Published online Aug 7, 2015. doi: 10.3748/wjg.v21.i29.8817
Peer-review started: January 29, 2015
First decision: June 2, 2015
Revised: June 18, 2015
Accepted: July 3, 2015
Article in press: July 3, 2015
Published online: August 7, 2015
Processing time: 198 Days and 18.1 Hours
AIM: To examine renal expression of organic anion transporter 5 (Oat5) and sodium-dicarboxylate cotransporter 1 (NaDC1), and excretion of citrate in rats with acute extrahepatic cholestasis.
METHODS: Obstructive jaundice was induced in rats by double ligation and division of the common bile duct (BDL group). Controls underwent sham operation that consisted of exposure, but not ligation, of the common bile duct (Sham group). Studies were performed 21 h after surgery. During this period, animals were maintained in metabolic cages in order to collect urine. The urinary volume was determined by gravimetry. The day of the experiment, blood samples were withdrawn and used to measure total and direct bilirubin as indicative parameters of hepatic function. Serum and urine samples were used for biochemical determinations. Immunoblotting for Oat5 and NaDC1 were performed in renal homogenates and brush border membranes from Sham and BDL rats. Immunohistochemistry studies were performed in kidneys from both experimental groups. Total RNA was extracted from rat renal tissue in order to perform reverse transcription polymerase chain reaction. Another set of experimental animals were used to evaluate medullar renal blood flow (mRBF) using fluorescent microspheres.
RESULTS: Total and direct bilirubin levels were significantly higher in BDL animals, attesting to the adequacy of biliary obstruction. An important increase in mRBF was determined in BDL group (Sham: 0.53 ± 0.12 mL/min per 100 g body weight vs BDL: 1.58 ± 0.24 mL/min per 100 g body weight, P < 0.05). An increase in the urinary volume was observed in BDL animals. An important decrease in urinary levels of citrate was seen in BDL group. Besides, a decrease in urinary citrate excretion (Sham: 0.53 ± 0.11 g/g creatinine vs BDL: 0.07 ± 0.02 g/g creatinine, P < 0.05) and an increase in urinary excretion of H+ (Sham: 0.082 ± 0.03 μmol/g creatinine vs BDL: 0.21 ± 0.04 μmol/g creatinine, P < 0.05) were observed in BDL animals. We found upregulations of both proteins Oat5 and NaDC1 in brush border membranes where they are functional. Immunohistochemistry technique corroborated these results for both proteins. No modifications were observed in Oat5 mRNA and in NaDC1 mRNA levels in kidney from BDL group as compared with Sham ones.
CONCLUSION: Citrate excretion is decreased in BDL rats, at least in part, because of the higher NaDC1 expression. Using the outward gradient of citrate generated by NaDC1, Oat5 can reabsorb/eliminate different organic anions of pathophysiological importance.
Core tip: Organic anion transporter 5 (Oat5) is an organic anion/dicarboxylate exchanger which has impact on renal excretion of hormones, drugs and xenobiotics. The primary function of sodium-dicarboxylate cotransporter 1 (NaDC1) is to reabsorb filtered Krebs cycle intermediates, such as citrate. We found upregulations of both transporters and a decrease in urinary citrate excretion in bile duct-ligated rats. Citrate excretion is decreased at least in part, because of the higher NaDC1 expression. Using the outward gradient of citrate generated by NaDC1, Oat5 can reabsorb/eliminate different organic anions of pathophysiological importance. Attention might be paid for those drugs transported by this protein because their pharmacokinetics may be altered during cholestasis.
- Citation: Brandoni A, Torres AM. Expression of renal Oat5 and NaDC1 transporters in rats with acute biliary obstruction. World J Gastroenterol 2015; 21(29): 8817-8825
- URL: https://www.wjgnet.com/1007-9327/full/v21/i29/8817.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i29.8817
Kidneys perform an essential function in the removal and reabsorption of organic anions from circulation[1]. Several transport proteins are implicated in the renal tubular secretion and reabsorption of endogenous and exogenous compounds[2].
Organic anion transporter 5 [(Oat5), Slc22a19] has been characterized as an organic anion/dicarboxylate exchanger[2-5]. This protein is localized in the brush border membrane of proximal tubule straight segment (S3)[4,5]. Oat5 has been reported to interact with many anionic drugs, such as bumetanide, furosemide, penicillin G and non-steroidal anti-inflammatory drugs[3-5]. The function of the organic anion exchanger Oat5 in renal cells under pathological conditions has not been fully elucidated yet.
