Published online Feb 14, 2015. doi: 10.3748/wjg.v21.i6.1956
Peer-review started: June 12, 2014
First decision: August 6, 2014
Revised: October 31, 2014
Accepted: January 8, 2015
Article in press: January 8, 2015
Published online: February 14, 2015
Processing time: 244 Days and 22.3 Hours
AIM: To review the current literature for the specific clinical characteristics of inflammatory bowel disease (IBD) associated with primary sclerosing cholangitis (PSC).
METHODS: A systematical review for clinical characteristics of IBD in PSC was performed by conducting a broad search for “primary sclerosing cholangitis” in Pubmed. “Clinical characteristics” were specified into five predefined subthemes: epidemiology of IBD in PSC, characteristics of IBD in PSC (i.e., location, disease behavior), risk of colorectal cancer development, IBD recurrence and de novo disease after liver transplantation for PSC, and safety and complications after proctocolectomy with ileal pouch-anal anastomosis. Papers were selected for inclusion based on their relevance to the subthemes, and were reviewed by two independent reviewers. Only full papers relevant to PSC-IBD were included. Additionally the references of recent reviews for PSC (< 5 years old) were scrutinized for relevant articles.
RESULTS: Initial literature search for PSC yielded 4704 results. After careful review 65 papers, comprising a total of 11406 PSC-IBD patients, were selected and divided according to subtheme. Four manuscripts overlapped and were included in two subthemes. Prevalence of IBD in PSC shows a large variance, ranging from 46.5% to 98.7% with ulcerative colitis (UC) being the most common type (> 75%). The highest IBD rates in PSC are found in papers reviewing both endoscopic and histological data for IBD diagnosis. Although IBD in PSC is found to be a quiescent disease, pancolitis occurs often, with rates varying from 35% to 95%. Both backwash ileitis and rectal sparing are observed infrequently. The development of dysplasia or colorectal carcinoma is increased in PSC-IBD; the cumulative 10 years risk varying between 0% and 11%. Exacerbation of IBD is common after liver transplantation for PSC and de novo disease is seen in 1.3% to 31.3% of PSC-IBD patients. The risk for development of pouchitis in PSC-IBD is found to be significant, affecting 13.8% to 90% of the patients after proctocolectomy with ileo anal-pouch anastomosis.
CONCLUSION: IBD in primary sclerosing cholangitis represents a distinct phenotype that differs from UC and Crohn’s disease and therefore requires specialized management.
Core tip: Inflammatory bowel disease (IBD) in primary sclerosing cholangitis (PSC) is observed to have different characteristics in comparison with conventional IBD without PSC. Based on these differences a distinct phenotype of IBD is suspected. Existing literature was reviewed for clinical characteristics of IBD in PSC yielding 65 studies consisting of 11406 PSC-IBD patients. A large variation for reported characteristics was found, which seem to be related to case ascertainment. This emphasizes the importance of full colonoscopy and biopsies to accurately diagnose IBD in PSC. Overall however, IBD in PSC shows large differences in comparison with conventional IBD, therefore representing a distinct phenotype.
- Citation: de Vries AB, Janse M, Blokzijl H, Weersma RK. Distinctive inflammatory bowel disease phenotype in primary sclerosing cholangitis. World J Gastroenterol 2015; 21(6): 1956-1971
- URL: https://www.wjgnet.com/1007-9327/full/v21/i6/1956.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i6.1956
Primary sclerosing cholangitis (PSC) is a chronic cholestatic liver disease characterized by inflammation and fibrosis of both the intrahepatic and extrahepatic bile ducts[1]. There is no curative therapy available and in most cases, PSC leads to liver cirrhosis and liver failure ultimately requiring liver transplantation[2,3]. In the United States and Western Europe, the reported incidence of PSC is 0.9 and 1.3 per 100.000/year, the prevalence is 8.5 and 13.6 per 100000[4,5]. There is a strong relationship between PSC and inflammatory bowel disease (IBD). Approximately 50% to 80% of the patients with PSC are also diagnosed with IBD, finding ulcerative colitis (UC) in approximately 80% of these cases and Crohn’s disease (CD) or unclassified IBD (IBD-U) in the remaining 20%[6]. In patients with IBD, PSC is found much less common. Approximately 2.4% to 7.5% of UC patients and 3.4% of CD patients are diagnosed with PSC[7]. With the established relationship between PSC and IBD[1,8], several studies aimed to characterize the clinical course and features of concomitant IBD and its differences from conventional UC and CD[9-12]. These studies showed increasing evidence that PSC-IBD may represent a distinctive phenotype in addition to the established and defined phenotypes UC and CD[11]. The IBD in PSC is frequently reported as a pancolitis, relatively often with rectal sparing. Although colitis in PSC tends to follow a quiescent course, patients with PSC-IBD are found to have a relatively high risk of developing colorectal carcinoma compared to IBD patients without PSC[13]. However, while evidence for distinct PSC-IBD phenotype is increasing, its reported characteristics vary. In this systematic review of the literature we therefore aimed to describe the specific characteristics of the PSC-IBD phenotype based on the most recent literature. We reviewed prevalence of IBD in PSC, its phenotypic characteristics and the risk of development of colorectal malignancy. In addition we looked at IBD associated with PSC in relation to its clinical course or de novo presentation after orthotopic liver transplantation (OLT) and we reviewed the safety of proctocolectomy and incidence of pouchitis reported for PSC-IDB. Finally, we discussed the shared and non-shared genetic factors to further highlight the distinct nature of this phenotype. Insight into how PSC-IBD differs from general IBD, will help clinicians in the field of IBD in their approach for this specific group of patients.
We first defined relevant subthemes related to the subject of IBD in PSC based on clinical observations and recent literature.
Epidemiology of IBD in PSC: Reported incidence, methods of case ascertainment and geographical variation.
Phenotypic features of IBD in PSC: Disease localization, activity, and its association with backwash ileitis and rectal sparing.
Colorectal cancer risk in PSC-IBD: The incidence and risk in comparison with conventional IBD.
PSC-IBD in relation to liver transplantation: Disease behavior after OLT and the occurrence of de novo IBD.
Ileal pouch anal anastomosis in PSC-IBD: Safety of the procedure and pouch complications.
A broad literature search using the keyword “primary sclerosing cholangitis” was performed in PubMed (http://www.ncbi.nlm.nih.gov/pubmed), an online database comprising more than 23 million citations for biomedical literature from MEDLINE, life sciences journals, and online books. Relevant papers addressing the defined subthemes were selected based on title and abstract. Papers not found relevant by both reviewers (BdV and MJ) were excluded. When in disagreement, papers were discussed and included or excluded based on mutual agreement. We only included full papers relevant to PSC-IBD. Additionally the references of recent review papers (< 5 years) concerning PSC-IBD were scrutinized for relevant articles. Full papers of the selected abstracts were reviewed by BdV. References in manuscripts specifically assessing the IBD phenotype in PSC were hand-searched to identify other relevant papers.
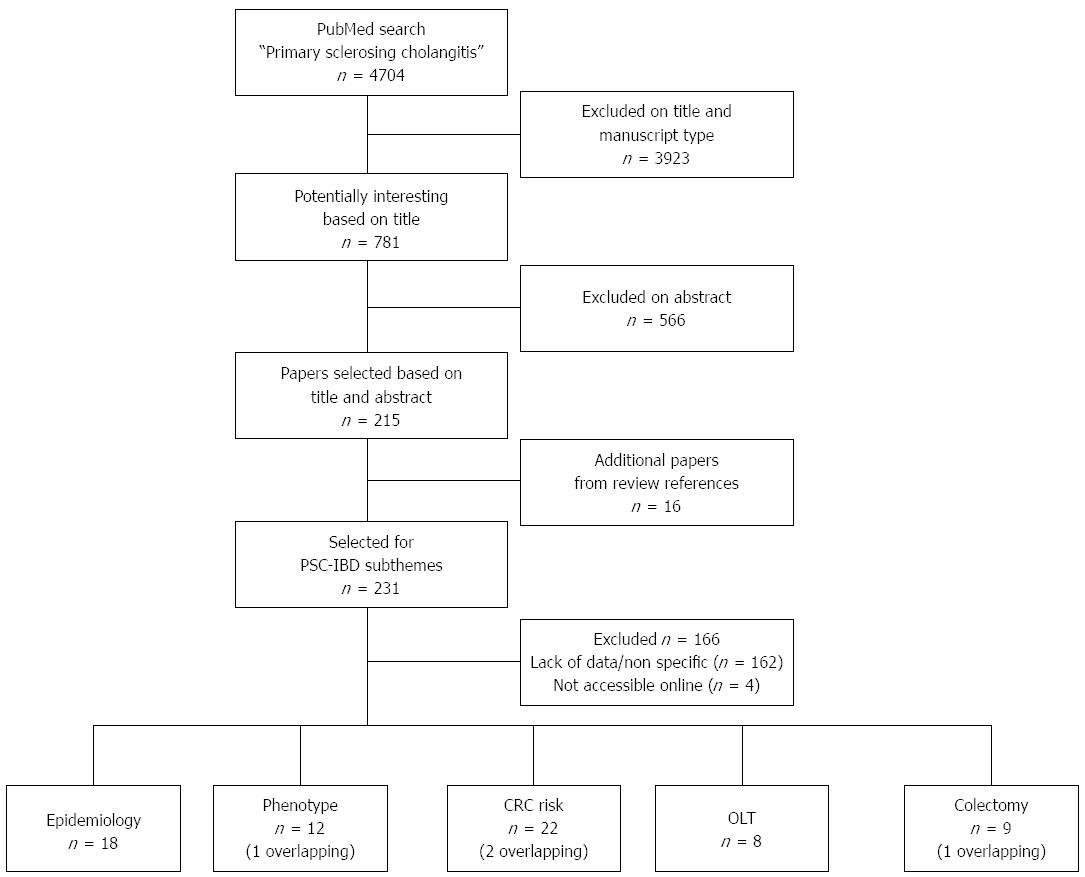
The literature search on “PSC” yielded 4704 results. After reviewing titles and manuscript type (excluding reviews), a total of 781 papers were selected for assessment of their abstracts. After careful review, 215 papers were selected and divided into a specific subtheme. The scrutinizing of references from reviews and PSC-IBD manuscripts yielded another 16 papers. After reading the full text, 65 papers, comprising a total of 11406 patients with PSC-IBD, were selected for the review (Figure 1). Criteria for study selection were study size, relevance to the subthemes, and specification of data (i.e., number of patients with backwash ileitis, localization of IBD). Four papers[11,14-16] overlapped, and were included in two subthemes.
