Published online Jul 21, 2015. doi: 10.3748/wjg.v21.i27.8314
Peer-review started: December 23, 2014
First decision: February 10, 2015
Revised: April 1, 2015
Accepted: May 21, 2015
Article in press: May 21, 2015
Published online: July 21, 2015
Processing time: 212 Days and 3.5 Hours
AIM: To characterize high-mobility group protein 1-toll-like receptor 4 (HMGB1-TLR4) and downstream signaling pathways in intestinal ischemia/reperfusion (I/R) injury.
METHODS: Forty specific-pathogen-free male C57BL/6 mice were randomly divided into five groups (n = 8 per group): sham, control, anti-HMGB1, anti-myeloid differentiation gene 88 (MyD88), and anti-translocating-chain-associating membrane protein (TRIF) antibody groups. Vehicle with the control IgG antibody, anti-HMGB1, anti-MyD88, or anti-TRIF antibodies (all 1 mg/kg, 0.025%) were injected via the caudal vein 30 min prior to ischemia. After anesthetization, the abdominal wall was opened and the superior mesenteric artery was exposed, followed by 60 min mesenteric ischemia and then 60 min reperfusion. For the sham group, the abdominal wall was opened for 120 min without I/R. Levels of serum nuclear factor (NF)-κB p65, interleukin (IL)-6, and tumor necrosis factor (TNF)-α were measured, along with myeloperoxidase activity in the lung and liver. In addition,morphologic changes that occurred in the lung and intestinal tissues were evaluated. Levels of mRNA transcripts encoding HMGB1 and NF-κB were measured by real-time quantitative PCR, and levels of HMGB1 and NF-κB protein were measured by Western blot. Results were analyzed using one-way analysis of variance.
RESULTS: Blocking HMGB1, MyD88, and TRIF expression by injecting anti-HMGB1, anti-MyD88, or anti-TRIF antibodies prior to ischemia reduced the levels of inflammatory cytokines in serum; NF-κB p65: 104.64 ± 11.89, 228.53 ± 24.85, 145.00 ± 33.63, 191.12 ± 13.22, and 183.73 ± 10.81 (P < 0.05); IL-6: 50.02 ± 6.33, 104.91 ± 31.18, 62.28 ± 6.73, 85.90 ± 17.37, and 78.14 ± 7.38 (P < 0.05); TNF-α, 43.79 ± 4.18, 70.81 ± 6.97, 52.76 ± 5.71, 63.19 ± 5.47, and 59.70 ± 4.63 (P < 0.05) for the sham, control, anti-HMGB1, anti-MyD88, and anti-TRIF groups, respectively (all in pg/mL).Antibodies also alleviated tissue injury in the lung and small intestine compared with the control group in the mouse intestinal I/R model. The administration of anti-HMGB1, anti-MyD88, and anti-TRIF antibodies markedly reduced damage caused by I/R, for which anti-HMGB1 antibody had the most obvious effect.
CONCLUSION: HMGB1 and its downstream signaling pathway play important roles in the mouse intestinal I/R injury, and the effect of the TRIF-dependent pathway is slightly greater.
Core tip: Intestinal mucosal barrier injury induced by intestinal ischemia/reperfusion is often the basis for a poor prognosis in many diseases. Despite extensive investigative efforts,the underlying mechanism remains a subject of debate, and there are currently no effective methods for its prevention or control. The findings reported here suggest that the high-mobility group protein 1-toll-like receptor 4 axis and the two downstream signaling pathways play important roles in ischemia/reperfusion injury and show potential therapeutic value for their blockade. This study has an important clinical significance, which could improve the survival rate and reduce complications in critically ill patients.
- Citation: Wang J, He GZ, Wang YK, Zhu QK, Chen W, Guo T. TLR4-HMGB1-, MyD88- and TRIF-dependent signaling in mouse intestinal ischemia/reperfusion injury. World J Gastroenterol 2015; 21(27): 8314-8325
- URL: https://www.wjgnet.com/1007-9327/full/v21/i27/8314.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i27.8314
Ischemia/reperfusion (I/R) injury is a syndrome in which serious cellular structural damage and functional metabolic disorders occur and organ function is further aggravated when blood flow that was halted curing ischemia is restored after a period of time[1-4].The gut is an important functional organ for the immune and endocrine systems, and it also serves as a protective barrier. Intestinal I/R injury is one of the main triggers that can lead to a systemic inflammatory response syndrome, acute lung injury, acute respiratory distress syndrome, and multiple organ dysfunction syndrome[5-8]. Although extensive investigative efforts have focused on characterizing the pathogenesis of distant organ injury induced by intestinal I/R, the underlying mechanism remains the subject of debate, and there are currently no effective methods for its prevention or control.
