Published online Feb 14, 2015. doi: 10.3748/wjg.v21.i6.1945
Peer-review started: March 26, 2014
First decision: April 28, 2014
Revised: July 31, 2014
Accepted: September 18, 2014
Article in press: September 19, 2014
Published online: February 14, 2015
Processing time: 322 Days and 11.7 Hours
AIM: To review literature on efficacy and safety of octreotide-long-acting repeatable (LAR) used at doses higher than the Food and Drug Administration (FDA)-approved 30 mg/mo for treatment of neuroendocrine tumors (NETs).
METHODS: We searched PubMed and Cochrane Library from 1998-2012, 5 conferences (American Society of Clinical Oncology, Endocrine Society, European Neuroendocrine Tumor Society, European Society for Medical Oncology, North American Neuroendocrine Tumor Society) from 2000-2013 using MeSH and keyterms including neuroendocrine tumors, carcinoid tumor, carcinoma, neuroendocrine, and octreotide. Bibliographies of accepted articles were also searched. Two reviewers reviewed titles, abstracts, and full-length articles. Studies that reported data on efficacy and safety of ≥ 30 mg/mo octreotide-LAR for NETs in human subjects, published in any language were included in the review.
RESULTS: The search identified 1086 publications, of which 238 underwent full-text review (20 were translated into English); 17 were included in the review. Studies varied in designs, subjects, octreotide-LAR regimens, and definition of outcomes. Eleven studies reported use of higher doses to control symptoms and tumor progression, although symptom severity and formal quality-of-life analysis were not quantitatively measured. Ten studies reported efficacy, describing 260 subjects with doses ranging from 40 mg/mo or 30 mg/3 wk up to 120 mg/mo. Eight studies reported expert clinical opinion that supported dose escalation of octreotide-LAR up to 60 mg/mo for symptom control and suggested increased doses may be effective at preventing tumor progression. Eight studies reported safety; there was no evidence of increased toxicity associated with doses of octreotide-LAR > 30 mg/mo.
CONCLUSION: As reported in this review, octreotide-LAR at doses > 30 mg/mo is being prescribed for symptom and tumor control in NET patients. Furthermore, expert clinical opinion provided support for escalation of somatostatin analogs for refractory hormonal symptoms.
Core tip: Although octreotide-long-acting repeatable (LAR) is Food and Drug Administration approved for alleviating severe diarrhea/flushing associated with metastatic carcinoid tumors at doses ≤ 30 mg every 4 wk, our review found that several studies within the published literature described the use of above-label doses of octreotide-LAR for symptom and tumor control of neuroendocrine tumors. Expert clinical opinion, as reported in this review, supports escalation of somatostatin analogs for patients with refractory hormonal symptoms.
- Citation: Broder MS, Beenhouwer D, Strosberg JR, Neary MP, Cherepanov D. Gastrointestinal neuroendocrine tumors treated with high dose octreotide-LAR: A systematic literature review. World J Gastroenterol 2015; 21(6): 1945-1955
- URL: https://www.wjgnet.com/1007-9327/full/v21/i6/1945.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i6.1945
Gastrointestinal neuroendocrine tumors (NETs) are rare and generally slow-growing neoplasms that secrete various peptides and neuroamines, some of which cause clinical symptoms[1]. The annual incidence of NETs is approximately 40-50 cases per million, which is about 1%-2% of all gastrointestinal malignancies. These tumors may be classified in multiple ways but are often divided into carcinoid tumors and pancreatic neuroendocrine tumors (pNET). Symptoms are often nonspecific, leading in many cases to delayed diagnosis.
First-line systemic therapy for NETs often consists of somatostatin analogs (SSAs) such as octreotide acetate (Sandostatin®; Novartis Pharmaceutical Company, East Hanover, NJ, United States) or lanreotide (Somatuline®; Ipsen Pharmaceuticals, Paris, France). These drugs, initially developed to palliate the symptoms of carcinoid syndrome, have an inhibitory effect on secretion of gastrointestinal hormones (e.g., serotonin). Accumulating data indicate that SSAs are also capable of inhibiting NET growth[2,3]. Currently, the maximum FDA-approved dosage and administration of octreotide long-acting repeatable (LAR), indicated for severe diarrhea/flushing episodes associated with metastatic carcinoid tumors and VIPomas, is 30 mg every 4 wk[4].
A recent physician expert consensus panel highlighted the appropriateness of using standard dose SSAs for control of hormonal symptoms and tumor growth in patients with advanced carcinoid tumors, as well as increasing dose/frequency of SSAs in treatment of refractory carcinoid syndrome[2]. The panel also recommended that increase in the dose/frequency of SSAs be considered for patients with radiographic progression, particularly in cases where disease was previously stabilized at a lower dose. Additionally, in clinical practice, octreotide-LAR is sometimes prescribed at above-label doses but evidence for this practice has not been systematically assessed. Motivated by the recent expert panel consensus and anecdotal evidence from clinical practices, our aim was to systematically review the published literature on efficacy and safety of octreotide-LAR used at doses higher than the Food and Drug Administration (FDA)-approved 30 mg and/or administered at a frequency greater than every 4 wk for management of NETs.
We searched PubMed and the Cochrane Library databases and five conferences: American Society of Clinical Oncology (ASCO) annual meeting and ASCO Gastrointestinal Cancers Symposium, Endocrine Society (ENDO), European Neuroendocrine Tumor Society (ENETS), European Society for Medical Oncology (ESMO), and North American Neuroendocrine Tumor Society (NANETS). In supplementing our search, we also reviewed reference lists of records included for data abstraction and asked for article nominations from physician experts that were part of a NET treatment consensus panel[2].
We searched PubMed using the keywords and Medical Subject Headings terms: “neuroendocrine tumors”, “carcinoid tumor”, and “carcinoma, neuroendocrine”, and “octreotide”. Our search was limited to human subjects and studies published from 1998 to present. The search year limit of “1998” was imposed based on octreotide acetate LAR (Sandostatin LAR) original approval date (http://www.accessdata.fda.gov/). In addition to the terms used in the PubMed search, the Cochrane Library was also searched using word combinations of “octreotide” and limited to Cochrane Reviews and Protocols. Conference abstracts were searched using an expanded version of search terms listed above: neuroendocrine tumor, neuroendocrine tumour(s), neuroendocrine carcinoma(s), carcinoid tumor(s), carcinoid tumour(s), carcinoid syndrome, octreotide, and sandostatin. The conference search was limited to studies in human subjects and published in 2000 or later.
