Published online Aug 21, 2015. doi: 10.3748/wjg.v21.i31.9358
Peer-review started: March 10, 2015
First decision: April 23, 2015
Revised: May 21, 2015
Accepted: June 26, 2015
Article in press: June 26, 2015
Published online: August 21, 2015
Processing time: 164 Days and 1.6 Hours
AIM: To examine the effect of aqueous fructus aurantii immaturus (FAI) extracts on the intestinal plexus of cathartic colons.
METHODS: Cathartic colons were induced in rats with dahuang, a laxative used in traditional Chinese medicine. Once the model was established (after approximately 12 wk), rats were administered mosapride (1.54 mg/kg) or various doses of aqueous FAI extracts (1-4 g/kg) for 14 d. Transit function was assessed using an ink propulsion test. Rats were then sacrificed, and the ultramicrostructure of colonic tissue was examined using transmission electron microscopy. The expression of the 5-hydroxytryptamine receptor 4 (5-HTR4) and neurofilament-H was assessed in colon tissues using real-time PCR, Western blot, and immunohistochemistry.
RESULTS: Mosapride and high dose (4 g/kg) of aqueous FAI extracts significantly improved the bowel movement in cathartic colons compared to untreated model colons as measured by the intestinal transit rate (70.06 ± 7.25 and 72.02 ± 8.74, respectively, vs 64.12 ± 5.19; P < 0.05 for both). Compared to controls, the ultramicrostructure of cathartic colons showed signs of neural degeneration. Treatment with mosapride and aqueous FAI extracts resulted in recovery of ultrastructural pathology. Treatment with mosapride alone upregulated the gene and protein expression of 5-HTR4 compared to untreated controls (P < 0.05 for both). Treatment with aqueous FAI extracts (≥ 2 g/kg) increased 5-HTR4 mRNA levels (P < 0.05), but no change in protein level was observed by Western blot or immunohistochemistry. The mRNA and protein levels of neurofilament-H were significantly increased with mosapride and ≥ 2 g/kg aqueous FAI extracts compared to controls (P < 0.05 for all).
CONCLUSION: Aqueous FAI extracts and mosapride strengthen bowel movement in cathartic colons via increasing the expression of 5-HTR4 and neurofilament-H.
Core tip: Bowel movements in cathartic colon can be strengthened with mosapride. However, recent studies show that aqueous fructus aurantii immaturus (FAI) extracts, a traditional Chinese medicine, can also strengthen bowel movement, and are widely used to treat gastrointestinal symptoms. The aim of this study was to identify the mechanism by which aqueous FAI extracts exert these effects. In a rat model of cathartic colons, treatment with mosapride and aqueous FAI extracts improved the intestinal transit rate, and increased the expression of 5-hydroxytryptamine receptor 4 and neurofilament-H.
- Citation: Wang SY, Liu YP, Fan YH, Zhang L, Cai LJ, Lv B. Mechanism of aqueous fructus aurantii immaturus extracts in neuroplexus of cathartic colons. World J Gastroenterol 2015; 21(31): 9358-9366
- URL: https://www.wjgnet.com/1007-9327/full/v21/i31/9358.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i31.9358
Slow transit constipation is a type of intractable constipation of an unknown etiology. The slowed colonic transmission results in functional constipation, thus affecting an individual’s quality of life[1]. Chronic use of anthraquinones or traditional Chinese laxatives, such as dahuang and fanxieye, can result in laxative-dependent defecation, also referred to as “cathartic colon”. Long-term laxative use causes damage to the intestinal myenteric plexus[2-4], resulting in movement disability within the colon and aggravation of constipation.
Mosapride is an agonist of 5-hydroxytryptamine receptor 4 (5-HTR4), and is widely used to treat constipation. It activates cholinergic neurons in the intestinal myenteric plexus to strengthen the movement of the digestive tract[5]. However, the traditional Chinese medicine fructus aurantii immaturus (FAI) has also been used to treat gastrointestinal diseases. Aqueous extracts of FAI, obtained from Citrus aurantium L. or C. sinensis Osbeck, have also been shown to strengthen the movement of the gastrointestinal tract[6-8]. The mechanism of action is not understood, but is thought to involve stimulation of substance P secretion from the nerve plexus[9], or activation of muscarinic acetylcholine[6] and histamine[10] receptors. It is not known whether aqueous FAI extracts also act on 5-HTR4.
The aim of the present study was to investigate the effects of aqueous FAI extracts in comparison to mosapride, and to evaluate the mechanism of action. To this end, a cathartic colon rat model was treated with mosapride and various doses of aqueous FAI extracts. The expression levels of 5-HTR4 and the structural protein neurofilament-H (NF-H) were then examined in the myenteric plexus of the intestinal wall.
Mosapride (5 mg; batch No. 2757C) was purchased from Dainippon Sumitomo Pharma Co., Ltd. (Chuo-ku, Osaka, Japan). Dahuang (batch No. 20130302) and FAI (batch No. 20131017) were produced by the Zhejiang Chinese Medical University Medical Pieces Co. Ltd. (Linan, Zhejiang, China). High-performance liquid chromatography analysis of the FAI indicated that it contained 0.79% hesperidin, 0.18% aloe emodin, 0.31% rheic acid, 0.28% rheum emodin, 0.35% chrysophanic acid, and 1.17% physcion.