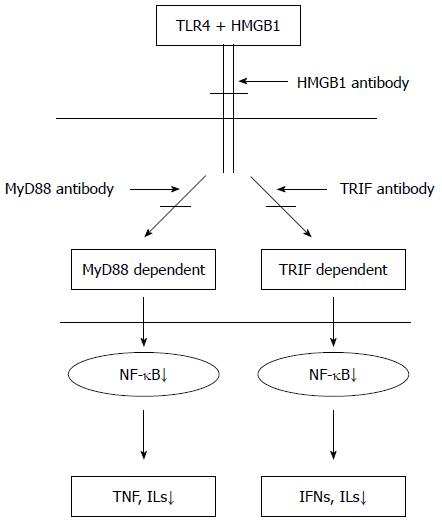
Recently, studies have suggested that I/R injury could be caused by interactions between toll-like receptor 4 (TLR4) and high-mobility group protein 1 (HMGB1), an endogenous TLR4 ligand. HMGB1 can activate TLR4, which can subsequently trigger downstream signal-transduction pathways[9]. The downstream signal transduction pathways initiated by TLR4 and HMGB1 are thought to include myeloid differentiation gene 88(MyD88)/TIR domain-containing adaptor protein (TIRAP)- and TIR domain-containing adaptor inducing interferon (IFN)-β (TRIF)/translocating-chain-associating membrane protein (TRAM)-dependent pathways[10]. In the process whereby TLR4 stimulates the release of inflammatory cytokines induced by endotoxin, MyD88- and TRIF-dependent pathways both play critical roles[11]. However, only a few studies have examined these pathways in aseptic/noninfectious I/R injury. Furthermore, whether intestinal I/R induces local and distant organ injury via these pathways has been poorly characterized. This study aimed to determine whether administering HMGB1 antibody could reduce tissue damage induced by intestinal I/R and to characterize the role of the HMGB1-TLR4 axis in I/R injury by administrating anti-HMGB1 antibody to block the binding of HMGB1 and TLR4 using a mouse intestinal I/R model. Additionally, to further investigate the MyD88/TIRAP or TRIF/TRAM pathways, we went on to characterize whether one pathway serves a dominant function in inducing a systemic inflammatory response and distant organ injury following intestinal I/R (Figure 1). This study has an important clinical significance that will inform efforts to identify an effective targeted therapy, which could improve the survival rate and reduce complications in critically ill patients.
Forty, male C57BL/6 mice that were specific-pathogen-free grade and weighed 24-26g were purchased from Beijing Vital River Laboratory Animal Technology Co. Ltd (scxk 2007-0001). The mice were housed under barrier-sustained conditions at a temperature of 25 °C and 50% humidity with 12 h light/dark cycles, and had free access to water and food for 2 wk prior to the operation. The mice were randomly divided into five groups (n = 8 for each group): sham, control (injected vehicle with the control IgG antibody), anti-HMGB1 (injected anti-HMGB1 antibody), anti-MyD88 (injected anti-MyD88 antibody), and anti-TRIF (injected anti-TRIF antibody). All mice were maintained in accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals. The research protocols were approved by the Academic Committee of Chinese Academy of Medical Sciences and Peking Union Medical College.
Prior to the operation, all mice were fasted overnight, but were allowed access to water ad libitum. Vehicle with the control IgG antibody (Abcam, Cambridge, United Kingdom), anti-HMGB1(Abcam), anti-MyD88(Abcam), or anti-TRIF (Biolegend, San Diego, CA, United States) antibodies (all 1 mg/kg, 0.025%) were injected via the caudal vein 30 min prior to ischemia[12,13]. The dose used was based on references and the results of a preliminary experiment. The mice were anesthetized with an injection of 1% sodium pentobarbital (50 mg/kg, ip). A midline incision was performed to bluntly separate the superior mesenteric artery(SMA). In addition to the sham group, the SMA was occluded for 60 min with a noninvasive artery clamp, followed by reperfusion for 60 min (according to the results of preliminary experiment). In the sham group, the abdominal cavity was only opened for 120 min without I/R. All animals were euthanized by barbiturate overdose (150 mg/kg pentobarbital sodium, ip) for tissue collection.
Blood: After the operation, blood was collected from the eye socket vein and centrifuged at 3000 rpm for 15 min at 4 °C. Next, serum was separated and stored at -80 °C for further analysis.
Tissue: After mice were sacrificed, the left lower lobe of the lung, a 3-cm proximal section of the jejunum, and a 3-cm distal section of the ileum were excised, rinsed in ice-cold normal saline, and dried on filter paper for histologic examination. The right liver, left upper lobe, right lung, a 6-cm proximal section of the jejunum, and a 6-cm distal section of the ileum were stored at -80 °C after being quickly immersed in liquid nitrogen for all other measurements.
Samples of the intestine and lung were fixed in 10% formalin solution and sectioned (4 μm) after dehydration, cleaning, and paraffin embedding. The sections were flattened, mounted, and heated on blank glass slides. Histologic evaluations were performed by hematoxylin and eosin staining and pathologic examination.
Lung histologic scoring system was applied as described[14]. Lung injury was scored in each sample according to the following four items: alveolar congestion, hemorrhage, infiltration or aggregation of neutrophils in the airspace or vessel wall, and thickness of the alveolar wall. A score of 0 represented normal findings and scores of 1, 2, 3, and 4 represented mild (< 25%), moderate (25%-50%), severe (50%-75%), and very severe (> 75%) lung involvement, respectively. The overall score was based on the sum of all scores.
The degree of intestinal injury was evaluated according to the scoring system developed by Anthony Stallion et al[15] (Table 1).The scale ranges from 0 to 4. No injury is scored as 0, with grade 4 depicting transmural necrosis of the intestine. The important components of injury include loss of villus height, infiltration of lymphocytes, and degree of necrosis.
| Grade | Features |
| 0 | Normal,villus to crypt ratio 5-6:1, minimal number of lymphocytes and plasma cells, tall columnar surface epithelial cells |
| 1 | Epithelial cell degenerative changes (cuboidal,vacuolated) but intact, mild increase of lymphocytes and plasma cells in lamina propria |
| 2 | Decreased villus height, yielding villus to crypt ratio ≤ 1, epithelial cell necrosis or erosions, more chronic inflammation in lamina propria ± neutrophils, glandular dilatation |
| 3 | Villi effaced (flat surface), epithelial cell necrosis or erosions, pseudomembrane may appear on surface, glandular destruction, inflammation extending deep to muscle layer |
| 4 | Transmural changes (all of above plus change in muscle layer) |
Tissue MPO activity was determined in the lung and liver. The left upper lobe of lung and liver were harvested, rinsed, blotted dry, and frozen at -80 °C. The samples were measured using an MPO detection assay according to the supplier’s specifications (Jiancheng Bio, Nanjing, China). Tissue MPO activity is expressed as activity units per gram of protein.
