Published online Feb 7, 2015. doi: 10.3748/wjg.v21.i5.1479
Peer-review started: August 5, 2014
First decision: August 27, 2014
Revised: September 9, 2014
Accepted: October 15, 2014
Article in press: October 15, 2014
Published online: February 7, 2015
Processing time: 189 Days and 1.4 Hours
AIM: To investigate the interaction between Xiaotan Sanjie (XTSJ) decoction and interleukin-8 (IL-8) and its effect on adhesion, migration and invasion of SGC-7901 gastric cancer cells.
METHODS: SGC-7901 gastric cancer cells were exposed to serum containing XTSJ decoction and/or IL-8 (1 ng/mL). SGC-7901 cell adhesion to fibronectin, an extracellular matrix component, was detected using the Cell Counting Kit-8. Migration and invasion abilities of SGC-7901 cells were detected by scratch wound and Transwell chamber assays. Then, protein (immunofluorescence and Western blot) and mRNA levels (quantitative polymerase chain reaction) of cluster of differentiation 44 (CD44), a cell adhesion molecule, were measured in 72-h-cultured SGC-7901 cells.
RESULTS: Cell adhesion was promoted by IL-8 (P = 0.001), but was inhibited by XTSJ decoction (P = 0.0001). Similarly, IL-8 promoted SGC-7901 cell invasion (P = 0.003), and XTSJ decoction inhibited cell invasion (P = 0.001). IL-8 induced SGC-7901 cell migration, but this was inhibited by XTSJ decoction. IL-8 up-regulated CD44 protein (P = 0.028) and mRNA expression (P = 0.002), whereas XTSJ decoction inhibited CD44 protein expression (P = 0.0001), but not mRNA expression (P = 0.275). An interaction between XTSJ decoction and IL-8 was confirmed in the invasion (P = 0.001) and CD44 mRNA expression of SGC-7901 cells (P = 0.010), but not in cell adhesion (P = 0.051).
CONCLUSION: XTSJ decoction may inhibit adhesion, migration and invasion of gastric cancer cells, which is partly associated with down-regulation of IL-8.
Core tip: SGC-7901 gastric cancer cells were used to evaluate the effects of Xiaotan Sanjie (XTSJ) decoction on adhesion, migration and invasion of gastric cancer cells and the role of interleukin-8 (IL-8) in these effects in vitro. This study focused on the interaction between XTSJ decoction and IL-8. XTSJ decoction significantly inhibited adhesion, migration and invasion of SGC-7901 cells, which was partly associated with down-regulation of IL-8. The results reflect the therapeutic potential of XTSJ decoction for preventing metastasis of gastric cancer.
- Citation: Shi J, Wei PK. Xiaotan Sanjie decoction inhibits interleukin-8-induced metastatic potency in gastric cancer. World J Gastroenterol 2015; 21(5): 1479-1487
- URL: https://www.wjgnet.com/1007-9327/full/v21/i5/1479.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i5.1479
Gastric cancer is one of the most aggressive tumors due to its invasion and metastasis abilities[1]. The key steps of invasion and metastasis include tumor cell transformation, growth, angiogenesis, invasion, dissemination in the circulation, and subsequent adhesion and colonization of secondary organs[2,3]. Therefore, the adhesion, migration and invasion capacities of cancer cells are regarded as important prerequisites of cancer metastasis[4].
Interleukin-8 (IL-8), a significant regulatory autocrine factor within the tumor microenvironment, is likely to be produced by a variety of cancer cells[5]. IL-8 may be strongly correlated with gastric cancer and play an important role in the progression of gastric cancer[6]. Furthermore, IL-8 promotes adhesion, migration and invasion of SGC-7901 gastric cancer cells in vitro[7,8]. All these results indicate that IL-8 may be a promoter of adhesion, migration and invasion in gastric cancer.
Traditional Chinese medicine (TCM) has confirmed preventive effects against gastric cancer metastasis[9]. Xiaotan Sanjie (XTSJ) decoction is an empirical compound formula based on the phlegm theory of gastric cancer, which is an academic theory within TCM. XTSJ decoction can prevent progression of gastric cancer in vitro and in vivo. Although the underlying mechanisms of this effect are still not fully understood, several potential pathways have been identified, such as decreasing lymphatic microvessel density[10-12]. We reported that XTSJ decoction significantly decreases the protein expression of IL-8 and its receptors in tumor xenografts and normal tissues adjacent to the tumor[13]. Therefore, we speculated that XTSJ decoction could inhibit adhesion, migration and invasion of gastric cancer by decreasing IL-8 expression. The purpose of this study was to provide direct information on the role of XTSJ decoction and the interaction between IL-8 and XTSJ decoction in adhesion, migration and invasion of gastric cancer.