The sodium-dicarboxylate cotransporter 1 [NaDC1), Slc13a2] is located in the apical membrane of the S1, S2, and S3 segments of proximal renal tubule[6,7]. The primary function of this transporter is to reabsorb filtered Krebs cycle intermediates[7,8]. These compounds, such as succinate, citrate and α-ketoglutarate are important substrates for renal metabolism because they account for 10%-15% of oxidative metabolism in the kidney[8,9]. Furthermore, Krebs cycle intermediates are involved in the maintenance of the outward dicarboxylate gradient that is crucial for the normal function of Oat exchanger proteins at both membrane domains, apical (such as Oat5) and basolateral (such as Oat1 and Oat3)[1,2].
Jaundice in chronic bile duct-ligated rats has been associated with functional and metabolic disturbances of the kidney[10]. Altered absorption, distribution and elimination of drugs have been described in this pathology[11,12].
The purpose of the current study was to examine the effects of acute extrahepatic cholestasis on the expression of Oat5 and NaDC1 in rats, and the contribution of these effects on renal excretion of citrate.
Male Wistar rats (110-130 d) were used throughout the study. The animal protocol was designed to minimize pain or discomfort to the animals. Animals were cared for in accordance with the principles and guidelines for the care and use of laboratory animals, recommended by the National Academy of Sciences and published by the National Institute of Health (NIH publication 7th edition revised 1996) and recommended by regulations of the local ethics committee. All experimental procedures were approved by the Faculty of Biochemical and Pharmaceutical Sciences Institutional Animal Care and Use Committee (Res. No. 637/2012).
Surgical procedure of bile duct ligation (BDL group) was performed as previously described[13-15]. A parallel group of Sham rats was processed. Studies were performed 21 h after surgery. During this period, animals were maintained in metabolic cages in order to collect urine. The urinary volume was determined by gravimetry.
Animals were anaesthetized with sodium thiopental (70 mg/kg body weight, ip). Blood was withdrawn by cardiac puncture from Sham and bile duct (BDL) animals. These samples were used to measure total and direct bilirubin, and creatinine serum levels.
The urine samples were used to determine the urinary levels of citrate, creatinine, glucose and proteins, alkaline phosphatase activity and pH.
Creatinine clearance was calculated employing the following formula: [Creatinine]urine× Urine flow/[Creatinine]plasma
Biochemical analyses were performed with optimized spectrophotometric techniques, employing commercial kits (Wiener Laboratory, Rosario, Argentina) except for citrate measurements that were performed using a citric acid enzymatic kit (Boehringer Mannheim/R-Biopharm, Darmstadt, Germany).
BBM from Sham (n = 4) and BDL (n = 4) rats were isolated from kidneys by Mg/EGTA precipitation as previously described[13]. For each experimental group, four different preparations were made. Protein quantification of samples was performed using the method of Sedmak and Grossberg[16].