The serum concentrations of interleukin (IL)-6,tumor necrosis factor (TNF)-α, and nuclear factor (NF)-κB p65 were determined using ELISA kits (ME044,ME055, and ME053, respectively; eBioscience Inc., San Diego, CA, United States) according to the manufacturer’s protocols.
Total RNA was extracted from terminal ileum and lung tissue specimens using E.Z.N.A.® Total RNA KitII (Omega Bio-Tek Inc., Norcross, GA, United States). The purity of RNA was tested by spectrophotometric analysis. Reverse transcription PCR amplification was conducted with MyCycler PCR (Bio-Rad Laboratories, Inc., Hercules, CA, United States), in accordance with the illustrations of the GoScriptTM Reverse Transcription System (A5000; Promega, Madison, WI, United States). Utilizing 2 μL of reverse transcriptase products, real-time quantitative PCR was performed in a final volume of 20 μL using the gene-specific primers. The following primers designed with Primer Express Software were used: 5′-TTAGTCCCAGCGAAGGCTAT-3′ (forward) and 5′-CAAGTTTCCTGAGCAATCCA-3′ (reverse) for mouse HMGB1; 5′-GCTACACAGAGGCCATTGAA-3′ (forward) and 5′-GTGGAGGAAGACGAGAGAGG-3′ (reverse) for mouse NF-κB; 5′-GGCATTTCACTGCTTGATGT-3′ (forward) and 5′-TGACATTCCCATGAAACCTC-3′ (reverse) for mouse MyD88; 5′-TCAGAGAGTCCATCATTCGG-3′(forward) and5′-TACACGCCCACTCTTCTGAG-3′ (reverse) for mouse TRIF; 5′-GCAAGTTCAACGGCACAG-3′(forward) and 5′-CGCCAGTAGACTCCACGAC-3′ (reverse) for mouse GAPDH. Amplification was processed on Roche LightCycler® 480 as follows: 10 min at 95 °C, followed by 40 cycles of 15 s at 95 °C, 60 s at 60 °C, and then the melting curve was determined. Gene transcripts were quantified with SYBR green PCR master mix (Applied Biosystems of Thermo Fisher Scientific, Waltham, MA, United States). Data were calculated by the 2-∆∆CT method and presented as fold change of transcripts for the HMGB1 and NF-κB in the ileum and lung tissue of other groups compared with sham-operated mice (defined as 1.0-fold). Mouse GAPDH was used as an internal control. The relative expression of the target gene was normalized to the level of GAPDH in the same cDNA.
Total protein extract was prepared, and samples were separated using 10% SDS-PAGE. Proteins were then transferred to nitrocellulose membranes, which were then blocked for 2 h in 5% nonfat dry milk. The membranes were then incubated overnight in anti-HMGB1 (1:1000; Abcam), NF-κB (1:1000; Cell Signaling Technology, Inc.,Danvers,MA, United States) and β-actin (1:1000; Santa Cruz Biotechnology, Inc., Dallas, TX,United States) primary antibodies. Membranes were washed in PBST and incubated in horseradish-peroxidase-conjugated mouse secondary antibody in 5% nonfat milk (1:10000; Santa Cruz Biotechnology, Inc.) for 1 h at room temperature. Protein bands were visualized by chemiluminescence.
Quantitative data are presented as mean ± SD. Statistical software SPSS l9.0(IBM Corp., Armonk, NY, United States)was used to test the homogeneity of variance. Multiple comparisons were performed with one-way analyses of variance followed by a least-significant difference tests.Statistical significance was determined by analyzing the data with the nonparametric Kruskal-Wallis test, followed by the Mann-Whitney test for the histologic score. Statistical significance was set at P < 0.05.
The statistical methods of this study were reviewed by Hai-Long Li from Department of Health Statistics of Peking Union Medical College.
Serum levels of IL-6, TNF-α and NF-κB p65 in the control, anti-HMGB1, anti-MyD88, and anti-TRIF groups were significantly higher than in the sham group (P < 0.05) (Table 2). However, the increased levels of inflammatory cytokines in the anti-HMGB1, anti-MyD88, and anti-TRIF groups were significantly lower than in the control group (P < 0.05). Furthermore, the degrees of reduction in the anti-MyD88 and anti-TRIF groups were lower than in the anti-HMGB1 group (P < 0.05). There was a slight but nonsignificant increase in the anti-MyD88 group compared with the anti-TRIF group.
| Group(n = 8) | p65 (pg/mL) | IL-6 (pg/mL) | TNF-α(pg/mL) |
| Sham | 104.64 ± 11.89 | 50.02 ± 6.33 | 43.79 ± 4.18 |
| Control | 228.53 ± 24.85a | 104.91 ± 31.18a | 70.81 ± 6.97a |
| Anti-HMGB1 | 145.00 ± 33.63ab | 62.28 ± 6.73ab | 52.76 ± 5.71ab |
| Anti-MyD88 | 191.12 ± 13.22abc | 85.90 ± 17.37abc | 63.19 ± 5.47abc |
| Anti-TRIF | 183.73 ± 10.81abc | 78.14 ± 7.38abc | 59.70 ± 4.63abc |
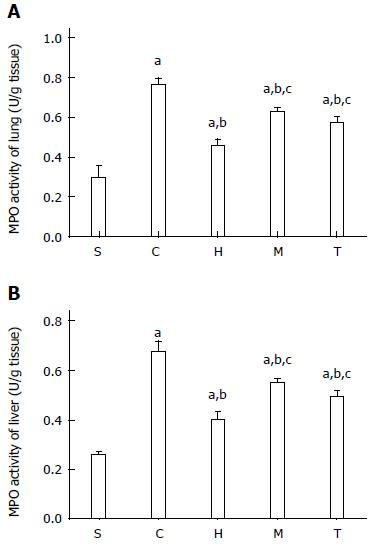
The levels of MPO activity (an enzyme marker for activated neutrophils) in the liver and lung in the control and antibody-treatment groups were significantly higher than in the sham group (P < 0.05) (Figure 2, Table 3). The MPO levels in the three antibody-treatment groups were lower than in the control group (P < 0.05). The anti-HMGB1 group, which showed the largest reduction among the three experimental groups, showed a significant difference compared with the anti-MyD88 and anti-TRIF groups (P < 0.05). However, there was no significant change between the anti-MyD88 and anti-TRIF groups.
