Published online Sep 7, 2015. doi: 10.3748/wjg.v21.i33.9793
Peer-review started: April 7, 2015
First decision: April 23, 2015
Revised: May 29, 2015
Accepted: June 26, 2015
Article in press: June 26, 2015
Published online: September 7, 2015
Processing time: 155 Days and 7.8 Hours
Pancreatic hemangioma is a rare type of benign vascular tumor. Low clinical suspicion and inability of current cross sectional imaging techniques to differentiate it from other pancreatic lesions, contribute to the difficulty in making the correct diagnosis. Without a definitive diagnosis, and due to concern for malignancy, in many instances, surgery is performed. We report a case of pancreas cavernous hemangioma in an 18-year-old female. The patient presented with three-month history of epigastric pain. Physical examination and routine blood tests were normal. Abdominal Computed Tomography scan revealed a 5 cm × 6 cm complex non-enhancing cystic mass in the head of pancreas. Magnetic resonance imaging, endoscopic ultrasonography (EUS) and EUS guided fine needle aspiration cytology were non-diagnostic. Because of uncontrolled symptoms, the patient underwent surgical resection. Histopathology and Immunohistochemical staining confirmed the diagnosis of cavernous hemangioma of pancreas.
Core tip: Pancreas hemangioma is a very rare pathological finding in adulthood. It may mimic other more common pancreatic lesions. Diagnosis is difficult, due to its rarity, and the absence of typical radiological findings of hemangioma. It should be considered in the differential diagnosis of cystic, mixed and solid pancreatic lesions. Surgical resection is the effective treatment in symptomatic cases, while providing a final diagnosis, however, surgery may not be necessary at all.
- Citation: Mondal U, Henkes N, Henkes D, Rosenkranz L. Cavernous hemangioma of adult pancreas: A case report and literature review. World J Gastroenterol 2015; 21(33): 9793-9802
- URL: https://www.wjgnet.com/1007-9327/full/v21/i33/9793.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i33.9793
Pancreatic cavernous hemangioma in adulthood is a very rare condition. To date, only 20 cases have been reported in the literature. Hemangioma mimics common pancreatic lesions, and modern imaging may not adequately characterize this benign condition. The following case is that of an 18-year-old female who underwent surgical resection of a large head of pancreas lesion, with a final diagnosis of cavernous hemangioma. A review of clinicopathological and radiographic features of all cases reported in the English literature to date is included.