Identified articles were screened in three phases, article title and abstract review, first full article review, and second full article review, and conference abstracts in one phase. Articles were advanced to the next phase of screening only if they met the screening criteria or if it was unclear if they met the screening criteria in the prior screening phase. The first and second screening phases were done by a single reviewer and the third by two reviewers. In the first phase, titles and abstracts of identified studies were screened for data in human subjects on the drug (octreotide-LAR) and condition (neuroendocrine tumors) of interest. If an article that passed this screening phase was not in English, it was translated and moved to phase two (first full article review). In phase two, studies were screened for use of octreotide acetate LAR at doses > 30 mg/4 wk. In phase three, an article was excluded if an outcome of interest (efficacy, safety, and/or expert clinical opinion) was not reported or if the article was a case report, since case reports present data on too few patients to be able to draw any generalizable conclusions. Conference abstracts were screened by a single reviewer in one phase. Studies that passed the screening were abstracted. All screening and abstraction of the identified studies was done using specialized software[5].
The outcomes of interest included reports of efficacy/effectiveness and safety surrounding the use of octreotide-LAR at doses higher than 30 mg or administered at a frequency greater than 4 wk. We also reviewed expert clinical opinion statements surrounding the use of above-label use of octreotide-LAR. Measures of efficacy included symptom burden, disease markers, tumor response, time to progression of disease, requirements for additional intervention, and survival. Measures of safety included frequency of various adverse effects.
The weighted Kappa inter-rater reliability was calculated between the two reviewers at the screening phase using an independently-screened sample of articles, considering that a Kappa value ranging from 0.81 to 0.99 is interpreted to indicate “almost perfect agreement”[6]. After determining that the inter-rater reliability was high (i.e., > 0.81)[6], the remaining articles were divided between the two reviewers. Data were also first independently extracted from a sample of accepted articles by the two reviewers, and any resultant inconsistencies were discussed. The remaining articles were then split up between the two reviewers to finalize data extraction. Further accuracy of data abstraction was assured by ongoing consultation between the reviewers and the research team, such that ambiguities were resolved via consensus. The Oxford’s Centre for Evidence-based Medicine Levels of Evidence (OCEBM) was used to assign a quality of evidence grade of 1 to 5 to each included study, with 1 indicated a study with the strongest scientific basis for support of conclusions and 5 the weakest (e.g., expert opinion)[7].
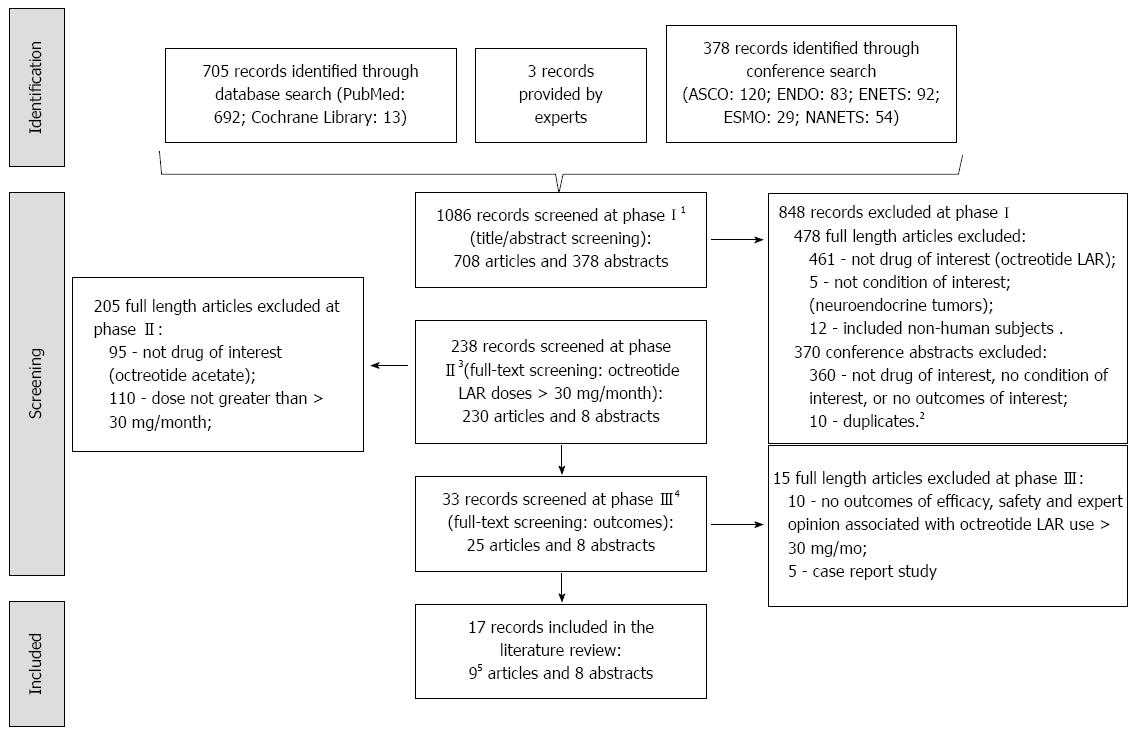
The PubMed and the Cochrane Library databases searches, conducted on 12/9/12, yielded 705 articles: 692 were identified in PubMed, 13 in the Cochrane Library database, and 3 more provided by experts (Figure 1). A search of conferences resulted in 116 abstracts identified from the ASCO meetings (87 from 2000-2012 annual meetings; 29 from 2004-2013 Gastrointestinal Cancers Symposiums), 83 from 2000- 2012 ENDO meetings, 92 from 2004, 2006, 2009- 2012 ENETS meetings, 29 from 2000 and 2002-2012 ESMO meetings, and 54 from 2008-2012 NANETS meetings. An update to the search was conducted in June 2013 to identify articles in the ASCO 2013 meeting, which resulted in 4 more abstracts. A total of 708 articles and 378 conference abstracts were screened.
