Published online Jul 21, 2015. doi: 10.3748/wjg.v21.i27.8304
Peer-review started: January 16, 2015
First decision: March 10, 2015
Revised: March 26, 2015
Accepted: May 4, 2015
Article in press: May 4, 2015
Published online: July 21, 2015
Processing time: 188 Days and 16.5 Hours
In China, acupuncture has been considered an effective method for treating gastrointestinal (GI) dysfunction diseases for thousands of years. In fact, acupuncture has gained progressive acceptance from both practitioners and patients worldwide. However, the therapeutic effects and underlying mechanisms in treating GI dysfunction have not yet been established due to a lack of systematic and comprehensive review articles. Therefore, the aim of this review is to discuss the efficacy of acupuncture as a treatment for GI dysfunction and the associated underlying mechanisms. A search of PubMed was conducted for articles that were published over the past 10 years using the terms “acupuncture”, “gastrointestine”, and other relevant keywords. In the following review, we describe the effect and underlying mechanisms of acupuncture on GI function from the perspectives of GI motility, visceral sensitivity, the GI barrier, and the brain-gut axis. The dual regulatory effects of acupuncture may manifest by promoting gastric peristalsis in subjects with low initial gastric motility, and suppressing peristalsis in subjects with active initial motility. In addition, the regulation of acupuncture on gastric motility may be intensity-dependent. Our findings suggest that further studies are needed to investigate the effects and more systematic mechanisms in treating GI dysfunction, and to promote the application of acupuncture for the treatment of GI diseases.
Core tip: Acupuncture has been used as an appropriate adjunctive treatment for gastrointestinal (GI) dysfunction diseases. However, the therapeutic effects and underlying mechanisms in treating GI dysfunction have not yet been established due to a lack of systematic and comprehensive review articles. This review clarifies the effects and underlying mechanisms of acupuncture on GI function from various perspectives including GI motility, visceral sensitivity, the GI barrier, and the brain-gut axis. In addition, the dual regulatory effects and the intensity-dependent nature of acupuncture on GI motility are discussed.
- Citation: Li H, He T, Xu Q, Li Z, Liu Y, Li F, Yang BF, Liu CZ. Acupuncture and regulation of gastrointestinal function. World J Gastroenterol 2015; 21(27): 8304-8313
- URL: https://www.wjgnet.com/1007-9327/full/v21/i27/8304.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i27.8304
Acupuncture has been an integral part of traditional Chinese medicine (TCM), with a history tracing back more than 3000 years[1]. In principle, acupuncture is a method by which yin and yang can come into balance with one another and qi can flow harmoniously throughout the body[2]. In addition, De-qi, a composite of unique needle sensations in patients, including soreness, numbness, heaviness, fullness, warmth, coolness, tingling, and dull pain, is an efficacy predictor and parameter for assessing clinical effectiveness of acupuncture[3]. However, only when qi is achieved will acupuncture be recognized as effective in the process of treating diseases.
In China, acupuncture, a therapy that involves inserting the tips of thin, stainless steel needles through the skin at specific points, has been widely used in clinical practice to treat various diseases and physiological disorders. The procedure can be accomplished by manual manipulation or electrical stimulation. Manual acupuncture involves the manipulation of the inserted needles by hand, such as lifting, thrusting, twisting, twirling, or other complex combinations[4]. Electroacupuncture (EA) is a modification of the traditional acupuncture and involves stimulating acupoints with an electrical pulse instead of manual manipulations[5].
Gastrointestinal (GI) diseases are a significant burden to society. In 2004, GI diseases were reported to affect an estimated 60 to 70 million United States citizens, and to total approximately $142 billion in direct and indirect costs[6]. To alleviate this burden, it is necessary to identify an economical means of treating GI diseases. Consequently, increased attention has been paid to studying the efficacy and related mechanisms of acupuncture in treating GI diseases.
Acupuncture is beneficial as an alternative therapy for the management of chemotherapy-induced nausea[7], postoperative nausea and vomiting[8,9], peptic ulcer disease[10] and postoperative ileus[11], as well as other functional disorders[12-14] including irritable bowel syndrome (IBS)[15], constipation[16], and diarrhea[17]. These effects of acupuncture occur through regulation of GI motility, protection of the stomach mucosa, and a decrease in visceral sensitivity[18-20]. For example, acupuncture at zusanli (ST-36), a classic acupoint, may improve upper and lower abdominal symptoms and restore impaired gastric slow waves, which are induced by rectal distension (RD), via the vagal pathway[21]. Moreover, EA at Foot-Yangming meridian (SMFY) may enhance gastric motility and improve gastric mucosal blood flow by regulating the concentration of motilin and somatostatin in sinus ventriculi and bulbus medullae[22].
A search of PubMed articles published between the beginning of 2005 and 2015 was conducted using the search term “acupuncture” in combination with the terms “gastrointestinal motility”, “gastric mucosa”, “intestinal mucosa”, “intestinal barrier”, “visceral sensitivity”, “brain-gut axis”, or “brain-gut peptide”. Sixty-five articles associated with acupuncture and GI regulation were selected from the publications obtained through the conduct of the literature search. In this review, four aspects of the regulatory effects of acupuncture on the GI tract are discussed including the modulation of GI motility, the GI barrier, visceral sensitivity, and the brain-gut axis.
GI motility maintains healthy digestive function through the contraction and expansion of smooth muscle in different sections of the GI tract[23,24]. The characteristic motility pattern in the interdigestive period is referred to as an interdigestive migrating contraction[25]. In research, gastric myoelectrical activity (GMA), multichannel electrogastrography (EGG), and gastric pressure are often used to assess GI motility[26-30]. Acupuncture may have regulatory effects on GI motility, especially on the motility of the stomach and colon (Table 1).
| Organ | Subjects | Methods | Findings | Ref. |
| Stomach | Normal mice | EA at ST-36 | ST-36-gastric motility ↑ | [48] |
| EA at CV-12 | CV-12-gastric motility ↓ | |||
| Rats with gastric mucosal damage | EA at acupoints in | Gastric motility ↑ | [22] | |
| Foot-Yangming Meridian | ||||
| Normal mice | EA at CV-12 | Gastric motility ↓ | [29] | |
| Normal rats | EA at ST-36 | Gastric motility ↑ | [30] | |
| Dogs with impaired gastric motility induced by RD | EA at ST-36 | Gastric motility ↑ | [18] | |
| Healthy human | Manual acupuncture at | Normogastria ↓ | [32] | |
| both PC-6 and ST-36 | Bradygastria ↑ | |||
| Colon | Conscious dogs | Manual acupuncture at acupoints in large intestine meridian or ST-25, BL-25, GV-1 | Only GV-1-colonic motility ↓ | [37] |
| Conscious rats | EA at ST-36 | Colonic motility ↑ | [50] | |
| Rats with stress | EA at ST-36 | Colonic motility ↓ | [35] |
According to TCM, one characteristic of acupuncture is that it has opposite regulatory effects during different physiological conditions. For example, acupuncture may promote gastric peristalsis in subjects with low initial gastric motility, but it may suppress peristalsis in subjects with active initial motility[31].