Literature search and evaluation of reviews yielded 18 papers with a total of 6589 included PSC-IBD patients. Data concerning epidemiology of PSC and associated IBD are summarized in Table 1.
| Ref. | Country | Period | Incidence1 | PSC | Male Gender | PSC-IBD | PSC-UC | PSC-CD | PSC-IBD-U | Diagnosis IBD |
| Rabinovitz et al[100], 1990 | United States | NA | NA | 66 | NA | 47 (71.2) | 39 (83.0) | 8 (17.0) | 0 | IBD23 |
| Farrant et al[19], 1991 | United Kingdom | 1972-1989 | NA | 126 | 78 (61.9) | 85 (67.5) | 83 (97.6) | 2 (2.4) | NA | IBD2 |
| Schrumpf et al[18], 1994 | Norway | 1975-1989 | NA | 77 | 51 (66.2) | 76 (98.7) | 58 (76.3) | 11 (14.5) | 7 (9.2) | IBD23 |
| Escorsell et al[17], 1994 | Spain | 1984-1988 | 0.07 | 43 | 26 (60.5) | 20 (46.5) | 19 (95.0) | 1 (5.0) | NA | IBD4 |
| Broomé et al[2], 1996 | Sweden | ? - 1992 | NA | 305 | 195 (63.9) | 249 (81.6) | 220 (88.4) | 20 (8.0) | 9 (3.6) | IBD2 |
| Boberg et al[4], 1998 | Norway | 1986-1995 | 1.31 | 17 | 12 (70.6) | 12 (70.6) | 9 (75.0) | 2 (16.7) | 1 (8.3) | IBD4 |
| Ponsioen et al[20], 2002 | The Netherlands | 1979-1999 | NA | 174 | 105 (60.3) | 114 (65.5) | 83 (72.8) | 28 (24.6) | 3 (2.6) | IBD4 |
| Bambha et al[5], 2003 | United States | 1976-2000 | 0.90 | 22 | 15 (68.3) | 16 (72.7) | 12 (75.0) | 3 (18.8) | 1 (5.0) | IBD2 |
| Kingham et al[101], 2004 | United Kingdom | 1984-2003 | 0.91 | 53 | 33 (62.3) | 33 (62.3) | 30 (90.9) | 3 (19.1) | 0 | IBD4 |
| Bergquist et al[102], 2005 | Sweden | 1984-1999 | NA | 145 | 100 (69.0) | 126 (86.9) | 107 (84.9) | 19 (15.1) | 0 | IBD23 |
| Tischendorf et al[103], 2007 | Germany | 1978-2004 | NA | 273 | 195 (71.4) | 172 (63.0) | 141 (82.0) | 29 (16.9) | 2 (1.2) | IBD23 |
| Bergquist et al[104], 2007 | Sweden | 1984-2004 | NA | 246 | 165 (67.1) | 195 (79.3) | 166 (85.1) | 21 (10.8) | 8 (4.1) | IBD23 |
| Kaplan et al[14], 2007 | Canada | 2000-2005 | 0.92 | 49 | 27 (55.1) | 36 (73.5) | 17 (47.2) | 19 (52.8) | 0 | IBD23 |
| Card et al[3], 2008 | United Kingdom | 1987-2002 | 0.41 | 223 | 141 (63.2) | 108 (48.4) | 67 (62.0) | 13 (12.0) | 28 (25.9) | IBD4 |
| Lindkvist et al[105], 2010 | Sweden | 1992-2005 | 1.22 | 199 | 142 (71.4) | 152 (76.4) | 129 (84.9) | 17 (11.2) | 5 (3.3) | IBD4 |
| Bowlus et al[106], 2010 | United States | 1995-2009 | NA | 6767 | 4475 (66.1) | 4637 (68.5) | 3067 (66.1) | 1090 (23.5) | 480 (10.4) | IBD4 |
| Toy et al[107], 2011 | United States | 2000-2006 | 0.41 | 169 | 101 (59.8) | 109 (64.5) | 95 (87.2) | 13 (11.9) | 1 (0.9) | IBD4 |
| Boonstra et al[22], 2013 | The Netherlands | 2000-2007 | 0.50 | 590 | 375 (63.6) | 402 (68.1) | 308 (76.6) | 78 (19.4) | 16 (4.0) | IBD23 |
PSC is a rare disease with a reported incidence between 0.07 and 1.31 per 100000[4,17]. The prevalence of associated IBD ranged from 46.5%[17] to 98.7%[18], with UC being the most common type (> 75%) in PSC-IBD (Table 1). CD in PSC-IBD is found in approximately 16% of PSC patients (range: 2.4%[19]-24.8%[20]). IBD-U is the least common IBD-type in PSC, seldom representing more than 5%. The actual percentages are expected to be even lower, as studies on IBD-U with and without associated PSC show that part of the initial IBD-U diagnosis is later re-classified as either UC or CD[15,21].
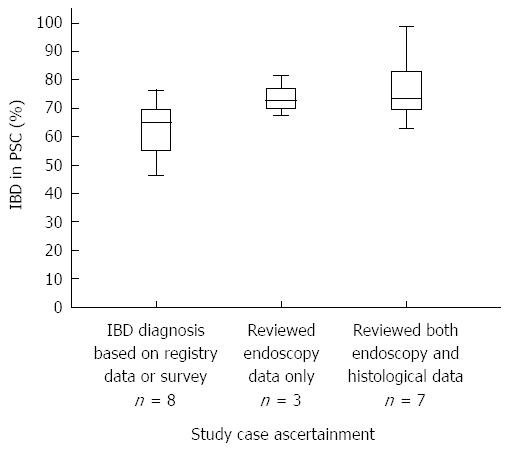
Varying IBD rates found in PSC: A recent large Dutch population-based study showed lower incidence rates for both PSC and PSC-IBD when compared with most other studies[22]. This emphasizes the importance of large multi-center studies with proper case ascertainment in studying rare diseases. In this review, all but two of the selected studies concerning epidemiology reviewed cholangiography or MRCP imaging to confirm the PSC diagnosis. Endoscopic imaging was reviewed in ten (55.5%) studies, of which seven also reviewed histological data to ascertain the method of IBD diagnosis in the included patients (Table 1). In the remaining eight studies, the presence of IBD in PSC was established or excluded based on registry data, physician surveys, or notes in medical file without reviewing original endoscopy or histology. Combined study population of these groups consisted of 1446 (endoscopy and histology), 453 (endoscopy only) and 7645 (no review of original endoscopy or histology) PSC patients respectively. The studies that used both endoscopic and histological confirmation for IBD diagnosis seem to show a higher median percentage of IBD in PSC when compared with studies that only used endoscopy or did not review diagnostic workup for IBD (Figure 2).
Geographic variation IBD - PSC: Geographic variation in the incidence of PSC and associated IBD has been reported, with highest rates found in Western Europe and North America (Table 1) and lower rates in Asia[23-25]. Several studies from Asia reported an incidence of IBD in PSC ranging between 20% and 34%[24,26-28].
Key points: Approximately 70% of patients with PSC have associated IBD, predominantly UC. The highest rates of IBD in PSC were observed in papers using strict case ascertainment criteria. Large geographic differences in the incidence of PSC and associated IBD between east and west are reported.
Frequently documented characteristics of IBD in PSC include a mild or quiescent disease course and a high rate of pancolitis[9,29,30]. The association with backwash ileitis and rectal sparing in PSC-IBD is also suggested, but less consistently. The present review included twelve studies (1217 patients with PSC-IBD) describing features of IBD in PSC, which are summarized in Table 2. In seven (58.3%) studies a comparison with conventional IBD was made.
| Ref. | IBD (n) | PSC-IBD (n) | Proctitis | Leftsided | Pancolitis | Backwash | Rectal Sparing | Diagnosis IBD | |||||
| IBD | PSC-IBD | IBD | PSC-IBD | IBD | PSC-IBD | IBD | PSC-IBD | IBD | PSC-IBD | ||||
| Olsson et al[9], 1991 | 1445 | 55 | 552 (38.2) | 3 (5.5) | NA | NA | 893 (61.8) | 52 (94.5) | NA | NA | NA | NA | IBD12 |
| Loftus et al[11], 2005 | 142 | 71 | NA | NA | NA | NA | 76 (53.5) | 60 (84.5) | 10 (7.0) | 20 (28.2) | 8 (5.6) | 34 (47.9) | IBD12 |
| Kaplan et al[14], 2007 | 0 | 36 | NA | NA | NA | NA | NA | 17 (47.2) | NA | 4 (11.1) | NA | 2 (5.6) | IBD12 |
| Sokol et al[15], 2008 | 150 | 75 | 138 (92.0) | 68 (90.7) | 130 (86.7) | 68 (90.7) | 91 (60.7) | 49 (65.3) | 36 (24.0) | 14 (18.7) | 20 (13.3) | 15 (20.0) | IBD12 |
| Joo et al[31], 2009 | 40 | 40 | 0 | 0 | 14 (35.0) | 3 (7.5) | 18 (45.0) | 34 (85.0) | 3 (7.5) | 4 (10.0) | 10 (25.0) | 11 (27.5) | IBD12 |
| Sano et al[32], 2010 | 60 | 20 | 18 (30.0) | 1 (5.0) | 19 (31.7) | 1 (5.0) | 21 (35.0) | 7 (35.0) | NA | NA | NA | NA | IBD12 |
| Ye et al[25], 2011 | 63 | 21 | NA | NA | NA | NA | 35 (55.6) | 20 (95.2) | 2 (3.2) | 9 (42.9) | 1 (1.6) | 8 (38.1) | IBD12 |
| Jørgensen et al[33], 2012 | 0 | 110 | NA | NA | NA | 3 (2.7) | NA | 60 (54.5) | NA | 17 (15.5) | NA | 73 (66.4) | IBD12 |
| O'toole et al[35], 2012 | 2649 | 103 | 209 (7.9) | 1 (1.0) | 649 (24.5) | 23 (22.3) | 663 (25.0) | 56 (54.4) | NA | NA | NA | NA | IBD12 |
| Boonstra et al[12], 2012 | 0 | 380 | NA | 9 (2.4) | NA | 34 (8.9) | NA | 219 (57.6) | NA | NA | NA | NA | IBD12 |
| Boonstra et al[12], 20123 | 80 | 80 | 4 (5.0) | 2 (2.5) | 16 (20.0) | 2 (2.5) | 35 (43.8) | 52 (65.0) | 2 (2.5) | 4 (5.0) | 1 (1.3) | 8 (10.0) | IBD12 |
| Schaeffer et al[34], 2013 | 0 | 97 | NA | 0 | NA | 17 (17.5) | NA | 42 (43.3) | NA | NA | NA | NA | IBD12 |
| Sinakos et al[30], 2013 | 0 | 129 | NA | NA | NA | 16 (12.4) | NA | 76 (58.9) | NA | 15 (11.6) | NA | 31 (24.0) | IBD12 |
| Mean | 28.8% | 13.4% | 39.6% | 18.8% | 47.5% | 64.7% | 12.3% | 16.7% | 9.9% | 30.9% | |||
Disease activity: Out of the twelve included studies, eight (66.6%) characterized the clinical course of IBD in PSC. All eight studies classified the IBD’s overall activity as mild[9,11,15,30-34]. Every included study reviewed endoscopic and histological data to confirm IBD diagnosis (Table 2). In addition, four studies also assessed histological severity of inflammation in different segments of the colon, none finding severe inflammation in PSC-IBD[31-34]. The majority of these studies found that prevalence for inflammation was highest in the right-sided colon and lowest toward the distal colon. Both Joo et al[31] and Sano et al[32] reported this was significantly different (0.019 and 0.034 respectively) in matched IBD-controls, with histologically graded inflammation being highest in the proximal colon and lowest in the rectum for PSC-IBD. In accordance with these results, two studies[12,33] reported a significant predominance of right-sided inflammation in PSC-IBD observed during endoscopy.