In the first phase of screening, 848 of 1086 records were excluded from further screening (Figure 1). Twenty articles were translated before phase two (2 from Spanish, 6 from Italian, 4 from Japanese, 1 from French, 6 from Hungarian, and 1 from Danish). In phase two, 205 of 230 full-length articles were excluded due to lack of data on the use of octreotide acetate LAR at doses > 30 mg/mo. In the final phase, 15 of 33 additional articles (10 did not discuss outcomes of interest and 5 were case reports) were excluded. Bibliographies of the 10 accepted full-length articles were screened for additional studies of interest but no additional studies were identified. Eight of the 378 identified abstracts passed the screening and were abstracted. The weighted kappa for the two reviewers was 0.94[6]. During abstraction, it was determined that 2 of the 10 included articles reported data on the same patients, hence only the most relevant and recent of the two studies (Woltering 2006), which reported data on outcomes of interest, was retained in this review.
The review consisted of 17 studies, including 8 conference abstracts and 9 articles (Table 1). Four were clinical studies, one was a report of a modified Delphi process[2], one was a systematic review[21], two were non-systematic reviews[8,9] and one was a letter to the editor[10]. Seven conference abstracts reported on clinical studies and one a randomized phase III trial. Eight studies were performed in the United States, two in Italy, one in both the United States and Europe, and the location for the remaining 6 was not reported. Ten studies considered efficacy, 8 addressed safety, and eight provided expert clinical opinion. OCEBM grades ranged from 2b to 5, with 9 studies out of 17 scoring 4 or 5.
| Ref. (OCEBM) | Country, timeframe | Study design | Study subjects | Doses of SLAR administered (number of patients or number of doses) | Reasons for high dose SLAR2 | Relevant outcomes reported |
| Strosberg et al[2] 2013 (5) | United States | Modified delphi process | 404 patient scenarios of patients with unresectable metastatic well-differentiated carcinoid tumors | Subset of patient scenarios treated with SLAR (frequency: every 2 wk, 3 wk, 4 wk; dosing 30 mg, 40 mg, 60 mg, 90 mg, 120 mg) | NA/NR | Expert clinical opinion |
| Colao et al[8] 2010 (5) | Italy | Literature review | Pituitary tumors and NETs | NA/NR | NA/NR | Expert clinical opinion |
| Oberg et al[9] 2004 (5) | Europe, United States | Literature review | Relating to pts. with GEPNETs | NA/NR | NA/NR | Expert clinical opinion |
| Yao et al[10] 2008 (5) | United States | Letter to editor | Relating to pts. with NETs | NA/NR | NA/NR | Expert clinical opinion |
| Anthony et al[11] 2011 (3b) | United States, 2000-2006 | Retrospective multicenter medical chart review | 392 pts. with functioning NETs (with or without CS), treated with SLAR ≥ 4 mo | 10 mg (22 doses), 20 mg (224), 30 mg (316), 40 mg (78), 50 mg (16), 60 mg (42) | Lack of efficacy | Efficacy, safety |
| Chadha et al[12] 2009 (2b) | United States, 2002-2007 | Retrospective single-center medical chart review | 54 pts. with GEPNETs treated with SLAR 20-30 mg | Median high dose of SLAR 40 mg, ranging 40-90 mg/mo (30 pts.) | Control of symptoms, treatment of tumor | Efficacy, safety, Expert clinical opinion |
| Costa et al[13] 20061 (4) | 2005 | Retrospective case series | 9 pts. with progressive metastatic NETs treated with SLAR 20 mg/mo | 20 mg/mo (9 pts.); 30 mg/mo, 40 mg/mo (3 pts.) | Treatment of tumor | Efficacy, Expert clinical opinion |
| Ferolla et al[14] 2012 (2b) | Italy | Multicenter open-label non-randomized prospective clinical trial | 28 pts. with well-differentiated NETs, progressing at standard dose SLAR ≥ 6 mo | 30 mg/28 d initially for 6-32 mo (28 pts.); 30 mg/21 d (28 pts.) for 4-48 mo | Control of symptoms, treatment of tumor, other | Efficacy, safety |
| Koumarianou et al[15] 20101(4) | 2008-2009 | Retrospective case series | 13 pts. with pretreated progressive metastatic GEPNETs | 30 mg/3 wk (12 pts.) | Treatment of tumor | Efficacy, safety |
| Markovich et al[16] 20121 (4) | Retrospective clinical trial | 31 pts. with pretreated progressing disseminated NETs | 20 mg/mo initially (29 pts.); 30 mg/mo (18); 40 mg/mo (13) long acting octreotide | Control of symptoms, treatment of tumor, other | Efficacy, safety | |
| Valle et al[17] 20011 (4) | Retrospective single-center case series | 8 pts. with metastatic carcinoid syndrome | Initially 20 mg/mo (5 pts), 15 mg/mo, 60/mo, 20/2 wk (all 1); escalated to 20 mg/3 wk, 30 mg/3 wk, 50 mg/4 wk, 120 mg/4 wk (4 pts.) | Control of symptoms | Efficacy, safety | |
| Weber et al[18] 20121 (4) | United States, 2000-2010 | Retrospective single-center medical chart review | 337 pts. with metastatic small-bowel carcinoid tumors, treated with SLAR | 27% (99 pts.) with increased high dose; common max doses were 40 mg/4 wk (37 pts.), 60 mg/mo (34), 30 mg/3 wk (17) | Control of symptoms, tumor progression, other | Efficacy |
| Wolin et al[19] 20131 (2b) | Multicenter randomized phase III clinical trial | 110 pts. with unresponsive NET symptoms to standard dose SLAR | 40 mg/28 d (57 pts.) | Control of symptoms | Efficacy, safety | |
| Woltering et al[20] 2006 (3) | United States | Non-randomized prospective clinical trial | 40 pts. with carcinoid syndrome on stable doses of SLAR for ≥ 3 mo | 20 mg (8 pts.), 30 mg (19), 60 mg (13) SLAR/mo | Control of symptoms | Efficacy |
| Ludlam et al[21] 2011 (3a) | 1965-2010 | Systematic literature review | Relating to pts. with NETs | NA/NR | Control of symptoms | Safety |
| Strosberg et al[22] 20131 (2b) | United States, 2004-2010 | Retrospective multicenter medical chart review | 271 pts. with carcinoid and pancreatic NETs, treated with octreotide-LAR | 40% (n = 82) of carcinoid pts had high dose: common max doses of 40 mg/mo (39%), 40 mg/3 wk (18%), 30 mg/2 wk (17%); and 23% (15) of pNET pts: 40 mg/mo (33%), 30 mg/2 wk (27%), 60 mg/mo (27%) | Control of symptoms, treatment of tumor, other | Expert clinical opinion |
| Xu et al[23] 20121 (3b) | United States, 1999-2007 | Retrospective analysis of SEER-Medicare claims | 355 pts. with NETs | 26 pts. (7.3%) with ≤ 10 mg initially, of which 3.9% increased to > 40 mg; 91 (25.6%) with 11-20 mg initially, 5.5% increased to 31-40 mg and 4.4% to > 40 mg; 147 (41.4%) with 21-30 mg initially, 11.6% increased to 31-40 mg and 10.9% to > 40 mg; 65 (18.3%) with 31-40 mg initially, 86.2% increased to 31-40 mg and 13.9% to > 40 mg; 26 (7.3%) with > 40 mg initially, 100% increased to > 40 mg; 134 pts. (37.7%) escalated to > 30 mg/mo during 1st year of therapy | NA/NR | Expert clinical opinion |
The efficacy of high dose octreotide-LAR was reported in 10 studies, of which 4 were full-length articles[11-20] (Table 2). Doses studied ranged from a minimum of either 40 mg per month or 30 mg per 3 wk up to a maximum of 120 mg per month. The studies describe over 260 subjects who received increased doses of octreotide-LAR (the exact number cannot be stated with certainty, as some studies did not report the number of patients treated with above-label doses and grouped them with other patients).