One study involving 65 healthy volunteers found that manual acupuncture at ST-36 and neiguan (PC-6) decreased normogastria and increased bradygastria; however, the study did not provide evidence to support that acupuncture was more effective than sham acupuncture[32]. Moreover, the results of several clinical studies indicated that acupuncture at hegu (LI-4) and PC-6 inhibited the excessive GI motility induced by mosapride citrate, and enhanced the suppression of loperamide-induced conditions, but these effects were not observed under normal conditions[33-35]. Similar outcomes have been noted in laboratory settings. Acupuncture applied at PC-6 or ST-36 in rats significantly enhanced gastric motility, while stimulating zhongwan (CV-12) significantly suppressed gastric motility[36]. Iwa et al[37] suggested that in rats, EA at ST-36 not only promoted gastric peristalsis, but also inhibited the acceleration of stress-induced colonic transit due to restraint. When acupuncture was applied at the changqiang acupoint (GV-1), colonic motility was also inhibited in healthy dogs through a decrease in the total duration and frequency of the contractions[38]. The results of these studies indicate that acupuncture has a dual regulatory effect on GI motility and supports the TCM theory that acupuncture restores the balance between the yin and yang[39].
The pathways, transmitters, and modulators of the nervous system are important factors in the regulation of GI motility (Table 2). In diabetic rats, EA at ST-36 has been shown to normalize contractions of the gastric antrum. This effect was partly related to an enhanced SCF/c-kit pathway which plays a critical role in maintaining the survival and proliferation of cells of Cajal (ICCs)[40,41]. ICCs, which act as a pacemaker in the GI tract, play a crucial role in the generation of slow waves and the control of gastric movements[42]. In rats with burns, EA at ST-36 improved postprandial gastric dysrhythmia, and delayed gastric liquid emptying via the enhancement of vagal activity, which indicated that the accelerative effect of EA on gastric motility might be blocked after a vagotomy[43]. Acupuncture at the acupoints in the limb promoted gastric motility via a supra-spinal reflex that activated the vagal nerve fibers, while the same stimulus to the abdomen resulted in the reverse effect via a spinal reflex that activated sympathetic nerve fibers[44-46]. Moreover, EA at ST-36 has been found to enhance gastric motility via the efferent parasympathetic pathway, whereas stimulating CV-12 has been found to inhibit GI motility via the efferent sympathetic pathway[47,48]. Furthermore, the inhibitory effect of EA on gastric motility may be intensity-dependent. In wild-type mice, EA at an intensity of 1 mA at CV-12 produced no significant effect on gastric motility. In contrast, gastric motility was significantly inhibited by EA stimulation at intensities of 2 mA and 4 mA. However, the intensity-dependent characteristic disappeared following the blockage of the TRPV1 channel, which indicated that TRPV1 was partially involved in the EA modulation of gastric motility[29]. Strong manual acupuncture stimulation applied in rats at ST-36 or CV-12 produced a more significant enhancement or inhibition on gastric motility compared with slight stimulation. In this case, the intensity of manual acupuncture stimulation depended on the twisting frequency of needle manipulations and De-qi sensation. And the effect was more likely to be mediated via A-delta and C-afferent fibers[49]. Similarly, Iwa et al[50] reported that EA applied in freely moving conscious rats might promote distal colonic motility and accelerate colonic transit via a sacral efferent parasympathetic pathway. The stimulatory effect of EA on stress-induced delayed gastric emptying was blocked by pretreatment with an intracerebroventricular injection of kynurenic acid, a glutamate receptor antagonist, which suggested that the glutamate receptor was involved in the regulation of gastric motility[51]. Glutamatergic receptors are classified as either N-methyl-D-aspartate (NMDA) receptors or non-NMDA receptors[52]. NMDA receptors, which are associated with vagal afferent nerve fibers, may play a critical role in mediating gastric motility. When anesthetized rats were microinjected with AP5, an antagonist of NMDA receptors, the increased gastric pressure that was induced by low frequency electric stimulation at ST-36 significantly decreased[30,53]. In addition, acupuncture could improve the GI symptoms associated with stress by up-regulating the expression of hypothalamic oxytocin (OXT), which is an anti-stressor agent[54].
| Function | Subjects | Stimulation | Result | Mechanism | Ref. | |
| Method | Region | |||||
| Gastric motility | Dogs with RD | EA | ST-36 | Increase | Vagal activity | [18] |
| opioid pathway | ||||||
| Healthy volunteers | MA | ST-36 | Increase | Vagal pathway | [21] | |
| Rats | EA | Foot-Yangming Meridian | Increase | Brain-gut peptide | [22] | |
| Wild-type mice | EA | CV-12 | Decrease | TRPV1 receptor | [29] | |
| Rats | EA | ST-36 | Increase | NMDA receptors | [30] | |
| Colonic motility | Rats | EA | ST-36 | Increase | Parasympathetic efferent pathway | [50] |
| Rats | TENS | Hind limbs | Decrease | OXT expression | [54] | |
The GI barrier, which is a component of GI defense, protects the epithelium from harmful microbes and toxins[55]. Impairment of the mucosal barrier primarily results from mucosal ischemia/reperfusion injury, inflammatory response, excess gastric acid secretion and bacterial infection[56-60].