Disease localization in PSC-IBD: Localization and extent of IBD were determined by endoscopy in the majority of studies (Table 2). Involvement of the whole colon is more common in PSC-IBD (35%[32]-95%[25], mean 64.7%) than in IBD controls (25%[35]-62%[9], mean 47.5%). Several authors have found these differences to be significant[11,12,25]. The reported rates of left sided colitis in PSC-IBD varies between studies (2.5%[12]-90.7%[15], mean 18.8%), but are lower than in IBD without concomitant PSC (20%[12]-86.7%[15], mean 39.6%). The overall reported occurrence of proctitis-only is low for PSC-IBD in most studies (1%[35]-5.5%[9], Table 2) and less common in comparison with IBD controls (5%[12]-38%[9]).
Backwash ileitis: Backwash ileitis (BWI) is an inflammation of the ileum due to diminished ileocecal valve function in severe UC, allowing for retrograde flow of colonic content and inflammation of the ileum[36]. The reported rates of BWI found for PSC-UC range between 5.0%-42.9% (mean 16.7%). In UC without PSC involvement of the ileum is infrequent, with reported rates ranging from 2.5%[12] to 24%[15] (mean 12.3%, Table 2).
Rectal sparing: Rectal sparing (RS) is described as rectal mucosa in UC or CD which is completely or partially spared from active or chronic inflammation in comparison to the more proximal colon[37]. The frequency of RS in PSC-IBD is specified in eight studies (Table 2). The reported incidence shows a large variation ranging from 5.6%[14] and 66.4%[33] (mean 30.9%) in PSC-IBD, compared with 1.6%[25] and 25%[31] (mean 9.9%) in IBD without PSC.
PSC-CD: The localization of PSC-CD is specified in five studies[11,12,14], two of which focused on the PSC-CD phenotype[38,39]. Colonic involvement in PSC-CD is reported most often (36.8%[14]-82.1%[38] followed by involvement of both ileum and colon (21.8%[12]-57.9%[14]). Isolated ileal involvement is rare in PSC-CD (2%[39]-5%[12]). The rate of ileitis is similar or lower in PSC-CD compared with CD controls[12,38,39]. Rectal sparing is not significantly increased in PSC-CD[12,38] and isolated upper gastrointestinal CD is reported in only two patients[15,39]. The disease activity in PSC-CD is characterized as similar or less active then in CD controls, with lower rates of stricturing and penetrating disease seen during endoscopy[12,38,39].
Key points: IBD associated with PSC is characterized by a quiescent course with a predominance for mild right-sided inflammation and a proximal- or pancolitis. Although less common than pancolitis, both backwash ileitis and rectal sparing are more often found in PSC-UC compared with UC. In addition, strictures and penetrating disease were found to be less common in PSC-CD than in CD without PSC.
The increased risk of development of colorectal cancer (CRC) in IBD has been widely recognized for several decades[40-44]. Both disease extent and duration of IBD have been identified as factors associated with this increased cancer risk[41,43,44]. An additional risk for development of dysplasia and CRC in PSC-IBD was observed by several authors[45-48]. A total of twenty-two studies assessing CRC risk in PSC-IBD were reviewed, comprising a total of 2936 patients with PSC-IBD (Table 3).
| Ref. | IBD (n) | PSC-IBD (n) | Country | Dysplasia | Risk/incidence | Proximal colon1 | Age dysplasia diagnosis (yr) | IBD duration (yr) | Diagnosis IBD | |||||
| IBD | PSC-IBD | IBD | PSC-IBD | IBD | PSC-IBD | IBD | PSC-IBD | IBD | PSC-IBD | |||||
| Broomé et al[45], 1995 | 80 | 40 | Sweden | 7 (8.8) | 11 (27.5) | CR = 10 yr: 2% | CR = 10 yr: 9% | NA | NA | NA | NA | 26.02 | 18.02 | IBD34 |
| Gurbuz et al[49], 1995 | 0 | 35 | United States | NA | 13 (37.1) | NA | CR = 10 yr: 0% | NA | NA | NA | 51.42 | NA | 12.22 | IBD34 |
| Brentnall et al[56], 1996 | 25 | 20 | United States | 4 (16) | 9 (45) | NA | OR = 4.9 | NA | NA | NA | NA | NA | 20.02 | IBD34 |
| Loftus et al[29], 1996 | 0 | 143 | United States | NA | 27 (18.9) | NA | CR = 20 yr: 17% | NA | 42.9%5 | NA | 41.06 | NA | NA | IBD34 |
| Kornfeld et al[48], 1997 | 0 | 58 | Sweden | NA | 5 (8.6) | NA | NA | NA | NA | NA | NA | NA | NA | IBD7 |
| Leidenius et al[50], 1997 | 45 | 45 | Finland | 4 (8.9) | 13 (28.9) | CR = 10 yr: 3% | CR = 10 yr: 11% | NA | NA | NA | NA | 17.52 | 11.06 | IBD34 |
| Marchesa et al[54], 19978 | 1185 | 27 | United States | 145 (12.2) | 18 (66.7) | NA | RR = 10.4 | 40.8%5 | 100.0%5 | NA | NA | NA | NA | IBD4 |
| Shetty et al[84], 1999 | 196 | 132 | United States | 11 (5.6) | 33 (25) | RR = 1 | RR = 3.15 | 20.0%5 | 76.5%5 | NA | NA | NA | NA | IBD34 |
| Lindberg et al[46], 2001 | 103 | 19 | Sweden | 31 (30.1) | 12 (63.2) | CR = 20 yr: 16% | CR = 20 yr: 32% | 32.3% | 69.2% | NA | NA | NA | NA | IBD34 |
| Bergquist et al[108], 2002 | 0 | 477 | Sweden | NA | 35 (7.3) | NA | SIR = 10.3 | NA | NA | NA | NA | NA | NA | IBD34 |
| Loftus et al[11], 2005 | 142 | 71 | United States | 15 (10.6) | 18 (25.4) | CP = 10 yr: 20% | CP = 10 yr: 53% | 50%5 | 28.6%5 | NA | NA | 12.16 | 12.76 | IBD34 |
| Kaplan et al[109], 2007 | 0 | 45 | Canada | NA | 5 (11.1) | NA | IR = 3.1 | NA | NA | NA | NA | NA | NA | IBD34 |
| Lepistö et al[16], 2008 | 389 | 52 | Finland | 63 (16.2) | 17 (32.7) | CR = 20 yr: 0.39 | CR = 20 yr: 0.43 | NA | NA | NA | NA | NA | NA | IBD4 |
| Sokol et al[15], 2008 | 150 | 75 | France | 2 (1.3) | 10 (13.3) | CR = 25 yr: 1.5% | CR = 25 yr: 25.6% | 0.0%5 | 100.0%5 | NA | NA | 44.42 | 17.42 | IBD34 |
| Terg et al[51], 2008 | 64 | 39 | Argentina | 2 (3.1) | 7 (17.9) | CR = 10 yr: 2.0% | CR = 10 yr: 11% | NA | NA | NA | NA | NA | NA | IBD34 |
| Claessen et al[52], 2009 | 0 | 126 | The Netherlands | NA | 35 (27.8) | NA | CR = 10 yr: 9% | NA | 63.0%5 | NA | NA | NA | 12.66 | IBD34 |
| Lindström et al[38], 2011 | 46 | 28 | Sweden | 3 (6.5) | 9 (32.1) | NA | OR = 6.78 | 0.0% | 66.0% | NA | NA | 10.06 | 12.06 | IBD34 |
| Braden et al[58], 2012 | 216 | 166 | United Kingdom | 14 (6.5) | 14 (8.4) | CIR = 2.9% | CIR = 7.5% | 30.0% | 35.7% | NA | NA | NA | NA | IBD34 |
| Imam et al[53], 2012 | 0 | 784 | United States | NA | 10 (1.3) | NA | I = 0.4/yr | NA | 60.0% | NA | 37.42 | NA | NA | IBD34 |
| Jess et al[55], 2012 | 47374 | NA | Denmark | 329 (0.7) | 9 (?)9 | RR = 1.07 (UC) | RR = 9.13 | 38.0% | 100.0% | 71.66 | 64.06 | NA | 13.76 | IBD7 |
| de Valle et al[79], 2012 | 0 | 152 | Sweden | NA | 3 (2.0) | NA | SIR = 4.31 | NA | NA | NA | NA | NA | NA | IBD34 |
| Boonstra et al[22], 2013 | 722 | 402 | The Netherlands | 7 (1) | 19 (4.7) | SIR = 1.2 | SIR = 8.6 | NA | NA | 596 | 39.06 | 4.06 | 15.06 | IBD34 |
CRC incidence and location in PSC-IBD: The 10-year cumulative risk for the development of colonic dysplasia and CRC in PSC-IBD is reported in five studies[45,49-52] and varies between 0%[49] and 11%[50]. In these studies the data on dysplasia or CRC development for IBD without PSC is limited, showing a 10 year cumulative risk of approximately 2%[45,50,51]. The observed percentage of included PSC-IBD patients developing dysplasia and CRC shows large differences, ranging from 1.3%[53] to 66.7%[54]. For the IBD controls, these percentages vary from 0.7%[55] to 30.1%[46]. The mean time interval between diagnosis of IBD and development of CRC in PSC-IBD ranges between 12.2[49] and 20[56] years (Table 3). In comparison, in IBD without PSC the mean interval varies between 17.5[50] and 44.4[15] years. The median age at which dysplasia or CRC presented itself was specified in three studies on PSC-IBD and in one study on IBD, being lower in the former (Table 3). For IBD the development of CRC in the recto-sigmoid has been described more common in UC whereas CD shows a more equal distribution between right sided and left sided colonic malignancy[57]. Dysplasia or CRC in PSC-IBD frequently develops in the proximal colon with reported rates varying between 28.6%[11] and 100%[55]. For IBD a proximal development of CRC and dysplasia is found less common (0%[15]-50%[11], Table 3).
CRC in PSC-CD and PSC without IBD: Limited data is available on the risk of CRC development in PSC-CD patients as it is less prevalent, and often only represents a few cases within PSC-IBD cohorts. Furthermore, disease characteristics of IBD in PSC can make it difficult to distinguish between UC and CD, resulting in a change of diagnosis during follow-up. A study by Lindström et al[38], which compared 28 PSC-CD patients with 46 CD controls found an increased risk of CRC development in PSC-CD that was comparable to the risk in PSC-UC. In contrast, a study by Braden et al[58] found a lower risk in PSC-CD compared with PSC-UC by looking at CRC development in 166 PSC-IBD (35 PSC-CD) and 216 IBD controls (102 CD). Risk of CRC development in PSC patients without IBD was found to be low[22,29,48,52].