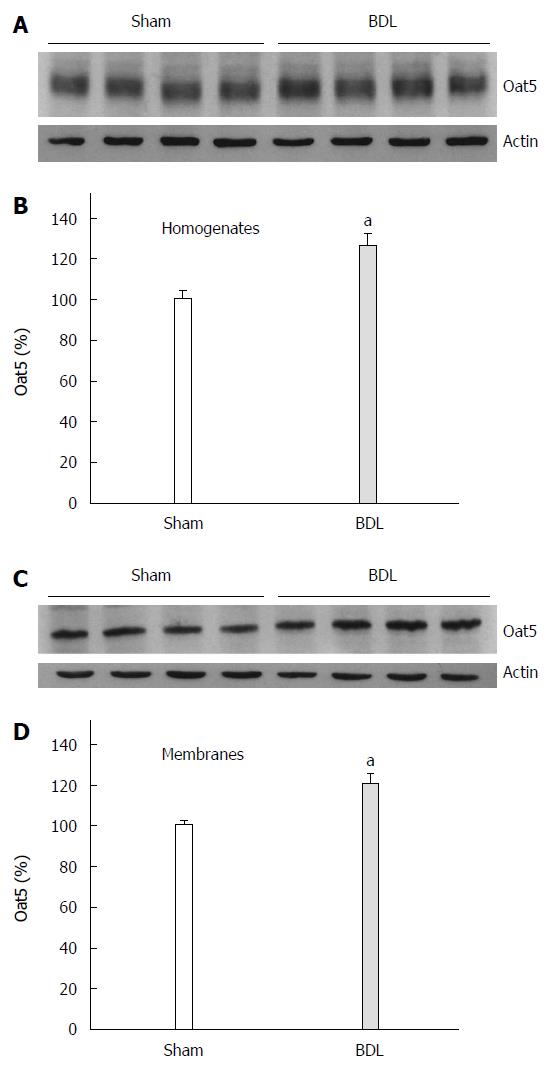
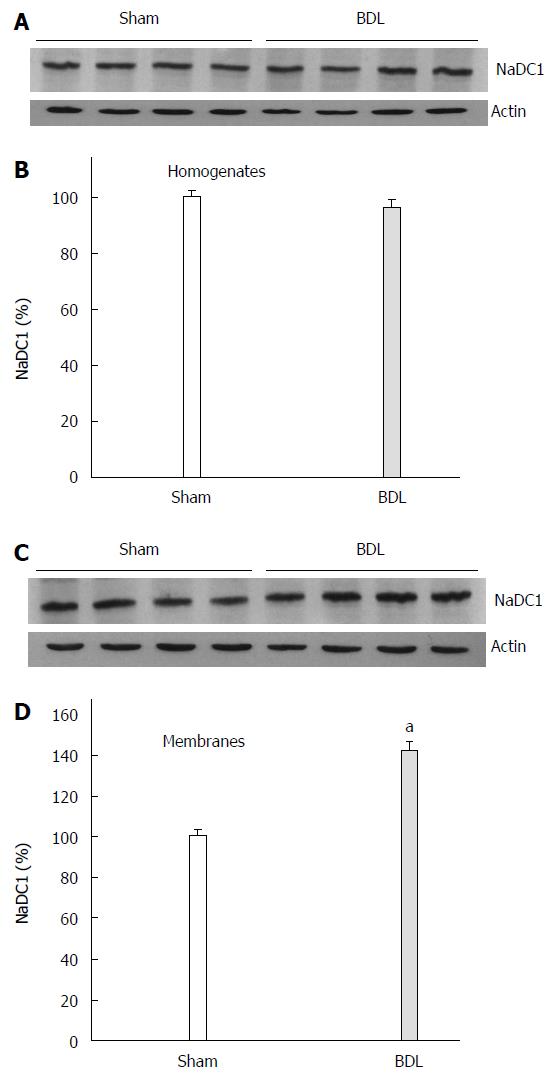
Immunoblotting for Oat5 and NaDC1 were performed in renal homogenates (20 μg of protein) and BBM (10 μg of protein) as previously described[13-15]. The membranes were incubated overnight at 4 °C with a non-commercial rabbit polyclonal antibodies against rat Oat5 (at a dilution of 1:800) or against rat NaDC1 (at a dilution of 1:800) or a commercial mouse monoclonal antibody against human β-actin (at a dilution of 1:800). Specificity of Oat5 and NaDC1 antibodies has been described elsewhere[4,6]. Blots were processed for detection using a commercial kit (ECL enhanced chemiluminescence system, Amersham, Buckinghamshire, United Kingdom). To verify equal protein loading and transfer between lanes, Ponceau Red and antibody against human β-actin were used as previously reported[13-15,17]. The abundance of Oat5 and NaDC1 were normalized to β actin. The relative protein expression for Oat5 or NaDC1 was expressed as percentage, considering the mean Sham value as the 100%.
The immunohistochemistry studies were performed as previously described[13,15,18,19]. Kidneys from the different experimental groups were briefly perfused with saline, followed by perfusion with periodate-lysine-paraformaldehyde solution (0.0375 M phosphate buffer (pH 6.2) containing 0.01 M NaIO4, 0.075 M lysine, 2% paraformaldehyde), through a cannula inserted in the abdominal aorta. The kidney slices were immersed in periodate-lysine-paraformaldehyde solution at 4 °C overnight. After that, the tissue was embedded in paraffin and paraffin sections were cut.
After deparaffining, some sections were used for routine haematoxylin-eosin staining, while others were incubated with 3% H2O2 for 15 min (in order to eliminate endogenous peroxidase activity) to perform Oat5 and NaDC1 renal immunohistochemistry. Then, the sections were incubated with blocking serum for 30 min and after that with non-commercial rabbit polyclonal antibody against rat Oat5 (diluted 1:100[19]) or against rat NaDC1 (diluted 1:500[19]) overnight at 4 °C. The sections were rinsed with Tris-Buffered Saline containing 1% Tween (TBST). Right after, the sections were incubated with horseradish peroxidase (HPR) conjugated secondary antibody against rabbit immunoglobulin for 1 h. So as to detect HPR labelling, a peroxidase substrate solution with diaminobenzidine (0.05% diaminobenzidine in TBST with 0.05% H2O2) was used. The sections were counterstained with hematoxylin before being examined under a light microscope. Controls using preimmune serum, antiserum absorbed with excess synthetic peptide, or omission of primary or secondary antibody revealed no labelling.
As previously described by Bulacio et al[20], total RNA was extracted from rat renal tissue using Trizol reagent (Invitrogen, Carlsbad, CA, United States) following the manufacturer’s instructions. Samples were stored at -80 °C until used.
The cDNA was synthesized using SuperScript™ First-Strand Synthesis System for reverse-transcriptase polymerase chain reaction (RT-PCR) (Invitrogen, Carlsbad, CA, United States) according to manufacturer’s instructions. cDNA samples were kept at -20 °C until assayed.