An 18-year-old Hispanic female was referred to our Institution by her primary care physician, for the evaluation of a “pancreatic cystic lesion” found on abdominal computed tomography (CT) scan, after she presented with complaints of worsening epigastric pain for three months. The pain was described as stabbing and severe, radiating to the back, occurring postprandial. Nausea and emesis were associated with pain. Analgesia was only partially achieved with the use of prescription oral narcotics.
The patient’s medical history included childhood treated pulmonary tuberculosis. There was no history of acute pancreatitis or abdominal trauma. Patient had no prior surgeries. She was on no medications, particularly has never been prescribed oral contraceptives, nor did she ever use over the counter drugs. Family history was non-contributory. Physical examination was normal. Complete blood counts, complete metabolic panel, serum amylase and lipase, coagulation panel, fasting lipid profile, serum CA 19-9 and carcinoembryonic antigen (CEA) levels were normal.
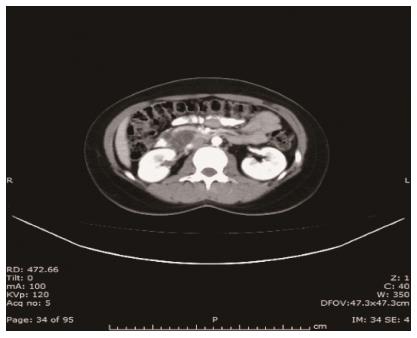
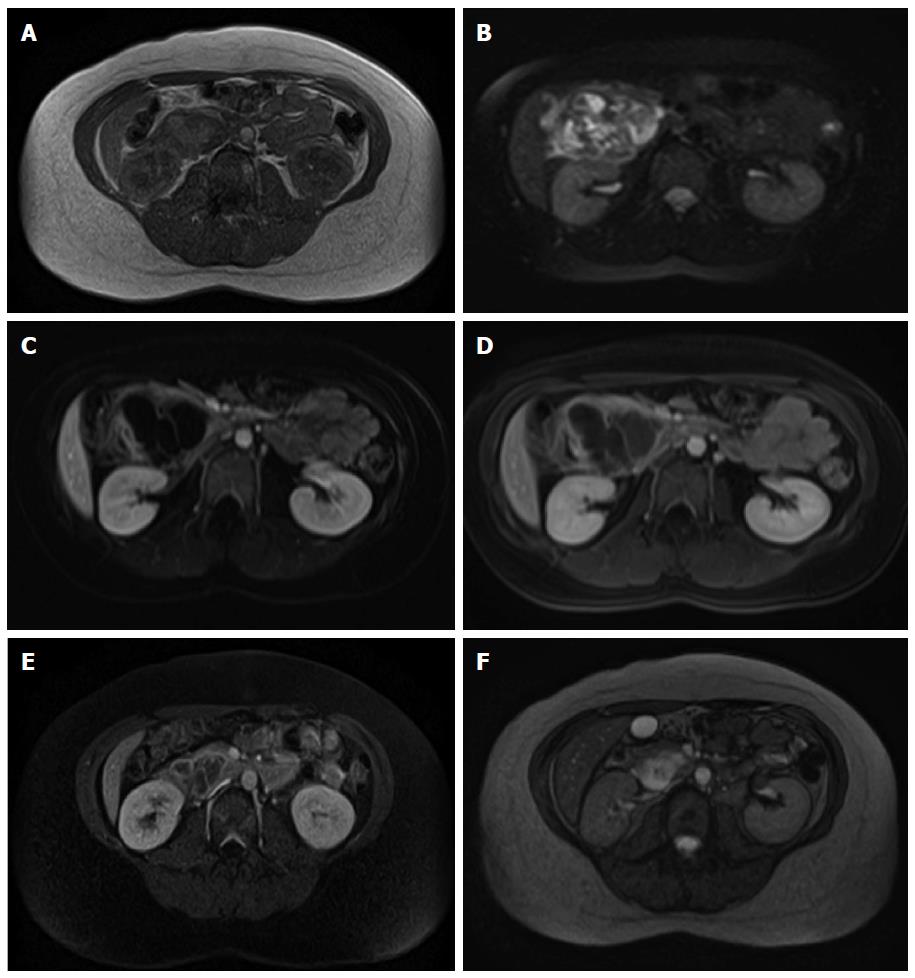
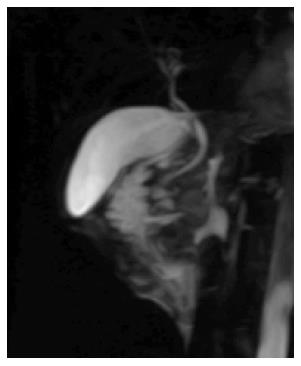
CT revealed a well-defined cystic mass with solid components in the head of pancreas. The lesion did not show contrast enhancement (Figure 1). Magnetic resonance imaging (MRI) with and without intravenous gadolinium showed a 5.3 cm × 6.2 cm, heterogeneous, multi-septated, predominantly cystic mass in the head and uncinate process of the pancreas, with variable T1 and T2 signal intensity (Figure 2). Foci of T1 hyperintensity were thought to be due to hemorrhagic or proteinaceous contents. The mass exhibited septal enhancement with maximum septal wall thickness of 2 mm. Post-contrast dynamic images showed peripheral hyperintensity with some indication of progressive centripetal enhancement. There was no ductal, vascular or nodal involvement. Magnetic resonance cholangiopancreatography (MRCP) demonstrated no ductal dilation (Figure 3).
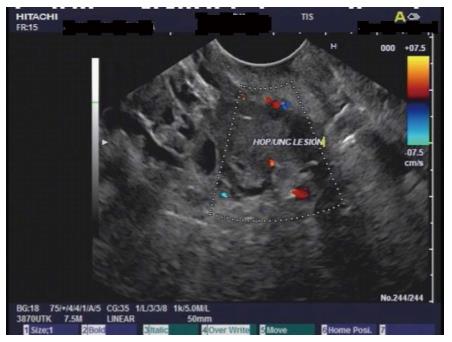
Endoscopic ultrasonography (EUS) showed a well-circumscribed complex structure with fluid and solid components arising from the uncinate process and extending to the head of pancreas (Figure 4). The lesion was devoid of Doppler signal. The surrounding parenchyma appeared normal. There was no ductal or vascular involvement. No peri-pancreatic lymph nodes were seen. The liver, gall bladder, spleen, left adrenal and kidneys appeared normal. Fine needle aspiration (FNA) using a 22G ProCore Cook Needle (Cook Medical Inc., Winston-Salem, NC, United States) was performed, and a total of five passes with adequate sampling was obtained in the presence of a cytopathologist. No bleeding occurred during or after the procedure, and patient had an uneventful post-procedure recovery.