Key points: The development of dysplasia and CRC in PSC-IBD is increased and has predominance for right-sided localization in comparison to conventional IBD. Based on limited results, presentation of dysplasia or CRC in PSC-IBD at a younger age is suggested. It remains unclear whether the interval between IBD diagnosis and dysplasia or CRC development was shorter.
In PSC patients after orthotopic liver transplantation (OLT), IBD exacerbations and even development of de novo IBD have been reported by a number of studies, despite continuous use of immunosuppressive medication[59-62]. To review IBD disease activity and de novo presentation in PSC-IBD after OLT, eight studies comprising 519 patients with PSC-IBD were included. Papers concerning both autoimmune hepatitis and PSC were scrutinized for PSC-IBD data only. An overview of the selected papers is presented in Table 4.
| Ref. | n | Pre-OLT | Post-OLT | ||||||
| PSC only | PSC-IBD | Intact colon1 | De novo IBD | Exacerbation | Colectomy | Refractory IBD | Median follow-up (yr) | ||
| Dvorchik et al[65], 2002 | 192 | 0 | 192 | 169 (88.0) | NA | 22 (13.0) | 33 (19.5) | 22 (66.7) | 5.93 |
| Haagsma et al[59], 2003 | 48 | 25 (52.1) | 23 (47.9) | 23 (100.0) | 6 (24.0) | 9 (39.1) | 1 (4.3) | 0 | 7.2 |
| Verdonk et al[88], 2006 | 60 | 15 (25.0) | 45 (75.0) | 45 (100.0) | 3 (20.0) | NA | NA | NA | 6.1 |
| Cholongitas et al[62], 20072 | 56 | 18 (32.1) | 38 (67.9) | 33 (68.8) | 3 (16.6) | 17 (51.5) | 7 (16.7) | 3 (42.9) | 2.8 |
| Moncrief et al[61], 2010 | 59 | 16 (27.1) | 42 (71.2) | 32 (76.2) | 5 (31.3) | 13 (40.6) | 6 (18.8) | 4 (66.7) | 5.6 |
| Joshi et al[60], 2011 | 110 | 36 (32.7) | 74 (67.3) | 65 (87.8) | 6 (16.7) | 33 (50.8) | 7 (10.8) | 6 (85.7) | 6.53 |
| Navaneethan et al[63], 2012 | 77 | 0 | 77 | 58 (75.3) | NA | 5 (8.6) | 9 (15.5) | 3 (33.3) | 5.0 |
| Mosli et al[64], 2013 | 105 | 77 (73.3) | 28 (26.7) | 24 (85.7) | 1 (1.3) | 6 (25.0) | 2 (8.3) | 0 | 7.33 |
IBD Exacerbations and de novo disease after OLT for PSC: Exacerbation of IBD after OLT is estimated to occur in 8.6%[63] to 51.5%[62] of the patients, whereas de novo IBD is seen less frequently, varying from 1.3%[64] to 31.3%[61] in patients transplanted for PSC. Long term outcome for IBD in PSC is reported in seven studies, with colectomy rates after OLT varying from 4.3%[59] to 19.5%[65] (Table 4). Treatment-refractory IBD as an indication for colectomy ranged from 0%[59] to 85.7%[60].
Key points: Approximately 32.6% of patients with PSC-IBD experience an exacerbation of IBD after their liver transplantation. In addition, approximately 18.3% of patients develop de novo IBD after liver transplantation for PSC.
Restorative proctocolectomy with ileal pouch anal anastomosis (IPAA) is performed for colonic dysplasia or treatment-refractory disease in UC with and without PSC. A long term complication after IPAA is the development of an acute or chronic inflammation of the ileal pouch[66]. Several reports have associated PSC-UC with a more frequent development of pouchitis in comparison with UC without liver involvement[67-70]. We reviewed nine studies that researched pouchitis in PSC-IBD after proctocolectomy, comprising a total of 379 PSC-IBD patients with IPAA (Table 5).
| Ref. | Country | IBD (n) | PSC-IBD (n) | IPAA | Pouchitis | Chronic pouchitis | Pouch failure | Diagnosis | ||||
| IBD | PSC-IBD | IBD | PSC-IBD | IBD | PSC-IBD | IBD | PSC-IBD | |||||
| Kartheuser et al[74], 1993 | United States | NA | 40 | NA | 40 | NA | 19 (47.5) | NA | NA | NA | NA | D1 |
| Penna et al[69], 1996 | United States | 1043 | 54 | 1043 | 54 | 336 (32.2) | 34 (63.0) | ? (15.0)2 | ? (60.0)2 | NA | NA | D3 |
| Aitola et al[68], 1998 | Finland | 63 | 10 | 63 | 10 | 19 (30.2) | 9 (90.0) | 7 (11.1) | 7 (70.0) | NA | NA | D3 |
| Gorgun et al[71], 2005 | United States | 260 | 65 | 260 | 65 | 31 (11.9) | 9 (13.8) | 31 (11.9) | 9 (13.8) | 19 (7.3) | 1 (1.5) | D345 |
| Abdelrazeq et al[67], 2007 | United Kingdom | 182 | 16 | 182 | 16 | 53 (29.1) | 11 (68.8) | 18 (9.9) | 9 (56.3) | NA | NA | D345 |
| Lepistö et al[16], 2008 | Finland | 389 | 52 | 389 | 52 | 101 (26.0) | 25 (48.1) | NA | NA | 13 (3.3) | 2 (3.8) | D1 |
| Wasmuth et al[72], 2010 | Norway | 178 | 11 | 178 | 11 | 94 (52.8) | 8 (72.0) | 17 (9.6) | 4 (36.4) | 20 (11.2) | 1 (9.1) | D34 |
| Mathis et al[73], 2011 | United States | NA | 100 | NA | 100 | NA | 64 (64.0) | NA | 16 (16.0) | NA | 3 (3.0) | D345 |
| Block et al[70], 2013 | Norway | 113 | 48 | 62 | 31 | 20 (32.3) | 27 (87.1) | 8 (12.9) | 20 (64.5) | 4 (6.5) | 5 (16.1) | D345 |
Pouchitis incidence: The reported overall incidence of pouchitis (ranging from an isolated episode to chronic pouchitis) for PSC-UC ranges from 13.8%[71] to 90%[68] compared with 11.9%[71] to 52.8%[72] in UC without PSC. In addition, the number of patients with chronic pouchitis in PSC-IBD varies from 13.8%[71] to 70%[68]. Chronic pouchitis is found in 9.6%[72] to 15%[69] of the IBD patients after proctocolectomy and IPAA. Apart from the incidence of pouchitis, pouch failure during follow up was assessed in five studies, which all showed similar results (Table 5). Pouch failure was seen in 1.5%[71] to 16.1%[70] of the PSC-IBD patients and in 3.3%[16] to 11.2%[72] of the IBD patients after IPAA. Safety of proctocolectomy with IPAA in PSC-IBD was discussed in three studies, with the majority describing it as a safe procedure[73,74].
Key points: Pouchitis after IPAA is more frequently found in PSC-IBD patients compared with IBD patients. Rate of pouch failure seems similar for IBD patients with and without PSC. Although data are limited, proctocolectomy with IPAA is deemed safe in PSC-IBD patients by the majority of studies.
In this review we have characterized the clinical features of IBD associated with PSC. In addition, we aimed to provide data to assist clinicians in the IBD field, to aid management for this specific group. PSC-IBD can be said to represent a distinct phenotype that differs from both UC and CD.
The prevalence for IBD found in PSC is high, being present in approximately 70% of the cases, with a large variation found between studies. The most common type is UC which represents > 75% of IBD found in PSC, followed by the less common CD and infrequently seen IBD-U. In contrast, a relative high prevalence of CD was found in a study by Kaplan et al[14] reporting 38.8% CD in the total of included patients with PSC. As possible explanations the authors suggested an increasing trend of awareness of PSC in IBD other than UC and the high prevalence of CD found (1.5 times that of UC) in the province of Alberta, Canada. Geographic differences could explain the large variation found in IBD prevalence in PSC. We found a high prevalence of IBD in PSC patients (> 60%) in Europe and North America, compared with lower rates of approximately 30% IBD in PSC patients in Asia. In most studies from Asia on PSC-IBD however, endoscopic and histological data were not specified for the IBD diagnosis, making case ascertainment unreliable. A more recent Japanese study[32] establishing IBD diagnosis in PSC based on both endoscopic and histological data, showed an IBD incidence of 68%, similar to the percentage found in studies from Europe and North America. Although these results were based on a small study population and need to be validated by future large multicenter studies, they do suggest that case ascertainment affects the prevalence of IBD found in PSC. To explore whether reported rates of IBD in PSC are influenced by method of case ascertainment, a comparison between included epidemiological studies was made. Strikingly, the highest rates of IBD in PSC were found in studies using the most stringent criteria, reviewing both endoscopic and histology data, to exclude or establish the presence of IBD in PSC. In addition, the lowest prevalence of IBD in PSC was reported in studies using registry data, physician surveys or notes in the medical file without reviewing original endoscopy or histology. These results are in accordance with the observation that IBD in PSC can be present without clinical signs and with normal endoscopic appearance[33,75], resulting in underdiagnosis[45]. This observation stresses the importance of histological diagnosis in PSC-IBD. In contrast, one of the largest PSC population-based studies to date, shows a relatively low rate of IBD in PSC patients (68.1%), while reviewing both histology and endoscopy[22]. Our findings can therefore not fully explain the large variation found in IBD prevalence in PSC, but they do stress the importance of full endoscopy combined with random biopsies to determine the presence of IBD in PSC patients.
The present review found that IBD in PSC patients is characterized by a mild disease activity, seldom showing severe inflammation. The prevalence of colonic inflammation follows a proximal to distal distribution, with most activity found in the cecum and ascending colon. A pancolitis is seen in > 60% of IBD associated with PSC. Isolated distal colon involvement in PSC-IBD is seen in < 20%, with isolated proctitis being uncommon. IBD in PSC can therefore be characterized by pancolitis and or right-sided colon involvement. While the exact mechanism of this distribution is unknown, a possible contributing factor was proposed by Schaeffer et al[34], who found differences in localization based on initial disease presentation in PSC-IBD. PSC diagnosis followed by IBD development was more often associated with right sided colonic inflammation, while pancolitis was found more frequent in IBD developing before PSC diagnosis.