Acupuncture may be helpful in restoring GI barrier injury by regulating the neuron-endocrine-immune system and antagonizing the inflammatory response. In duodenal ulcer patients, acupuncture has been shown to reduce the acid output and to achieve symptomatic relief[61]. EA at ST-36 provided protective effects against gut injury and mucosal barrier dysfunction in hemorrhaged rats by activating the cholinergic anti-inflammatory-dependent pathway and enteric glial cells which are known to secrete pro-epidermal growth factor and thereby increase epithelial restitution[62-65]. The anti-inflammatory effect of acupuncture has the potential to significantly prevent postoperative intra-abdominal adhesion formation, which is a leading cause of small bowel obstruction[66]. The loss of intestinal barrier function and epithelial cell integrity induced by gut ischemia/reperfusion injury could promote the systemic production of various inflammatory mediators and activate leukocytes, which may lead to remote organ injury[67]. In rats with ischemia/reperfusion injury, the anti-inflammatory effect of EA at ST-36 has been shown to prevent the injury to the intestinal barrier and remote organs via the activation of the α7 subunit of nicotinic receptor and the cholinergic anti-inflammatory-dependent pathway[68].
Stress-induced mucosal diseases occur with local ischemia[69]. In rats with stress-induced gastric ulcers, EA at ST-36 aided in the repair of the stomach mucosa by increasing the concentration of epidermal growth factor in gastric mucosal tissue[70,71]. The protective effect of EA on the gastric mucosa may be related to an increase in the expression of the intestinal trefoil factor gene and SS-R1mRNA in gastric mucosal tissue, which may promote mucosal restoration[20,72,73]. As a neurotransmitter, nitric oxide (NO) has both protective and deleterious effects on the gastric mucosa, depending on the type of nitric oxide synthase (NOS, i.e., NOS1, NOS2, or NOS3)[74]. EA applied at ST-36 in rats has been shown to increase the expression of NOS1, but decrease the expression of NOS2 and NOS3, resulting in the protection of the gastric mucous[75]. Prior research has indicated that excess gastric acid secretion may cause GI mucosal lesions[60]. Furthermore, plasma beta-endorphin (β-EP) and somatostatin (SS) inhibit acid secretion[76,77]. Following the intragastric administration of a mixed amino acid meal to dogs, EA at ST-36, PC-6, and pishu (BL-20) suppressed acid secretion and significantly increased β-EP and SS simultaneously. Moreover, the inhibition of acid secretion by EA was significantly greater than inhibition observed following sham EA[78].
IBS is characterized by chronic or recurrent abdominal pain, discomfort, and/or altered bowel habits (i.e., diarrhea, constipation or both) that often interfere with the daily life of a patient[79,80]. Clinically, acupuncture has been acknowledged as an effective treatment for abdominal pain in IBS patients, although some acupuncture trials failed to demonstrate superiority over a placebo treatment in patients who have IBS[81-83].
Chronic visceral hypersensitivity (CVH) is an important and characteristic feature of IBS. In rats, EA at bilateral points of ST-36 and shangjuxu (ST-37) significantly attenuated CVH that was induced by neonatal colon irritation. This effect was not observed following sham-EA at ST-36 and ST-37 without electrical stimulation, or following EA at control points, shenmai (BL-62), and the tail, which not only confirmed the acupoint specificity but also accounted for the placebo effect of EA[84].
The antihyperalgesic effect of acupuncture may be mediated via the opioidergic, adrenergic, and serotonergic pathways in both the central and peripheral nervous systems. In rats with heterotypic intermittent stress (HIS), EA delivered at acupoint ST-36 for 30 min in both hind limbs significantly attenuated the hypersensitive responses to colorectal distention compared with sham EA treatment. In contrast, the analgesic effects were blocked by pretreatment with naloxone, an opioid receptor antagonist, which suggested that the opioid pathway was involved in the regulation of visceral hypersensitivity by acupuncture[85]. Serotonin (5-HT) hyperactivity is known to increase visceral sensitivity in the enteric nervous system. This is supported by the effectiveness of the 5-HT3 receptor antagonist in treating IBS[86]. Several studies have shown that EA at ST-36 decreased visceral sensitivity and produced an analgesic effect in rats with CVH via the serotonergic pathway[87,88]. In response to colorectal distension, Chu et al[89] used the abdominal electromyogram (EMG) as the measurement index for visceral hypersensitivity in rats with CVH, and found that the inhibitory effect of EA at ST-36 on visceral hypersensitivity may be mediated by the 5-HT3 receptor in colon tissue. However, a study performed by Liu et al[90] suggested that EA at tianshu (ST-25) and ST-37 reduced the concentration of 5-HT by activating the 5-HT4 receptor, which indicated that the 5-HT3 receptor may not be involved in the regulation of the visceral pain threshold in CVH rats. These conflicting results may be partially due to the application of different acupuncture points in the two experiments. When EA was applied at ST-36 and ST-37 in CVH rats, the abdominal withdrawal reflex (AWR) decreased, and the pain threshold pressure (PTP) increased. Furthermore, the high expression levels of phosphorylated NMDA receptor subunit (pNR1) in the spinal cords (L4-L5 segments) of CVH rats was markedly attenuated by EA treatment[84]. Aside from the peripheral nervous system, the central nervous system plays an important role in the regulation of visceral sensitivity by EA. Corticotropin-releasing hormone (CRH) has been shown to increase rectal sensitivity, which was inhibited by the administration of α-helical CRH, an antagonist of the CRH receptor[91,92]. In IBS model rats, EA at ST-37 significantly decreased the visceral sensitivity and hypothalamic CRH levels compared with the untreated IBS model rats. Therefore, it is possible that EA can moderate visceral sensitivity by regulating the hypothalamic CRH concentrations in the rodent IBS model[93].
The brain-gut axis is a bridge that connects the CNS and GI tract[94]. By means of the brain-gut axis, signals from the brain influence the sensory, motor, and secretory modalities, as well as the microbiota of the gut. Conversely, visceral messages from the microbiota may influence brain functions[95].
The brain-gut axis involves the CNS, autonomic nervous system (ANS), hypothalamic-pituitary axis (HPA) and brain-gut peptides. A functional magnetic resonance imaging (fMRI) study of the human brain demonstrated that manual acupuncture at ST-36 modulated neural activity at multiple levels in the cerebro-cerebellar and limbic systems[96]. Further, the anterior cingulate cortex, prefrontal cortices, and the caudate tail may play a role in processing gastric perceptions in functional dyspepsia (FD) patients. Acupuncture could deactivate the primary somatosensory area and the cerebella, and could activate the visual-related cortex[97].