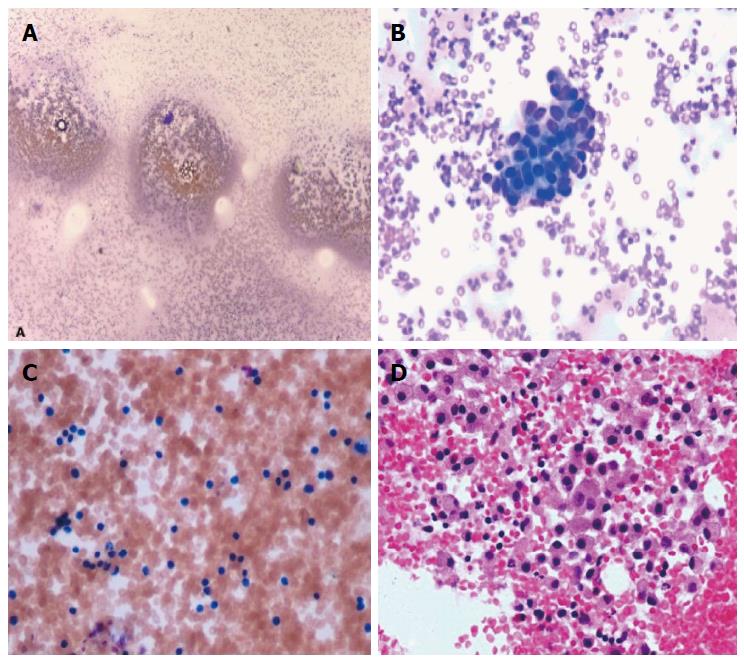
Microscopy of the direct smear from FNA showed abundant red blood cells and a few clusters of cells that had a cohesive nature, but no atypia (Figure 5). Some areas had increased lymphocytes, suggesting an inflammatory process. The cell block showed clusters of histiocytes, some of which had light brown material in their cytoplasm, suggestive of hemosiderin deposition. Focal fibrotic fragments of tissue were seen, without distinct vascular structures being identified. No granulomatous features were seen. Cyst fluid carcinoembryonic antigen (CEA) level was < 0.5 ng/mL, and amylase level was 13 U/L. Gram stain, acid-fast bacilli, and alpha 1 antitrypsin stains were negative. CAM 5.2 was negative. Vimentin was positive in macrophages, but no epithelioid cells were identified. Overall, the fine needle aspiration findings suggested a benign process most consistent with an inflammatory benign cyst. Due to the presence of a few cohesive clusters of cells, a neoplastic cystic lesion (serous cystadenoma or solid cystic papillary tumor) could not entirely be ruled out.
The case was reviewed in the multidisciplinary Pancreatic Tumor Board. A recommendation was made to continue close clinical monitoring, and a consensus from pathologists, radiologists, gastroenterologists, and surgeons reiterated the benign etiology of the lesion. Over the next few weeks, the patient experienced worsening nausea, vomiting and abdominal pain, refractory to narcotics and antiemetics. Subsequently, the patient limited her oral intake and lost 20 pounds of body weight. The patient was eventually taken to surgery. Intra-operatively, the pancreatic lesion was found to be inseparable from the duodenum. Pylorus preserving pancreaticoduodenectomy with cholecystectomy and resection of 17 lymph nodes were performed.
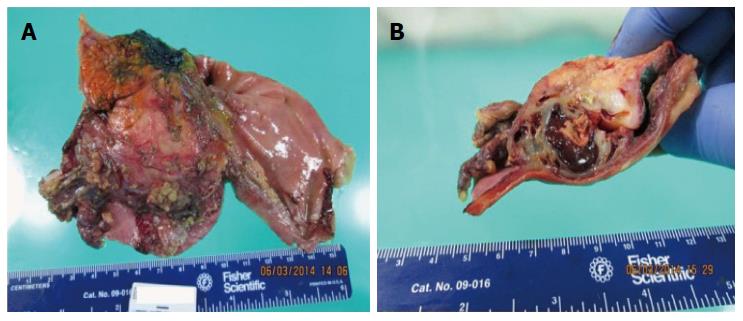
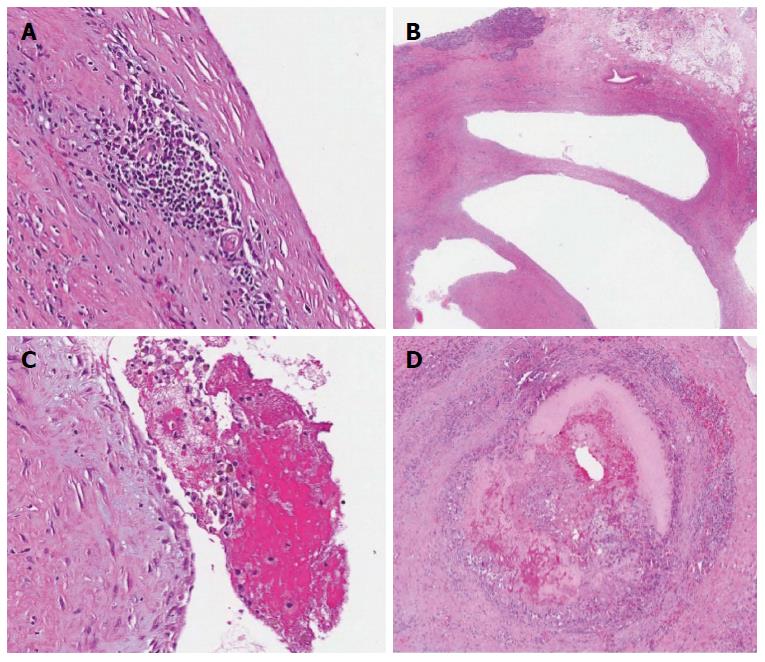
The gross pathology revealed a pink-brown, partly hemorrhagic, multi-loculated cystic mass, 6 cm × 4 cm × 3.5 cm in size, with intracystic hemorrhage and no mucinous component (Figure 6). On microscopic examination (Figure 7), a single layer of flattened cells lined the blood filled cystic spaces, and had uniform nuclei and no atypia. The vessels lumina contained macrophages, some with hemosiderin within. Aggregates of chronic inflammatory cells were present in the surrounding fibrous tissue. One vessel had an organizing thrombus, also containing fibrin and foamy macrophages. On immunohistochemical (IHC) staining, the lining was positive for CD31, focally positive for CD34, and negative for cytokeratin (AE1/AE3) supporting the diagnosis of hemangioma. All 17 lymph nodes had normal histology.