To prepare the aqueous extracts, FAI was soaked for 30 min in water (8-10 ×V), and then boiled for 30 min. Next, water was added (3-5 ×V), and the solution was boiled for an additional 25 min. The decoction was concentrated to 1 g/mL crude drug using rotary evaporators (SENCO R-201; Shanghai Zhicheng Biological Technology Co., Ltd., Shanghai, China) and sterilized.
Eighty-two specific pathogen-free male Sprague-Dawley rats (200 ± 10 g) were purchased from Shanghai Xipuer-bikai Experimental Animal Co., Ltd. (Shanghai, China) and housed for 1 wk under a 12 h light/dark cycle at 22-24 °C with 50%-60% humidity and a noise level < 50 db. Prior to experimentation, rats were allowed free access to food and tap water. All the procedures involving animals were conducted in accordance with the ethical principles adopted by the Animal Experimental Center of Zhejiang Chinese Medical University and were approved by the Ethics Committee on Animal Experiments at Zhejiang Chinese Medical University.
The experimental group of rats (n = 70) received daily oral administration of 15 mL dahuang at an initial dose of 200 mg/kg (13.3 mg/mL). The dose increased by 200 mg/kg each day until 50% of the rats exhibited loose stools. This occurred when the dosage was about 2400 mg/kg per day, which was maintained until the loose stools disappeared in 80% of the rats. In the next stage, the dosage was again increased by 200 mg/kg per day until 50% of the rats exhibited loose stools again. The final dosage of dahuang was 3800 mg/kg per day (at 253.3 mg/mL). The time to establish the laxative-dependent slow transit constipation model was 12 wk. Food and water were not limited during the modeling procedure. Animals in the control group (n = 12) received daily oral administration of 15 mL/kg normal saline.
Two animals died during establishment of the cathartic colon model; thus, the remaining 68 rats were divided into the following five treatment groups: model (n = 12); mosapride (n = 14); low-dose (1 g/kg) aqueous FAI extract (FAI-L; n = 14); medium-dose (2 g/kg) aqueous FAI extract (FAI-M; n = 14); high-dose (4 g/kg) aqueous FAI extract (FAI-H; n = 14). The treatment consisted of daily oral administration of 15 mL/kg per day for 2 wk; normal saline was administrated to the control and model groups.
The dose of mosapride was 1.54 mg/kg according to the surface area conversion[11], which is equivalent to 6.2 × the human adult dosage used in clinical work. The doses of 1 g/kg, 2 g/kg, and 4 g/kg aqueous FAI extract are equivalent to 6.2 ×, 12.4 ×, and 24.8 × the human adult dosage, respectively, used in clinical work.
To evaluate the intestinal transit rate (ITR), animals received an oral administration of 2 mL carbonic ink. After 40 min, the rats were anesthetized with 350 mg/kg chloral hydrate and the complete intestinal tract, from the pylorus to the terminal rectum, was removed. Without applying tension, the lengths of the whole intestinal tract, small intestine, large intestine, and ink propulsion were measured. The percentage of blackened intestinal tracts was calculated: ITR (%) = pushing length/total length × 100.
Colonic tissue located 1 cm from the anus was collected from one randomly selected rat in each group, cut into 1 mm3 portions, and fixed for 24 h in 2.5% buffered glutaraldehyde for transmission electron microscopy (TEM). From all rats, colonic tissue (1 cm) dissected approximately 2 cm from the anus was fixed in 4% buffered neutral formalin and stored at 4 °C for subsequent immunohistochemical analyses. Colonic tissue (2 cm) located approximately 4 cm from the anus was rapidly frozen in liquid nitrogen for PCR and Western blot analyses.
Following fixation in 2.5% buffered glutaraldehyde solution, colonic tissue samples were washed with phosphate buffered saline (PBS) for 30 min, fixed for 1 h in 1% osmic acid, washed in PBS again, and dehydrated through a graded ethanol series. Specimens were embedded in epoxy resin (Epon 812) and stained with methylene blue. Sections were cut using an ultramicrotome (HM335E; Microm GmbH, Waldrof, Germany), and stained with uranyl acetate and lead citrate. Sections were imaged under a transmission electron microscope (H-7650; Hitachi, Ltd., Tokyo, Japan).