Brain-gut peptides, which are distributed along the GI tract and in the central nervous system, are involved in the modulation of GI tract processing[22,94]. Tachykinins, as exemplified by substance P (SP), participate in important physiological processes in GI systems including smooth muscle contractility, epithelial secretion, and proliferation[98]. A reduction in the secretion of SP and vasoactive intestinal polypeptide (VIP), an inhibitor of GI motility, could contribute to the major effects of EA when treating rats with IBS[99]. Motilin and cholecystokinin (CCK) are peptides that are known to be potent regulators of GI motility. In a study by Niu et al[47], EA at ST-36 significantly increased the secretion of motilin and CCK, which could be the mechanism by which acupuncture enhanced the GI myoelectrical activity of conscious rabbits[100]. Further, evidence suggested that the orexigenic peptides, including ghrelin and neuropeptide Y (NPY), could be down-regulated by EA and could stimulate decreased food intake in rats[101].
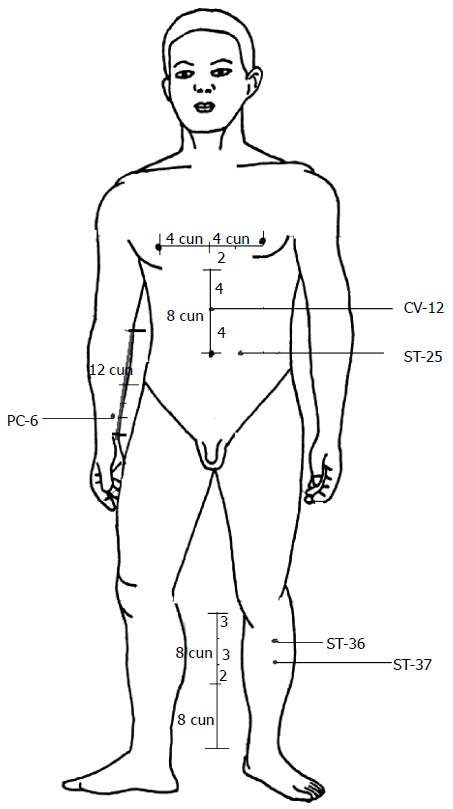
Although acupuncture has been used as an appropriate adjunctive treatment for GI dysfunction diseases, the underlying mechanisms have not been clearly understood. Currently, there are no systematic and comprehensive review articles that clarify the regulatory effect of acupuncture on GI function. In this review, we discuss the regulation on GI function through acupuncture from the perspectives of GI motility, the GI barrier, visceral sensitivity, and the brain-gut axis. The results of studies in both humans (Table 3) and animals suggest that acupuncture has the potential to treat GI disorders by regulating GI motility, the GI barrier, visceral sensitivity, and the brain-gut axis. In addition, the dual regulatory effects and the intensity-dependent nature of acupuncture on GI motility are discussed. According to classical acupuncture theory, acupoints are important reflex points of visceral dysfunction. Different acupoints have various therapeutic effects. ST-36, PC-6, ST-37, CV-12, and ST-25 are the primary acupoints used in the treatment of patients or animals with GI disorders (Figure 1), which suggests the specificity of acupoints. The duration and frequency of acupuncture manipulation are important parameters for the success of the therapy. Thus future studies should incorporate optimized methodologies that account for the selection of acupoints and stimulation parameters. The mechanisms underlying the beneficial effects of acupuncture may be associated with the pathways and transmitters of the nervous system. However, the mechanisms behind the involvement of brain-gut peptides in acupuncture and GI regulation have not been established because there are no known antagonists available to serve as controls in experimental studies. Although manual acupuncture and EA are both effective in regulating GI disorders, it is still unclear which method is more effective in certain conditions. Further investigation of the efficacy of acupuncture on multiple targets may be more likely to inspire scientific interest. Furthermore, to elucidate the roles and mechanisms of acupuncture in GI regulation and new therapeutic approaches for treating various GI diseases, proper controls are required in future studies.
| Trial design | Subjects | Method | Acupoints | Result | Ref. |
| Randomized controlled trial | Healthy persons administered with mosapride citrate | Manual acupuncture | LI-4 and PC-6 | GI motility ↓ | [32] |
| Non-randomized controlled trial | Healthy persons administered with loperamide | Manual acupuncture | LI-4 and PC-6 | GI motility ↑ | [33] |
| Non-randomized controlled trial | Healthy persons | Manual acupuncture | LI-4 and PC-6 | Intestinal motility - | [34] |
| Randomized controlled trial | Healthy volunteers | Manual acupuncture | ST-36 | GI motility ↑ | [21] |
| Randomized controlled trial | Healthy volunteers | Manual acupuncture | ST-36 and PC-6 | Normogastria↓ | [31] |
| Bradygastria ↑ |
| 1. | Han JS, Ho YS. Global trends and performances of acupuncture research. Neurosci Biobehav Rev. 2011;35:680-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 127] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 2. | Kaptchuk TJ. Acupuncture: theory, efficacy, and practice. Ann Intern Med. 2002;136:374-383. [PubMed] |
| 3. | Kong J, Gollub R, Huang T, Polich G, Napadow V, Hui K, Vangel M, Rosen B, Kaptchuk TJ. Acupuncture de qi, from qualitative history to quantitative measurement. J Altern Complement Med. 2007;13:1059-1070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 272] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 4. | Xing W, Li Q. Effects of different manipulations of acupuncture on electrical activity of stomach in humans. J Tradit Chin Med. 1998;18:39-42. [PubMed] |
| 5. | Hsieh CL, Lin JG, Li TC, Chang QY. Changes of pulse rate and skin temperature evoked by electroacupuncture stimulation with different frequency on both Zusanli acupoints in humans. Am J Chin Med. 1999;27:11-18. [PubMed] |
| 6. | Everhart JE, Ruhl CE. Burden of digestive diseases in the United States part I: overall and upper gastrointestinal diseases. Gastroenterology. 2009;136:376-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 432] [Article Influence: 25.4] [Reference Citation Analysis (1)] |