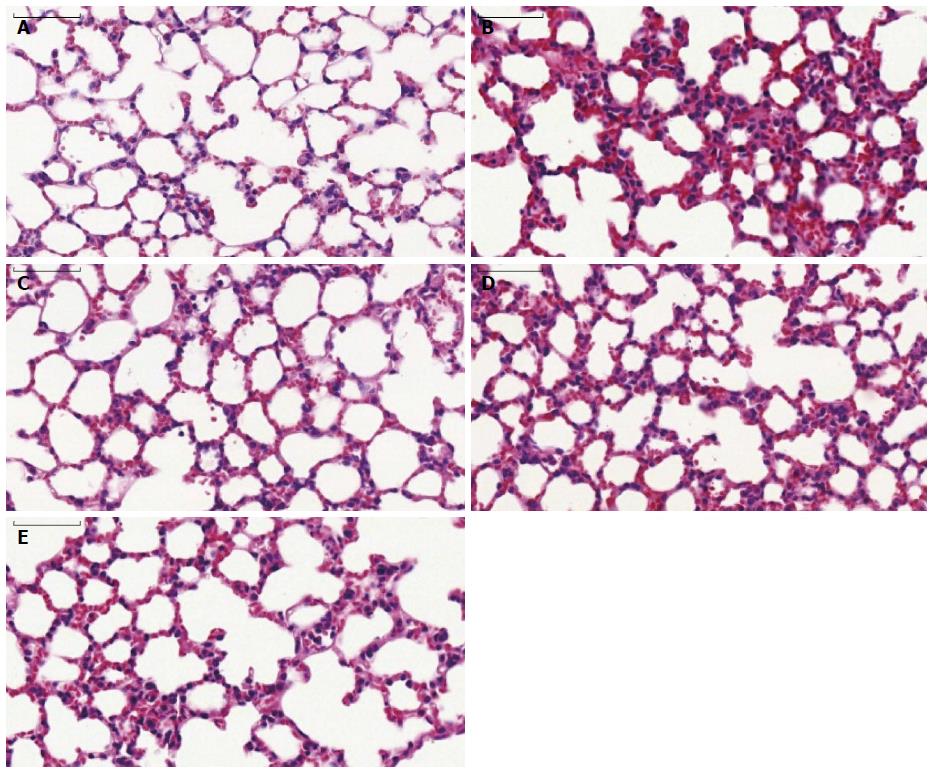
Pathologic changes of lung: As shown in Figure 3A, mice in the sham group had normal lung tissue with a complete and clear alveolar structure; neither edema nor alveolar hemorrhage was observed. Lung tissue from the control group showed features of substantial lung injury, including alveolar epithelial damage, fracture and fusion, alveolar septal thickening, interstitial and intra-alveolar edema with patchy hemorrhage, vascular congestion and some collapsed alveoli, as well as notable inflammatory cell infiltration (Figure 3B). Compared with the control group, tissue injury was markedly attenuated by HMGB1 antibody administration. Alveoli were well aerated and a mild neutrophil infiltration in the interstitium could be observed (Figure 3C). The degree of tissue injury in the anti-MyD88 and anti-TRIF treated groups was significantly lower than in the control group (P < 0.05), but was markedly more serious than in the anti-HMGB1 group (Figure 3D, E, Table 4). There was a trend towards a reduction in lung injury in the anti-TRIF group compared with the anti-MyD88 group, but this difference was not statistically significant.