RNAiso Plus (9108; Takara Bio, Inc., Otsu, Shiga, Japan) was used to extract RNA from frozen tissue samples, and the concentration of RNA was measured using a trace nucleic acid analyzer (Thermo Fisher Scientific, Waltham, MA, United States). RNA (1 μg/μL) was reverse transcribed to cDNA using a PrimeScript RT reverse transcription kit (RR036A; Takara Bio Inc.). Amplification reactions were as follows: 2 μL cDNA, 10 μL SYBR Premix Ex Taq II, 0.4 μL ROX II, 0.8 μL forward and reverse primers (10 μmol/L), and 6.0 μL dH2O. The two-step amplification method was performed on a real-time PCR system (7500; Applied Biosystems of Thermo Fisher Scientific): initial denaturation at 95 °C for 30 s, followed by 40 cycles of denaturation at 95 °C for 5 s and annealing and extension at 60 °C for 30 s. A final melting curve protocol was performed to confirm the specificity of the primers. Primers were designed and synthesized by Shenggong Biology and Engineering Co., Ltd. (Shanghai, China) (Table 1). GAPDH was used as the normalization control, and the 2-ΔΔCT method was used to calculate the relative expression of target genes.
| Gene | Primer sequence | Amplification length (bp) |
| NF-H | Forward: 5'-GCCCTCACCAAACAGGAAT-3' | 147 |
| Reverse: 5'-GCGTTCAGCAATACATCACG-3' | ||
| 5-HTR4 | Forward: 5'-GCCTTCTACATCCCGTTTCTC-3' | 180 |
| Reverse: 5'-CTTGGCTGCTTTGGTCTCTG-3' | ||
| GAPDH | Forward: 5'-GGCACAGTCAAGGCTGAGAATG-3' | 252 |
| Reverse: 5'-ATGGTGGTGAAGACGCCAGTA-3' |
Colon tissue samples (50 mg) were lysed and homogenized in 200 μL lysis buffer and centrifuged at 10000 g for 10 min at 4 °C. The concentration of protein in the supernatant was determined using a BCA protein assay kit (P0012; Beyotime Technology Co., Ltd., Jiangsu, China). Proteins were separated by SDS-PAGE using an electrophoresis apparatus (PowerPac 3000; Bio-Rad Laboratories, Inc., Hercules, CA, United States), and then transferred to PVDF membranes. The membranes were blocked in skim milk for 2 h and then incubated overnight with GAPDH (sc-365062; 1:500), 5-HTR4 (sc-32564; 1:200) (Santa Cruz Biotechnology, Inc., Dallas, TX, United States), and NF-H (#2836, 1:500; Cell Signaling Technology, Inc., Danvers, MA, United States) primary antibodies. Then, the membranes were incubated in horseradish peroxidase-conjugated goat-anti-mouse (sc-2005) or donkey-anti-goat (sc-2020) IgGs (Santa Cruz Biotechnology, Inc.) for 2 h at room temperature. Proteins were visualized with enhanced chemiluminescence (GE Healthcare, Little Chalfont, United Kingdom) and quantified using Quantity One 4.6.2 software (Bio-Rad Laboratories, Inc.). Protein expression was normalized to GAPDH.
The formalin-fixed colon tissues were embedded in paraffin and sectioned at a thickness of 4 μm. Sections were prepared for immunostaining using a two-step Envision method involving high-pressure antigen retrieval and quenching of endogenous peroxidase activity with hydrogen peroxide. Sections were incubated with primary antibodies (anti-NF-H, 1:80; anti-5-HTR4, 1:30), followed by horseradish peroxidase-conjugated secondary antibodies, and visualized with diaminobenzidine with hematoxylin counterstaining. The slides were imaged using a Nikon Eclipse 80i optical microscope (Nikon Corp., Tokyo, Japan). Positive staining was defined by the presence of brown-colored staining in the cytoplasm. IPP 6.0 color image analysis software (Media Cybernetics, Rockville, MD, United States) was used to identify the mean integrated optical density (IOD) from five randomly selected positive areas using the formula: IOD = ∑ area (positive expression) × density (mean IOD in this area).
All analyses were performed using SPSS 17.0 statistical software (SPSS Inc., Chicago, IL, United States). Comparisons between groups were conducted using repeated measures analysis of variance followed by a least significant difference test in the case of equal variance, otherwise a Dunnett’s T3 method was used. All data are expressed as the mean ± SD. P < 0.05 was considered statistically significant.
Cathartic colons were successfully established in 68/70 animals administered dahuang; two animals died during the 12-wk period. Model animals were thinner than controls, with substantially less defecation and hard stools.
Of the 68 animals with cathartic colons, one rat from the FAI-M group died during the study. All cathartic colon groups had significantly shorter ITR than control animals (P < 0.05 for all) (Table 2). Compared to the model group, mosapride treatment and high-dose FAI significantly improved the ITR (P < 0.05 for both).