| Ref. | Symptoms | Disease markers2 | Tumor response/Disease progression |
| Anthony et al[11] 2011 | NA/NR | NA/NR | Complete response rates: 2% (20 mg), 1% (30 mg), 0% (40 mg), 2% (60 mg); partial response: 6% (20mg), 8% (30 mg), 4% (40 mg), 10% (60 mg); stable disease: 57% (20 mg), 57% (30 mg), 55% (40 mg), 50% (60 mg); progression: 21% (20 mg), 25% (30 mg), 18% (40 mg), 29% (60 mg) |
| Chadha et al[12] 2009 | NA/NR | NA/NR | Median time to intervention was 2.9 mo (conventional dose group) vs 17.7 mo (high-dose) (P = 0.12) |
| Costa et al[13] 20061 | NA/NR | NA/NR | After evidence of progressive disease in liver, disease stabilization was achieved with increase to 30 mg and to 40 mg/mo |
| Ferolla et al[14] 2012 | Complete normalization: 40%; partial symptom control: 60%; flushing (normalized in 71.4%, improved in 28.6%), diarrhea (70%, 30%); pain (disappeared in 33%; improved in 67%); bronchospasm improved in 100%; hypoglycemia improved in 100%; weakness/well-being improved in all pts | 30% of pts. with elevated markers responded to higher dose; significant response to high dose SLAR was in 30% of pts. with high CgA, 57.1% of pts. with high 5-HIAA, and 100% of pts. with high gastrin; median time-to-biochemical progression was 30 mo (SLAR 30 mg/21 d) vs 14 mo (standard dose) (P < 0.01) | Partial response in 7.2%, stable disease in 92.8%; median time to progression was 30 mo. (SLAR 30 mg/21 d) vs 9 mo. (standard dose) (P < 0.0001); |
| Koumarianou et al[15] 20101 | NA/NR | NA/NR | 75% (9/12) with > 50% tumor size reduction, and 25% had stable disease; median PFS was 24.6 wk |
| Markovich et al[16] 20121 | NA/NR | NA/NR | Partial response in 3.2%, stable disease in 80.7%, progressed in 16.1%; tumor growth control in 83.9%, pts. had biochemical response and symptom relief (results not broken down by dose) |
| Valle et al[17] 20011 | Improvement in flushing, diarrhea, and bronchospasm (results not broken down by dose) | pts. with dose-escalation of SLAR had increased 5-HIAA, suggesting increased disease activity | |
| Weber et al[18] 20121 | In pts., with increased doses of SLAR for refractory CS, 62% had improvement in diarrhea and 56% had improvement in flushing | NA/NR | NA/NR |
| Wolin et al[19] 20131 | At month 6, symptom response was 21% (9/43) in pasireotide-LAR vs 27% (12/45) in ocrteotide-LAR arms (OR = 0.73; 95%CI: 0.27-1.97; P = 0.53); 36.5% ± 29.1% had a reduction in diarrhea/d and a 49.4% ± 36.7% in flushing/2 wk | NA/NR | Median PFS was 6.8 mo in octreotide-LAR vs 11.8 mo pasireotide-LAR arms (HR = 0.46; P = 0.045) |
| Woltering et al[20] 2006 | Flushing not controlled in 0% (20 mg), 11.1% (30 mg), vs 7.1% (60 mg) SLAR groups (P = 1.0); diarrhea not controlled in 0% of pts. (20 mg), 27.8% (30 mg), vs 30.8% (60 mg) groups (P = 0.3182) | Mean absolute serum CgA: 53.1 (20 mg SLAR), 65.8 (30 mg), 70.7 (60 mg) (P = 0.9847); mean absolute plasma CgA: 56.6 (20 mg), 66.2 (30 mg), 65.2 (60 mg) (P = 0.9092) | NA/NR |
Chadha et al[12] retrospectively studied high dose octreotide-LAR used to treat 30 patients with GEP-NETs. Compared to 24 subjects receiving conventional dose octreotide-LAR therapy, there was a trend (P = 0.12) toward increased time before further treatment intervention was required in this group but no effect on survival (P = 0.48). Ferolla[14] was the only prospective trial of high dose octreotide-LAR, examining the effect of 30 mg every 3 wk on tumor progression, serum markers and symptoms. The comparator group was historical and consisted of the same subjects receiving octreotide-LAR 30 mg/mo. Control of symptoms was seen with increased dose of octreotide-LAR, with 40% noting normalization and 60% noting control of symptoms (flushing, diarrhea, and bronchospasm). The time to tumor progression and the time to biochemical progression (increase in serum CGA, serum gastrin and/or urinary 5-HIAA) were both significantly delayed (P < 0.01). The study reported that “weakness and well-being improved in all patients” but a specific tool for measuring quality of life was not described. One of the larger studies[11] reported physician practice patterns in medical management of 392 patients with NETs. Data were reported by doses delivered rather than by patients treated, so it was not possible to compare this study to the other studies of efficacy that used more standard methodology. Tumor responses to doses of octreotide-LAR 20 and 30 mg were similar to those at doses of 40 and 60 mg. Woltering[20] examined whether CgA levels correlated with octreotide-LAR dose and symptoms in 40 patients with carcinoid syndrome, and determined that CgA levels were similar for those receiving octreotide-LAR 20 mg, 30 mg or 60 mg/mo.