| 7. | Anders EF, Findeisen A, Lode HN, Usichenko TI. Acupuncture for treatment of acute vomiting in children with gastroenteritis and pneumonia. Klin Padiatr. 2012;224:72-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Liodden I, Howley M, Grimsgaard AS, Fønnebø VM, Borud EK, Alraek T, Norheim AJ. Perioperative acupuncture and postoperative acupressure can prevent postoperative vomiting following paediatric tonsillectomy or adenoidectomy: a pragmatic randomised controlled trial. Acupunct Med. 2011;29:9-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 9. | Glickman-Simon R, Tessier J. Guided imagery for postoperative pain, energy healing for quality of life, probiotics for acute diarrhea in children, acupuncture for postoperative nausea and vomiting, and animal-assisted therapy for mental disorders. Explore (NY). 2014;10:326-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Wang H, Wang CY, Zhang JS, Sun L, Sun JP, Tian QH, Jin XL, Yin L. Acupuncture therapy for experimental stomach ulcer and c-Fos expression in rats. World J Gastroenterol. 2005;11:5517-5520. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Ng SS, Leung WW, Mak TW, Hon SS, Li JC, Wong CY, Tsoi KK, Lee JF. Electroacupuncture reduces duration of postoperative ileus after laparoscopic surgery for colorectal cancer. Gastroenterology. 2013;144:307-313.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 130] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 12. | Chon TY, Lee MC. Acupuncture. Mayo Clin Proc. 2013;88:1141-1146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 99] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 13. | Zeng F, Qin W, Ma T, Sun J, Tang Y, Yuan K, Li Y, Liu J, Liu X, Song W. Influence of acupuncture treatment on cerebral activity in functional dyspepsia patients and its relationship with efficacy. Am J Gastroenterol. 2012;107:1236-1247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 92] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 14. | Ma TT, Yu SY, Li Y, Liang FR, Tian XP, Zheng H, Yan J, Sun GJ, Chang XR, Zhao L. Randomised clinical trial: an assessment of acupuncture on specific meridian or specific acupoint vs. sham acupuncture for treating functional dyspepsia. Aliment Pharmacol Ther. 2012;35:552-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 15. | MacPherson H, Bland M, Bloor K, Cox H, Geddes D, Kang’ombe A, Reynolds J, Stamuli E, Stuardi T, Tilbrook H. Acupuncture for irritable bowel syndrome: a protocol for a pragmatic randomised controlled trial. BMC Gastroenterol. 2010;10:63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Li Y, Zheng H, Zeng F, Zhou SY, Zhong F, Zheng HB, Chen M, Jing XH, Cai YY, Jia BH. Use acupuncture to treat functional constipation: study protocol for a randomized controlled trial. Trials. 2012;13:104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Sun JH, Wu XL, Xia C, Xu LZ, Pei LX, Li H, Han GY. Clinical evaluation of Soothing Gan and invigorating Pi acupuncture treatment on diarrhea-predominant irritable bowel syndrome. Chin J Integr Med. 2011;17:780-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 18. | Chen J, Song GQ, Yin J, Koothan T, Chen JD. Electroacupuncture improves impaired gastric motility and slow waves induced by rectal distension in dogs. Am J Physiol Gastrointest Liver Physiol. 2008;295:G614-G620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 82] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 19. | Xu GY, Winston JH, Chen JD. Electroacupuncture attenuates visceral hyperalgesia and inhibits the enhanced excitability of colon specific sensory neurons in a rat model of irritable bowel syndrome. Neurogastroenterol Motil. 2009;21:1302-e125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 20. | Li XP, Yan J, Yi SX, Chang XR, Lin YP, Yang ZB, Huang A, Hu R. Effect of electroacupunture on gastric mucosal intestinal trefoil factor gene expression of stress-induced gastric mucosal injury in rats. World J Gastroenterol. 2006;12:1962-1965. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (10)] |
| 21. | Liu J, Huang H, Xu X, Chen JD. Effects and possible mechanisms of acupuncture at ST36 on upper and lower abdominal symptoms induced by rectal distension in healthy volunteers. Am J Physiol Regul Integr Comp Physiol. 2012;303:R209-R217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 22. | Lin YP, Yi SX, Yan J, Chang XR. Effect of acupuncture at Foot-Yangming Meridian on gastric mucosal blood flow, gastric motility and brain-gut peptide. World J Gastroenterol. 2007;13:2229-2233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 23. | Yin J, Chen JD. Roles of interstitial cells of Cajal in regulating gastrointestinal motility: in vitro versus in vivo studies. J Cell Mol Med. 2008;12:1118-1129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 24. | Sallam HS, McNearney TA, Chen JD. Acupuncture-based modalities: novel alternative approaches in the treatment of gastrointestinal dysmotility in patients with systemic sclerosis. Explore (NY). 2014;10:44-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | Kusano M, Hosaka H, Kawada A, Kuribayashi S, Shimoyama Y, Zai H, Kawamura O, Yamada M. Gastrointestinal motility and functional gastrointestinal diseases. Curr Pharm Des. 2014;20:2775-2782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | Ruckebusch Y, Pairet M, Becht JL. Origin and characterization of migrating myoelectric complex in rabbits. Dig Dis Sci. 1985;30:742-748. [PubMed] |
| 27. | McNearney T, Lin X, Shrestha J, Lisse J, Chen JD. Characterization of gastric myoelectrical rhythms in patients with systemic sclerosis using multichannel surface electrogastrography. Dig Dis Sci. 2002;47:690-698. [PubMed] |
| 28. | Wang SB, Chen SP, Gao YH, Luo MF, Liu JL. Effects of electroacupuncture on cardiac and gastric activities in acute myocardial ischemia rats. World J Gastroenterol. 2008;14:6496-6502. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 29. | Yu Z, Cao X, Xia Y, Ren B, Feng H, Wang Y, Jiang J, Xu B. Electroacupuncture Stimulation at CV12 Inhibits Gastric Motility via TRPV1 Receptor. Evid Based Complement Alternat Med. 2013;2013:294789. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 30. | Gao X, Qiao Y, Jia B, Jing X, Cheng B, Wen L, Tan Q, Zhou Y, Zhu B, Qiao H. NMDA Receptor-Dependent Synaptic Activity in Dorsal Motor Nucleus of Vagus Mediates the Enhancement of Gastric Motility by Stimulating ST36. Evid Based Complement Alternat Med. 2012;2012:438460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 31. | Ren BB, Yu Z, Xu B. [Overview of the two-way regulatory effect of acupuncture on gastrointestinal motility]. Zhongguo Zhenjiu. 2012;32:765-768. [PubMed] |
| 32. | Witt CM, Meissner K, Pach D, Thiele C, Lüdtke R, Ghadiyali Z, Deter HC, Zimmermann-Viehoff F. Stimulation of gastric slow waves with manual acupuncture at acupuncture points ST36 and PC6--a randomized single blind controlled trial. Neurogastroenterol Motil. 2012;24:438-445, e211-e212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 33. | Shin KM, Park JE, Lee S, Choi SM, Ahn YC, Lee JW, Kim JH, Son CG. Effect of siguan acupuncture on gastrointestinal motility: a randomized, sham-controlled, crossover trial. Evid Based Complement Alternat Med. 2013;2013:918392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 34. | DS Oh, SY Jung, AR Kim, WC Kang, JE Park, CM Koo, JY Choi, HJ Jung, SM Choi, CG Son. A crossover clinical trial to determine the effect of Siguan (four gates) points on GI motility suppressed by loperamide administration. J Korean Oriental Med. 2008;29:1-6. |