SGC-7901 human gastric cancer cells were purchased from the cell bank of the Chinese Academy of Sciences (Shanghai, China). SGC-7901 cells were grown in RPMI-1640 medium (Wisent, Canada) supplemented with 10% fetal bovine serum (FBS, Tianhang, Hangzhou, China), 1% penicillin/streptomycin and 1% L-glutamine, and maintained at 37 °C in a humidified chamber containing 5% CO2.
All decoction components were purchased from Shanghai Leiyunshang Pharmacy Co., Ltd (China). The decoction (10.3 g/mL) was extracted by Shanghai Changzheng Hospital Manufacturing Laboratory (Shanghai, China). High-performance liquid chromatography-DAD (HPLC-DAD) fingerprinting was used to control the quality of the decoction. We used 0.9% normal saline to dilute the extraction to a predetermined concentration.
Twenty male Sprague Dawley rats (200-220 g) were purchased from Shanghai Slac Laboratory Animal CO., Ltd (Shanghai, China) and maintained in specific pathogen-free (SPF) conditions for 1 wk before the experiment. The rats were randomly divided into two groups (blank serum group and XTSJ decoction-containing serum group) and had free access to food and water until 12 h before blood collection. The blank serum group (n = 10) was treated with 0.9% normal saline, and the XTSJ decoction-containing serum group (n = 10) was treated with XTSJ decoction (61.8 g/kg each time, 10 times the equivalent dose used in humans) via intragastric administration (4 mL each time, twice a day for three consecutive days). All rats were anesthetized by intraperitoneal injection of 1% pentobarbital sodium (40 mg/kg), and then blood was collected from the abdominal aorta 1 h after the final intragastric administration. These blood samples were placed at 4 °C for 4 h and centrifuged at 3000 r/min for 15 min. After separation, the sera from the same group were mixed well, heated to inactivation in a 56 °C water bath for 30 min, filtered through a 0.22 μm membrane filter and then stored at -70 °C. Two sera were named the blank serum and XTSJ decoction serum, respectively.
First, we established a blank group (pure culture medium) and blank serum group (10% blank serum) to investigate the influence of blank serum. Four groups were subsequently established according to various interventions: blank group (pure culture medium), IL-8 group (1.0 ng/mL IL-8), XTSJ group (10% XTSJ decoction serum) and XTSJ + IL-8 group (10% XTSJ decoction serum + 1.0 ng/mL IL-8).
Fibronectin is an extracellular matrix component. We analyzed the attachment of SGC-7901 cells to fibronectin using the Cell Counting Kit-8 (Dojindo, Japan). Briefly, 96-well plates were coated with fibronectin 100 μg (Sigma, United States) overnight at 4 °C. After three washes with phosphate-buffered saline (PBS) solution containing 1% bovine serum albumin (BSA) to block nonspecific cell adhesion, 1 × 105 cells/well were added in the presence of the various interventions for 2 h. A formazan generation-inducing reagent, WST-8 (10 μL), was then added to the cells after washing with PBS. The cells were cultured for a further 4 h. Colorimetric absorbance was measured by a microplate reader at 450 nm to obtain an optical density (OD) value. OD ultimate value = OD measured value – OD blank value.
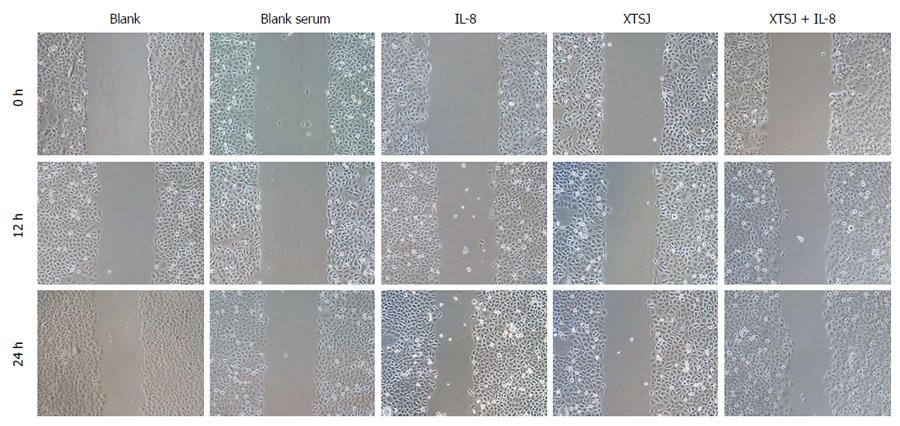
Cell migration was evaluated with a scratch wound assay. SGC-7901 cells (2 × 105 cells/well) were seeded in a 6-well plate. A scratch was made with a 10 μL pipette tip in a confluent cell monolayer. After washing twice, various interventions were added in serum-free medium. The wells were photographed at the beginning of the experiment and after 12 h and 24 h using an Olympus CK40-F200 inverted microscope (Olympus, Tokyo, Japan). Digital images were obtained with a MicroFire digital camera driven by PictureFrame imaging software.