Six conference abstracts reported efficacy of high dose octreotide-LAR[13,15-19]. Costa[13] analyzed characteristics and clinical outcomes in patients with progressive metastatic NETs treated with octreotide-LAR. The authors noted that “after evidence of objective progressive disease in liver, a second response with disease stabilization was achieved with octreotide dosage increase from 30 mg to 40 mg every 4 wk” in two subjects that received high dose octreotide-LAR. Koumarianou[15] studied efficacy of octreotide-LAR (30 mg/3 wk) combination therapy in 13 subjects with NETs. There was no comparator group. Disease remained stable in 25% (3/12) and 75% showed over > 50% reduction in tumor size. The median progression free survival in evaluable patients was 24.6 wk. Markovich[16] studied the efficacy of long acting octreotide (including octreotide-LAR, Octreotide-Depot and Octreotide-LONG) 30-40 mg/mo in heavily pretreated patients with disseminated NETs. Subjects (n = 29) initially received long-acting octreotide 20 mg/mo and the dose was escalated to 30 mg/mo (n = 18) or 40 mg/mo (n = 13). The reason for dose escalation was disease progression in 19 and lack of symptom control in 12. Those receiving long-acting octreotide alone (n = 14) did not have a response. However, tumor growth was controlled in 25 subjects (80.7%). Median time to progression in the total group was 18 mo. The report did not indicate what percentage of subjects specifically received octreotide-LAR vs other forms of long acting octreotide. Valle et al[17] determined patient acceptability and control of carcinoid-related symptoms in 8 patients receiving octreotide-LAR, two of which started on high dose. Three subjects required dose escalation to high dose octreotide-LAR to control symptoms. Valle et al[17] reported “patients who required continued dose-escalation of Sandostatin LAR showed evidence of increased urinary 5-HIAA suggesting increased disease activity.” Weber et al[18] determined the frequency of “above-label” octreotide-LAR dosing and outcomes. Of 337 subjects treated with octreotide-LAR for metastatic NETs at a single tertiary care institution, 99 (27%) received high dose. The indications for dose increase were worsening carcinoid syndrome (60 patients), radiographic progression (33 patients), and rising urine 5-HIAA (6 patients). Among patients whose doses were increased for refractory carcinoid syndrome, flushing improved in 56% and diarrhea improved in 62%. Finally, a randomized phase III clinical trial by Wolin et al[19] showed that treatment with octreotide-LAR to 40 mg/4 wk led to better control of diarrhea and flushing at 6 mo after starting treatment. Progression free survival was 6.8 mo. The comparator arm in this trial was a novel long-acting somatostatin analog (pasireotide-LAR 60 mg/4 wk), which showed a longer progression free survival (11.8 mo).
Considering these studies together, there is a trend supporting the use of octreotide-LAR at above-label doses to control symptoms and tumor progression.
The safety of high dose octreotide-LAR was reported in 8 studies[11,12,14-17,19,21] (Table 3). There was no increased toxicity compared to conventional dose therapy reported in 3 of the studies that examined safety[12,17,21]. Wolin et al[19] compared octreotide-LAR treatment arm to patients using pasireotide-LAR, and showed the two arms have a similar safety profile (diarrhea: 7% vs 9%; abdominal pain: 9% vs 2%) except for the higher frequency of hyperglycemia (0% vs 11%) in the pasireotide group, and 4 (7%) and 7 (13%) patients that discontinued due to adverse events. Overall, our review shows that adverse events were not well described. This may be because octreotide-LAR has a favorable safety profile and that modest increases in the dose may not lead to significant increases in toxicity, or because the studies were too small to identify rare events.
| Ref. | Safety |
| Anthony et al[11] 2011 | NA/NR (adverse events not broken down by SLAR dose); of 392 pts., 8.7% had hyperglycemia, 6.4% had cholelithiasis, 2.8% had cholecystitis, 2.3% had steatorrhea, and 1.5% had hypoglycemia, and 22% had ≥ 1 adverse event during SLAR use |
| Chadha et al[12] 2009 | p 4129: “No treatment related toxicities were reported.” |
| Ferolla et al[14] 2012 | p 329: “No additional toxicity was recorded for the schedule treatment with LAR 30 mg every 21 d when compared with standard LAR dose and no treatment discontinuation or dose modification was required. Adverse events observed in patients in treatment with LAR 30 mg every 21 d were diarrhea in 1, abdominal pain in 1, cholelithiasis in 2, pyrexia in 1 patient. No constipation, dizziness, arterial hypertension or any other adverse event was observed.” |
| Koumarianou et al[15] 20101 | “Patients reported no significant symptoms related to treatment adverse events. Two patients experienced a grade I neutropenia and one patient a grade II thrombocytopenia. One patient did not receive treatment due to persistent nausea and vomiting resistant to metoclopramide.” |
| Ludlam et al[21] 2011 | p 838: “A trial designed to compare two dose levels of octreotide LAR (30 and 40 mg/mo) highlighted the ability of octreotide LAR to control diarrhea in patients with active or prior chemotherapy-induced diarrhea. Fewer patients in the 40 mg/mo group compared with those in the 30 mg/mo group experienced severe diarrhea (62% vs 48%; P = 0.14), required intravenous fluid (32% vs 19%; P = 0.10), or had diarrhea-related unscheduled healthcare visits (42% vs 28%; P = 0.11). Most importantly, adverse events were balanced between the two groups.” |
| Markovich et al[16] 20121 | "Tolerability of long-acting octreotide in a dose of 30-40 mg was satisfactory for all pts.” |
| Valle et al[17] 20011 | "All patients found the long-acting analogue acceptable and none requested a change back to conventional daily octreotide. Sandostatin LAR is an alternative somatostatin analogue that is highly acceptable to patients; doses may be safely escalated if required.” |
| Wolin et al[19] 20131 | “Hyperglycemia (11% vs 0%), diarrhea (9% vs 7%), and abdominal pain (2% vs 9%) were the most common grade 3/4 AEs in the pasireotide-LAR (P) vs octreotide-LAR (O) arms in the core phase, and 7 (13%) and 4 (7%) patients discontinued due to AE. P and O showed a similar safety profile except for the higher frequency of hyperglycemia in P.” |
Expert clinical opinions were reported in 8 articles[2,8-10,12,13,22,23] (Table 4). Both Oberg et al[9] and Strosberg et al[2] supported the idea of increasing octreotide-LAR dose up to 60 mg/mo for control of symptoms. Chadha et al[12], Colao et al[8], and Costa et al[13] further suggested that increased doses of octreotide-LAR may be effective for tumor progression. Strosberg et al[22] reported above-label dosing of octreotide-LAR is common in NCCN institutions, and the primary indication is refractory carcinoid syndrome. Yao et al[10] cautioned that until there are appropriate studies (i.e., randomized controlled trials) completed, escalating doses of octreotide-LAR should be done only for control of symptoms. Xu et al[23] reported that a substantial number of patients in this study required doses greater than the FDA approved dose of 30 mg/mo. Overall, our review indicated that clinical experts support dose escalation of octreotide-LAR.