| Group (n = 6) | Alveolar congestion | Hemorrhage | Neutrophil infiltration | Alveolar wall thickness | Total score |
| Sham | 1.18 ± 0.21 | 0.63 ± 0.27 | 0.23 ± 0.08 | 0.17 ± 0.08 | 2.22 ± 0.64 |
| Control | 3.82 ± 0.18 | 2.72 ± 0.18 | 1.85 ± 0.15 | 2.37 ± 0.23 | 10.75 ± 0.73a |
| Anti-HMGB1 | 2.05 ± 0.12 | 1.02 ± 0.26 | 0.47 ± 0.10 | 0.55 ± 0.14 | 4.08 ± 0.60ab |
| Anti-MyD88 | 3.07 ± 0.16 | 2.13 ± 0.26 | 0.87 ± 0.18 | 1.08 ± 0.23 | 7.15 ± 0.77abc |
| Anti-TRIF | 2.93 ± 0.16 | 1.92 ± 0.18 | 0.77 ± 0.14 | 1.02 ± 0.16 | 6.63 ± 0.62abc |
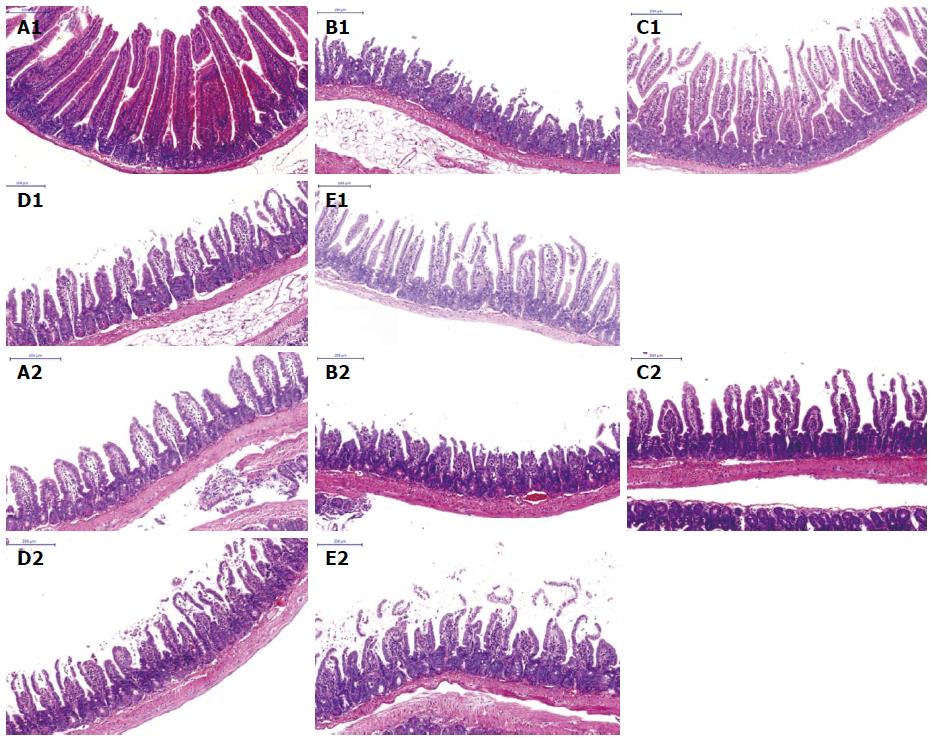
Pathologic changes of the jejunum and ileum: By light microscopy, small intestine specimens of the sham group showed unchanged morphologic structures with an integrated mucosal epithelial, clear tissue layers, no congestion, and inflammatory cell infiltration. Compared with the sham group, the control group showed obvious tissue injury based on mucosal necrosis, erosion, edema, and hyperemia, as well as the reduction of intestinal villi height and mucosal thickness, regional bleeding in the mucosa and lamina propria, and a large amount of inflammatory cell infiltration. However, the anti-HMGB1 group showed slight damage in the villus apex and less inflammatory cell infiltration in the mucosa. The anti-HMGB1 group showed obvious attenuation of small intestinal injury compared with the control group. Our findings for intestinal injury in the anti-MyD88 and anti-TRIF groups were in accordance with the findings in lung (Figure 4, Table 5).
The mRNA expression of HMGB1 and NF-κB in the lung and the ileum in the control and antibody-treatment groups were all significantly higher than the sham group (all P < 0.05) (Tables 6 and 7). The increase in the control group was the most obvious, and was significantly higher than the anti-HMGB1 group (P < 0.05). The levels in the anti-MyD88 and anti-TRIF groups were significantly lower than in the control group (P < 0.05), but were the lowest in the anti-HMGB1 group. There was a slight increase in the anti-MyD88 group compared with the anti-TRIF group, but this was not significant. The mRNA expression of MyD88 in ileum in the anti-HMGB1 group and the anti-MyD88 group were all significantly lower than in the control group and the anti-TRIF group (P < 0.05); The mRNA expression of TRIF in ileum in the anti-HMGB1 group and the anti-TRIF group were all significantly lower than in the control group and the anti-MyD88 group (P < 0.05).
| Group (n = 6) | HMGB1 | NF-κB | MyD88 | TRIF |
| Sham | 1.04 ± 0.19 | 1.03 ± 0.21 | 1.01 ± 0.10 | 1.01 ± 0.11 |
| Control | 2.67 ± 0.30a | 2.04 ± 0.29a | 2.55 ± 0.16a | 3.05 ± 0.10a |
| Anti-HMGB1 | 1.89 ± 0.18ab | 1.42 ± 0.23ab | 2.37 ± 0.08ab | 2.90 ± 0.13ab |
| Anti-MyD88 | 2.35 ± 0.31abc | 1.77 ± 0.18abc | 2.30 ± 0.16ab | 3.04 ± 0.13ac |
| Anti-TRIF | 2.29 ± 0.28abc | 1.70 ± 0.13abc | 2.53 ± 0.07acd | 2.85 ± 0.12abd |
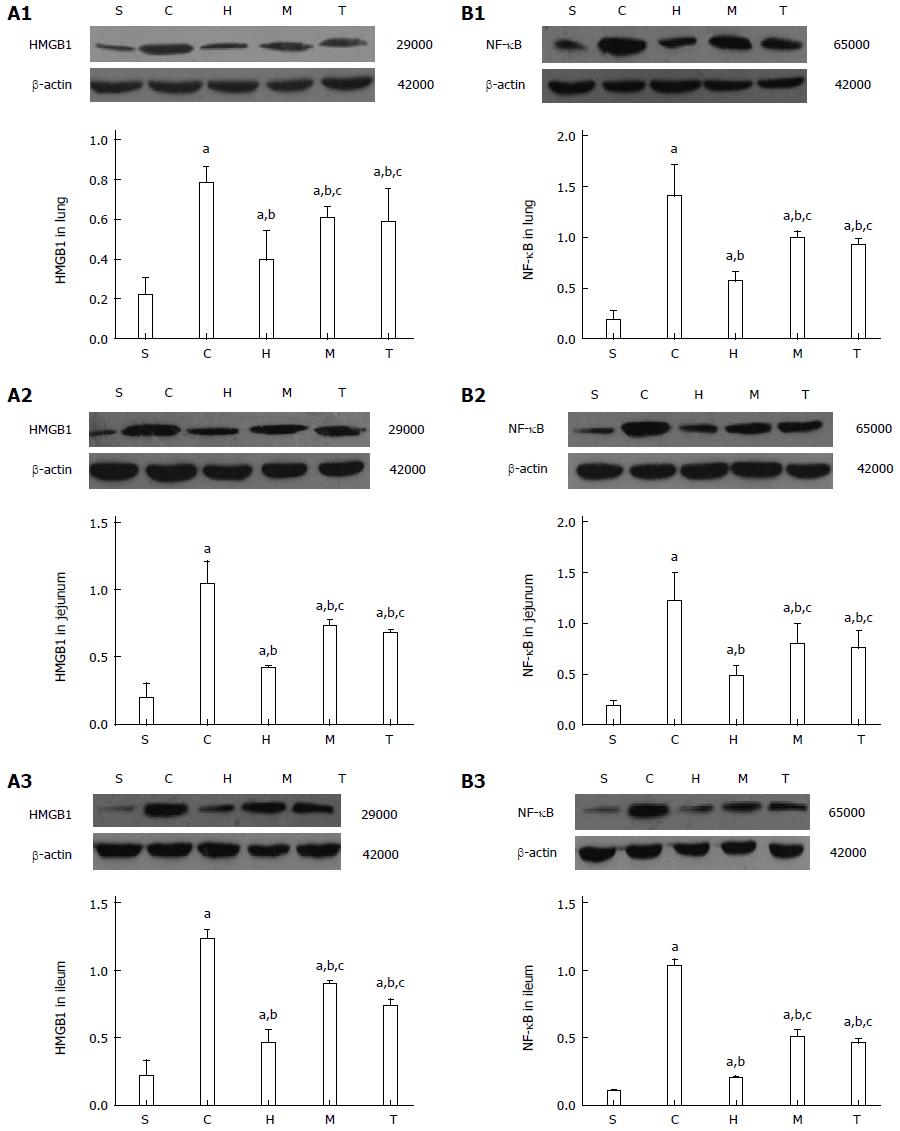