| Group | n | Pushing length (cm) | Total length (cm) | ITR |
| Control | 12 | 84.35 ± 10.27 | 107.82 ± 10.83 | 78.14 ± 4.26 |
| Model | 12 | 59.51 ± 11.08 | 92.40 ± 13.14 | 64.12 ± 5.19b |
| Mosapride | 14 | 82.40 ± 11.46 | 117.43 ± 11.48 | 70.06 ± 7.25bc |
| FAI-L | 14 | 70.50 ± 17.30 | 101.90 ± 17.04 | 68.76 ± 7.48b |
| FAI-M | 13 | 75.74 ± 16.39 | 110.93 ± 13.16 | 67.78 ± 8.00b |
| FAI-H | 14 | 84.71 ± 13.53 | 117.73 ± 13.60 | 72.02 ± 8.74ad |
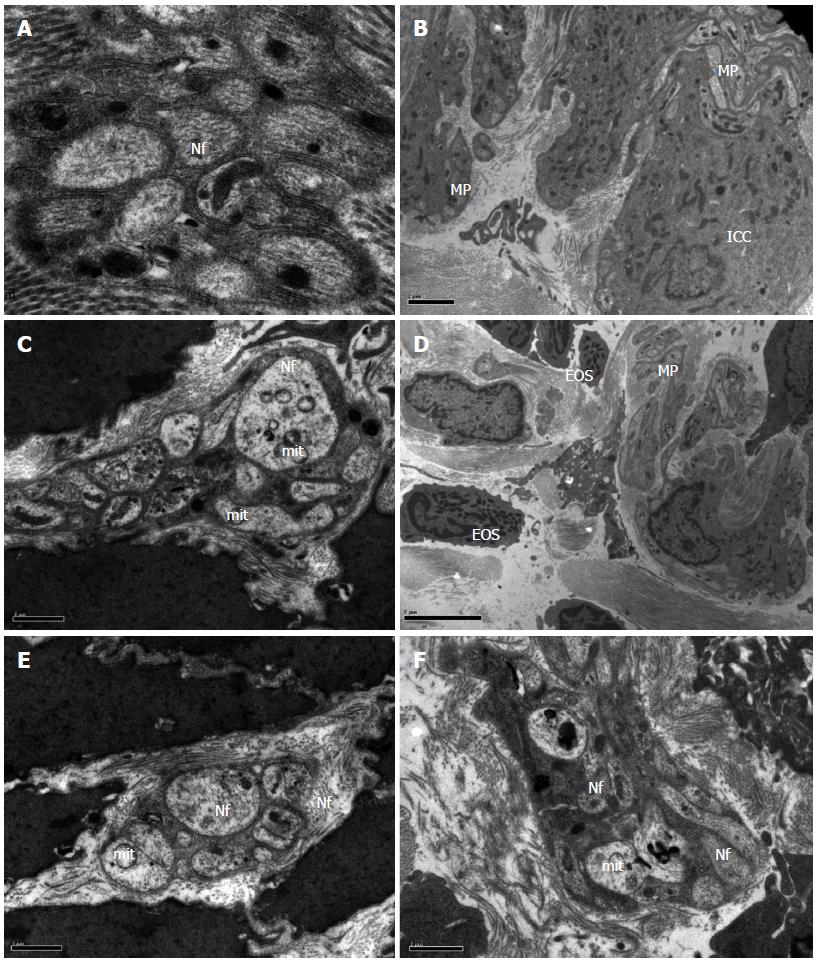
In the control group, neurons in the colonic tissue had a normal morphology and contained abundant rough endoplasmic reticulum, free ribosomes, mitochondria, and neurofilaments (Figure 1A and B). Cathartic colons in model animals showed signs of neurodegeneration, with eosinophil infiltration, fractured collagenous fibers with disordered arrangement, vacuoles in swollen mitochondria, and autophagic vacuoles in the cytoplasm (Figure 1C and D). In addition, sparse neurofilaments and debris of eosinophils, which contained a large amount of lipofuscin, could be seen in the muscular layer and myenteric plexus. Interstitial cells of Cajal appeared shrunken. In contrast, these changes were not observed in animals receiving mosapride treatment or FAI (Figure 1E and F).
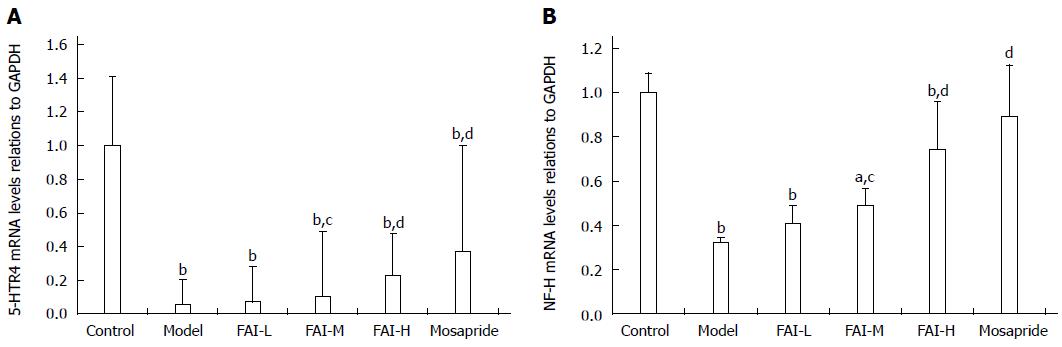
Expression of 5-HTR4 and NF-H mRNAs was significantly reduced in cathartic colons compared to normal controls (both P < 0.01) (Figure 2). However, treatment with mosapride or ≥ 2 g/kg aqueous FAI extracts (medium- and high-dose groups) significantly upregulated the expression compared to the model group (P < 0.05 for all).