| Ref. | Quoted expert clinical opinion |
| Chadha et al[12] 2009 | p 4130: "Our experience and those of others have shown that S-LAR can provide disease control to prolong time needed for liver- directed therapies and systemic therapies. Dose escalation provides an opportunity to spare patients from morbidities associated with these procedures and systemic therapy." |
| Colao et al[8] 2010 | p 290: “The dosages of SSAs currently used are probably insufficient to determine control of hormone secretion and tumor growth both in acromegaly and NETs.” |
| Costa et al[13] 20061 | "Dose increase with octreotide LAR should be offered to those patients who progress after achieving a first objective response with SS analogues." |
| Oberg et al[9] 2004 | p 970: "As a general rule, if the total IR dose is 200-600 μg/d, LAR 20 mg should be tried, and if total IR dose is 750-1500 μg/d, LAR 30 mg should be tried. The LAR doses range from 20 to 60 mg/28 d. Supplementary administration with the IR form of octreotide in patients escaping anti-secretory response is often required during long-term treatment with LAR. If it is necessary to give the patient rescue doses of IR octreotide three or four times per week, increase the LAR dose to 30 mg/4 wk, or reduce the interval between administrations of the depot formulation (e.g., 20 mg/3 wk). Furthermore, the temporal occurrence of hypersecretion during the 4-week dosing interval should be considered. For example, if the rescue s.c. therapy is required during the week before the next injection of LAR, then a reduction of the dosing interval by 1 wk is advisable. On the other hand, if the need for rescue medication occurs sporadically throughout the month then increasing the dose stepwise by 10 mg/mo up to 60 mg/mo should be tried. Doses of LAR > 60 mg/mo are rarely of added value." |
| Strosberg et al[2] 2013 | p 5: "In patients with uncontrolled secretory symptoms, increasing the dose/frequency of SSAs is appropriate (median rating, 8), particularly among patients who had previously responded to a lower dose. The panel considered dose escalations of octreotide LAR up to 60 mg/4 wk (median rating, 7) or up to 40 mg/3 wk (median rating, 7) to be reasonable adjustments for refractory carcinoid syndrome. Increasing the dose/frequency of SSAs may be considered in patients with radiographic progression, particularly those whose disease was previously stabilized at a lower dose. The panel considered an increase in dose/frequency up to 40 mg/3 or 4 wk to be reasonable (median rating: 4-5.5). There is a lack of evidence that increasing the dose/frequency of SSAs slows radiographic progression." |
| Strosberg et al[22] 20131 | “Above label dosing of octreotide LAR is common in NCCN institutions. The primary indication is refractory carcinoid syndrome.” |
| Xu et al[23] 20121 | "Our analyses showed that patients frequently required the escalation of octreotide LAR dose during their course of illness. A substantial number of patients required doses greater than the FDA approved dose of 30 mg/mo." |
| Yao et al[10] 2008 | p 338: "Although octreotide has proven to be a safe and efficacious drug for carcinoid syndrome, it nonetheless can cause adverse events including steatorrhea, cholelithiasis, and hyperglycemia. Until conclusive data from randomized studies are available to show that octreotide has a disease-stabilizing effect and at what does this effect occurs in humans, we advocate titrating the octreotide LAR dose according to symptoms rather than to an arbitrary blood level." |
There is a general trend supporting the use of high dose octreotide-LAR for control of symptoms and limited data supporting the use of high-dose octreotide for control of tumor progression in patients with NETs. There were no published data identified suggesting increased toxicity of octreotide-LAR at above FDA-approved dosage and frequency of administration. The lack of data may imply that there is no significant toxicity, which is consistent with reports of higher doses used in acromegaly without significant toxicity[21], or that the studies were too small to identify uncommon adverse events. Several studies provided expert clinical opinion statements, mostly endorsing use of above-label doses of octreotide-LAR for patients with symptom or tumor progression.
There were 4 articles and 6 abstracts that reported on the efficacy of octreotide-LAR > 30 mg/mo in over 260 patients[11-20]. Ferolla et al[14] showed efficacy of octreotide-LAR > 30 mg/mo, indicating that biochemical and tumor progression were more rapid with lower dose using historical controls. The key results reported by Weber et al[18], demonstrating that octreotide-LAR is commonly prescribed in doses or schedules above the label-recommended dose and frequency for refractory carcinoid syndrome, are now available in greater detail in a full-length article by Strosberg et al[24]. No studies showed a negative effect on efficacy of high dose octreotide-LAR. In most studies, higher octreotide-LAR doses were used as rescue or salvage therapy, and it may be that response rates would be higher in treatment-naïve patients.