After intestinal I/R injury, protein levels of HMGB1 and NF-κB in the lung, jejunum, and ileum in the control and antibody-treatment groups were significantly increased compared with the sham group, but were significantly lower in the anti-HMGB1, anti-MyD88, and anti-TRIF groups compared with the control group (all P < 0.05) (Figure 5). The degrees of reduced injury in the anti-MyD88 and anti-TRIF groups were lower than in the anti-HMGB1 group (P < 0.05), and there was no significant difference between the anti-MyD88 and anti-TRIF groups,though there was a slight trend towards attenuation in the anti-TRIF group compared with the anti-MyD88 group.
Surgery, trauma, shock, transplantation, and many other diseases often induce tissue I/R. I/R results in the recruitment and activation of a number of downstream adaptor proteins and signaling kinases[16]. Previous studies have shown that high levels of molecular oxygen and overproduction of toxic oxygen radicals play critical roles in the pathologic process of I/R injury[17]. Wu et al[18] summarized that during renal I/R injury, reactive oxygen species not only directly inhibit the reperfusion of renal tissue, but more importantly induce and amplify inflammatory responses via oxidative stress, thereby increasing renal injury and apoptosis. In additional, the study by Quesnelle et al[19] shows that hepatic I/R injury involves numerous pathophysiologic processes.For example, during I/R injury, reactive oxygen species are generated in excess, which leads to oxidative stress and results in cell injury and death. Furthermore, reactive oxygen species, along with oxidative stress, are an essential mediator in the release of HMGB1. As the gut is sensitive to ischemia, intestinal mucosal barrier injury induced by intestinal I/R can often be the basis for a poor prognosis in many diseases, but its specific mechanism remains unclear. Recently, studies have found that the expression of HMGB1 and TLR4 in heart, brain, liver, and kidney are increased during I/R and the HMGB1-TLR4 axis might be the most important trigger of the inflammatory response to I/R injury in many organs[20-23].
TLRs, which can recognize molecules such as danger-associated molecular patterns, are a family of transmembrane proteins that can bind to a range of microbial products and can also recognize endogenous ligands to mediate the secretion of cytokines and the generation of natural immune responses[24-26]. HMGs were originally identified as a type of highly conserved DNA binding proteins that are widely expressed in mammals. Within the nucleus, HMGs participate in the construction and stability of nucleosomes and also regulate gene transcription; outside the nucleus, HMGs mediate inflammatory reactions, and promote cell differentiation and tumor growth[27,28]. Among HMGs, HMGB1 has the closest association with I/R injury. HMGB1 can be released by monocytes/macrophages, and has been shown to bind to TLR4 after I/R[29,30]. The results of the current study show that the protein and mRNA levels of HMGB1 in the small intestine and lung and levels of serum inflammatory cytokines, including TNF-α and IL-6, in mice subjected to intestinal I/R were increased significantly compared with sham-operated mice. Administering HMGB1 antibody prior to ischemia resulted in the marked attenuation of protein and mRNA levels of HMGB1 and inflammatory factors compared with the control group. These findings confirm that the HMGB1-TLR4 axis plays an important role in local and distant organ damage induced by I/R.