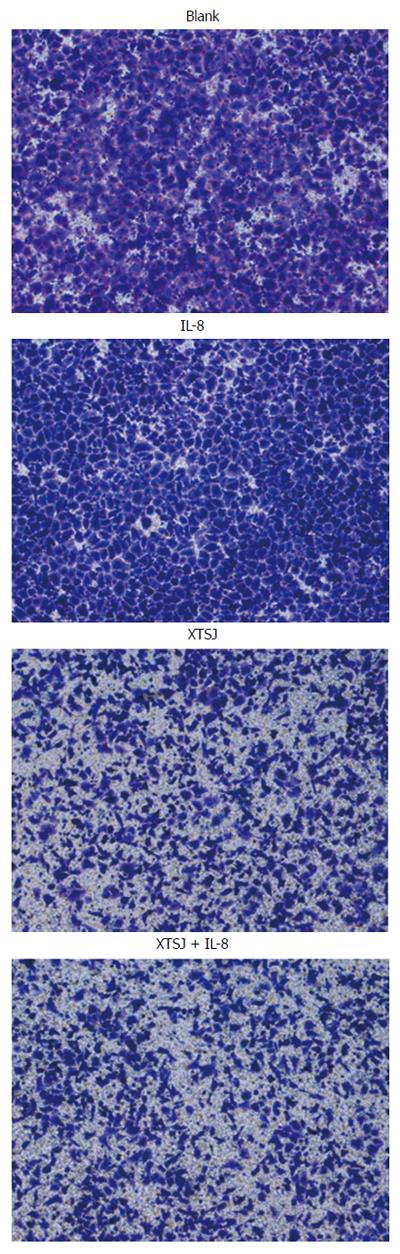
We examined the invasion ability of SGC-7901 cells using Transwell chambers (Corning, United States) according to the manufacturer’s protocol. Briefly, SGC-7901 cells (8 × 104) were seeded in the upper chamber containing a thin layer of Matrigel basement membrane matrix. Thereafter, 600 μL culture medium and various interventions were added to the lower chamber. After 24 h incubation, the cells remaining on the upper side of the membrane (noninvasive cells) were removed with a cotton swab. The cells that had attached to the lower side of the membrane (invasive cells) were fixed with 4% paraformaldehyde for 15 min and then stained with a crystal violet cell colony staining kit (GenMed, China) according to the manufacturer’s protocol. The results are expressed as the mean number of cells invading four random microscopic fields (magnification, × 10).
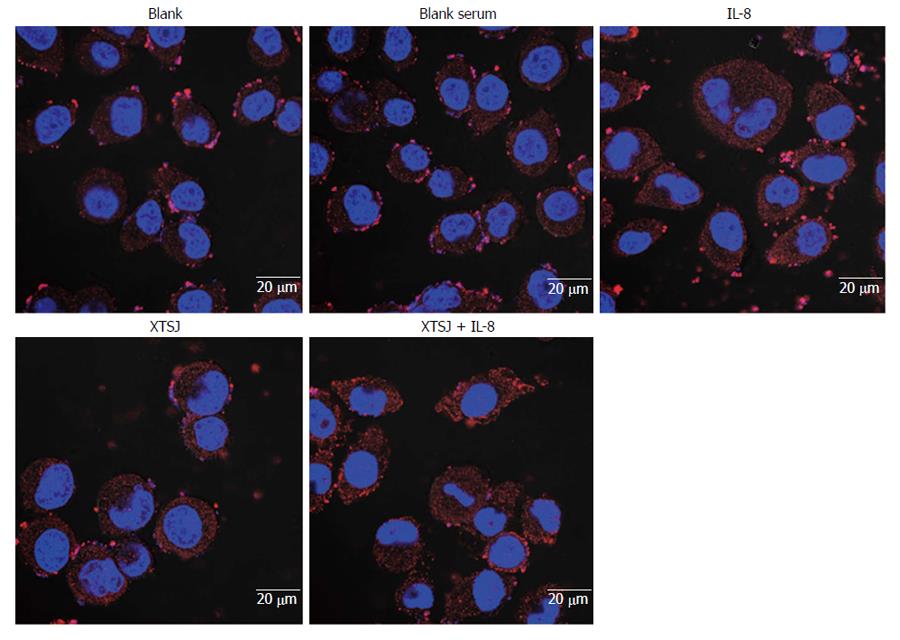
SGC-7901 cells (2 × 105) were seeded onto coverslips in 6-well plates and cultured with the various interventions for 72 h. The cells were then fixed in 4% paraformaldehyde, permeabilized with 0.5% Triton-100 for 10 min, incubated in blocking buffer, and cultured with CD44 rabbit monoclonal antibody (1:80, Epitomics, United States) at 4 °C overnight, followed by the Cy3-conjugated AffiniPure goat anti-rabbit IgG (H + L) (1:1000 dilution, Proteintech Group, China) for an additional 1 h. Cell nuclei were labeled with 4′,6-diamidino-2-phenylindole (DAPI, Thermo Scientific, United States). The coverslips were then mounted on a glass slide and visualized under a laser confocal scanning microscope (LSM710, Zeiss, Germany).