Although a relation between backwash ileitis (BWI) and PSC-UC has been documented, it is not undisputed. Some studies for example, reported high rates[11,25], which in turn could not be confirmed by other studies[12,31]. While more frequently found in PSC-UC compared with IBD without PSC, BWI seems to be an uncommon feature of PSC-UC as it is only found in approximately 16% of the patients. Comparison between rates observed in PSC-UC is complicated by a large variation found between studies, which can be explained by differences in BWI diagnosis based on definition. Some studies, for example, noted all histological changes of the ileum as BWI[11,25], while others specified between active inflammation and chronic inflammation and only used the former for the observed BWI rates[31]. Apart from differences in definition, the localization and degree of colonic inflammation have also been associated with prevalence of BWI. Haskell et al[36], reporting a 17% prevalence of BWI in UC (n = 200), noted the importance of correlating the presence of histological cecal colonic inflammation with ileitis findings in UC to distinguish these from ileal involvement in CD. In addition, several studies reported on the relation between the degree of inflammatory activity of the whole colon or cecum and the incidence of BWI[31,36,76]. In three (37.5%) of these studies[12,31,33], cecal inflammation was correlated with findings of BWI in the ileum. Jørgensen et al[33] found a significantly (P = 0.017) higher degree of cecal inflammation in patients with BWI. Interestingly, these three studies all reported BWI rates < 12% for UC in PSC, representing the lower limits of total rates found. While the high rates of right-sided and complete colonic involvement make an association between PSC-UC and BWI seem plausible, the overall percentage was found to be low. Available data on BWI are conflicting and studies differ in histological and diagnostic criteria. Our results emphasize the need to correlate histological findings in the ileum with cecal findings in order to accurately asses the presence of BWI in PSC-UC. Nonetheless, based on recent literature, BWI is more frequently observed PSC-UC compared with UC without PSC.
Similar to BWI, rectal sparing (RS) was reported as a common feature of IBD in PSC[11,30], together with extensive colitis and a predominance for right-sided inflammation[33]. Based on the reviewed papers, RS seems to be present in approximately one third of the patients with PSC-IBD, but large inconsistencies between studies are found. In most studies, the presence of RS was based on either endoscopic or histological data, but this approach has several limitations. Endoscopic imaging in quiescent disease, for example, could give the impression of macroscopic rectal sparing but does not exclude microscopic inflammation. Furthermore, a history of recent local steroid therapy can influence the endoscopic appearance of rectal mucosa, but only two studies[11,12] took this into account. Finally, discrepancies in diagnosis of RS have been reported, showing rectal involvement in resected specimens despite frequently found rectal sparing in biopsies[37]. A comparison between rates of RS in PSC-IBD patients and rates found in IBD controls also yielded conflicting results. While all studies comparing RS incidence between PSC-IBD and IBD controls found higher rates in the former group, differences were significant in only two studies[11,25]. In contrast, Joo et al[31] found almost equal rates of RS in both UC and UC with associated PSC in their review of histological specimens. Both these results and the previously stated limitations regarding the diagnosis of RS, make it difficult to see RS as a distinct feature of the PSC-IBD phenotype. Diagnosis of true RS requires both endoscopic and histological absence of inflammation with the exclusion of recent (< 6 mo) local steroid therapy.
Based on limited results from small subgroup populations, we found that the characteristics of CD in PSC are similar to those found in UC associated with PSC. Disease activity is mild with an observed lower frequency of stricturing and penetrating disease compared with conventional CD. An isolated colitis is most common, being present in 55% of the patients with PSC-CD. Notably, the severity of PSC-CD may also be less, compared with PSC associated with UC. This was suggested by two studies[39,39,77] that found higher rates of small duct PSC in CD compared with PSC-UC, with one also finding a non-significant (P = 0.06) trend for increased survival[39].
PSC as a third and separate risk factor for CRC in concomitant IBD was first proposed by Broomé et al[78] in 1992 and was subsequently confirmed by several authors[22,38,46]. Other studies, however, have questioned this finding. They found that PSC did not constitute an additional risk of CRC[11,29,79] and proposed that PSC-IBD characteristics, i.e., pancolitis and mild sub-clinical disease, facilitate the two previously mentioned risk factors for CRC development in IBD and explain the higher rates of CRC in PSC-IBD[11].
A comparison between the estimated risk of dysplasia and CRC development in PSC-IBD poses a challenge, as the use of statistical analyses and output parameters greatly varies between studies (Table 3). The most common reported format for colonic dysplasia and CRC development is the cumulative 10 year risk, which ranges from 0% to 11% for PSC-IBD. In all studies combined, development of dysplasia or CRC is observed in approximately 24% of patients with PSC and associated IBD. In contrast, in IBD controls the development of dysplasia or CRC is observed in 9% of the patients, with a reported estimated cumulative 10 years risk of 2%. In addition, a recent large French prospective study on neoplasia in IBD found an even lower rate of patients developing colonic neoplasia. In this study 0.3% of 19486 patients developed high grade dysplasia or CRC[80].
The included studies on both IBD and PSC-IBD differed greatly with respect to reported rates of dysplasia and CRC development. These differences are likely caused by the retrospective nature of the studies and by differences between centers regarding referral bias. Furthermore, discrepancies in the definition of dysplasia were found. Some studies reported all types of dysplasia, including low grade dysplasia, whereas other studies only registered colorectal cancer. While the progression of low grade dysplasia to advanced neoplasia in IBD has been shown to be low[81,82], it is worth noting that an increased risk of progression in PSC-IBD has been observed[83] making its inclusion justifiable. Based on these results and by taking study limitations into account, an increased risk of development of dysplasia and CRC in PSC-IBD can be confirmed. Future studies would benefit from a standard statistical output parameter to determine the risk of development of CRC in PSC-IBD.
Apart from an increased risk of CRC, a shorter interval between diagnosis of IBD and development of CRC in PSC-IBD was also reported[47]. The combined mean interval between development of IBD and colonic dysplasia or CRC found in patients with PSC-IBD is 19 years compared with 26 years for IBD patients without PSC. The observed median interval however, shows the opposite, with equal or even lower median intervals in IBD compared with PSC-IBD (Table 3). Although data on the age of both PSC-IBD patients and IBD patients without PSC at diagnosis of CRC were scarce, a lower median age at diagnosis of CRC was suggested for the former group. Based on these results, it is unclear whether the age of presentation and the interval between IBD diagnosis and development of large bowel neoplasia are lower in PSC-IBD compared with IBD. As both a younger age at CRC diagnosis and the efficacy of early surveillance in PSC-IBD have been confirmed and disputed[22,53], further studies are warranted.
As noted earlier, in PSC-IBD, a predisposition for development of right-sided colonic dysplasia and CRC has been observed[46,54,55,84]. While the reported percentages vary, a high predominance (> 60%) for dysplasia and CRC development in the proximal colon is confirmed by the majority of studies. The exact cause of increased dysplasia and CRC development in PSC-IBD however, remains unknown. A proposed mechanism is a cytotoxic and carcinogenic effect of secondary bile acids accumulating in the proximal colon due to defective small intestine reabsorption in cholestatic liver disease[85]. This assumption is strengthened by the association found between high concentrations of bile acids and the development of colon carcinoma in colitis[86].
The clinical course of IBD in PSC after liver transplantation was both observed to stabilize[87] or to worsen[88]. In all studies combined, we found that approximately 32% of patients with PSC-IBD experience an exacerbation after OLT. For the development of de novo IBD after receiving a liver transplantation for PSC this was approximately 18%. These results are slightly lower than those presented in a recent review by Singh et al[89], who found IBD to worsen in a third of PSC-IBD patients after OLT. The different rates found for exacerbation and de novo presentation of IBD after liver transplantation for PSC have been contributed to the type of immunosuppressive medication, with lower rates reported for cyclosporin A and azathioprine compared with tacrolimus[59,88,90]. A proposed mechanism is the stronger suppression of interleukin-2 production by T-cells in tacrolimus compared to cyclosporin A, resulting in an inability to activate a regulatory response[59]. A subsequent study by the same group was not able to find a drug specific effect for tacrolimus on regulatory T cells in noninflamed colon mucosa of OLT patients[91]. Although further (pathophysiological) studies are required, the use of cyclosporin and azathioprine over tacrolimus seems to have a more favorable outcome on IBD after OLT for PSC, and should therefore be considered.
An early study by Cangemi et al[92] showed no beneficial effect of proctocolectomy on the progression or development of complications in PSC patients. In addition, careful consideration was urged in choosing proctocolectomy with ileostomy as the treatment of choice for colonic disease in PSC-UC, as it resulted in frequent development of parastomal varices. The safety and complications of proctocolectomy with ileal pouch-anal anastomosis (IPAA) in PSC-IBD were infrequently reported. While two out of three studies described proctocolectomy to be safe in PSC-IBD finding no increased mortality, a significant number of patients experienced postoperative morbidity (29% and 39% respectively[73,74]). Reported complications ranged from urinary tract and wound infections to bacterial cholangitis or hepatic decompensation. While peristomal varices are a common problem in ileostomy after proctocolectomy[93], none of the included studies demonstrated development of perianastomotic varices in IPAA. A less favorable outcome in PSC-IBD after colectomy was suggested, however, by a more recent study in which one-third of the patients progressed to OLT or death within an mean of 2.6 years[94]. In addition, this study stressed the importance of pre-operative assessment of liver function, finding a low preoperative platelet count and a low albumin level to be related to worse outcomes.
A frequently reported long term complication of IPAA in both IBD and PSC-IBD is pouchitis. Symptoms of pouchitis reported in the included studies are (bloody) diarrhea, abdominal cramping, urgency, malaise, and fever[95,96]. The definitions and diagnostic criteria for pouchitis however differed substantially between studies, making comparisons difficult. For instance, most authors found increased overall rates of pouchitis in PSC-UC, while Gorgun et al[71], who only looked at chronic pouchitis, found lower rates (13.8%) that were comparable to UC controls. Based on the included studies, we found that approximately 60% of PSC-UC patients experienced at least one episode of pouchitis after proctocolectomy with IPAA. This is more frequent when compared with conventional IBD after IPAA where pouchitis is observed in approximately one-third of the patients. Chronic pouchitis after IPAA is observed less frequently, but it still occurred more often in PSC-IBD when compared to IBD without PSC. Although pouchitis was found more common in PSC-IBD after IPAA, the rate of pouch failure does not seem to be increased and is comparable to IBD without PSC.
From a molecular perspective there is also increasing evidence that PSC-IBD is distinct from UC and CD. Large scale genetic studies performed within the International IBD Genetics Consortium have identified 163 independent genetic loci to be associated with IBD. The majority of these loci are shared between both UC and CD[97]. Similar studies have been performed within the International PSC study group, and they identified 16 independent loci outside the HLA region[98]. Mathematical modeling of these genetic variants showed that PSC is genetically more similar to UC than to CD which is comparable to the clinical observation. A striking finding of these studies is that only 50% of the PSC risk loci are shared with IBD. Based on power calculations and the fact that more than 70 % of the PSC patients had concomitant IBD one would have expected that many more of the 163 IBD loci would be associated with PSC than is currently observed[98,99]. These intriguing findings suggest that from a genetic perspective IBD-PSC is different from both CD and UC.
Based on our findings, several recommendations can be made concerning futures studies and the management of IBD associated with PSC.