Our review of 4 articles and 4 abstracts, evaluating over 220 patients[11,12,14-17,19,21], found no evidence of increased toxicity associated with doses of octreotide-LAR > 30 mg/mo. None of the studies included in this review reported significant toxicity, although these studies did not report power analyses or a priori calculations of sample size.
Expert clinical opinion expressed in 6 of 8 articles[2,8,9,12,13,22] supported the use of higher doses of octreotide-LAR when lower doses are inadequate to control disease. One study reported that above-label octreotide-LAR is commonly prescribed in NCCN institutions[22]. Most experts suggested that higher doses should be used in cases where there is tumor progression or lack of symptom control on lower doses. Yao et al[10] questioned the use of higher doses due to lack of high-level evidence supporting this practice.
The strength of this review lies in its comprehensive search, review, and synthesis of global literature on above the FDA-approved label dosage and frequency of administration of octreotide-LAR in patients with NETs. This study also has limitations. The data on symptom improvement are limited by lack of quantitative measurements of symptom severity and absence of formal quality of life analysis. Nine of the 17 included studies were retrospective, and retrospective studies may be subject to recall and reporting biases. The only prospective trial reporting efficacy data in a full-length article[14] used historical controls, which may limit estimation of effects. Wolin et al[19] reported on a randomized clinical trial of pasireotide-LAR (comparator arm) vs octreotide-LAR 40 mg/28 d. However, this trial did not assess other doses of octreotide-LAR. The studies included in this review varied in design, patient population, octreotide-LAR regimens, and definitions of outcomes, and the data were reported in several ways across the reviewed studies. Thus, the heterogeneity of these data made it difficult to compare directly between studies and prevented us from conducting a meta-analysis. OCEBM grades ranged from 2b to 5, with 9 studies out of 17 scoring 4 or 5, indicating a relatively low quality of evidence (grade C)[7]. Of the 10 studies that reported data on efficacy and of the 8 that reported data on safety, 5 and 4 were small (< 100 patients) retrospective studies, respectively. Finally, in this study, we were unable to assess separately the effects of octreotide-LAR co-therapy vs monotherapy on safety and efficacy outcomes given the limited data for co-therapy in this review.
Future research efforts should focus on establishing efficacy and safety of prescribing octreotide-LAR at doses higher than the FDA-approved 30 mg/mo for management of neuroendocrine tumors using generalizable randomized controlled trial study designs. Studies comparing the safety of octreotide-LAR in patients treated with doses < 30 mg/mo vs those treated with above-label regimens of octreotide-LAR are also warranted.
In conclusion, this systematic literature review suggests that above-label doses of octreotide-LAR are being used frequently for the management of NETs in clinical practice and excess toxicity has not been observed in the reviewed studies. In most articles reviewed, above-label octreotide-LAR appears to be prescribed in patients with disease progression or uncontrolled symptoms while on standard dose therapy. Expert clinical opinion, as reported in this review, supports escalation of SSAs for patients with refractory hormonal symptoms. However, given limited published information on this topic, the safety and efficacy of above-label doses and/or frequency of octreotide-LAR in treatment of NETs warrants further evaluation.
Gastrointestinal neuroendocrine tumors (NETs) are rare and generally slow-growing neoplasms that secrete various peptides and neuroamines, some of which cause clinical symptoms. These tumors may be classified in multiple ways but are often divided into carcinoid tumors and pancreatic neuroendocrine tumors. First-line systemic therapy for NETs often consists of somatostatin analogs (SSAs) such as octreotide acetate or lanreotide.
The maximum Food And Drug Administration-approved dosage and administration of octreotide long-acting repeatable (LAR), indicated for severe diarrhea/flushing episodes associated with metastatic carcinoid tumors and VIPomas, is 30 mg every 4 wk. However, in clinical practice, octreotide-LAR is sometimes prescribed at above-label doses but evidence for this practice has not been systematically assessed.
This systematic literature review suggests that above-label doses of octreotide-LAR are being used frequently for the management of NETs in clinical practice and excess toxicity was not observed in the reviewed studies. In most articles reviewed, above-label octreotide-LAR appears to be prescribed in patients with disease progression or uncontrolled symptoms while on standard dose therapy. Expert clinical opinion, as reported in this review, supports escalation of SSAs for patients with refractory hormonal symptoms. However, given limited published information on this topic, the safety and efficacy of above-label doses and/or frequency of octreotide-LAR in treatment of NETs warrants further evaluation.
The strength of this study lies in its comprehensive search, review, and synthesis of global literature on above the FDA-approved label dosage and frequency of administration of octreotide-LAR in patients with NETs. Thus, the findings summarized in this review may inform clinicians who manage patients with NETs and motivate future research in this field.
This is an informative, well conducted and documented review about a focused topic on the use of above-label doses of octreotide-LAR in patients with NETs.