Postoperatively, the patient was monitored in the Intensive Care unit for two days. By second day, she was tolerating oral intake of liquids. She was discharged home on the 3rd post-operative day. Six months after surgery, she remained symptom free.
Vascular tumors (hemangioma, lymphangioma, hemolymphangioma, hemangioendothelioma, hemangiopericytoma, hemangioblastoma and angiosarcoma) constitute only 0.1% of the pancreatic tumors[1]. Hemangiomas are vascular tumors composed of blood vessels lined by endothelial cells. Visceral hemangiomas can be found in various organs including the brain, parotid, thorax, liver, spleen, adrenal, retroperitoneum and gastrointestinal tract, in isolation, or with synchronous cutaneous lesions[2]. Hemangioma is the most common benign tumor of the liver, however, pancreas hemangiomas are extremely rare, both in adult and pediatric population.
A PubMed and Google Scholar search of the literature using the keywords “pancreas hemangioma” was performed, and pertinent articles and their references were reviewed. Since 1960, a total of twenty-one cases have been reported in nineteen articles (Table 1)[3-19]. Mundinger et al[20] in their 2009 article, stated that a number of nine cases have been described in the medical literature prior to 1939. In our review, cases of hemolymphangioma, lymphangioma and vascular malformations were excluded. Hemangiomas with retroperitoneal origin involving the pancreas were also excluded. The aim of the present review is to provide a summary of the epidemiological and clinical characteristics of this rare tumor.
| No. | Year | Country | Author | Age (yr)/sex | Site/size (cm) | Preoperative diagnosis | Treatment | Follow up |
| 1 | 1939 | - | Ranstrom | 61/F | Head/7 | Found at autopsy | - | |
| 2 | 1960 | France | Ringoir | - | - | - | Surgery | |
| 3 | 1961 | France | Ringoir | 71/F | Head/15 | - | Retrocolic gastroenterostomy, vagotomy | |
| 4 | 1972 | France | Colardyn | 42/F | Body tail/- | - | Fat free diet, anticholinergic1 | |
| 5 | 1985 | France | Mangin | 62/F | Head to tail/20 | Multicystic heterogenous structure | Laparotomy with observation1 | |
| 6 | 1991 | Japan | Kobayashi | 30/M | Head/20 | Cavernous hemangioma suggested on US/MRI | Pancreatoduodenectomy | |
| 7 | 1991 | Germany | Dageforde | 79/F | Body tail/6 | Observation1 | ||
| 8 | 2003 | Taiwan | Chang | 70/F | Body tail/4 | Heterogenous hypervascular solid mass. Serous/cystic adenoma/adenocarcinoma? | Subtotal pancreatectomy | Healthy at 14 mo |
| 9 | 2006 | Austria | Plank | 36/M | Head/3 | Hypervascular mass. Nonfunctioning neuroendocrine tumor | Hemangioma suspected during laparotomy, confirmed by intraoperative US. Not resected1 | |
| 10 | 2008 | China | Xu | - | - | Three cases reported | - | |
| 11 | 2009 | United States | Mundinger | 45/F | Head/5.5 | Hypodensity mass in CT. Benign (duplication cyst, paraganglioma, cystic GIST) cyst | Frozen section: benign cyst. PPPD performed | |
| 12 | 2010 | - | Jarboui | 60/F | - | - | - | |
| 13 | 2011 | Israel | Weidenfeld | 73/F | Head/5 | Cyst with solid part | Whipple’s procedure | |
| 14 | 2011 | Malaysia | Lee | 49/F | Neck/5 | Non-enhancing septated cyst. Mucious cyst with malignant features | Central partial pancreatectomy and gastrostomy | Symptom free at 6 mo |
| 15 | 2012 | Germany | Kersting | 53/M | Head/8 | Non-enhancing mass. Adenocarcinoma? | Extirpation of the tumor | |
| 16 | 2013 | China | Zhi-hua | 23/F | Head/5.4 | Non enhancing multilocular cyst with fluid fluid levels | Subtotal pancreatectomy | Healthy, no recurrence at 1 yr |
| 17 | 2013 | United Kingdom | Malik | 70/F | Head/8 | Giant hemangioma suggested on CT | PPPD | Fine at 6 wk |
| 18 | 2014 | United Kingdom | Williamson | 78/F | Head/4 | Hemangioma per EUS | Observation1 | |
| 19 | 2014 | Japan | Naito | 40/F | Body tail/10 | Multilocular septated cystic mass | Pancreatectomy | No recurrence at 6 yr |
| 20 | 2015 | United States | Mondal | 18/F | Head/6 | Benign cyst | PPPD | Fine at 6 mo |
The average age of the patients at diagnosis is 48.3 years (range: 18-79 years). Our patient is the youngest adult case reported. Infantile hemangiomas of pancreas are very rare, but they involute by early childhood[21]. Male to female ratio is 1:3. Other pancreatic tumors e.g., mucinous cystic neoplasm (MCN), intraductal papillary mucinous neoplasm (IPMN) and solid pseudopapillary neoplasm (SPN) also show female predominance. Male to female ratio of 1:5 is reported in cavernous hemangioma of the liver. Although an association between the proliferation of hepatic cavernous hemangiomas and increased levels of estrogen (from contraceptives, pregnancy) is reported, no definite causal relationship has been proven[22].