RT-PCR for Oat5, NaDC1 and 18S rRNA as housekeeping gene was performed on cDNA samples using the BOECO TC-SQ Thermal Cycler (Boeckel Co., GmbH Co., KG, Hamburg, Germany). Reaction conditions used for the PCR were: initial denaturation for 2 min at 94 °C, denaturation for 15 s at 94 °C, annealing for 30 s at 55 °C and elongation for 60 s at 72 °C. The final elongation step was 72 °C for 10 min. The specific PCR primers used were: for Oat5: 5’-GGAGGCAGCAGAGACAAAAC-3’ (forward) and 5’-TTGCTCCTCCTAATGATGCC-3’ (reverse), for NaDC1: 5’-GAACGATAAGATGCCCTGGA-3’ (forward) and 5’-TGAAGACAGATGGCTTGTGC-3’ (reverse), and for 18S rRNA: 5’-CGCGGTTCTATTTTGTTGGT-3’ (forward) and 5’-AGTCGGCATCGTTTATGGTC-3’ (reverse).
RT-PCR products were then resolved by electrophoresis in a 1.2% agarose gel stained with SYBR Safe™ and visualized using the Safe Imager™ blue light transilluminator. For semiquantitative measurement, images of the gels were acquired and quantification of the optical density (OD) of the bands was performed. The Oat5/18S rRNA or NaDC1/18S rRNA product ratio was calculated and used as an index of Oat5 or NaDC1 mRNA expression, respectively. The relative mRNA expression for Oat5 or NaDC1 was expressed as percentage, considering the mean Sham value as the 100%.
Another set of experimental animals (Sham, n = 4; BDL, n = 4) were used to evaluate Medullar renal blood flow (mRBF) using fluorescent microspheres as previously described[14,21-23]. The mRBF were calculated by the formula: renal flow (mL/min) = fl/flref × R (mL/min), where fl is the fluorescence of renal tissue, flref is the fluorescence of reference blood flow sample, and R is the withdrawal rate of reference blood flow sample.
Chemicals were purchased from Sigma (St. Louis, Missouri, United States) and were analytical grade pure.
Results are expressed as mean ± SE. Statistical analysis was performed using an unpaired t-test. When variances were not homogeneous a Welch’s correction was employed. P values less to 0.05 were considered significant. For these analyses GraphPad software was used. The statistical review of the study was performed by a biomedical statistician.
Total and direct bilirubin levels were significantly higher in BDL animals, attesting to the adequacy of biliary obstruction (Table 1).
An important increase in mRBF was determined in BDL group (mL/min per 100 g body weight; Sham, n = 4: 0.53 ± 0.12; BDL, n = 4: 1.58 ± 0.24, P < 0.05).
Table 2 shows an increase in the urinary flow of BDL animals. There were no significant differences between groups in creatinine clearance, glucose and protein urinary excretion, and the activity of alkaline phosphatase. An important decrease in urinary levels of citrate was seen in BDL group. Besides, a decrease in urinary citrate excretion and an increase in urinary excretion of H+ were observed in BDL animals.
| Sham | BDL | |
| (n = 4) | (n = 4) | |
| Urine flow (μL/min per 100 g bw) | 2.10 ± 0.24 | 3.87 ± 0.57a |
| Creatinine clearance (mL/min per 100 g bw) | 0.65 ± 0.03 | 0.53 ± 0.06 |
| Glucose (g/g creatinine) | 0.17 ± 0.10 | 0.17 ± 0.07 |
| Protein (g/g creatinine) | 1.15 ± 0.08 | 1.15 ± 0.10 |
| Alkaline phosphatase (U/g creatinine) | 266 ± 14 | 253 ± 20 |
| Citrate concentration (g/L) | 0.71 ± 0.16 | 0.07 ± 0.03a |
| Citrate excretion (g/g creatinine) | 0.53 ± 0.11 | 0.07 ± 0.02a |
| Proton excretion (μmol/g creatinine) | 0.082 ± 0.03 | 0.21 ± 0.04a |
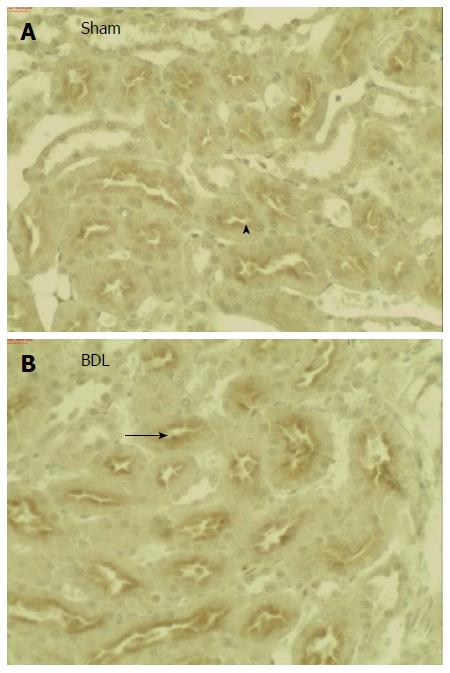
Figure 1 shows a significant increase in Oat5 protein expression in homogenates as well as in BBM in BDL group. Oat5 renal expression was also assessed by immunohistochemistry technique. As it is shown in Figure 2, strong Oat5 labelling was associated with the apical membrane domains in proximal tubule cells. Oat5 staining of proximal tubule cells was increased in BDL group, consistent with the density observed by Western blotting studies in each experimental group.