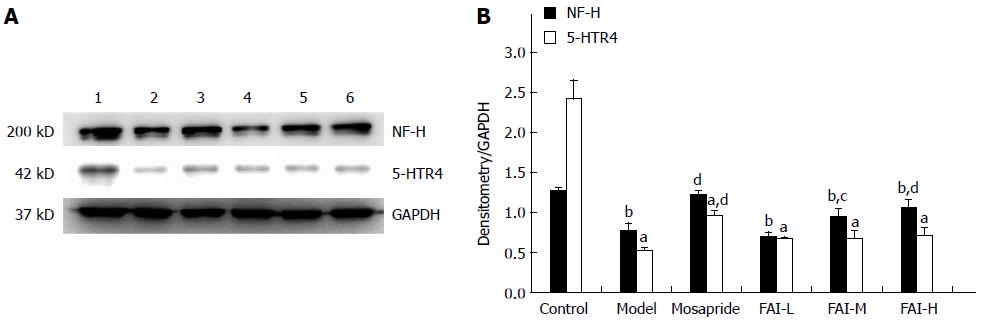
Expression of 5-HTR4 and NF-H proteins was significantly reduced in cathartic colons compared to normal controls, as assessed by Western blot (P < 0.05 for both) (Figure 3). Treatment with mosapride significantly upregulated 5-HTR4 and NF-H (P < 0.01 for both); however, only NF-H expression was increased significantly in the FAI-M and FAI-H groups (P < 0.05).
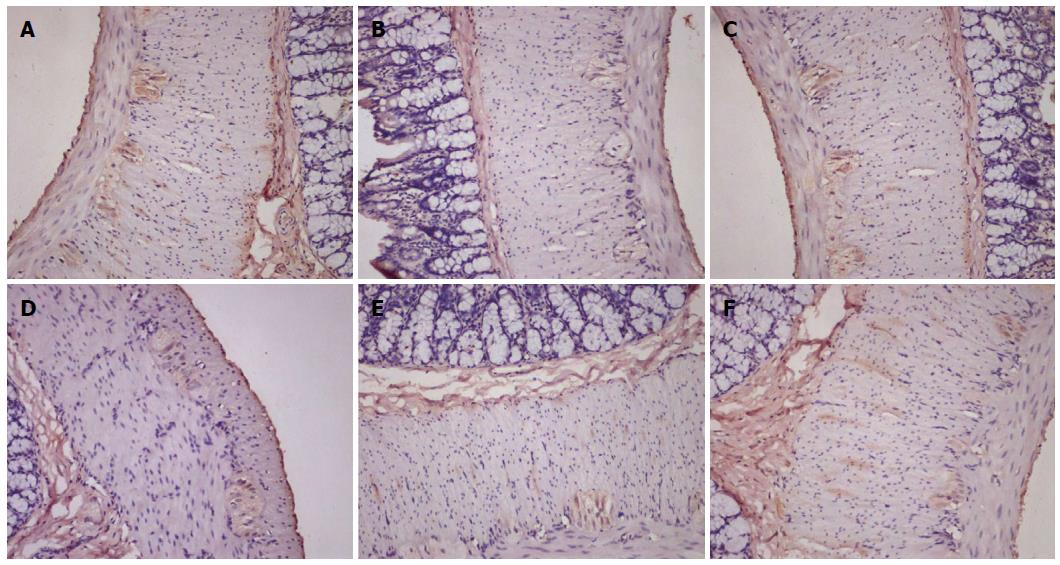
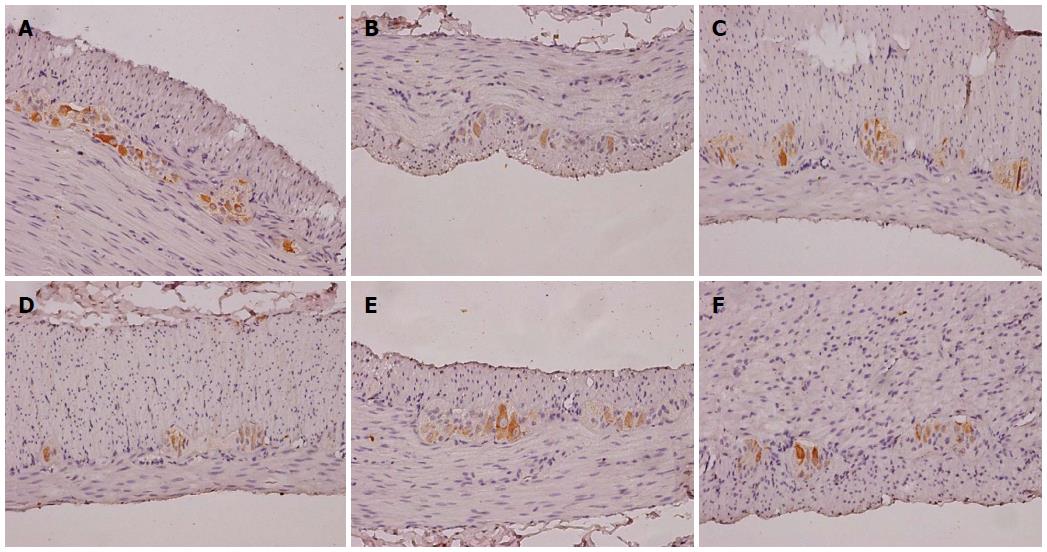
5-HTR4-positive cells were observed within the mucous layer, submucosal plexus, muscular layer, and myenteric plexus (Figure 4). Semi-quantitative analysis of expression revealed that 5-HTR4 expression was significantly reduced in cathartic colons compared to control animals (P < 0.01 for all) (Table 3). However, only mosapride treatment resulted in a significant increase in expression compared to the model group (P < 0.05).