| 35. | Yim YK, Kang WC, Cho JH, Shin JW, Lee NH, Choi SM, Koo ST, Park KS, Son CG. Crossover clinical trial to determine the effect of manual acupuncture at Siguan points (bilateral LI4 and LR3) on intestinal motility in healthy subjects. Am J Chin Med. 2007;35:209-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 36. | Yang ZK, Wu ML, Xin JJ, He W, Su YS, Shi H, Wang XY, Hu L, Jing XH, Litscher G. Manual acupuncture and laser acupuncture for autonomic regulations in rats: observation on heart rate variability and gastric motility. Evid Based Complement Alternat Med. 2013;2013:276320. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 37. | Iwa M, Nakade Y, Pappas TN, Takahashi T. Electroacupuncture elicits dual effects: stimulation of delayed gastric emptying and inhibition of accelerated colonic transit induced by restraint stress in rats. Dig Dis Sci. 2006;51:1493-1500. [PubMed] |
| 38. | Kim HY, Hahm DH, Pyun KH, Lee HJ, Nam TC, Shim I. Effect of traditional acupuncture on proximal colonic motility in conscious dogs. J Vet Med Sci. 2006;68:603-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (1)] |
| 39. | Takahashi T. Mechanism of acupuncture on neuromodulation in the gut--a review. Neuromodulation. 2011;14:8-12; discussion 12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 103] [Article Influence: 6.4] [Reference Citation Analysis (1)] |
| 40. | Herlin T, Ternowitz T, Kragballe K. Calcium efflux changes in neutrophils from patients with severe atopic dermatitis. Acta Derm Venereol Suppl (Stockh). 1985;120:50-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 41. | Tong W, Jia H, Zhang L, Li C, Ridolfi TJ, Liu B. Exogenous stem cell factor improves interstitial cells of Cajal restoration after blockade of c-kit signaling pathway. Scand J Gastroenterol. 2010;45:844-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 42. | Hirst GD, Edwards FR. Electrical events underlying organized myogenic contractions of the guinea pig stomach. J Physiol. 2006;576:659-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 67] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 43. | Song J, Yin J, Sallam HS, Bai T, Chen Y, Chen JD. Electroacupuncture improves burn-induced impairment in gastric motility mediated via the vagal mechanism in rats. Neurogastroenterol Motil. 2013;25:807-e635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 44. | Noguchi E. Acupuncture regulates gut motility and secretion via nerve reflexes. Auton Neurosci. 2010;156:15-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 45. | Li YQ, Zhu B, Rong PJ, Ben H, Li YH. Neural mechanism of acupuncture-modulated gastric motility. World J Gastroenterol. 2007;13:709-716. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 79] [Cited by in RCA: 90] [Article Influence: 4.7] [Reference Citation Analysis (2)] |
| 46. | Sato A, Sato Y, Suzuki A, Uchida S. Neural mechanisms of the reflex inhibition and excitation of gastric motility elicited by acupuncture-like stimulation in anesthetized rats. Neurosci Res. 1993;18:53-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 170] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 47. | Niu WX, He GD, Liu H, Qin XY. Effects and probable mechanisms of electroacupuncture at the Zusanli point on upper gastrointestinal motility in rabbits. J Gastroenterol Hepatol. 2007;22:1683-1689. [PubMed] |
| 48. | Su YS, He W, Wang C, Shi H, Zhao YF, Xin JJ, Wang XY, Shang HY, Hu L, Jing XH. “Intensity-response” effects of electroacupuncture on gastric motility and its underlying peripheral neural mechanism. Evid Based Complement Alternat Med. 2013;2013:535742. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 49. | Su YS, Yang ZK, Xin JJ, He W, Shi H, Wang XY, Hu L, Jing XH, Zhu B. Somatosensory Nerve Fibers Mediated Generation of De-qi in Manual Acupuncture and Local Moxibustion-Like Stimuli-Modulated Gastric Motility in Rats. Evid Based Complement Alternat Med. 2014;2014:673239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (1)] |
| 50. | Iwa M, Matsushima M, Nakade Y, Pappas TN, Fujimiya M, Takahashi T. Electroacupuncture at ST-36 accelerates colonic motility and transit in freely moving conscious rats. Am J Physiol Gastrointest Liver Physiol. 2006;290:G285-G292. [PubMed] |
| 51. | Iwa M, Nakade Y, Pappas TN, Takahashi T. Electroacupuncture improves restraint stress-induced delay of gastric emptying via central glutaminergic pathways in conscious rats. Neurosci Lett. 2006;399:6-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 52. | Travagli RA, Gillis RA, Rossiter CD, Vicini S. Glutamate and GABA-mediated synaptic currents in neurons of the rat dorsal motor nucleus of the vagus. Am J Physiol. 1991;260:G531-G536. [PubMed] |
| 53. | Bouzioukh F, Tell F, Jean A, Rougon G. NMDA receptor and nitric oxide synthase activation regulate polysialylated neural cell adhesion molecule expression in adult brainstem synapses. J Neurosci. 2001;21:4721-4730. [PubMed] |
| 54. | Yoshimoto S, Babygirija R, Dobner A, Ludwig K, Takahashi T. Anti-stress effects of transcutaneous electrical nerve stimulation (TENS) on colonic motility in rats. Dig Dis Sci. 2012;57:1213-1221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 55. | Boltin D, Niv Y. Pharmacological and alimentary alteration of the gastric barrier. Best Pract Res Clin Gastroenterol. 2014;28:981-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 56. | Chang JX, Chen S, Ma LP, Jiang LY, Chen JW, Chang RM, Wen LQ, Wu W, Jiang ZP, Huang ZT. Functional and morphological changes of the gut barrier during the restitution process after hemorrhagic shock. World J Gastroenterol. 2005;11:5485-5491. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 82] [Cited by in RCA: 85] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 57. | Zhang LH, Yao CB, Gao MQ, Li HQ. Gastric mucosal injury due to hemorrhagic reperfusion and efficacy of Salvia miltiorrhizae extract F and cimetidine. World J Gastroenterol. 2005;11:2830-2833. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 58. | Verbeke L, Farre R, Verbinnen B, Covens K, Vanuytsel T, Verhaegen J, Komuta M, Roskams T, Chatterjee S, Annaert P. The FXR agonist obeticholic acid prevents gut barrier dysfunction and bacterial translocation in cholestatic rats. Am J Pathol. 2015;185:409-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 178] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 59. | Schuster AT, Homer CR, Kemp JR, Nickerson KP, Deutschman E, Kim Y, West G, Sadler T, Stylianou E, Krokowski D. Chromosome-associated protein d3 promotes bacterial clearance in human intestinal epithelial cells by repressing expression of amino Acid transporters. Gastroenterology. 2015;148:1405-1416.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (1)] |