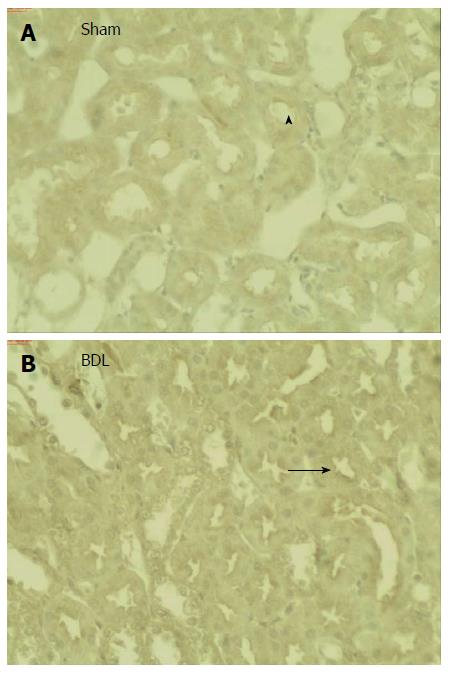
Figure 3 shows a higher abundance of NaDC1 in BBM from BDL rats while no difference was observed in NaDC1 protein expression in homogenates. Immunohistochemistry showed labelling of NaDC1 associated with apical plasma membranes of proximal tubule of Sham and BDL rat kidneys (Figure 4). BDL rats showed an increased apical NaDC1 expression, corroborating the data obtained by Western blotting.
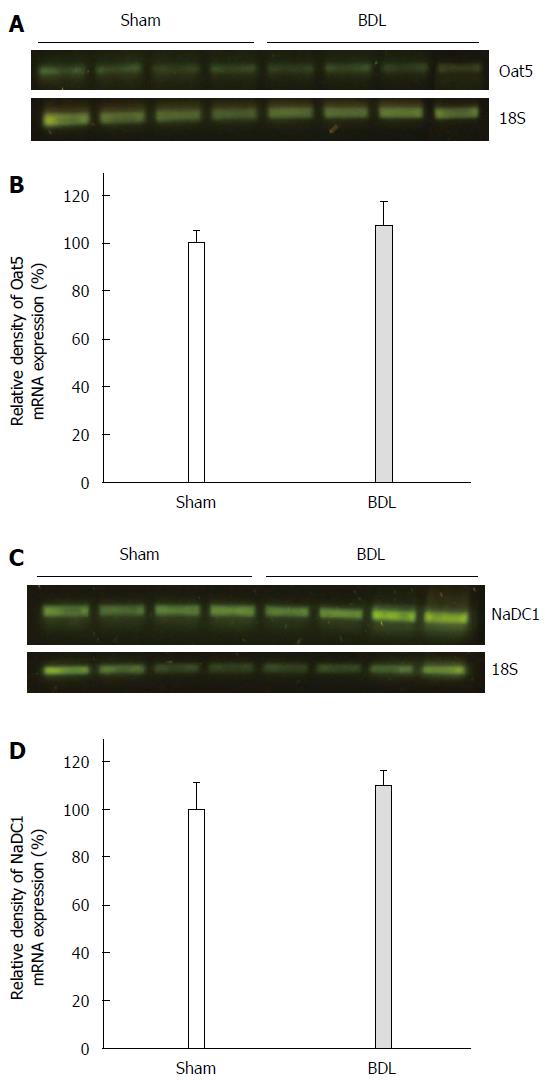
Oat5 and NaDC1 mRNA levels were determined by RT-PCR. As shown in Figure 5, no modifications were observed in Oat5 mRNA and in NaDC1 mRNA levels in kidney from BDL group as compared with Sham ones.
Cholestasis has been demonstrated to alter the transport of compounds such as bile salts and of other organic anions in liver and kidneys[11,12]. Renal function is also impaired during cholestasis as previously reported[10,13,15,18]. In this study we observed an increase in mRBF in BDL rats. This increase might lead to an important wash out of the cortico-medullar gradient. Moreover, we found an increased urinary volume that might be explained by the wash out of the cortico-medullar gradient and by the presence in the urine of a greater amount of osmotically active solutes such as bile acids[24,25] that could not be eliminated by the liver. This finding suggests intrarenal blood flow distribution with increased medullary blood flow following acute bile duct ligation as other authors have described after chronic bile duct ligation in rats[10].