| 1. | Vinik AI, Woltering EA, Warner RR, Caplin M, O’Dorisio TM, Wiseman GA, Coppola D, Go VL. NANETS consensus guidelines for the diagnosis of neuroendocrine tumor. Pancreas. 2010;39:713-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 193] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 2. | Strosberg JR, Fisher GA, Benson AB, Malin JL, Anthony LB, Arslan B, Gibbs JF, Greeno E, Iyer RV, Kim MK. Systemic treatment in unresectable metastatic well-differentiated carcinoid tumors: consensus results from a modified delphi process. Pancreas. 2013;42:397-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Rinke A, Müller HH, Schade-Brittinger C, Klose KJ, Barth P, Wied M, Mayer C, Aminossadati B, Pape UF, Bläker M. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J Clin Oncol. 2009;27:4656-4663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1609] [Cited by in RCA: 1789] [Article Influence: 105.2] [Reference Citation Analysis (0)] |
| 4. | Sandostatin LAR Depot [prescribing information]. East Hanover, New Jersey: Novartis Pharmaceuticals Corporation; 2011. Accessed 7/30/14. Available from: http://www.accessdata.fda.gov/scripts/cder/drugsatfda/. |
| 5. | DistillerSR (Version 2.0). (2012) Ottawa, Ontario, Canada: Evidence Partners Incorporated. Available from: http://systematic-review.net. |
| 6. | Viera AJ, Garrett JM. Understanding interobserver agreement: the kappa statistic. Fam Med. 2005;37:360-363. [PubMed] |
| 7. | OCEBM Levels of Evidence Working Group. "The Oxford 2011 Levels of Evidence". Oxford Centre for Evidence-Based Medicine. Available from: http://www.cebm.net/index.aspx?o=5653. |
| 8. | Colao A, Faggiano A, Pivonello R. Somatostatin analogues: treatment of pituitary and neuroendocrine tumors. Prog Brain Res. 2010;182:281-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Oberg K, Kvols L, Caplin M, Delle Fave G, de Herder W, Rindi G, Ruszniewski P, Woltering EA, Wiedenmann B. Consensus report on the use of somatostatin analogs for the management of neuroendocrine tumors of the gastroenteropancreatic system. Ann Oncol. 2004;15:966-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 379] [Cited by in RCA: 367] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 10. | Yao JC, Kvols LK. Octreotide LAR in carcinoid: how to dose? Pancreas. 2008;37:337-338; author reply 338-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 11. | Anthony L, Vinik AI. Evaluating the characteristics and the management of patients with neuroendocrine tumors receiving octreotide LAR during a 6-year period. Pancreas. 2011;40:987-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Chadha MK, Lombardo J, Mashtare T, Wilding GE, Litwin A, Raczyk C, Gibbs JF, Kuvshinoff B, Javle MM, Iyer RV. High-dose octreotide acetate for management of gastroenteropancreatic neuroendocrine tumors. Anticancer Res. 2009;29:4127-4130. [PubMed] |
| 13. | Costa L, Quintela A, Rodrigues T, Pinto A, Távora I. Somatostatin Analogues to Control Disease Progression in Neuroendocrine Tumors (NET): A Review of Patients’ Characteristics and Clinical Outcome. In: 3rd Annual ENETS Conference, March 22-24, 2006, Prague, Czech Republic. Available from: http://www.karger.com/Article/Pdf/93339. |
| 14. | Ferolla P, Faggiano A, Grimaldi F, Ferone D, Scarpelli G, Ramundo V, Severino R, Bellucci MC, Camera LM, Lombardi G. Shortened interval of long-acting octreotide administration is effective in patients with well-differentiated neuroendocrine carcinomas in progression on standard doses. J Endocrinol Invest. 2012;35:326-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 30] [Reference Citation Analysis (0)] |
| 15. | Koumarianou A, Zilos A, Syrios J, Kanakis G, Antoniou S, Liaskos K, Dokou A, Pectasides D, Tsavaris N, Kaltsas G. Metronomic Combination Therapy Including Temozolamide, Bevacizumab and Somatostatin Analogue for the Treatment of Malignant Gastroenteropancreatic Neuroendocrine Tumors. In: 7th Annual ENETS Conference, March 11-12, 2010, Berlin, Germany. Available from: http://www.karger.com/Article/Abstract/313935. |
| 16. | Markovich A, Emelianova G, Gorbounova V, Bruzgin V, Orel N. Long Acting Octreotide in Pts with Disseminated Neuroendocrine Tumors. In: ESMO 2012 Congress, September 28-October 2, 2012, Vienna, Austria. Available from: http://annonc.oxfordjournals.org/content/supplemental. |
| 17. | Valle JW, Clayton A, Danson S, Burgess A, Meehan M, Hawkins RE. The Use of Sandostation-LAR in the Treatment of Carcinoid Syndrome Experience from a Single Centre. In: 37th Annual Meeting of the American Society of Clinical Oncology (ASCO), May 12-15, 2001, San Francisco, CA. Accessed December 2012. Available from: http://www.asco.org/ASCOv2/Meetings/Abstracts. |
| 18. | Weber JM, Feldman M, Kvols L, Strosberg JR. Above-label doses of octreotide-LAR in patients with metastatic small intestinal carcinoid tumors. In: 48th American Society of Clinical Oncology (ASCO) Annual Meeting 2012, June 1-5, 2012, Chicago, IL. Available from: http://www.asco.org/ASCOv2/Meetings/Abstracts. |
| 19. | Wolin EM, Jarzab B, Eriksson B, Walter T, Toumpanakis C, Morse M, Tomassetti P, Weber M, Fogelman DR, Ramage J. A multicenter, randomized, blinded, phase III study of pasireotide LAR versus octreotide LAR in patients with metastatic neuroendocrine tumors (NET) with disease-related symptoms inadequately controlled by somatostatin analogs [abstract]. In: 2013 American Society of Clinical Oncology Annual Meeting; 2013 May 31-June 4; Chicago, IL. J Clin Oncol. 2013;31 suppl:abstract 4031. |
| 20. | Woltering EA, Hilton RS, Zolfoghary CM, Thomson J, Zietz S, Go VL, Vinik AI, Vinik E, O’Dorisio TM, Mamikunian G. Validation of serum versus plasma measurements of chromogranin a levels in patients with carcinoid tumors: lack of correlation between absolute chromogranin a levels and symptom frequency. Pancreas. 2006;33:250-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 21. | Ludlam WH, Anthony L. Safety review: dose optimization of somatostatin analogs in patients with acromegaly and neuroendocrine tumors. Adv Ther. 2011;28:825-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Strosberg JR, Bobiak S, Zornosa CC, Choti MA, Bergsland EK, Benson AB, Bloomston M, Kulke M, Shah MH, Ya JC. Dosing patterns for octreotide LAR in neuroendocrine tumor (NET) patients: NCCN NET outcomes database [abstract]. J Clin Oncol. 2013;31 suppl:abstract 4142. |
| 23. | Xu Y, Shih YT, Leary C, Shen C, Yao JC. Dosing patterns of octreotide LAR among elderly patients with neuroendocrine tumors: Analysis of the SEER-Medicare database. In: 48th American Society of Clinical Oncology (ASCO) Annual Meeting 2012, June 1-5, 2012, Chicago, IL. Available from: http://www.asco.org/ASCOv2/Meetings/Abstracts. |
| 24. | Strosberg J, Weber J, Feldman M, Goldman J, Almhanna K, Kvols L. Above-Label Doses of Octreotide-LAR in Patients With Metastatic Small Intestinal Carcinoid Tumors. Gastrointest Cancer Res. 2013;6:81-85. [PubMed] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Gevers TJG, Gonzalez-Reimers E, Paydas S S- Editor: Qi Y L- Editor: A E- Editor: Liu XM