive mechanism after injuries, like intestinal inflammation for the self-preservation of the epithelial layer. Furthermore, in colorectal cancer determination the extent of quiescent cells retaining a maintained clonogenic potential may provide further insight into the etiology of tumorous colonic disorders and develop the basis of novel, efficient anti-cancer therapies.