NF-H-positive cells were found primarily within the myenteric plexus (Figure 5). Compared with the control group, semi-quantitative analysis showed significantly decreased NF-H protein expression in the cathartic colons (P < 0.05 for all) (Table 3). Treatment with mosapride and high-dose FAI significantly increased NF-H expression compared to model animals (P < 0.05 for both).
Pathologic changes in the enteric nervous system are responsible for slow transit constipation[12]. Together, the myenteric plexus, innervating the smooth muscle, and the submucosal plexus, innervating the intestinal mucosa, regulate gastrointestinal function[13]. Clinical evidence indicates that degeneration of the myenteric plexus is the primary pathologic finding[2-4], possibly due to increased neuronal apoptosis. Thus, slow transit constipation is not simply a functional disease, but may also represent an enteric neuropathy[14,15].
In the present study, a rat model of slow transit constipation was induced by chronic administration of dahuang, demonstrated by the reduced ITR in ink propulsion tests. Ultrastructural examination revealed that a possible mechanism for this effect was loss of ganglion in the myenteric plexus and a decrease in neurofilaments, which is consistent with our previous research[16] and reports of others[17]. Indeed, a previous study showed that the expression of the neurotrophin receptor p75 is increased in the intestinal wall of cathartic colon[18], which is known to mediate neuronal apoptosis[19]. Furthermore, the presence of eosinophil infiltration suggests that an additional inflammatory component may contribute to the observed reduction in intestinal function.
Gastrointestinal motility is enhanced with mosapride treatment, as shown in clinical studies[20-24] and in animal models[25-27]. The findings of the present study are consistent with this, as mosapride treatment significantly increased the ITR in animals with cathartic colons. Moreover, this recovery of function was accompanied by increased expression of 5-HTR4 and NF-H, which is consistent with previous studies[5,28]. This study shows that functional recovery with aqueous FAI extracts may occur via a similar mechanism, as treatment with a high dose significantly increased transcription of both 5-HTR4 and NF-H. The upregulation of NF-H expression by mosapride and aqueous FAI extracts is indicative of neuronal repair within the intestinal wall of cathartic colons. However, whereas mosapride increased both 5-HTR4 and NF-H protein expression, FAI led to upregulation of only NF-H protein. As aqueous FAI extracts increased the transcription of 5-HTR4, it is also possible that they affected related functional RNAs, such as microRNAs or long noncoding RNAs, to regulate 5-HTR4 at the post-transcriptional level[29].
It has been demonstrated that 5-HTR4 agonists play an important role in the development and survival of intestinal neurons[30-32]. Therefore, these agonists may represent a new therapeutic tool to treat enteric nervous system-deficiency diseases[33]. The results presented here indicate that aqueous FAI extracts could also be therapeutic in these cases. However, further studies are needed to establish an exact mechanism for the observed recovery of intestinal function. Importantly, the composition of the aqueous FAI extracts is complex[34]. Therefore, the active component(s) have yet to be identified.
Slow transit constipation can be alleviated by treatment with mosapride and aqueous FAI extracts; both of which promote repair of the myenteric plexus and upregulate transcription of 5-HTR4 and NF-H. Therefore, patients with laxative-dependent constipation may benefit from administration of either mosapride or aqueous FAI extracts.
The authors would like to thank Professor Wang Li from the Electron Microscope Center at the Medical College of Zhejiang University for assistance with TEM experiments.
In China, fructus aurantii immaturus (FAI) is widely used to treat various kinds of gastrointestinal diseases. Aqueous FAI extracts promote movement of the gastrointestinal tract, though the mechanism remains unknown.
Evidence suggests that irritant laxatives can damage the enteric nervous system, resulting in laxative-dependent constipation, also known as cathartic colon. This type of slow transit constipation can be treated with agonists of the 5-hydroxytryptamine receptor 4 (5-HTR4). Aqueous FAI extracts have a similar effect, though the mechanism is not known.
This study shows that mosapride and aqueous FAI extracts strengthen bowel movement in rat cathartic colon and promote myenteric plexus repair. Whereas mosapride increases expression of 5-HTR4 and neurofilament-H (NF-H) protein, aqueous FAI extracts only result in an increase of NF-H protein.