The HMGB1-TLR4-mediated signaling pathway mainly activates NF-κB, which plays a critical role in the release of inflammatory factors through two downstream pathways, including MyD88- and TRIF-dependent pathways[31,32]. The MyD88/TIRAP pathway causes the release of various inflammatory factors, such as IL-1, IL-6, IL-8, IL-10, IL-12, IL-18, and TNF-α, mainly through the activation of NF-κB[32]. The TRIF/TRAM pathway mainly depends on the activation of IFN regulatory factor 3, followed by the expression of IFN-β and IFN-induced genes (such as IP-10) and the activation of transcription factors (NF-κB) that induce the synthesis and release of inflammatory factors[32]. In the present study, the mRNA levels of MyD88 and TRIF in ileum of mice subjected to I/R were obviously increased, and the MyD88/TIRAP and TRIF/TRAM pathways could be blocked effectively by injecting anti-MyD88 or anti-TRIF antibody, respectively. Previous studies reported that the contribution of these two downstream pathways is not equivalent in organ I/R injury. For example, the MyD88-dependent pathway was confirmed to play a key role in kidney I/R injury by comparing renal tubular epithelial cells between MyD88-deficient and wildtype animals[33]. Furthermore, the MyD88-dependent pathway might play a more important role in aseptic inflammatory reactions, such as rheumatoid arthritis. However, the TRIF-dependent pathway might play a dominant role in the process of nerve cell degeneration induced by brain I/R[34,35]. However, in intestinal I/R injury, whether there is a dominant pathway activated by TLR4 that drives pathology has not been reported. The results of the current study demonstrate that the serum levels of inflammatory cytokines IL-6, TNF-α, and NF-κB p65 in anti-HMGB1, anti-MyD88, and anti-TRIF groups were significantly lower than in the control group. Additionally, the degree of reduction in the anti-HMGB1 group was the most obvious (perhaps because HMGB1 has an essential role, and downstream molecules MyD88 and TRIF might have partial redundancy). However, there was a slight increase in the anti-MyD88 group compared with the anti-TRIF group, but this difference was not significant. Consistent changes were observed in lung and small intestinal morphology and levels of MPO activity in the liver and lung. Thus, we can make some preliminary conclusions.First, HMGB1-TLR4 plays an important function in intestinal I/R injury in mice by triggering MyD88- and TRIF-dependent downstream signaling pathways.Second, these two downstream signaling pathways both play important roles, but the effect of the TRIF-dependent pathway might be slightly greater.Finally, the tissue damage caused by I/R was obviously alleviated by antibody administration.
The NF-κB family contains important transcription factors that play critical roles in regulating the expression of groups of genes involved in immune and inflammatory responses[36]. MyD88- and TRIF-dependent pathways can both activate NF-κB to induce the release of inflammatory cytokines. The results of the present study show that the mRNA levels of NF-κB in the lung and ileum in the anti-HMGB1, anti-MyD88, and anti-TRIF groups were significantly lower than those in the control group. The degrees of reduction in the anti-MyD88 and anti-TRIF groups were lower than that in the anti-HMGB1 group. Measurements of NF-κB protein levels were in accordance with those of mRNA expression. These findings further suggest that HMGB1-TLR4 is key in local and distant tissue injury induced by intestinal I/R, and that the two downstream pathways both affect protein and mRNA expression.
The protein and mRNA levels of HMGB1 in lung and small intestine were markedly attenuated by administration of anti-HMGB1, anti-MyD88, and anti-TRIF antibodies. There are several possible reasons for this finding. HMGB1 can stimulate the secretion of inflammatory factors, such as TNF-α, IL-6, IL-1β, and IL-8.In addition, inflammatory factors secreted after HMGB1 stimulation could promote monocyte/macrophage secretion of HMGB1. Therefore, HMGB1 could form a positive feedback loop to cause inflammatory signal cascade amplification[33]. Blocking the MyD88/TIRAP and TRIF/TRAM pathways by injecting anti-MyD88 and anti-TRIF antibodies, respectively, can partially inhibit the expression of inflammatory factors (e.g., NF-κB, IL-6, and TNF-α), and the reduction these, in some contexts, can attenuate levels of HMGB1 expression.
Blocking HMGB1, MyD88 and TRIF expression by injecting anti-HMGB1, anti-MyD88, or anti-TRIF antibodies prior to ischemia can significantly reduce the levels of inflammatory cytokines in serum, including TNF-α, IL-6 and NF-κB, and can also alleviate tissue injury in the lung and small intestine compared with the control group in the mouse intestinal I/R model. The findings presented here also suggest that the HMGB1-TLR4 axis and the two downstream signaling pathways all play important roles in I/R injury and show the potential therapeutic value of blocking these pathways. Interventions to prevent the release of HMGB1 and to block the two downstream signaling pathways could become a new clinical therapeutic method to reduce intestinal I/R injury in the future.
In conclusion, HMGB1 and its downstream signaling pathways play important roles in the mouse intestinal I/R injury, and the effect of the TRIF-dependent pathway tends to be slightly larger. The administration of anti-HMGB1, anti-MyD88, and anti-TRIF antibodies significantly reduce the damage caused by I/R, and the effect of the anti-HMGB1 antibody is the most obvious.
We sincerely thank our technician Mr. De-Tian Wang for help with pathology and Mrs. Ya-Ling Dou for help with PCR. We also express our thanks to Zhang Rui, Jiong-Xian Yang,and Zi-Jian Li for help with the animal experiments.
The intestine is an important organ with immune, endocrine, and barrier functions. Intestinal ischemia reperfusion (I/R) injury is believed to lead to systemic inflammatory response syndrome, acute lung injury, acute respiratory distress syndrome, and multiple organ dysfunction syndrome, but the specific mechanism remains controversial. The interaction of toll like receptor 4(TLR4) and high-mobility group protein1 (HMGB1) and the two downstream signaling pathways have gained attention in organ injury induced by I/R.