The most common presenting symptom is epigastic pain (47%). Nausea, abdominal distention, jaundice, fever, episodic dizziness and palpitations and gastrointestinal bleeding may be associated to pain. No reported case had episodes of pancreatitis or family history of pancreas diseases. Rarely, especially in young patients, cavernous hemangiomas of the pancreas may be observed in the setting of von Hippel-Lindau disease[23]. Abnormalities of the serum liver tests and thrombocytopenia may be present but no correlation between the size or of the lesions location and symptomatology was noted. In fact, these tumors can be as large as 20 cm and arising from the head of pancreas, yet they do not cause much pain or obstructive features. On the contrary, two of the smallest reported lesions, presented with obstructive jaundice (No. 8, 9). Average reported tumor size is 8.9 cm (range: 3-20 cm), with the majority (70%) involving the head of the pancreas. Hemangiomas are indolent (No. 13, 14) and do not have malignant potential. Why our patient presented with rather short duration of symptoms is unknown. Whether hemorrhage, thrombosis or necrosis within the cyst occurred and led to symptoms is not known.
Transabdominal ultrasonography (US) is reported to detect hemangiomas in 9 cases. All these lesions were more than 5 cm in size. Lesions were well demarcated, appeared overall hyper- or iso-echogenic to rest of the pancreas, and had mixed echogenicity. A 20 cm mass in the head of pancreas was diagnosed as a giant hemangioma on US (No. 6). The absence of a fine speckled internal echo pattern may suggest a vascular tumor as opposed to a mucinous cystic tumor[24]. Intra-operative US was helpful in diagnosing pancreatic hemangioma in one case and prevented pancreatectomy (No. 9). Our Endoscopic ultrasound findings were similar to those described by Lee (No. 14). We identified a cystic mass with thick septations and solid components. Doppler signal was absent within the lesion. The overall sonographic appearance was suggestive of an inflammatory or mucinous cyst. Willamson (No. 18) made a diagnosis of pancreas hemangioma based on endosonographic characteristics, which allowed a conservative approach to the management of his reported case, and surgery was avoided. Hemorrhage, thrombosis, necrosis, shunting and vascularity of the solid component within, may account for poor Doppler signal in most pancreatic hemangiomas[9].
Typically, hemangiomas are strongly contrast enhancing in the arterial phase of conventional contrast-enhanced CT imaging[7]. Peripheral irregular enhancement with central non-enhancement in venous phase, and progressive filling-in during the delayed phases of dynamic CT scan is typically described in hepatic hemangiomas. Based on these features, preoperative diagnosis of pancreatic giant hemangioma was suggested only in one case (No. 17). A non-enhancing three-phase contrast CT scan does not exclude hemangioma. Of the cases that reported pre-contrast CT findings, five lesions (No. 8, 9, 11, 17, 19) were heterogeneous (with both, high and low attenuation), multi-loculated cysts and five (No. 13, 14, 16, 18, 20) were hypo-attenuating “cysts” with solid components and peripheral micro- or speckled calcifications. Post-contrast CT showed poorly enhanced or unenhanced, hypo-vascular, multi-loculated, cystic tumors in some cases (No. 6, 11, 14, 15, 16, 20) and hyper-enhancement in others (No. 8, 9, 13, 17, 18, 19).
Hemangioma on MRI appears as a lobulated, hypo-intense mass in T1 -weighted images, and shows moderate hyperintensity signal in T2 -weighted images, while after intravenous gadolinium administration, there is marked enhancement[25,26]. Four articles (No. 6, 9, 16, 19) reported MRI findings of pancreas hemangiomas. Low T1W signal attenuation in (No. 6, 9, 16) and high T2W signal attenuation in (No. 16, 19) were seen on non-contrast MRI. MRI was able to diagnose a giant hemangioma of pancreas (No. 6) without further work-up. In our case, in the post-contrast dynamic imaging, there was some evidence of centripetal filling-in. No dilatation of the pancreatic and the common bile ducts was seen on MRCP in our patient.
Since radiological imaging cannot characterize these lesions, almost all complex cystic lesions of the pancreas undergo endoscopic or per-cutaneous fine needle aspiration (FNA) to establish a diagnosis. Hemangiomas can be highly vascular. Fatality related to biopsy of hepatic hemangioma has been reported[27], therefore concern regarding FNA sampling of these lesions should rise. If the radiographic characteristics of hemangioma are absent on imaging, the risk of bleeding remains concerning following lesion sampling or during surgery. On the other hand, the article reporting 38 patients with sampled hepatic hemangiomas, along with several other small series, revealed needle biopsy to be safe[28]. FNA was performed in pancreas hemangiomas (No. 14, 17, 18), and there was no reported bleeding. We performed EUS guided FNA using a 22G ProCore Cook Needle (Cook Medical Inc., Winston-Salem, NC, United States) with 5 passes without associated bleeding.