| 60. | Schubert ML. Gastric secretion. Curr Opin Gastroenterol. 2014;30:578-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 61. | Sodipo JO, Falaiye JM. Acupuncture and gastric acid studies. Am J Chin Med. 1979;7:356-361. [PubMed] |
| 62. | Du MH, Luo HM, Hu S, Lv Y, Lin ZL, Ma L. Electroacupuncture improves gut barrier dysfunction in prolonged hemorrhagic shock rats through vagus anti-inflammatory mechanism. World J Gastroenterol. 2013;19:5988-5999. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 43] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 63. | Shi X, Zhong Y, Yao J, Hu S, Wang L, Litscher G. The influence of zusanli and nonmeridian acupuncture points on the survival rate and intestinal tissue features after fatal hemorrhagic shock in rats. Evid Based Complement Alternat Med. 2013;2013:750620. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 64. | Hu S, Zhao ZK, Liu R, Wang HB, Gu CY, Luo HM, Wang H, Du MH, Lv Y, Shi X. Electroacupuncture activates enteric glial cells and protects the gut barrier in hemorrhaged rats. World J Gastroenterol. 2015;21:1468-1478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 65. | Van Landeghem L, Chevalier J, Mahé MM, Wedel T, Urvil P, Derkinderen P, Savidge T, Neunlist M. Enteric glia promote intestinal mucosal healing via activation of focal adhesion kinase and release of proEGF. Am J Physiol Gastrointest Liver Physiol. 2011;300:G976-G987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 121] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 66. | Du MH, Luo HM, Tian YJ, Zhang LJ, Zhao ZK, Lv Y, Xu RJ, Hu S. Electroacupuncture ST36 prevents postoperative intra-abdominal adhesions formation. J Surg Res. 2015;195:89-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (1)] |
| 67. | Wu R, Dong W, Ji Y, Zhou M, Marini CP, Ravikumar TS, Wang P. Orexigenic hormone ghrelin attenuates local and remote organ injury after intestinal ischemia-reperfusion. PLoS One. 2008;3:e2026. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 79] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 68. | Hu S, Du MH, Luo HM, Wang H, Lv Y, Ma L, Lin ZL, Shi X, Gaischek I, Wang L. Electroacupuncture at Zusanli (ST36) Prevents Intestinal Barrier and Remote Organ Dysfunction following Gut Ischemia through Activating the Cholinergic Anti-Inflammatory-Dependent Mechanism. Evid Based Complement Alternat Med. 2013;2013:592127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 69. | Laine L, Takeuchi K, Tarnawski A. Gastric mucosal defense and cytoprotection: bench to bedside. Gastroenterology. 2008;135:41-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 536] [Cited by in RCA: 496] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 70. | Shi JJ, Huang LF. [Effects of transcutaneous electrical acupoint stimulation of “Zusanli” (ST 36) on gastric mucosal injury in exercise stress-induced gastric ulcer rats]. Zhenci Yanjiu. 2013;38:181-185. [PubMed] |
| 71. | Yang ZB, Yan J, Zou XP, Yi SX, Chang XR, Lin YP, Li XP. Enhanced expression of epidermal growth factor receptor gene in gastric mucosal cells by the serum derived from rats treated with electroacupuncture at stomach meridian acupoints. World J Gastroenterol. 2006;12:5557-5561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 72. | Hoffmann W. Trefoil factors TFF (trefoil factor family) peptide-triggered signals promoting mucosal restitution. Cell Mol Life Sci. 2005;62:2932-2938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 164] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 73. | Cho CH. Current roles of nitric oxide in gastrointestinal disorders. J Physiol Paris. 2001;95:253-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 74] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 74. | Sun JP, Pei HT, Jin XL, Yin L, Tian QH, Tian SJ. Effects of acupuncturing Tsusanli (ST36) on expression of nitric oxide synthase in hypothalamus and adrenal gland in rats with cold stress ulcer. World J Gastroenterol. 2005;11:4962-4966. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 75. | Fykse V, Coy DH, Waldum HL, Sandvik AK. Somatostatin-receptor 2 (sst2)-mediated effects of endogenous somatostatin on exocrine and endocrine secretion of the rat stomach. Br J Pharmacol. 2005;144:416-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 76. | Lenz HJ, Klapdor R, Hester SE, Webb VJ, Galyean RF, Rivier JE, Brown MR. Inhibition of gastric acid secretion by brain peptides in the dog. Role of the autonomic nervous system and gastrin. Gastroenterology. 1986;91:905-912. [PubMed] |
| 77. | Jin HO, Zhou L, Lee KY, Chang TM, Chey WY. Inhibition of acid secretion by electrical acupuncture is mediated via beta-endorphin and somatostatin. Am J Physiol. 1996;271:G524-G530. [PubMed] |
| 78. | Bouin M, Plourde V, Boivin M, Riberdy M, Lupien F, Laganière M, Verrier P, Poitras P. Rectal distention testing in patients with irritable bowel syndrome: sensitivity, specificity, and predictive values of pain sensory thresholds. Gastroenterology. 2002;122:1771-1777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 345] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 79. | Thompson WG, Longstreth GF, Drossman DA, Heaton KW, Irvine EJ, Müller-Lissner SA. Functional bowel disorders and functional abdominal pain. Gut. 1999;45 Suppl 2:II43-II47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 622] [Cited by in RCA: 832] [Article Influence: 30.8] [Reference Citation Analysis (1)] |