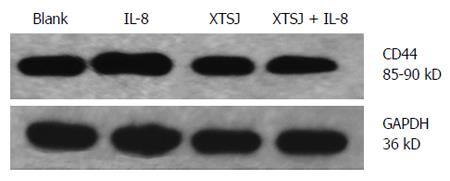
SGC-7901 cells (2 × 105) were seeded in 6-well plates and cultured for 72 h with various interventions. The cells were subsequently collected and decomposed by 150 μL loading buffer. Proteins in the total cell lysate were separated by SDS–PAGE (10% separation gel, 5% spacer gel) and electrotransferred to polyvinylidene difluoride membranes (Bio-Rad, United States). Blotted membranes were placed in blocking solution for 1 h at room temperature. CD44 rabbit monoclonal antibody (1:250, Epitomics, United States) was used to probe blots overnight at 4 °C. After washing thoroughly, the film was incubated with anti-rabbit IgG-HRP secondary antibody (1:1000, Santa Cruz, CA, United States) for 1 h at room temperature, and then visualized using the ECL method. Blots were exposed to plain X-ray film in a darkroom. Grayscale reconstruction was performed by Image J software, and the density of CD44 vs GAPDH protein (first antibody: mouse anti-human GAPDH monoclonal antibody from Sungene, China; secondary antibody: goat anti-mouse IgG-horse radish peroxidase from Santa Cruz, CA, United States), as an internal control protein, was calculated. All experiments were repeated three times.
SGC-7901 cells (1 × 105) were collected after 72 h cultivation with various interventions. In brief, total RNA in the cells was extracted using TRIzol reagent (TaKaRa, Japan) according to the manufacturer’s protocol and reverse-transcribed. Quantitative reverse transcription-polymerase chain reaction (RT-PCR) was performed with SYBR Green chemistry in a real-time PCR system (Bio-Rad iQ5, United States). Cycling conditions consisted of one cycle of 95 °C for 2 min, 95 °C for 15 s, 60 °C for 20 s, 72 °C for 20 s and then 40 cycles of 72 °C for 30 s. The PCR primers used for amplification are shown in Table 1. GAPDH mRNA was co-amplified as an internal control. Based on the 2-ΔΔCt value, relative levels of CD44 mRNA expression were calculated.
| mRNA | Sense primer sequence | bp |
| hGAPDH-F | 5’-GGGTGTGAACCATGAGAAGTATG-3’ | 145 |
| hGAPDH-R | 5’-GATGGCATGGACTGTGGTCAT-3’ | |
| CD44-F | 5’-ATGGACAAGTTTTGGTGGCA-3’ | 230 |
| CD44-R | 5’-CAGGTCTCAAATCCGATGCTC-3’ |
Statistical analysis was performed using SPSS 13.0. All data are expressed as the mean ± SD. One-way analysis of variance (ANOVA) was used to analyze the difference between the blank group and the blank serum group. ANOVA with a factorial design was used to assess the interaction between XTSJ decoction and IL-8 and their effects on invasion, adhesion and CD44 expression. A difference was considered statistically significant when the corresponding P value was ≤ 0.05.
We investigated the effect of IL-8 and XTSJ decoction on the adhesion capacity of SGC-7901 cells to fibronectin. Blank serum had no significant influence on SGC-7901 cell adhesion (P = 0.814). IL-8 promoted cell adhesion (P = 0.001), but XTSJ decoction inhibited cell adhesion (P = 0.0001). However, no interaction was found between them (P = 0.051) (Table 2).
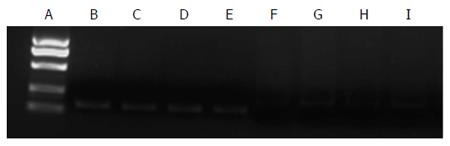
We investigated the effect of IL-8 and XTSJ decoction on the invasion capacity of SGC-7901 cells. The blank serum group was not different from the blank group (P = 0.872). IL-8 promoted cell invasion (P = 0.003), but XTSJ decoction inhibited cell invasion (P = 0.001). Of note, an interaction was observed between XTSJ decoction and IL-8 (P = 0.001): XTSJ decoction inhibited the invasion of SGC-7901 cells induced by IL-8 (Figure 1 and Table 2).
We investigated the effects of IL-8 and XTSJ decoction on the migration capacity of SGC-7901 cells. The scratch assay showed that blank serum had no significant influence on migration. XTSJ decoction inhibited migration. Although IL-8 induced SGC-7901 cell migration, this migration was inhibited by XTSJ decoction (Figure 2).