Angiography performed in cases of pancreatic hemangiomas showed either a poorly vascular (No. 6) or a hypervascular (No. 8) tumor without encasement of surrounding vascular structures. Arteriography does not provide additional useful information beyond CT/MRI, and is not routinely performed, unless selective embolization of feeding vessels is being considered as treatment. Technetium (Tc)-99 isotope study and positron-emission tomography (PET) scan have been found useful in diagnosing hemangiomas in other organs, but none has been reported in pancreas hemangioma cases.
As hemangiomas are essentially benign tumors, correct preoperative differentiation from other tumors could prevent surgical resection. On imaging, hemangiomas elicit characteristics that overlap with radiographic phenotypes of other common lesions of the pancreas. The list of radiological differential diagnoses for cystic-solid and cystic pancreatic lesions is broad (Table 2). Most cases proceeded to surgery without confirmatory diagnosis. The correct pre-operative or intra-operative diagnosis of hemangioma was made only in 5 out of 20 cases (No. 4, 5, 7, 9, 18), thus avoiding need for surgical resection.
| Cystic lesions | Sex/age/size/site | Imaging, cytology features |
| Pseudocysts | Any | Unilocular |
| Serous cystic neoplasms | F/60s/large/body or tail | Serous and mucinous cystadenoma similar on CT |
| Central fibrous scar with calcification | ||
| Septation | ||
| Multiple cysts lined by glycogen-rich epithelial cells that are positive for periodic acid Schiff and express cytokeratins | ||
| Mucinous cystic neoplasms | F/50-60s/> 10 cm/body or tail | Single multilocular cysts that do not communicate with the ductal system |
| Smooth shape, even wall, with or without (fine) septa with potential small nodules | ||
| Peripheral egg shell calcification | ||
| Cyst lined by columnar mucin-producing epithelial cells set within an ovarian-like stroma | ||
| Dysplasia and malignant potential | ||
| Express keratin, carcinoembryonic antigen and CA19-9, while CK20 and CDX2 (markers of intestinal differentiation) are negative | ||
| IPMN | M = F/70-80s/head | Intraductal proliferation of mucinous cells usually showing papillary projections |
| Dysplasia, invasion and malignant potential | ||
| The epithelium of IPMNs expresses keratins, CEA and CA19-9, with variable expression of MUC | ||
| Solid pseudopapillary neoplasms | Young F/> 10 cm/tail, head | Solid or cystic-solid hypervascular tumors, solid part enhances |
| Show pseudopapillae and pseudocysts | ||
| Stain for vimentin and beta catenin with partial reactivity to keratin | ||
| CD56 and synaptophysin stains may be positive, chromogranin negative | ||
| Cystic change of solid tumors | PDAC is rare < 50, M = F | Recognition of adjacent solid lesion (carcinoma) is the key to correct diagnosis of ductal adenocarcinoma |
| PDAC are fibrotic, hypo-vascular, ill defined border, early infiltration in peri-pancreatic fat, invasion of vascular structures and ducts | ||
| NET and Islet cell tumors are hyper-vascular and hyper-enhancing with positive staining for chromogranin and synaptophysin | ||
| Lymphoepithelial cysts | M/50-60s/ body or tail | Lined by squamous epithelium and surrounded by dense lymphoid tissue, possibly showing germinal centers |
| Vascular tumors (Lymphangiomas, hemangiomas, hemolymphangioma, hemangioendothelioma, angiosarcoma | F > M/large | Cystic-solid, encapsulated, multi-loculated, micro-cystic portions appear solid and enhancing |
| Vascular markers CD31, CD34 and factor VIII are positive in endothelial cells | ||
| Cytokeratins neg | ||
| Lymphatic marker D2-40 neg in hemangioma, positive in lymphangioma | ||
| Angiosarcomas, highly aggressive, a vascular channel lined by variably atypical endothelial cells | ||
| Metastatic from renal cell cancer | Hypervascular, invasive | |
| Accessory splenic tissue | Tail | hypervascular |
| Others | Rare solid tumors of the pancreas also include: mature teratoma, hamartoma, sarcoidosis, yolk sac tumor, acinar cell carcinoma, lymphoplasmatic sclerosing pancreatitis, primary pancreatic lymphoma., duodenal tumor, duplication cysts, paraganglioma, cystic GIST, glomus tumor, etc. |
Microscopically, hemangiomas are composed of an increased number of blood-filled ectatic capillaries lined by flat endothelium and separated by scant fibrous connective tissue stroma. Depending on the size of vascular spaces, they can be capillary or cavernous type. Vascular endothelial marker (CD31, CD34 and factor VIII-related antigen) positivity, along with negative results for cytokeratin (AE1/AE3, D2-40, CAM 5.2, MNF 116) immunohistochemical staining indicates hemangioma, lymphangioma and other benign vascular tumors. Lymphangioma may be easily excluded, as they consist of lymphatics (instead of blood vessels) filled or infiltrated with lymphocytes. Using electron microscopy (EM), lymphangioma was diagnosed in a resected pancreas tumor[29], however, EM has not been described in hemangioma. A precise histopathological diagnosis of hemangioma is very important, as other vascular tumors may require wider surgical margin at resection, adjunctive therapy and closer post-resection follow up. For instance, in hemolymphangioma, 10%-27% and 50%-100% recurrences are reported after complete and partial resection, respectively[30].