In this study we observed no differences in the biochemical parameters evaluated in urine, glucose and protein urinary excretion and the activity of alkaline phosphatase. At this point it is important to emphasize that there was no difference in the glomerular filtration rate, evaluated in this study by creatinine clearance, between Sham and BDL rats as it was previously described by us using inulin clearance[15].
We have previously demonstrated alterations on the expression of different transporters in rats suffering extrahepatic cholestasis[12-15,18].
The organic anion/dicarboxylate exchanger Oat5 is important for the transport of several organic anions, including steroids sulphates[26]. Little is known about renal expression of Oat5 under pathological conditions, particularly under obstructive jaundice. In this experimental model of extrahepatic cholestasis, we found an increase in Oat5 protein expression both in BBM and in homogenates in BDL group. In addition to immunoblotting, the immunohistochemical technique corroborated the increase in apical membrane expression of Oat5. No modifications were observed in mRNA levels for Oat5. These results suggest a decrease in Oat5 protein degradation. The Oat5 upregulation might lead to a higher elimination/reabsorption of its transported compounds. This is especially important for those drugs transported by this protein because their pharmacokinetics may be altered during cholestasis.
Sodium-coupled transporters, such as NaDC1, are responsible for the active transport of Krebs cycle intermediates, including succinate, α-ketoglutarate and citrate[7]. In this study, we observed that BDL rats have a higher renal expression of NaDC1 protein at apical membranes while no difference is observed in homogenates which might suggest an increased recruitment of preformed transporters into the membranes or an inhibition in the internalization of membrane transporters. Immunohistochemical technique corroborated these results, showing an increase in the staining for NaDC1 in apical membranes of proximal tubule cells from BDL animals. No modifications were observed in mRNA levels for NaDC1.
It has been proposed that NaDC1 and Oat5 have an important role in the late (S2 to S3) segments of proximal tubules. Using the outward gradient of succinate, citrate and α-ketoglutarate generated by NaDC1, Oat5 can reabsorb some organic anions, such as steroid sulphates that are glomerular filtrated or tubular secreted by multidrug resistance proteins such as Mrp2 and Mrp4[4]. NaDC1 cotransporter might also help to maintain the outward dicarboxylate gradient necessary for the correct function of Oat proteins both at apical (Oat5) and basolateral membranes (Oat1 and Oat3)[27]. Thus, Oat1 and Oat3 may contribute to the elimination of toxic metabolites such as bile acids and other potential toxins existing in extrahepatic cholestasis that could not be excreted into bile in this cholestatic model. Then, Oat5 may contribute to the elimination of these compounds as well as anionic drugs that are uptaken by Oat1 and Oat3.
Besides the upregulations of both apical proteins NaDC1 and Oat5, we have found an important decrease in citrate urine levels in BDL rats, at least in part, because of higher tubular citrate reabsorption.
The importance of extracellular or luminal pH in the alteration of citrate reabsorption is also emphasized[7,9]. We found an increase in urinary excretion of H+ in BDL rats that would favour the protonation of citrate3- to citrate2- making it a much stronger substrate for the dicarboxylate transporters[7]. Furthermore, this increase in urinary excretion of H+ in BDL rats might explain, at least in part, the increased renal expression of NaDC1 observed in BDL rats since it has been reported that this cotransporter is modulated by pH changes[7,9,28]. The accumulation of bilirubin, bile acids and other potential toxics existing in this experimental model of extrahepatic cholestasis may affect post-transcriptional mechanisms[29,30].
In summary, we present evidence that cholestasis induced by common bile duct ligation in the rat induces upregulations of both apical proteins Oat5 and NaDC1 in kidneys. The urinary excretion of citrate is decreased in BDL rats, probably because of higher NaDC1 expression. These modifications might be part of a likely adaptation leading to support normal renal tubular function by increasing important metabolites reabsorption, such as citrate, that have different important roles in proximal tubule cell metabolism. Besides, special attention might be paid for those drugs transported by Oat5 because their pharmacokinetics may be altered during cholestasis.
The authors thank to Professor H. Endou and to Professor N. Anzai (Department of Pharmacology and Toxicology, Kyorin University School of Medicine, Tokyo, Japan) for kindly providing Oat5 and NaDC1 specific antibodies. The authors also thank Wiener Lab Argentina for analytical kits.
Jaundice in chronic bile duct-ligated rats has been associated with functional and metabolic disturbances of the kidney. Altered absorption, distribution and elimination of drugs have been described in this pathology.