| 80. | Lembo AJ, Conboy L, Kelley JM, Schnyer RS, McManus CA, Quilty MT, Kerr CE, Drossman D, Jacobson EE, Davis RB. A treatment trial of acupuncture in IBS patients. Am J Gastroenterol. 2009;104:1489-1497. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 96] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 81. | Schneider A, Enck P, Streitberger K, Weiland C, Bagheri S, Witte S, Friederich HC, Herzog W, Zipfel S. Acupuncture treatment in irritable bowel syndrome. Gut. 2006;55:649-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 105] [Article Influence: 5.3] [Reference Citation Analysis (1)] |
| 82. | Xing J, Larive B, Mekhail N, Soffer E. Transcutaneous electrical acustimulation can reduce visceral perception in patients with the irritable bowel syndrome: a pilot study. Altern Ther Health Med. 2004;10:38-42. [PubMed] |
| 83. | Rafiei R, Ataie M, Ramezani MA, Etemadi A, Ataei B, Nikyar H, Abdoli S. A new acupuncture method for management of irritable bowel syndrome: A randomized double blind clinical trial. J Res Med Sci. 2014;19:913-917. [PubMed] |
| 84. | Tian SL, Wang XY, Ding GH. Repeated electro-acupuncture attenuates chronic visceral hypersensitivity and spinal cord NMDA receptor phosphorylation in a rat irritable bowel syndrome model. Life Sci. 2008;83:356-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 46] [Article Influence: 2.6] [Reference Citation Analysis (1)] |
| 85. | Zhou YY, Wanner NJ, Xiao Y, Shi XZ, Jiang XH, Gu JG, Xu GY. Electroacupuncture alleviates stress-induced visceral hypersensitivity through an opioid system in rats. World J Gastroenterol. 2012;18:7201-7211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 33] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 86. | Camilleri M, Northcutt AR, Kong S, Dukes GE, McSorley D, Mangel AW. Efficacy and safety of alosetron in women with irritable bowel syndrome: a randomised, placebo-controlled trial. Lancet. 2000;355:1035-1040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 406] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 87. | Tian XY, Bian ZX, Hu XG, Zhang XJ, Liu L, Zhang H. Electro-acupuncture attenuates stress-induced defecation in rats with chronic visceral hypersensitivity via serotonergic pathway. Brain Res. 2006;1088:101-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 88. | Wu JC, Ziea ET, Lao L, Lam EF, Chan CS, Liang AY, Chu SL, Yew DT, Berman BM, Sung JJ. Effect of electroacupuncture on visceral hyperalgesia, serotonin and fos expression in an animal model of irritable bowel syndrome. J Neurogastroenterol Motil. 2010;16:306-314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 89. | Chu D, Cheng P, Xiong H, Zhang J, Liu S, Hou X. Electroacupuncture at ST-36 relieves visceral hypersensitivity and decreases 5-HT(3) receptor level in the colon in chronic visceral hypersensitivity rats. Int J Colorectal Dis. 2011;26:569-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 90. | Liu HR, Wang XM, Zhou EH, Shi Y, Li N, Yuan LS, Wu HG. Acupuncture at both ST25 and ST37 improves the pain threshold of chronic visceral hypersensitivity rats. Neurochem Res. 2009;34:1914-1918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 91. | Lembo T, Plourde V, Shui Z, Fullerton S, Mertz H, Tache Y, Sytnik B, Munakata J, Mayer E. Effects of the corticotropin-releasing factor (CRF) on rectal afferent nerves in humans. Neurogastroenterol Motil. 1996;8:9-18. [PubMed] |
| 92. | Sagami Y, Shimada Y, Tayama J, Nomura T, Satake M, Endo Y, Shoji T, Karahashi K, Hongo M, Fukudo S. Effect of a corticotropin releasing hormone receptor antagonist on colonic sensory and motor function in patients with irritable bowel syndrome. Gut. 2004;53:958-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 209] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 93. | Wu HG, Liu HR, Zhang ZA, Zhou EH, Wang XM, Jiang B, Shi Z, Zhou CL, Qi L, Ma XP. Electro-acupuncture relieves visceral sensitivity and decreases hypothalamic corticotropin-releasing hormone levels in a rat model of irritable bowel syndrome. Neurosci Lett. 2009;465:235-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 94. | Bonaz B, Sabate JM. [Brain-gut axis dysfunction]. Gastroenterol Clin Biol. 2009;33 Suppl 1:S48-S58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 95. | Bonaz B. Inflammatory bowel diseases: a dysfunction of brain-gut interactions? Minerva Gastroenterol Dietol. 2013;59:241-259. [PubMed] |
| 96. | Hui KK, Liu J, Marina O, Napadow V, Haselgrove C, Kwong KK, Kennedy DN, Makris N. The integrated response of the human cerebro-cerebellar and limbic systems to acupuncture stimulation at ST 36 as evidenced by fMRI. Neuroimage. 2005;27:479-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 350] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 97. | Zeng F, Song WZ, Liu XG, Xie HJ, Tang Y, Shan BC, Liu ZH, Yu SG, Liang FR. Brain areas involved in acupuncture treatment on functional dyspepsia patients: a PET-CT study. Neurosci Lett. 2009;456:6-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 98. | Steinhoff MS, von Mentzer B, Geppetti P, Pothoulakis C, Bunnett NW. Tachykinins and their receptors: contributions to physiological control and the mechanisms of disease. Physiol Rev. 2014;94:265-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 480] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 99. | Wu HG, Jiang B, Zhou EH, Shi Z, Shi DR, Cui YH, Kou ST, Liu HR. Regulatory mechanism of electroacupuncture in irritable bowel syndrome: preventing MC activation and decreasing SP VIP secretion. Dig Dis Sci. 2008;53:1644-1651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 51] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 100. | Crawley JN, Corwin RL. Biological actions of cholecystokinin. Peptides. 1994;15:731-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 516] [Cited by in RCA: 491] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 101. | Tian N, Wang F, Tian DR, Zou Y, Wang SW, Guan LL, Shi YS, Chang JK, Yang J, Han JS. Electroacupuncture suppresses expression of gastric ghrelin and hypothalamic NPY in chronic food restricted rats. Peptides. 2006;27:2313-2320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Hu S, Kuai L S- Editor: Yu J L- Editor: Wang TQ E- Editor: Liu XM