CD44 is a cell adhesion molecule involved in gastric cancer cell adhesion and invasion. We investigated the protein and mRNA expression of CD44. Blank serum had no effect on CD44 protein (P = 0.420) or mRNA (P = 0.684), but IL-8 up-regulated CD44 protein (P = 0.028) and mRNA (P = 0.002) (Table 3, Figures 3, 4, 5). XTSJ decoction inhibited CD44 protein expression (P = 0.0001), but not mRNA expression (P = 0.275), and an interaction was observed between XTSJ decoction and IL-8 in mRNA expression (P = 0.010) (Table 3, Figures 3-5).
Gastric cancer was the third leading cause of cancer mortality worldwide in 2012, responsible for 723027 deaths[14]. Metastasis is the most fatal characteristic of gastric cancer. Chinese herbal medicines have proven effects in preventing and treating gastric cancer[9,15]. This study aimed to evaluate the effects of XTSJ decoction on the adhesion, migration and invasion of gastric cancer cells and the role of IL-8 in its mechanism of action. The results show that XTSJ decoction inhibited the adhesion, migration and invasion of gastric cancer cells accompanied by partial down-regulation of IL-8, suggesting that XTSJ decoction may be a promising therapeutic to inhibit metastasis of gastric cancer.
Phlegm is a special etiological factor widely used to explain the pathogenesis of cancer within the TCM paradigm. Given the properties of gastric cancer, it is believed that gastric cancer is closely related to phlegm. Phlegm contamination is regarded as the most fundamental cause and pathogenesis of gastric cancer. Therefore, resolving phlegm and dispersing nodules is suggested as the preferred treatment for gastric cancer[16]. XTSJ decoction (Chinese national patent number: 2006100290635) is modified on the basis of Daotan decoction (a classical decoction for eliminating phlegm) and is composed of Pinelliae rhizome, Rhizoma arisaematis, Poria cocos, Aurantii fructus immaturus, Citri reticulatae viride pericarpium, Scorpio, Scolopendra, Galli gigerii endothelium corneum, Fritillariae cirrhosae bulbus, Semen brassicae, and Glycyrrhiza uralensis Fisch. Several Chinese herbal components of XTSJ decoction, including Rhizoma arisaematis, Scolopendra and Scorpio, inhibit the growth of various cancers. Active components of these decoction constituents have be identified, such as polysaccharides and glycoproteins[17-22]. We found an obvious absorption peak at 280 nm in the HPLC-DAD fingerprinting of XTSJ decoction aqueous extract, suggesting that the XTSJ decoction aqueous extract may contain certain active anti-cancer components.
Distant metastasis is regarded as an important sign of poor prognosis in patients with gastric cancer (e.g., lymph node, peritoneum and liver metastasis)[23,24]. Tumor cell metastasis is a multistage and complex process. Adhesion, migration and invasion of cancer cells are important events in the metastatic process of cancer[25]. IL-8 is not only a member of the CXC chemokine family, but also a multifunctional inflammatory cytokine. IL-8 is likely produced by a variety of human cancer cells, including melanoma[26], squamous cell carcinoma[27], cervical cancer[28], ovarian cancer[29], non-small-cell lung cancer[30], colon cancer[31] and even gastric cancer[32]. IL-8 is strongly correlated with gastric cancer and plays an important role in the development and metastasis of gastric cancer by autocrine or paracrine mechanisms[6,33]. The IL-8 level is significantly associated with the depth of invasion, venous invasion and lymphatic invasion, and may be an independent prognostic factor in human gastric carcinomas[34]. In a previous study, we reported that IL-8 may enhance adhesion, migration and invasion of SGC-7901 human gastric cancer cells[7]. Similarly, using cDNA and siRNA transfectants, Kuai et al[8] reported that IL-8 plays an essential role in the adhesion, migration, invasion and chemosensitivity of human gastric cancer cells. In the current study, we found that adhesion, migration, invasion and CD44 protein and mRNA expression were promoted by IL-8. These results suggest that IL-8 may be a direct promoter of adhesion, migration and invasion in gastric cancer.
We previously reported that XTSJ decoction prevents progression of gastric cancer via various pathways. For example, XTSJ decoction inhibits the proliferation and promotes the apoptosis of gastric cancer cells[35,36], decreases the expression of proliferating cell nuclear antigen and epidermal growth factor receptor[37], reduces microsatellite instability[10], inhibits the formation of vasculogenic mimicry[11], and decreases lymphatic microvessel density in gastric tumor xenografts[12]. In the current study, XTSJ decoction inhibited adhesion, migration, invasion and CD44 protein expression. These results suggest that XTSJ decoction may be an inhibitor of the spread and metastasis of gastric cancer.