New therapeutic agents for the treatment of slow transit constipation are needed to counteract the increased use of laxatives in China, which can cause myenteric plexus damage. The findings of this study indicate that aqueous FAI extracts, a traditional Chinese medicine, may be effective for recovery of intestinal functional and enteric nervous system damage.
Cathartic colon is a condition resulting from long-term use of stimulant/irritant weight-control agents (e.g., phenolphthalein, cascara, castor oil, and senna extract). Prolonged misuse causes neuromuscular disruption in the intestine resulting in stimulant-dependent constipation.
This article describes the effect of aqueous FAI extracts on cathartic colons in rats. The results show that treatment increases the intestinal transit rate, promotes myenteric plexus recovery, and upregulates transcription of 5-HTR4 and NF-H, similar to what is observed with mosapride. In contrast, aqueous FAI extracts increase protein expression of NF-H only, whereas mosapride increases expression of both 5-HTR4 and NF-H protein.
| 1. | Shahid S, Ramzan Z, Maurer AH, Parkman HP, Fisher RS. Chronic idiopathic constipation: more than a simple colonic transit disorder. J Clin Gastroenterol. 2012;46:150-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 2. | Krishnamurthy S, Schuffler MD, Rohrmann CA, Pope CE. Severe idiopathic constipation is associated with a distinctive abnormality of the colonic myenteric plexus. Gastroenterology. 1985;88:26-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 3. | Schouten WR, ten Kate FJ, de Graaf EJ, Gilberts EC, Simons JL, Klück P. Visceral neuropathy in slow transit constipation: an immunohistochemical investigation with monoclonal antibodies against neurofilament. Dis Colon Rectum. 1993;36:1112-1117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 55] [Article Influence: 1.7] [Reference Citation Analysis (1)] |
| 4. | Wedel T, Roblick UJ, Ott V, Eggers R, Schiedeck TH, Krammer HJ, Bruch HP. Oligoneuronal hypoganglionosis in patients with idiopathic slow-transit constipation. Dis Colon Rectum. 2002;45:54-62. [PubMed] |
| 5. | Kawahara I, Kuniyasu H, Matsuyoshi H, Goto K, Obata K, Misawa H, Fujii H, Takaki M. Comparison of effects of a selective 5-HT reuptake inhibitor versus a 5-HT4 receptor agonist on in vivo neurogenesis at the rectal anastomosis in rats. Am J Physiol Gastrointest Liver Physiol. 2012;302:G588-G597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Huang ZH, Yang DZ, Wei YQ. Effect of atropine on the enhancing action of Fructus Aurantii Immaturus on the myoelectric activity of small intestine in dogs. Zhongguo Zhongxiyi Jiehe Zazhi. 1996;16:292-294. |
| 7. | Li XW, Ren JG. Experimental study on the coordinative application of Immature Bitter Orange and Bighead Atractylodes. Zhongguo Chuantong Yiyao Yanjiu. 2002;15:23-23. |
| 8. | Lee HT, Seo EK, Chung SJ, Shim CK. Effect of an aqueous extract of dried immature fruit of Poncirus trifoliata (L.) Raf. on intestinal transit in rodents with experimental gastrointestinal motility dysfunctions. J Ethnopharmacol. 2005;102:302-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Wang CF, Yang DZ. Study on the effect of fructus aurantii immaturus on myoelectric of gastrointestinal tract in rats. Dongnan Daxue Xuebao. 2001;20:153-154. |
| 10. | Liu LJ, Wei YQ. The regulation of histamine receptor antagonist in the effect of fructus aurantii immaturus on the motor activity of small intestine in mice. Dongnan Daxue Xuebao. 2001;20:144-146. |
| 11. | Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22:659-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3894] [Cited by in RCA: 5019] [Article Influence: 264.2] [Reference Citation Analysis (1)] |
| 12. | Bassotti G, De Giorgio R, Stanghellini V, Tonini M, Barbara G, Salvioli B, Fiorella S, Corinaldesi R. Constipation: a common problem in patients with neurological abnormalities. Ital J Gastroenterol Hepatol. 1998;30:542-548. [PubMed] |
| 13. | Furness JB. The enteric nervous system and neurogastroenterology. Nat Rev Gastroenterol Hepatol. 2012;9:286-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 856] [Cited by in RCA: 1143] [Article Influence: 81.6] [Reference Citation Analysis (0)] |
| 14. | Bassotti G, Villanacci V. Slow transit constipation: a functional disorder becomes an enteric neuropathy. World J Gastroenterol. 2006;12:4609-4613. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 73] [Cited by in RCA: 76] [Article Influence: 3.8] [Reference Citation Analysis (1)] |
| 15. | Bassotti G, Villanacci V, Creţoiu D, Creţoiu SM, Becheanu G. Cellular and molecular basis of chronic constipation: taking the functional/idiopathic label out. World J Gastroenterol. 2013;19:4099-4105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 34] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (1)] |