Organic anion transporter 5 (Oat5) is a protein exclusively localized in the kidney. Oat5 has been reported to interact with many anionic drugs of pharmacological interest. The function of Oat5 in renal cells under pathological conditions has not been fully elucidated yet. The sodium-dicarboxylate cotransporter 1 (NaDC1) is also located in the kidney. The primary function of this transporter is to reabsorb filtered Krebs cycle intermediates. These compounds, such as succinate, citrate and α-ketoglutarate are important substrates for renal metabolism because they account for 10%-15% of oxidative metabolism in the kidney.
The effects of acute extrahepatic cholestasis on the expression of Oat5 and NaDC1 in rats, and the contribution of these effects on renal excretion of citrate were examined in this basic research study.
This study shows that cholestasis induces upregulations of both apical proteins Oat5 and NaDC1 in kidneys and decreases the urinary excretion of citrate. Thus, special attention might be paid for the renal handling of citrate and of those therapeutic drugs transported by Oat5 because their plasma levels may be altered during cholestasis.
Cholestasis is a condition where bile cannot flow from the liver to the duodenum. The obstructive type of cholestasis is a mechanical blockage in the duct system that can occur from a gallstone or malignancy. A membrane transport protein is a membrane protein involved in the movement of ions, small molecules or macromolecules across a biological membrane. Krebs cycle or citric acid cycle is a series of chemical reactions used by all aerobic organisms to generate energy through the oxidation of acetate derived from carbohydrates, fats and proteins into carbon dioxide and chemical energy in the form of adenosine triphosphate.
This is a good descriptive study with important findings. Oat5 and NaDC1 are two carriers expressed in the apical membrane of renal proximal tubule cells. Oat5 is an organic anion/dicarboxylate exchanger which has impact on renal excretion of hormones, drugs and xenobiotics. The primary function of NaDC1 is to reabsorb filtered Krebs cycle intermediates, such as citrate. In the present work, they found upregulations of both transporters and a decrease in urinary citrate excretion in bile duct-ligated rats. Citrate excretion is decreased at least in part, because of the higher NaDC1 expression. Using the outward gradient of citrate generated by NaDC1, Oat5 can reabsorb/eliminate different organic anions of pathophysiological importance. Attention might be paid for those drugs transported by this protein because their pharmacokinetics may be altered during cholestasis.
| 1. | Burckhardt G, Pritchard JB. Organic anion and cation antiporters. The Kidney. Physiology and Pathophysiology. Volume 1, 3rd ed. Philadelphia: LIPPINCOTT WILLIAMS & WILKINS 2000; 193-222. |
| 2. | VanWert AL, Gionfriddo MR, Sweet DH. Organic anion transporters: discovery, pharmacology, regulation and roles in pathophysiology. Biopharm Drug Dispos. 2010;31:1-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 119] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 3. | Youngblood GL, Sweet DH. Identification and functional assessment of the novel murine organic anion transporter Oat5 (Slc22a19) expressed in kidney. Am J Physiol Renal Physiol. 2004;287:F236-F244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 77] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 4. | Anzai N, Jutabha P, Enomoto A, Yokoyama H, Nonoguchi H, Hirata T, Shiraya K, He X, Cha SH, Takeda M. Functional characterization of rat organic anion transporter 5 (Slc22a19) at the apical membrane of renal proximal tubules. J Pharmacol Exp Ther. 2005;315:534-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 68] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 5. | Kwak JO, Kim HW, Oh KJ, Ko CB, Park H, Cha SH. Characterization of mouse organic anion transporter 5 as a renal steroid sulfate transporter. J Steroid Biochem Mol Biol. 2005;97:369-375. [PubMed] |
| 6. | Sekine T, Cha SH, Hosoyamada M, Kanai Y, Watanabe N, Furuta Y, Fukuda K, Igarashi T, Endou H. Cloning, functional characterization, and localization of a rat renal Na+-dicarboxylate transporter. Am J Physiol. 1998;275:F298-F305. [PubMed] |
| 7. | Pajor AM. Molecular properties of the SLC13 family of dicarboxylate and sulfate transporters. Pflugers Arch. 2006;451:597-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 108] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 8. | Ho HT, Ko BC, Cheung AK, Lam AK, Tam S, Chung SK, Chung SS. Generation and characterization of sodium-dicarboxylate cotransporter-deficient mice. Kidney Int. 2007;72:63-71. [PubMed] |
| 9. | Zuckerman JM, Assimos DG. Hypocitraturia: pathophysiology and medical management. Rev Urol. 2009;11:134-144. [PubMed] |
| 10. | Kramer HJ. Impaired renal function in obstructive jaundice: roles of the thromboxane and endothelin systems. Nephron. 1997;77:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Klaassen CD, Aleksunes LM. Xenobiotic, bile acid, and cholesterol transporters: function and regulation. Pharmacol Rev. 2010;62:1-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 660] [Cited by in RCA: 594] [Article Influence: 37.1] [Reference Citation Analysis (11)] |