XTSJ decoction can significantly decrease the protein expression of IL-8 and its receptors in tumor xenografts and normal tissues adjacent to the tumor[13]. In light of the inhibitory effect of XTSJ decoction on IL-8 expression, we focused on the interaction between XTSJ decoction and IL-8 as one of the possible mechanisms for the metastatic inhibition of XTSJ decoction in gastric cancer. In the current study, an interaction between XTSJ decoction and IL-8 in SGC-7901 cell invasion was confirmed: XTSJ decoction inhibited IL-8-induced invasion of SGC-7901 cells. This suggests that XTSJ decoction may inhibit the invasion of gastric cancer cells through down-regulation of IL-8 protein in gastric cancer. CD44, as a polymorphic integral membrane glycoprotein, plays an important role in matrix adhesion[38]. In gastric adenocarcinoma, CD44 is highly expressed and is correlated with a poor prognosis in patients with the intestinal type of gastric adenocarcinoma[39]. We focused on the CD44 protein and mRNA expression as a biological marker of adhesion. For adhesion of SGC-7901 cells, the interaction between XTSJ decoction and IL-8 approached statistical significance (P = 0.051). In addition, an interaction between XTSJ decoction and IL-8 in CD44 mRNA expression was confirmed: XTSJ decoction inhibited IL-8-induced enhanced CD44 mRNA expression. These findings suggest that XTSJ decoction may also reduce the adhesion of gastric cancer cells through down-regulation of IL-8 protein. This interaction between XTSJ decoction and IL-8 in adhesion of gastric cancer cells warrants further investigation.
In summary, these findings suggest that XTSJ decoction may inhibit the metastatic potential of gastric cancer cells, including their adhesion, migration and invasion. These effects were partly associated with down-regulation of IL-8.
Traditional Chinese medicine (TCM) has seen increased acceptance in recent decades. in particular, its effects in the prevention and treatment of cancer have been confirmed. Xiaotan Sanjie (XTSJ) decoction, an empirical compound formula, can prevent progression of gastric cancer and decrease interleukin-8 (IL-8) protein expression in gastric tumor xenografts. IL-8 is a promoter of adhesion, migration and invasion in gastric cancer. Whether XTSJ decoction inhibits adhesion, migration and invasion of gastric cancer by decreasing IL-8 level remains unclear.
This study was performed to provide direct information on the effect of XTSJ decoction, the interaction between IL-8 and XTSJ decoction and their effects on the adhesion, migration and invasion of gastric cancer. Adhesion, migration and invasion abilities were detected by the Cell Counting Kit-8, scratch wound assay and Transwell chamber assay, respectively. The protein and mRNA levels of CD44 were measured. The results show that XTSJ decoction inhibits the adhesion, migration and invasion of gastric cancer, which was partly associated with down-regulation of IL-8.
This study suggested that XTSJ decoction inhibits adhesion, migration and invasion of SGC-7901 gastric cancer cells partly via down-regulation of IL-8.
The results indicate that IL-8 is partly involved in the inhibition of adhesion, migration and invasion of gastric cancer cells by XTSJ decoction. However, further investigation is needed to evaluate the interaction between IL-8 and XTSJ decoction in other metastatic pathways.
TCM has become well known for its effects in the prevention and treatment of cancer. XTSJ decoction is a traditional Chinese medicine modified on the basis of Daotan decoction (a classical decoction for eliminating phlegm).
To investigate the interaction between XTSJ decoction and IL-8 on adhesion, migration and invasion of gastric cancer cells, the authors used gastric cancer SGC-7901 cells exposed to the serum containing XTSJ decoction and/or IL-8. They found that cell adhesion, migration, invasion abilities and CD44 protein expression were promoted by IL-8, in contrast were inhibited by XTSJ decoction. XTSJ decoction had insignificant influence on CD44 mRNA expression, which was promoted by IL-8. These results suggested that XTSJ decoction could inhibit adhesion, migration and invasion of gastric cancer cells.