Unlike pediatric hemangiomas that mostly involute, adult pancreatic hemangiomas do not. The natural history of adult pancreas hemangiomas is unknown. They appear to be very slow growing and have no malignant potential. Over a 7 years period, a hemangioma grew from 2 cm to 5 cm (No. 14). Pancreatic hemangiomas, even when large, rarely obstruct or infiltrate adjacent structures. Although the possibility of future complications (spontaneous or traumatic rupture, bleeding, pancreatitis, infection, etc.) is present, a conservative approach can be considered, if confident diagnosis is made preoperatively. Five cases are reported to be managed by observation strategy. However, details regarding surveillance and outcomes are not known. Propranolol, corticosteroid, interferon alpha, vincristine, cyclophosphamide and radiotherapy have been used to successfully treat infantile hemangiomas, but their role in adult hemangioma treatment is not known.
Surgical excision seems to be a reasonable strategy for large hemangiomas when diagnostic uncertainty remains, for uncontrolled symptoms with failed conservative management, or to address complications from mass effect. All resected pancreas hemangioma cases had good outcome, with resolution of symptoms and no tumor recurrence. Naito (No. 19) reported surgical removal of a giant pancreatic hemangioma without recurrence at 6-year follow up. Preoperative suspicion, use of intra-operative ultrasound and frozen sections may help in determining the best surgical method, e.g., enucleation, other more conservative surgical techniques or an extensive resection.
Current imaging techniques cannot reliably diagnose pancreas hemangioma from other cystic neoplasm. Newer techniques like contrast enhanced US, PET scan, elastography, etc. may be tried in future. Early suspicion by clinicians and radiologists is crucial. Although rare, pancreatic hemangioma should be considered in the differential diagnosis.
An 18-year-old female with nausea, vomiting and epigastric pain for 3 mo and a 5 cm x 6 cm non-enhancing cystic mass in the head of pancreas on computed tomography scan.
Epigastric tenderness but no mass on examination.
Pancreatic neoplasm, pancreatic cyst
Complete blood counts, complete metabolic panel, serum amylase and lipase, coagulation panel, fasting lipid profile, serum CA 19-9 and carcinoembryonic antigen levels were normal.
Magnetic resonance imaging showed a 5.3 cm x 6.2 cm, heterogeneous, multi-septated, predominantly cystic mass in the head of the pancreas, with variable T1W and T2W signal intensity and differential diagnoses included: serous cystadenoma, solid pseudopapillary neoplasm, inflammatory cyst and mucinous cyst.
Endoscopic ultrasonography guided fine needle aspiration cytology was negative for malignancy; post resection histopathology (blood-filled cavernous spaces lined by flat endothelium) and immunohistochemistry (CD31, CD34 positive and AE1/AE3, CAM 5.2 negative) confirmed the diagnosis of hemangioma.
Pylorus preserving pancreatoduodenectomy.
Cavernous hemangioma of pancreas is a rare condition and preoperative diagnosis is difficult. Cases reported in English literature were reviewed to identify clinicopathological and radiological characteristics of these tumors.
Pancreas hemangiomas are rare benign tumors that mimic other more common lesions of pancreas. Confident preoperative diagnosis is challenging. Symptomatic patients need resection that also establishes the diagnosis.
This article describes the clinical, radiological and pathological features of a case of cavernous hemangioma of pancreas and also provides a literature review of the reported cases.