Large differences between IBD rates in PSC were found. Therefore, to accurately assess or exclude the presence of IBD in PSC, a full colonoscopy with multiple biopsies from every segment and terminal ileum is required. Furthermore, the additional diagnostic criteria and a uniform definition are needed to assess the actual presence of backwash ileitis and rectal sparing in PSC-IBD patients.
Studies on the risk of the development of colonic neoplasia in PSC-IBD would benefit from a standard statistical outcome allowing comparison. The increase in the development of large bowel neoplasia in PSC-IBD patients observed in these studies requires frequent surveillance colonoscopy. The optimal timing of the start of surveillance warrants further study as recent data were found to be contradictory.
The use of cyclosporin A and azathioprine instead of tacrolimus should be considered after liver transplantation for PSC, as the former were associated with a lower rate of IBD exacerbation and the development of de novo disease.
The safety and outcome of proctocolectomy in PSC-IBD depend upon the severity of liver disease, something which should always be assessed preoperatively. Ileal pouch-anal anastomosis reconstruction after colectomy in PSC-IBD was associated with a frequent development of pouchitis, and patients should be informed about this risk. Choosing for IPAA instead of ileostomy in PSC-IBD, however, seems favorable as the risk of development of local perianastomotic varices is low.
In conclusion, although IBD in PSC is common, there is a large variance in observed rates, which seems to be related to case ascertainment and, possibly, geographical variance. Based on the described clinical characteristics, the higher risk of development of colonic neoplasia, and the increasing molecular evidence, PSC-IBD should be regarded as a distinct phenotype of IBD, which differs from UC and CD and which therefore requires specialized management.
Inflammatory bowel disease (IBD) in Primary sclerosing cholangitis (PSC) often presents as quiescent disease affecting the whole colon. It is reported to be associated with backwash ileitis and rectal sparing. In addition an increased risk for the development of colorectal carcinoma has been described in several studies.
Based on differences in disease characteristics it has been suggested that IBD in PSC represents a distinct phenotype in addition to ulcerative colitis (UC) and Crohn’s disease. Studies looking at the characteristics for this phenotype however are limited.
While previous studies have summarized different aspects of IBD in PSC, studies providing a description of the PSC-IBD phenotype are limited. By reviewing existing literature on five relevant subthemes, an overview of the characteristics of the PSC-IBD phenotype is provided.
The results of this study suggest that IBD in PSC is different from conventional IBD and therefore represents a distinct phenotype which requires specialized management.
PSC is a chronic cholestatic liver disease characterized by inflammation and fibrosis of both the intrahepatic and extrahepatic bile ducts. There is no curative therapy available and in most cases, PSC leads to liver cirrhosis and liver failure ultimately requiring liver transplantation. In the majority of cases PSC is accompanied by IBD most often UC.
The authors provided a systemic review on PSC-IBD characteristics compare to UC and IBD. The review is interesting and well done.
| 1. | Chapman RW, Arborgh BA, Rhodes JM, Summerfield JA, Dick R, Scheuer PJ, Sherlock S. Primary sclerosing cholangitis: a review of its clinical features, cholangiography, and hepatic histology. Gut. 1980;21:870-877. [PubMed] |
| 2. | Broomé U, Olsson R, Lööf L, Bodemar G, Hultcrantz R, Danielsson A, Prytz H, Sandberg-Gertzén H, Wallerstedt S, Lindberg G. Natural history and prognostic factors in 305 Swedish patients with primary sclerosing cholangitis. Gut. 1996;38:610-615. [PubMed] |
| 3. | Card TR, Solaymani-Dodaran M, West J. Incidence and mortality of primary sclerosing cholangitis in the UK: a population-based cohort study. J Hepatol. 2008;48:939-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 94] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 4. | Boberg KM, Aadland E, Jahnsen J, Raknerud N, Stiris M, Bell H. Incidence and prevalence of primary biliary cirrhosis, primary sclerosing cholangitis, and autoimmune hepatitis in a Norwegian population. Scand J Gastroenterol. 1998;33:99-103. [PubMed] |
| 5. | Bambha K, Kim WR, Talwalkar J, Torgerson H, Benson JT, Therneau TM, Loftus EV, Yawn BP, Dickson ER, Melton LJ. Incidence, clinical spectrum, and outcomes of primary sclerosing cholangitis in a United States community. Gastroenterology. 2003;125:1364-1369. [PubMed] |
| 6. | Fausa O, Schrumpf E, Elgjo K. Relationship of inflammatory bowel disease and primary sclerosing cholangitis. Semin Liver Dis. 1991;11:31-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 190] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 7. | Chapman R, Fevery J, Kalloo A, Nagorney DM, Boberg KM, Shneider B, Gores GJ; American Association for the Study of Liver Diseases. Diagnosis and management of primary sclerosing cholangitis. Hepatology. 2010;51:660-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 888] [Cited by in RCA: 853] [Article Influence: 53.3] [Reference Citation Analysis (2)] |
| 8. | Wiesner RH, LaRusso NF. Clinicopathologic features of the syndrome of primary sclerosing cholangitis. Gastroenterology. 1980;79:200-206. [PubMed] |
| 9. | Olsson R, Danielsson A, Järnerot G, Lindström E, Lööf L, Rolny P, Rydén BO, Tysk C, Wallerstedt S. Prevalence of primary sclerosing cholangitis in patients with ulcerative colitis. Gastroenterology. 1991;100:1319-1323. [PubMed] |
| 10. | Lundqvist K, Broomé U. Differences in colonic disease activity in patients with ulcerative colitis with and without primary sclerosing cholangitis: a case control study. Dis Colon Rectum. 1997;40:451-456. [PubMed] |
| 11. | Loftus EV, Harewood GC, Loftus CG, Tremaine WJ, Harmsen WS, Zinsmeister AR, Jewell DA, Sandborn WJ. PSC-IBD: a unique form of inflammatory bowel disease associated with primary sclerosing cholangitis. Gut. 2005;54:91-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 515] [Cited by in RCA: 529] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 12. | Boonstra K, van Erpecum KJ, van Nieuwkerk KM, Drenth JP, Poen AC, Witteman BJ, Tuynman HA, Beuers U, Ponsioen CY. Primary sclerosing cholangitis is associated with a distinct phenotype of inflammatory bowel disease. Inflamm Bowel Dis. 2012;18:2270-2276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 167] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 13. | Eaton JE, Talwalkar JA, Lazaridis KN, Gores GJ, Lindor KD. Pathogenesis of primary sclerosing cholangitis and advances in diagnosis and management. Gastroenterology. 2013;145:521-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 306] [Article Influence: 23.5] [Reference Citation Analysis (36)] |
| 14. | Kaplan GG, Laupland KB, Butzner D, Urbanski SJ, Lee SS. The burden of large and small duct primary sclerosing cholangitis in adults and children: a population-based analysis. Am J Gastroenterol. 2007;102:1042-1049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 187] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 15. | Sokol H, Cosnes J, Chazouilleres O, Beaugerie L, Tiret E, Poupon R, Seksik P. Disease activity and cancer risk in inflammatory bowel disease associated with primary sclerosing cholangitis. World J Gastroenterol. 2008;14:3497-3503. [PubMed] |
| 16. | Lepistö A, Kärkkäinen P, Järvinen HJ. Prevalence of primary sclerosing cholangitis in ulcerative colitis patients undergoing proctocolectomy and ileal pouch-anal anastomosis. Inflamm Bowel Dis. 2008;14:775-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 42] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | Escorsell A, Parés A, Rodés J, Solís-Herruzo JA, Miras M, de la Morena E. Epidemiology of primary sclerosing cholangitis in Spain. Spanish Association for the Study of the Liver. J Hepatol. 1994;21:787-791. [PubMed] |
| 18. | Schrumpf E, Abdelnoor M, Fausa O, Elgjo K, Jenssen E, Kolmannskog F. Risk factors in primary sclerosing cholangitis. J Hepatol. 1994;21:1061-1066. [PubMed] |
| 19. | Farrant JM, Hayllar KM, Wilkinson ML, Karani J, Portmann BC, Westaby D, Williams R. Natural history and prognostic variables in primary sclerosing cholangitis. Gastroenterology. 1991;100:1710-1717. [PubMed] |
| 20. | Ponsioen CY, Vrouenraets SM, Prawirodirdjo W, Rajaram R, Rauws EA, Mulder CJ, Reitsma JB, Heisterkamp SH, Tytgat GN. Natural history of primary sclerosing cholangitis and prognostic value of cholangiography in a Dutch population. Gut. 2002;51:562-566. [PubMed] |
| 21. | Wells AD, McMillan I, Price AB, Ritchie JK, Nicholls RJ. Natural history of indeterminate colitis. Br J Surg. 1991;78:179-181. [PubMed] |
| 22. | Boonstra K, Weersma RK, van Erpecum KJ, Rauws EA, Spanier BW, Poen AC, van Nieuwkerk KM, Drenth JP, Witteman BJ, Tuynman HA. Population-based epidemiology, malignancy risk, and outcome of primary sclerosing cholangitis. Hepatology. 2013;58:2045-2055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 421] [Cited by in RCA: 531] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 23. | Shorbagi A, Bayraktar Y. Primary sclerosing cholangitis--what is the difference between east and west? World J Gastroenterol. 2008;14:3974-3981. [PubMed] |
| 24. | Takikawa H, Manabe T. Primary sclerosing cholangitis in Japan--analysis of 192 cases. J Gastroenterol. 1997;32:134-137. [PubMed] |
| 25. | Ye BD, Yang SK, Boo SJ, Cho YK, Yang DH, Yoon SM, Kim KJ, Byeon JS, Myung SJ, Yu CS. Clinical characteristics of ulcerative colitis associated with primary sclerosing cholangitis in Korea. Inflamm Bowel Dis. 2011;17:1901-1906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 26. | Ang TL, Fock KM, Ng TM, Teo EK, Chua TS, Tan JY. Clinical profile of primary sclerosing cholangitis in Singapore. J Gastroenterol Hepatol. 2002;17:908-913. [PubMed] |
| 27. | Tanaka A, Takamori Y, Toda G, Ohnishi S, Takikawa H. Outcome and prognostic factors of 391 Japanese patients with primary sclerosing cholangitis. Liver Int. 2008;28:983-989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 28. | Tanaka A, Tazuma S, Okazaki K, Tsubouchi H, Inui K, Takikawa H. Nationwide survey for primary sclerosing cholangitis and IgG4-related sclerosing cholangitis in Japan. J Hepatobiliary Pancreat Sci. 2014;21:43-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 85] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 29. | Loftus EV, Sandborn WJ, Tremaine WJ, Mahoney DW, Zinsmeister AR, Offord KP, Melton LJ. Risk of colorectal neoplasia in patients with primary sclerosing cholangitis. Gastroenterology. 1996;110:432-440. [PubMed] |