| 1. | Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13286] [Cited by in RCA: 13561] [Article Influence: 645.8] [Reference Citation Analysis (3)] |
| 2. | Jin X, Zhu Z, Shi Y. Metastasis mechanism and gene/protein expression in gastric cancer with distant organs metastasis. Bull Cancer. 2014;101:E1-12. [PubMed] |
| 3. | Goubran HA, Kotb RR, Stakiw J, Emara ME, Burnouf T. Regulation of tumor growth and metastasis: the role of tumor microenvironment. Cancer Growth Metastasis. 2014;7:9-18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 153] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 4. | Martin TA, Mason MD, Jiang WG. Tight junctions in cancer metastasis. Front Biosci (Landmark Ed). 2011;16:898-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 78] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 5. | Campbell LM, Maxwell PJ, Waugh DJ. Rationale and Means to Target Pro-Inflammatory Interleukin-8 (CXCL8) Signaling in Cancer. Pharmaceuticals (Basel). 2013;6:929-959. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 112] [Article Influence: 8.6] [Reference Citation Analysis (1)] |
| 6. | Kitadai Y, Haruma K, Mukaida N, Ohmoto Y, Matsutani N, Yasui W, Yamamoto S, Sumii K, Kajiyama G, Fidler IJ. Regulation of disease-progression genes in human gastric carcinoma cells by interleukin 8. Clin Cancer Res. 2000;6:2735-2740. [PubMed] |
| 7. | Ju D, Sun D, Xiu L, Meng X, Zhang C, Wei P. Interleukin-8 is associated with adhesion, migration and invasion in human gastric cancer SCG-7901 cells. Med Oncol. 2012;29:91-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Kuai WX, Wang Q, Yang XZ, Zhao Y, Yu R, Tang XJ. Interleukin-8 associates with adhesion, migration, invasion and chemosensitivity of human gastric cancer cells. World J Gastroenterol. 2012;18:979-985. [PubMed] [DOI] [Full Text] |
| 9. | Liu X, Hua BJ. [Effect of traditional Chinese medicine on quality of life and survival period in patients with progressive gastric cancer]. Zhongguo Zhongxiyi Jiehe Zazhi. 2008;28:105-107. [PubMed] |
| 10. | Ye M, Sun DZ, Wei PK. [Inhibitory effect of Xiaotan Sanjie Recipe on the microsatellite instability of orthotopic transplantation tumor in MKN-45 human gastric cancer nude mice]. Zhongguo Zhongxiyi Jiehe Zazhi. 2014;34:592-596. [PubMed] |
| 11. | Zhou W, Li YJ, Wei PK. [Effects of xiaotan sanjie recipe on vasculogenic mimicry of human gastric cancer xenografts in nude mice]. Zhongguo Zhongxiyi Jiehe Zazhi. 2011;31:532-536. [PubMed] |
| 12. | Pang B, Wei PK, Li YJ. [Effect of xiaotan sanjie recipe on expressions of VEGF-C and VEGFR-3 in nude mice with transplanted human gastric adenocarcinoma cell MKN-45]. Zhongguo Zhongxiyi Jiehe Zazhi. 2011;31:204-208. [PubMed] |
| 13. | Ju DW, Wei PK, Lin HM, Sun DZ, Yu S, Xiu LJ. [Effects of Xiaotan Sanjie Decoction on expressions of interleukin-8 and its receptors in gastric tumor xenografts and gastric tissue adjacent to the tumor in mice]. Zhongxiyi Jiehe Xuebao. 2010;8:74-79. [PubMed] |
| 14. | Ferlay J, Soerjomataram I, Ervik M, Forman D, Bray F, Dikshit R, Elser S, Mathers C, Rebelo M, Parkin DM. GLOBOCAN 2012 v1.0, cancer incidence and mortality worldwide. IARC CancerBase 2013-11, cited 2014-08-10. Available from: http: //globocan.iarc.fr/. |
| 15. | Cao ND, Zhao AG, Yang JK. [Survival time of advanced gastric cancer patients treated with integrated traditional Chinese and Western medicine therapy]. Zhongxiyi Jiehe Xuebao. 2010;8:116-120. [PubMed] |
| 16. | Shi J, Wei PK. [The phlegm theory of gastric cancer]. Zhongxiyi Jiehe Xuebao. 2011;9:581-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Kang SA, Park HJ, Kim MJ, Lee SY, Han SW, Leem KH. Citri Reticulatae Viride Pericarpium extract induced apoptosis in SNU-C4, human colon cancer cells. J Ethnopharmacol. 2005;97:231-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Chen XY, Xu XJ, Zhang LN, Zeng FB. Chain conformation and anti-tumor activities of phosphorylated (1→3)-β-d-glucan from Poria cocos. Carbohydr Polym. 2009;78:581-587. [RCA] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 126] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 19. | Huang Q, Zhang L. Preparation, chain conformation and anti-tumor activities of water-soluble phosphated (1→3)-α-d-glucan from Poria cocos mycelia. Carbohydr Polym. 2011;83:1363-1369. [RCA] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 20. | Chen YY, Chang HM. Antiproliferative and differentiating effects of polysaccharide fraction from fu-ling (Poria cocos) on human leukemic U937 and HL-60 cells. Food Chem Toxicol. 2004;42:759-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 92] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 21. | Kwon KB, Kim EK, Lim JG, Jeong ES, Shin BC, Jeon YS, Kim KS, Seo EA, Ryu DG. Molecular mechanisms of apoptosis induced by Scorpio water extract in human hepatoma HepG2 cells. World J Gastroenterol. 2005;11:943-947. [PubMed] |