| 16. | Fan YH, Zhou Y, Li YL, LV B, Zhang L, Huang Z. The effect of glial cell line derived neurotrophic factor (GDNF) to colonic myenteric plexus ultrastructural changes in slow transit constipation rats. Zhonghua Xiaohua Zazhi. 2009;845-846. |
| 17. | Zhang Y, Li HY. Colonic ultrastructural changes in rats with constipation. Beijing Zhongyiyao Daxue Xuebao. 2005;28:63-65. |
| 18. | Fan YH, Lu B, Wang M, Ni GB, Chen MT, Xu Y. Expression and significance of nerve growth factor receptor p75 in rats’ cathartic colonic wall. Zhonghua Xiaohua Zazhi. 2006;7:225-229. |
| 19. | Bamji SX, Majdan M, Pozniak CD, Belliveau DJ, Aloyz R, Kohn J, Causing CG, Miller FD. The p75 neurotrophin receptor mediates neuronal apoptosis and is essential for naturally occurring sympathetic neuron death. J Cell Biol. 1998;140:911-923. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 351] [Cited by in RCA: 426] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 20. | Mine Y, Yoshikawa T, Oku S, Nagai R, Yoshida N, Hosoki K. Comparison of effect of mosapride citrate and existing 5-HT4 receptor agonists on gastrointestinal motility in vivo and in vitro. J Pharmacol Exp Ther. 1997;283:1000-1008. [PubMed] |
| 21. | Liu Z, Sakakibara R, Odaka T, Uchiyama T, Uchiyama T, Yamamoto T, Ito T, Asahina M, Yamaguchi K, Yamaguchi T. Mosapride citrate, a novel 5-HT4 agonist and partial 5-HT3 antagonist, ameliorates constipation in parkinsonian patients. Mov Disord. 2005;20:680-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 134] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 22. | Ueno N, Inui A, Satoh Y. The effect of mosapride citrate on constipation in patients with diabetes. Diabetes Res Clin Pract. 2010;87:27-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 23. | Odaka T, Suzuki T, Seza A, Yamaguchi T, Saisho H. [Serotonin 5- HT4 receptor agonist (mosapride citrate)]. Nihon Rinsho. 2006;64:1491-1494. [PubMed] |
| 24. | Kanazawa M, Watanabe S, Tana C, Komuro H, Aoki M, Fukudo S. Effect of 5-HT4 receptor agonist mosapride citrate on rectosigmoid sensorimotor function in patients with irritable bowel syndrome. Neurogastroenterol Motil. 2011;23:754-e332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 25. | Inui A, Yoshikawa T, Nagai R, Yoshida N, Ito T. Effects of mosapride citrate, a 5-HT4 receptor agonist, on colonic motility in conscious guinea pigs. Jpn J Pharmacol. 2002;90:313-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | Makimoto N, Sakurai-Yamashita Y, Furuichi A, Kawakami S, Enjoji A, Kanematsu T, Taniyam K. In vivo assessment of acceleration of motor activity associated with acetylcholine release via 5-hydroxytryptamine4 receptor in dog intestine. Jpn J Pharmacol. 2002;90:28-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Okamura K, Sasaki N, Yamada M, Yamada H, Inokuma H. Effects of mosapride citrate, metoclopramide hydrochloride, lidocaine hydrochloride, and cisapride citrate on equine gastric emptying, small intestinal and caecal motility. Res Vet Sci. 2009;86:302-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 28. | Matsuyoshi H, Kuniyasu H, Okumura M, Misawa H, Katsui R, Zhang GX, Obata K, Takaki M. A 5-HT(4)-receptor activation-induced neural plasticity enhances in vivo reconstructs of enteric nerve circuit insult. Neurogastroenterol Motil. 2010;22:806-813, e226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 29. | Bai M, Zhu XZ, Zhang Y, Zhang S, Zhang L, Xue L, Zhong M, Zhang X. Anhedonia was associated with the dysregulation of hippocampal HTR4 and microRNA Let-7a in rats. Physiol Behav. 2014;129:135-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 30. | Liu MT, Kuan YH, Wang J, Hen R, Gershon MD. 5-HT4 receptor-mediated neuroprotection and neurogenesis in the enteric nervous system of adult mice. J Neurosci. 2009;29:9683-9699. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 288] [Cited by in RCA: 272] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 31. | Liu M, Gershon MD. Neuroprotective/trophic effects of 5-HT4 receptor stimulation on enteric neurons of mice. Neurogastroenterol Motil. 2006;18:780-781. |
| 32. | Gershon MD, Liu MT. Serotonin and neuroprotection in functional bowel disorders. Neurogastroenterol Motil. 2007;19 Suppl 2:19-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 33. | Takaki M, Goto K, Kawahara I. The 5-hydroxytryptamine 4 Receptor Agonist-induced Actions and Enteric Neurogenesis in the Gut. J Neurogastroenterol Motil. 2014;20:17-30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 34. | Starkenmann C, Niclass Y, Escher S. Volatile organic sulfur-containing constituents in Poncirus trifoliata (L.) Raf. (Rutaceae). J Agric Food Chem. 2007;55:4511-4517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Harmanci O, Luo HS, Pescatori M, Raju J, Shehata MMM, Tsai KW S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Liu XM