| 30. | Sinakos E, Samuel S, Enders F, Loftus EV, Sandborn WJ, Lindor KD. Inflammatory bowel disease in primary sclerosing cholangitis: a robust yet changing relationship. Inflamm Bowel Dis. 2013;19:1004-1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 31. | Joo M, Abreu-e-Lima P, Farraye F, Smith T, Swaroop P, Gardner L, Lauwers GY, Odze RD. Pathologic features of ulcerative colitis in patients with primary sclerosing cholangitis: a case-control study. Am J Surg Pathol. 2009;33:854-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 104] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 32. | Sano H, Nakazawa T, Ando T, Hayashi K, Naitoh I, Okumura F, Miyabe K, Yoshida M, Takahashi S, Ohara H. Clinical characteristics of inflammatory bowel disease associated with primary sclerosing cholangitis. J Hepatobiliary Pancreat Sci. 2011;18:154-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 33. | Jørgensen KK, Grzyb K, Lundin KE, Clausen OP, Aamodt G, Schrumpf E, Vatn MH, Boberg KM. Inflammatory bowel disease in patients with primary sclerosing cholangitis: clinical characterization in liver transplanted and nontransplanted patients. Inflamm Bowel Dis. 2012;18:536-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 106] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 34. | Schaeffer DF, Win LL, Hafezi-Bakhtiari S, Cino M, Hirschfield GM, El-Zimaity H. The phenotypic expression of inflammatory bowel disease in patients with primary sclerosing cholangitis differs in the distribution of colitis. Dig Dis Sci. 2013;58:2608-2614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 35. | O’Toole A, Alakkari A, Keegan D, Doherty G, Mulcahy H, O’Donoghue D. Primary sclerosing cholangitis and disease distribution in inflammatory bowel disease. Clin Gastroenterol Hepatol. 2012;10:439-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 36. | Haskell H, Andrews CW, Reddy SI, Dendrinos K, Farraye FA, Stucchi AF, Becker JM, Odze RD. Pathologic features and clinical significance of “backwash” ileitis in ulcerative colitis. Am J Surg Pathol. 2005;29:1472-1481. [PubMed] |
| 37. | Joo M, Odze RD. Rectal sparing and skip lesions in ulcerative colitis: a comparative study of endoscopic and histologic findings in patients who underwent proctocolectomy. Am J Surg Pathol. 2010;34:689-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 49] [Article Influence: 3.1] [Reference Citation Analysis (1)] |
| 38. | Lindström L, Lapidus A, Ost A, Bergquist A. Increased risk of colorectal cancer and dysplasia in patients with Crohn’s colitis and primary sclerosing cholangitis. Dis Colon Rectum. 2011;54:1392-1397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 39. | Halliday JS, Djordjevic J, Lust M, Culver EL, Braden B, Travis SP, Chapman RW. A unique clinical phenotype of primary sclerosing cholangitis associated with Crohn’s disease. J Crohns Colitis. 2012;6:174-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 41. | Gyde SN, Prior P, Macartney JC, Thompson H, Waterhouse JA, Allan RN. Malignancy in Crohn’s disease. Gut. 1980;21:1024-1029. [PubMed] |
| 42. | Ekbom A, Helmick C, Zack M, Adami HO. Increased risk of large-bowel cancer in Crohn’s disease with colonic involvement. Lancet. 1990;336:357-359. [PubMed] |
| 43. | Ekbom A, Helmick C, Zack M, Adami HO. Ulcerative colitis and colorectal cancer. A population-based study. N Engl J Med. 1990;323:1228-1233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1294] [Cited by in RCA: 1206] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 44. | Dawson IM, Pryse-Davies J. The development of carcinoma of the large intestine in ulcerative colitis. Br J Surg. 1959;47:113-128. [PubMed] |
| 45. | Broomé U, Löfberg R, Veress B, Eriksson LS. Primary sclerosing cholangitis and ulcerative colitis: evidence for increased neoplastic potential. Hepatology. 1995;22:1404-1408. [PubMed] |
| 46. | Lindberg BU, Broomé U, Persson B. Proximal colorectal dysplasia or cancer in ulcerative colitis. The impact of primary sclerosing cholangitis and sulfasalazine: results from a 20-year surveillance study. Dis Colon Rectum. 2001;44:77-85. [PubMed] |
| 47. | Thackeray EW, Charatcharoenwitthaya P, Elfaki D, Sinakos E, Lindor KD. Colon neoplasms develop early in the course of inflammatory bowel disease and primary sclerosing cholangitis. Clin Gastroenterol Hepatol. 2011;9:52-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 48. | Kornfeld D, Ekbom A, Ihre T. Is there an excess risk for colorectal cancer in patients with ulcerative colitis and concomitant primary sclerosing cholangitis? A population based study. Gut. 1997;41:522-525. [PubMed] |
| 49. | Gurbuz AK, Giardiello FM, Bayless TM. Colorectal neoplasia in patients with ulcerative colitis and primary sclerosing cholangitis. Dis Colon Rectum. 1995;38:37-41. [PubMed] |
| 50. | Leidenius MH, Färkkilä MA, Kärkkäinen P, Taskinen EI, Kellokumpu IH, Höckerstedt KA. Colorectal dysplasia and carcinoma in patients with ulcerative colitis and primary sclerosing cholangitis. Scand J Gastroenterol. 1997;32:706-711. [PubMed] |
| 51. | Terg R, Sambuelli A, Coronel E, Mazzuco J, Cartier M, Negreira S, Muñoz A, Gil A, Miguez C, Huernos S. Prevalence of primary sclerosing cholangitis in patients with ulcerative colitis and the risk of developing malignancies. A large prospective study. Acta Gastroenterol Latinoam. 2008;38:26-33. [PubMed] |
| 52. | Claessen MM, Vleggaar FP, Tytgat KM, Siersema PD, van Buuren HR. High lifetime risk of cancer in primary sclerosing cholangitis. J Hepatol. 2009;50:158-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 281] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 53. | Imam MH, Thackeray EW, Lindor KD. Colonic neoplasia in young patients with inflammatory bowel disease and primary sclerosing cholangitis. Colorectal Dis. 2013;15:198-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 54. | Marchesa P, Lashner BA, Lavery IC, Milsom J, Hull TL, Strong SA, Church JM, Navarro G, Fazio VW. The risk of cancer and dysplasia among ulcerative colitis patients with primary sclerosing cholangitis. Am J Gastroenterol. 1997;92:1285-1288. [PubMed] |
| 55. | Jess T, Simonsen J, Jørgensen KT, Pedersen BV, Nielsen NM, Frisch M. Decreasing risk of colorectal cancer in patients with inflammatory bowel disease over 30 years. Gastroenterology. 2012;143:375-81.e1; quiz e13-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 388] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 56. | Brentnall TA, Haggitt RC, Rabinovitch PS, Kimmey MB, Bronner MP, Levine DS, Kowdley KV, Stevens AC, Crispin DA, Emond M. Risk and natural history of colonic neoplasia in patients with primary sclerosing cholangitis and ulcerative colitis. Gastroenterology. 1996;110:331-338. [PubMed] |
| 57. | Choi PM, Zelig MP. Similarity of colorectal cancer in Crohn’s disease and ulcerative colitis: implications for carcinogenesis and prevention. Gut. 1994;35:950-954. [PubMed] |
| 58. | Braden B, Halliday J, Aryasingha S, Sharifi Y, Checchin D, Warren BF, Kitiyakara T, Travis SP, Chapman RW. Risk for colorectal neoplasia in patients with colonic Crohn’s disease and concomitant primary sclerosing cholangitis. Clin Gastroenterol Hepatol. 2012;10:303-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 59. | Haagsma EB, Van Den Berg AP, Kleibeuker JH, Slooff MJ, Dijkstra G. Inflammatory bowel disease after liver transplantation: the effect of different immunosuppressive regimens. Aliment Pharmacol Ther. 2003;18:33-44. [PubMed] |
| 60. | Joshi D, Bjarnason I, Belgaumkar A, O’Grady J, Suddle A, Heneghan MA, Aluvihare V, Rela M, Heaton N, Agarwal K. The impact of inflammatory bowel disease post-liver transplantation for primary sclerosing cholangitis. Liver Int. 2013;33:53-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 61. | Moncrief KJ, Savu A, Ma MM, Bain VG, Wong WW, Tandon P. The natural history of inflammatory bowel disease and primary sclerosing cholangitis after liver transplantation--a single-centre experience. Can J Gastroenterol. 2010;24:40-46. [PubMed] |
| 62. | Cholongitas E, Papatheodoridis GV, Zappoli P, Giannakopoulos A, Patch D, Marelli L, Shusang V, Kalambokis G, Shirling G, Rolando N. Combined HLA-DR and -DQ disparity is associated with a stable course of ulcerative colitis after liver transplantation for primary sclerosing cholangitis. Liver Transpl. 2007;13:552-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (1)] |
| 63. | Navaneethan U, Choudhary M, Venkatesh PG, Lashner BA, Remzi FH, Shen B, Kiran RP. The effects of liver transplantation on the clinical course of colitis in ulcerative colitis patients with primary sclerosing cholangitis. Aliment Pharmacol Ther. 2012;35:1054-1063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 64. | Mosli M, Croome K, Qumosani K, Al-Judaibi B, Beaton M, Marotta P, Chandok N. The effect of liver transplantation for primary sclerosing cholangitis on disease activity in patients with inflammatory bowel disease. Gastroenterol Hepatol (N Y). 2013;9:434-441. [PubMed] |
| 65. | Dvorchik I, Subotin M, Demetris AJ, Fung JJ, Starzl TE, Wieand S, Abu-Elmagd KM. Effect of liver transplantation on inflammatory bowel disease in patients with primary sclerosing cholangitis. Hepatology. 2002;35:380-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 75] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 66. | Moskowitz RL, Shepherd NA, Nicholls RJ. An assessment of inflammation in the reservoir after restorative proctocolectomy with ileoanal ileal reservoir. Int J Colorectal Dis. 1986;1:167-174. [PubMed] |
| 67. | Abdelrazeq AS, Kandiyil N, Botterill ID, Lund JN, Reynolds JR, Holdsworth PJ, Leveson SH. Predictors for acute and chronic pouchitis following restorative proctocolectomy for ulcerative colitis. Colorectal Dis. 2008;10:805-813. [PubMed] |
| 68. | Aitola P, Matikainen M, Mattila J, Tomminen T, Hiltunen KM. Chronic inflammatory changes in the pouch mucosa are associated with cholangitis found on peroperative liver biopsy specimens at restorative proctocolectomy for ulcerative colitis. Scand J Gastroenterol. 1998;33:289-293. [PubMed] |
| 69. | Penna C, Dozois R, Tremaine W, Sandborn W, LaRusso N, Schleck C, Ilstrup D. Pouchitis after ileal pouch-anal anastomosis for ulcerative colitis occurs with increased frequency in patients with associated primary sclerosing cholangitis. Gut. 1996;38:234-239. [PubMed] |
| 70. | Block M, Jørgensen KK, Oresland T, Lindholm E, Grzyb K, Cvancarova M, Vatn MH, Boberg KM, Börjesson L. Colectomy for patients with ulcerative colitis and primary sclerosing cholangitis - what next? J Crohns Colitis. 2014;8:421-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 71. | Gorgun E, Remzi FH, Manilich E, Preen M, Shen B, Fazio VW. Surgical outcome in patients with primary sclerosing cholangitis undergoing ileal pouch-anal anastomosis: a case-control study. Surgery. 2005;138:631-637; discussion 637-639. [PubMed] |