| 22. | Chung WT, Lee SH, Kim JD, Sung NS, Hwang B, Lee SY, Yu CY, Lee HY. Effect of the extracts from Glycyrrhiza uralensis Fisch on the growth characteristics of human cell lines: Anti-tumor and immune activation activities. Cytotechnology. 2001;37:55-64. [PubMed] |
| 23. | Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893-2917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 24. | Deng JY, Liang H. Clinical significance of lymph node metastasis in gastric cancer. World J Gastroenterol. 2014;20:3967-3975. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 142] [Cited by in RCA: 160] [Article Influence: 13.3] [Reference Citation Analysis (1)] |
| 25. | Trepat X, Chen Z, Jacobson K. Cell migration. Compr Physiol. 2012;2:2369-2392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 26. | Gabellini C, Trisciuoglio D, Desideri M, Candiloro A, Ragazzoni Y, Orlandi A, Zupi G, Del Bufalo D. Functional activity of CXCL8 receptors, CXCR1 and CXCR2, on human malignant melanoma progression. Eur J Cancer. 2009;45:2618-2627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 113] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 27. | Christofakis EP, Miyazaki H, Rubink DS, Yeudall WA. Roles of CXCL8 in squamous cell carcinoma proliferation and migration. Oral Oncol. 2008;44:920-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 44] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 28. | Wu S, Shang H, Cui L, Zhang Z, Zhang Y, Li Y, Wu J, Li RK, Xie J. Targeted blockade of interleukin-8 abrogates its promotion of cervical cancer growth and metastasis. Mol Cell Biochem. 2013;375:69-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 29. | Wang Y, Xu RC, Zhang XL, Niu XL, Qu Y, Li LZ, Meng XY. Interleukin-8 secretion by ovarian cancer cells increases anchorage-independent growth, proliferation, angiogenic potential, adhesion and invasion. Cytokine. 2012;59:145-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 30. | Luppi F, Longo AM, de Boer WI, Rabe KF, Hiemstra PS. Interleukin-8 stimulates cell proliferation in non-small cell lung cancer through epidermal growth factor receptor transactivation. Lung Cancer. 2007;56:25-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 174] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 31. | Ning Y, Manegold PC, Hong YK, Zhang W, Pohl A, Lurje G, Winder T, Yang D, LaBonte MJ, Wilson PM. Interleukin-8 is associated with proliferation, migration, angiogenesis and chemosensitivity in vitro and in vivo in colon cancer cell line models. Int J Cancer. 2011;128:2038-2049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 32. | Takagi A, Kamiya S, Koga Y, Ohta U, Kobayashi H, Shirai T, Harasawa S, Miwa T. Analysis of interleukin-8 secretion induced by Helicobacter pylori from the gastric epithelial cell line MKN45: a mechanism independent of the intensity of cytotoxicity. J Gastroenterol Hepatol. 1997;12:368-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 33. | Kitadai Y, Haruma K, Sumii K, Yamamoto S, Ue T, Yokozaki H, Yasui W, Ohmoto Y, Kajiyama G, Fidler IJ. Expression of interleukin-8 correlates with vascularity in human gastric carcinomas. Am J Pathol. 1998;152:93-100. [PubMed] |
| 34. | Kido S, Kitadai Y, Hattori N, Haruma K, Kido T, Ohta M, Tanaka S, Yoshihara M, Sumii K, Ohmoto Y. Interleukin 8 and vascular endothelial growth factor -- prognostic factors in human gastric carcinomas? Eur J Cancer. 2001;37:1482-1487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 110] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 35. | Li CJ, Wei PK, Yue BL. Study on the mechanism of xiaotan sanjie recipe for inhibiting proliferation of gastric cancer cells. J Tradit Chin Med. 2010;30:249-253. [PubMed] |
| 36. | Gui MW, Wei PK, Lu Y, Guo W, Qin ZF, Sun DZ. [Effects of Xiaotan Sanjie Decoction-containing serum on proliferation and apoptosis of human gastric cancer cells MKN-45]. Zhongxiyi Jiehe Xuebao. 2010;8:250-255. [PubMed] |
| 37. | Guo XD, Wei PK. [Effect of Xiaotan Sanjie Recipe on growth of transplanted tumor and expressions of proliferating cell nuclear antigen and epidermal growth factor receptor in tissue of gastric carcinoma of nude mice]. Zhongxiyi Jiehe Xuebao. 2007;5:432-436. [PubMed] |
| 38. | Aruffo A, Stamenkovic I, Melnick M, Underhill CB, Seed B. CD44 is the principal cell surface receptor for hyaluronate. Cell. 1990;61:1303-1313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1782] [Cited by in RCA: 1937] [Article Influence: 53.8] [Reference Citation Analysis (0)] |
| 39. | Ghaffarzadehgan K, Jafarzadeh M, Raziee HR, Sima HR, Esmaili-Shandiz E, Hosseinnezhad H, Taghizadeh-Kermani A, Moaven O, Bahrani M. Expression of cell adhesion molecule CD44 in gastric adenocarcinoma and its prognostic importance. World J Gastroenterol. 2008;14:6376-6381. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 43] [Cited by in RCA: 51] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Guo JM S- Editor: Ma YJ L- Editor: Webster JR E- Editor: Ma S