| 1. | England RJ, Woodley H, Cullinane C, McClean P, Walker J, Stringer MD. Pediatric pancreatic hemangioma: a case report and literature review. JOP. 2006;7:496-501. [PubMed] |
| 2. | Schulz AS, Urban J, Gessler P, Behnisch W, Kohne E, Heymer B. Anaemia, thrombocytopenia and coagulopathy due to occult diffuse infantile haemangiomatosis of spleen and pancreas. Eur J Pediatr. 1999;158:379-383. [PubMed] |
| 3. | Derom F, Ringoir S, Marlier R. [Two cases of intraabdominal hemangioma: liver and pancreas]. Acta Chir Belg. 1960;59:172-182. [PubMed] |
| 4. | Ringoir S, Derom F, Colle R, Mortier G. Hemangioma of the pancreas. Report of a case. Gastroenterology. 1961;41:43-45. [PubMed] |
| 5. | Colardyn F, Elewaut A, Van de Velde E, Barbier F. Hemangioma of the pancreas. Tijdschr Gastroenterol. 1972;15:260-267. [PubMed] |
| 7. | Kobayashi H, Itoh T, Murata R, Tanabe M. Pancreatic cavernous hemangioma: CT, MRI, US, and angiography characteristics. Gastrointest Radiol. 1991;16:307-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Chang WT, Lee KT, Yang SF. Cavernous hemangioma of the pancreas: report of a case. Pancreas. 2003;26:310-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Plank C, Niederle B, Ba-Ssalamah A, Schima W. Pancreatic hemangioma: imaging features with contrast-enhanced CT and with gadolinium- and mangafodipir-enhanced MRI. Eur J Radiol. 2006;57:59-62. [RCA] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Xu Q, Wang CF, Zhao P, Shan Y, Zhao DB, Liu Q. [The diagnosis and treatment of pancreatic cavernous hemangioma]. Zhonghua Yi Xue Za Zhi. 2008;88:28-30. [PubMed] |
| 12. | Jarboui S, Salem A, Gherib BS, Ben Moussa M, Rajhi H, Mnif N, Zaouche A. Hemangioma of the pancreas in a 60-year-old woman: a report of a new case. Gastroenterol Clin Biol. 2010;34:569-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Weidenfeld J, Zakai BB, Faermann R, Barshack I, Aviel-Ronen S. Hemangioma of pancreas: a rare tumor of adulthood. Isr Med Assoc J. 2011;13:512-514. [PubMed] |
| 14. | Lee J, Raman K, Sachithanandan S. Pancreatic hemangioma mimicking a malignant pancreatic cyst. Gastrointest Endosc. 2011;73:174-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Kersting S, Janot MS, Munding J, Suelberg D, Tannapfel A, Chromik AM, Uhl W, Bergmann U. Rare solid tumors of the pancreas as differential diagnosis of pancreatic adenocarcinoma. JOP. 2012;13:268-277. [PubMed] |
| 16. | Lu ZH, Wu M. Unusual features in an adult pancreatic hemangioma: CT and MRI demonstration. Korean J Radiol. 2013;14:781-785. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Malik M, Ahmed I, Kurban L. Pancreatic Hemangioma - A case Report. Gastroenterol Hepatol Res. 2013;2:545-548. [DOI] [Full Text] |
| 18. | Williamson JM, Finch-Jones M, Pope I. Endoscopic ultrasonography allowing expectant management of pancreatic haemangioma. Ann R Coll Surg Engl. 2014;96:e1-e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Naito Y, Nishida N, Nakamura Y, Torii Y, Yoshikai H, Kawano H, Akiyama T, Sakai T, Taniwaki S, Tanaka M. Adult pancreatic hemangioma: A case report. Oncol Lett. 2014;8:642-644. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Mundinger GS, Gust S, Micchelli ST, Fishman EK, Hruban RH, Wolfgang CL. Adult pancreatic hemangioma: case report and literature review. Gastroenterol Res Pract. 2009;2009:839730. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Takahashi K, Mulliken JB, Kozakewich HP, Rogers RA, Folkman J, Ezekowitz RA. Cellular markers that distinguish the phases of hemangioma during infancy and childhood. J Clin Invest. 1994;93:2357-2364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 483] [Cited by in RCA: 454] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 22. | Glinkova V, Shevah O, Boaz M, Levine A, Shirin H. Hepatic haemangiomas: possible association with female sex hormones. Gut. 2004;53:1352-1355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 105] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 23. | Hammel P, Beigelman C, Chauveau D, Resche F, Bougerolles E, Flejou JF, Bernades P, Delchier JC, Richard S. [Variety of pancreatic lesions observed in von Hippel-Lindau disease. Apropos of 8 cases]. Gastroenterol Clin Biol. 1995;19:1011-1017. [PubMed] |
| 24. | Balderramo DC, Di Tada C, de Ditter AB, Mondino JC. Hemolymphangioma of the pancreas: case report and review of the literature. Pancreas. 2003;27:197-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Semelka RC, Brown ED, Ascher SM, Patt RH, Bagley AS, Li W, Edelman RR, Shoenut JP, Brown JJ. Hepatic hemangiomas: a multi-institutional study of appearance on T2-weighted and serial gadolinium-enhanced gradient-echo MR images. Radiology. 1994;192:401-406. [PubMed] |
| 26. | Goshima S, Kanematsu M, Kondo H, Yokoyama R, Kajita K, Tsuge Y, Shiratori Y, Onozuka M, Moriyama N. Hepatic hemangioma: correlation of enhancement types with diffusion-weighted MR findings and apparent diffusion coefficients. Eur J Radiol. 2009;70:325-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 27. | Terriff BA, Gibney RG, Scudamore CH. Fatality from fine-needle aspiration biopsy of a hepatic hemangioma. AJR Am J Roentgenol. 1990;154:203-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 0.6] [Reference Citation Analysis (1)] |
| 28. | Tung GA, Cronan JJ. Percutaneous needle biopsy of hepatic cavernous hemangioma. J Clin Gastroenterol. 1993;16:117-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 29. | Murao T, Toda K, Tomiyama Y. Lymphangioma of the pancreas. A case report with electron microscopic observations. Acta Pathol Jpn. 1987;37:503-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 30. | Fang YF, Qiu LF, Du Y, Jiang ZN, Gao M. Small intestinal hemolymphangioma with bleeding: a case report. World J Gastroenterol. 2012;18:2145-2146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 41] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Cosen-Binker L, Sakata N, Sumi S S- Editor: Ma YJ L- Editor: A E- Editor: Wang CH