| 12. | Brandoni A, Hazelhoff MH, Bulacio RP, Torres AM. Expression and function of renal and hepatic organic anion transporters in extrahepatic cholestasis. World J Gastroenterol. 2012;18:6387-6397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Brandoni A, Torres AM. Characterization of the mechanisms involved in the increased renal elimination of bromosulfophthalein during cholestasis: involvement of Oatp1. J Histochem Cytochem. 2009;57:449-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Brandoni A, Quaglia NB, Torres AM. Compensation increase in organic anion excretion in rats with acute biliary obstruction: role of the renal organic anion transporter 1. Pharmacology. 2003;68:57-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Brandoni A, Villar SR, Picena JC, Anzai N, Endou H, Torres AM. Expression of rat renal cortical OAT1 and OAT3 in response to acute biliary obstruction. Hepatology. 2006;43:1092-1100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 16. | Sedmak JJ, Grossberg SE. A rapid, sensitive, and versatile assay for protein using Coomassie brilliant blue G250. Anal Biochem. 1977;79:544-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2217] [Cited by in RCA: 2133] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 17. | Romero-Calvo I, Ocón B, Martínez-Moya P, Suárez MD, Zarzuelo A, Martínez-Augustin O, de Medina FS. Reversible Ponceau staining as a loading control alternative to actin in Western blots. Anal Biochem. 2010;401:318-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 547] [Cited by in RCA: 634] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 18. | Brandoni A, Anzai N, Kanai Y, Endou H, Torres AM. Renal elimination of p-aminohippurate (PAH) in response to three days of biliary obstruction in the rat. The role of OAT1 and OAT3. Biochim Biophys Acta. 2006;1762:673-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 19. | Di Giusto G, Anzai N, Endou H, Torres AM. Oat5 and NaDC1 protein abundance in kidney and urine after renal ischemic reperfusion injury. J Histochem Cytochem. 2009;57:17-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Bulacio R, Hazelhoff MH, Torres AM. Renal expression and function of oat1 and oat3 in rats with vascular calcification. Pharmacology. 2012;90:66-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Quaglia NB, Brandoni A, Villar SR, Torres AM. Haemodynamic and tubular renal dysfunction in rats with sustained arterial calcinosis. Clin Exp Pharmacol Physiol. 2004;31:231-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 22. | Villar SR, Brandoni A, Torres AM. Time course of organic anion excretion in rats with bilateral ureteral obstruction: role of organic anion transporters (Oat1 and Oat3). Nephron Physiol. 2008;110:45-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | Di Giusto G, Torres AM. Renal blood flow measurement. Experimental Surgical Models in the Laboratory Rat. Boca Raton: Taylor and Francis Group, CRC Press 2009; 183-187. |
| 24. | Lee J, Azzaroli F, Wang L, Soroka CJ, Gigliozzi A, Setchell KD, Kramer W, Boyer JL. Adaptive regulation of bile salt transporters in kidney and liver in obstructive cholestasis in the rat. Gastroenterology. 2001;121:1473-1484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 119] [Article Influence: 4.8] [Reference Citation Analysis (11)] |
| 25. | Schlattjan JH, Winter C, Greven J. Regulation of renal tubular bile acid transport in the early phase of an obstructive cholestasis in the rat. Nephron Physiol. 2003;95:49-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 26. | Breljak D, Ljubojevic M, Balen D, Zlender V, Brzica H, Micek V, Kusan M, Anzai N, Sabolic I. Renal expression of organic anion transporter Oat5 in rats and mice exhibits the female-dominant sex differences. Histol Histopathol. 2010;25:1385-1402. [PubMed] |
| 27. | Anzai N, Kanai Y, Endou H. Organic anion transporter family: current knowledge. J Pharmacol Sci. 2006;100:411-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 155] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 28. | Aruga S, Pajor AM, Nakamura K, Liu L, Moe OW, Preisig PA, Alpern RJ. OKP cells express the Na-dicarboxylate cotransporter NaDC-1. Am J Physiol Cell Physiol. 2004;287:C64-C72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 29. | Zollner G, Fickert P, Fuchsbichler A, Silbert D, Wagner M, Arbeiter S, Gonzalez FJ, Marschall HU, Zatloukal K, Denk H. Role of nuclear bile acid receptor, FXR, in adaptive ABC transporter regulation by cholic and ursodeoxycholic acid in mouse liver, kidney and intestine. J Hepatol. 2003;39:480-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 138] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 30. | Donner MG, Schumacher S, Warskulat U, Heinemann J, Häussinger D. Obstructive cholestasis induces TNF-alpha- and IL-1 -mediated periportal downregulation of Bsep and zonal regulation of Ntcp, Oatp1a4, and Oatp1b2. Am J Physiol Gastrointest Liver Physiol. 2007;293:G1134-G1146. [PubMed] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Kim B S- Editor: Yu J L- Editor: A E- Editor: Liu XM