| 72. | Wasmuth HH, Tranø G, Endreseth BH, Wibe A, Rydning A, Myrvold HE. Primary sclerosing cholangitis and extraintestinal manifestations in patients with ulcerative colitis and ileal pouch-anal anastomosis. J Gastrointest Surg. 2010;14:1099-1104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 73. | Mathis KL, Benavente-Chenhalls LA, Dozois EJ, Wolff BG, Larson DW. Short- and long-term surgical outcomes in patients undergoing proctocolectomy with ileal pouch-anal anastomosis in the setting of primary sclerosing cholangitis. Dis Colon Rectum. 2011;54:787-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 74. | Kartheuser AH, Dozois RR, Wiesner RH, LaRusso NF, Ilstrup DM, Schleck CD. Complications and risk factors after ileal pouch-anal anastomosis for ulcerative colitis associated with primary sclerosing cholangitis. Ann Surg. 1993;217:314-320. [PubMed] |
| 75. | Broomé U, Löfberg R, Lundqvist K, Veress B. Subclinical time span of inflammatory bowel disease in patients with primary sclerosing cholangitis. Dis Colon Rectum. 1995;38:1301-1305. [PubMed] |
| 76. | Goldstein N, Dulai M. Contemporary morphologic definition of backwash ileitis in ulcerative colitis and features that distinguish it from Crohn disease. Am J Clin Pathol. 2006;126:365-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 70] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 77. | Rasmussen HH, Fallingborg JF, Mortensen PB, Vyberg M, Tage-Jensen U, Rasmussen SN. Hepatobiliary dysfunction and primary sclerosing cholangitis in patients with Crohn’s disease. Scand J Gastroenterol. 1997;32:604-610. [PubMed] |
| 78. | Broomé U, Lindberg G, Löfberg R. Primary sclerosing cholangitis in ulcerative colitis--a risk factor for the development of dysplasia and DNA aneuploidy? Gastroenterology. 1992;102:1877-1880. [PubMed] |
| 79. | de Valle MB, Björnsson E, Lindkvist B. Mortality and cancer risk related to primary sclerosing cholangitis in a Swedish population-based cohort. Liver Int. 2012;32:441-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 80. | Beaugerie L, Svrcek M, Seksik P, Bouvier AM, Simon T, Allez M, Brixi H, Gornet JM, Altwegg R, Beau P. Risk of colorectal high-grade dysplasia and cancer in a prospective observational cohort of patients with inflammatory bowel disease. Gastroenterology. 2013;145:166-175.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 252] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 81. | Navaneethan U, Jegadeesan R, Gutierrez NG, Venkatesh PG, Hammel JP, Shen B, Kiran RP. Progression of low-grade dysplasia to advanced neoplasia based on the location and morphology of dysplasia in ulcerative colitis patients with extensive colitis under colonoscopic surveillance. J Crohns Colitis. 2013;7:e684-e691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 82. | Jess T, Loftus EV, Velayos FS, Harmsen WS, Zinsmeister AR, Smyrk TC, Tremaine WJ, Melton LJ, Munkholm P, Sandborn WJ. Incidence and prognosis of colorectal dysplasia in inflammatory bowel disease: a population-based study from Olmsted County, Minnesota. Inflamm Bowel Dis. 2006;12:669-676. [PubMed] |
| 83. | Venkatesh PG, Jegadeesan R, Gutierrez NG, Sanaka MR, Navaneethan U. Natural history of low grade dysplasia in patients with primary sclerosing cholangitis and ulcerative colitis. J Crohns Colitis. 2013;7:968-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 84. | Shetty K, Rybicki L, Brzezinski A, Carey WD, Lashner BA. The risk for cancer or dysplasia in ulcerative colitis patients with primary sclerosing cholangitis. Am J Gastroenterol. 1999;94:1643-1649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 167] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 85. | Torres J, Pineton de Chambrun G, Itzkowitz S, Sachar DB, Colombel JF. Review article: colorectal neoplasia in patients with primary sclerosing cholangitis and inflammatory bowel disease. Aliment Pharmacol Ther. 2011;34:497-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 84] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 86. | Hill MJ, Melville DM, Lennard-Jones JE, Neale K, Ritchie JK. Faecal bile acids, dysplasia, and carcinoma in ulcerative colitis. Lancet. 1987;2:185-186. [PubMed] |
| 87. | Befeler AS, Lissoos TW, Schiano TD, Conjeevaram H, Dasgupta KA, Millis JM, Newell KA, Thistlethwaite JR, Baker AL. Clinical course and management of inflammatory bowel disease after liver transplantation. Transplantation. 1998;65:393-396. [PubMed] |
| 88. | Verdonk RC, Dijkstra G, Haagsma EB, Shostrom VK, Van den Berg AP, Kleibeuker JH, Langnas AN, Sudan DL. Inflammatory bowel disease after liver transplantation: risk factors for recurrence and de novo disease. Am J Transplant. 2006;6:1422-1429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 122] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 89. | Singh S, Loftus EV, Talwalkar JA. Inflammatory bowel disease after liver transplantation for primary sclerosing cholangitis. Am J Gastroenterol. 2013;108:1417-1425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 90. | Jørgensen KK, Lindström L, Cvancarova M, Karlsen TH, Castedal M, Friman S, Schrumpf E, Foss A, Isoniemi H, Nordin A. Immunosuppression after liver transplantation for primary sclerosing cholangitis influences activity of inflammatory bowel disease. Clin Gastroenterol Hepatol. 2013;11:517-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 91. | Verdonk RC, Haagsma EB, Jonker MR, Bok LI, Zandvoort JH, Kleibeuker JH, Faber KN, Dijkstra G. Effects of different immunosuppressive regimens on regulatory T-cells in noninflamed colon of liver transplant recipients. Inflamm Bowel Dis. 2007;13:703-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 92. | Cangemi JR, Wiesner RH, Beaver SJ, Ludwig J, MacCarty RL, Dozois RR, Zinsmeister AR, LaRusso NF. Effect of proctocolectomy for chronic ulcerative colitis on the natural history of primary sclerosing cholangitis. Gastroenterology. 1989;96:790-794. [PubMed] |
| 93. | Wiesner RH, LaRusso NF, Dozois RR, Beaver SJ. Peristomal varices after proctocolectomy in patients with primary sclerosing cholangitis. Gastroenterology. 1986;90:316-322. [PubMed] |
| 94. | Treeprasertsuk S, Björnsson E, Sinakos E, Weeding E, Lindor KD. Outcome of patients with primary sclerosing cholangitis and ulcerative colitis undergoing colectomy. World J Gastrointest Pharmacol Ther. 2013;4:61-68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 95. | Lohmuller JL, Pemberton JH, Dozois RR, Ilstrup D, van Heerden J. Pouchitis and extraintestinal manifestations of inflammatory bowel disease after ileal pouch-anal anastomosis. Ann Surg. 1990;211:622-627; discussion 627-629. [PubMed] |
| 96. | Kuisma J, Järvinen H, Kahri A, Färkkilä M. Factors associated with disease activity of pouchitis after surgery for ulcerative colitis. Scand J Gastroenterol. 2004;39:544-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 68] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 97. | Jostins L, Ripke S, Weersma RK, Duerr RH, McGovern DP, Hui KY, Lee JC, Schumm LP, Sharma Y, Anderson CA. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491:119-124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3979] [Cited by in RCA: 3709] [Article Influence: 264.9] [Reference Citation Analysis (1)] |
| 98. | Liu JZ, Almarri MA, Gaffney DJ, Mells GF, Jostins L, Cordell HJ, Ducker SJ, Day DB, Heneghan MA, Neuberger JM. Dense fine-mapping study identifies new susceptibility loci for primary biliary cirrhosis. Nat Genet. 2012;44:1137-1141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 245] [Cited by in RCA: 218] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 99. | Henriksen EK, Melum E, Karlsen TH. Update on primary sclerosing cholangitis genetics. Curr Opin Gastroenterol. 2014;30:310-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 100. | Rabinovitz M, Gavaler JS, Schade RR, Dindzans VJ, Chien MC, Van Thiel DH. Does primary sclerosing cholangitis occurring in association with inflammatory bowel disease differ from that occurring in the absence of inflammatory bowel disease? A study of sixty-six subjects. Hepatology. 1990;11:7-11. [PubMed] |
| 101. | Kingham JG, Kochar N, Gravenor MB. Incidence, clinical patterns, and outcomes of primary sclerosing cholangitis in South Wales, United Kingdom. Gastroenterology. 2004;126:1929-1930. [PubMed] |
| 102. | Bergquist A, Lindberg G, Saarinen S, Broomé U. Increased prevalence of primary sclerosing cholangitis among first-degree relatives. J Hepatol. 2005;42:252-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 82] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 103. | Tischendorf JJ, Hecker H, Krüger M, Manns MP, Meier PN. Characterization, outcome, and prognosis in 273 patients with primary sclerosing cholangitis: A single center study. Am J Gastroenterol. 2007;102:107-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 277] [Article Influence: 14.6] [Reference Citation Analysis (1)] |
| 104. | Bergquist A, Said K, Broomé U. Changes over a 20-year period in the clinical presentation of primary sclerosing cholangitis in Sweden. Scand J Gastroenterol. 2007;42:88-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 105. | Lindkvist B, Benito de Valle M, Gullberg B, Björnsson E. Incidence and prevalence of primary sclerosing cholangitis in a defined adult population in Sweden. Hepatology. 2010;52:571-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 137] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 106. | Bowlus CL, Li CS, Karlsen TH, Lie BA, Selmi C. Primary sclerosing cholangitis in genetically diverse populations listed for liver transplantation: unique clinical and human leukocyte antigen associations. Liver Transpl. 2010;16:1324-1330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 61] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 107. | Toy E, Balasubramanian S, Selmi C, Li CS, Bowlus CL. The prevalence, incidence and natural history of primary sclerosing cholangitis in an ethnically diverse population. BMC Gastroenterol. 2011;11:83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 83] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 108. | Bergquist A, Ekbom A, Olsson R, Kornfeldt D, Lööf L, Danielsson A, Hultcrantz R, Lindgren S, Prytz H, Sandberg-Gertzén H. Hepatic and extrahepatic malignancies in primary sclerosing cholangitis. J Hepatol. 2002;36:321-327. [PubMed] |
| 109. | Kaplan GG, Heitman SJ, Hilsden RJ, Urbanski S, Myers RP, Lee SS, Burak KW, Swain M, Panaccione R. Population-based analysis of practices and costs of surveillance for colonic dysplasia in patients with primary sclerosing cholangitis and colitis. Inflamm Bowel Dis. 2007;13:1401-1407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Kaya M, Sakai Y, Zeng Z S- Editor: Ma YJ L- Editor: A E- Editor: Ma S