Recently, studies have found that the expression of HMGB1 and TLR4 in heart, brain, liver, and kidney are increased during I/R, and the HMGB1-TLR4 axis might be the most important trigger of the inflammatory response to I/R injury in many organs.
Only a few studies have examined TLR4-HMGB1 axis and the downstream signaling pathway in aseptic/noninfectious I/R injury. Furthermore, whether intestinal I/R induces local and distant organ injury via these pathways has been poorly characterized. This study aimed to determine whether administering an HMGB1 antibody could reduce tissue damage induced by intestinal I/R and to characterize the role of the HMGB1-TLR4 axis in I/R injury by administrating an anti-HMGB1 antibody. Additionally, to further investigate the myeloid differentiation gene 88(MyD88)/TIR domain-containing adaptor protein- and TIR domain-containing adaptor inducing interferon (IFN)-β/translocating-chain-associating membrane protein-dependent pathways, the authors went on to characterize whether one pathway serves a dominant function in inducing a systemic inflammatory response and distant organ injury following intestinal I/R.
The study results suggest that interventions to prevent the release of HMGB1 and to block the two downstream signaling pathways could become a new clinical therapeutic method to reduce intestinal I/R injury in the future.Furthermore, it has an important clinical significance that could improve the survival rate and reduce complications in critically ill patients.
Intestinal I/R injury is associated with severe trauma, acute necrotizing pancreatitis, major surgery, extensive burns, and other stresses.HMGB1 is one of the endogenous ligands which can be recognized by and combine with TLR4, and is also the key factor of inflammation of aseptic injury. The MyD88-dependent pathway is the downstream signaling pathway of TLR4-HMGB1 axis in I/R injury and causes the release of various inflammatory factors, through the activation of nuclear factor-κB.The TIR domain-containing adaptor inducing IFN-β-dependent pathway is the other downstream signaling pathway of TLR4-HMGB1 axis in I/R injury and mainly depends on the activation of IFN regulatory factor 3, followed by the expression of IFN-β and IFN-induced genes (such as IP-10) and the activation of transcription factors (NF-κB).
This is a good descriptive study in which authors show that HMGB1 and its downstream signaling pathways play important roles in the mouse intestinal I/R injury. The results are interesting and suggest that interventions to prevent the release of HMGB1 and to block the two downstream signaling pathways could become a new clinical therapeutic method to reduce intestinal I/R injury in the future.
| 1. | Elias-Miró M, Jiménez-Castro MB, Rodés J, Peralta C. Current knowledge on oxidative stress in hepatic ischemia/reperfusion. Free Radic Res. 2013;47:555-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 157] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 2. | Liao YF, Zhu W, Li DP, Zhu X. Heme oxygenase-1 and gut ischemia/reperfusion injury: A short review. World J Gastroenterol. 2013;19:3555-3561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 32] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (1)] |
| 3. | Kougias P, Lau D, El Sayed HF, Zhou W, Huynh TT, Lin PH. Determinants of mortality and treatment outcome following surgical interventions for acute mesenteric ischemia. J Vasc Surg. 2007;46:467-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 4. | Slone EA, Fleming SD. Membrane lipid interactions in intestinal ischemia/reperfusion-induced Injury. Clin Immunol. 2014;153:228-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Ding HS, Yang J, Chen P, Yang J, Bo SQ, Ding JW, Yu QQ. The HMGB1-TLR4 axis contributes to myocardial ischemia/reperfusion injury via regulation of cardiomyocyte apoptosis. Gene. 2013;527:389-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 116] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 6. | Yang Z, Deng Y, Su D, Tian J, Gao Y, He Z, Wang X. TLR4 as receptor for HMGB1-mediated acute lung injury after liver ischemia/reperfusion injury. Lab Invest. 2013;93:792-800. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 77] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 7. | Sánchez-Miralles A, Castellanos G, Badenes R, Conejero R. [Abdominal compartment syndrome and acute intestinal distress syndrome]. Med Intensiva. 2013;37:99-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Guzel A, Kanter M, Guzel A, Yucel AF, Erboga M. Protective effect of curcumin on acute lung injury induced by intestinal ischaemia/reperfusion. Toxicol Ind Health. 2013;29:633-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Reino DC, Pisarenko V, Palange D, Doucet D, Bonitz RP, Lu Q, Colorado I, Sheth SU, Chandler B, Kannan KB. Trauma hemorrhagic shock-induced lung injury involves a gut-lymph-induced TLR4 pathway in mice. PLoS One. 2011;6:e14829. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 80] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 10. | Moresco EM, LaVine D, Beutler B. Toll-like receptors. Curr Biol. 2011;21:R488-R493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 381] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 11. | O’Neill LA, Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol. 2007;7:353-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1836] [Cited by in RCA: 2050] [Article Influence: 107.9] [Reference Citation Analysis (0)] |
| 12. | Kojima M, Tanabe M, Shinoda M, Yamada S, Miyasho T, Suda K, Hibi T, Obara H, Itano O, Kawachi S. Role of high mobility group box chromosomal protein 1 in ischemia-reperfusion injury in the rat small intestine. J Surg Res. 2012;178:466-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Sawa H, Ueda T, Takeyama Y, Yasuda T, Shinzeki M, Nakajima T, Kuroda Y. Blockade of high mobility group box-1 protein attenuates experimental severe acute pancreatitis. World J Gastroenterol. 2006;12:7666-7670. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 107] [Cited by in RCA: 109] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 14. | Kim DH, Chung JH, Son BS, Kim YJ, Lee SG. Effect of a neutrophil elastase inhibitor on ventilator-induced lung injury in rats. J Thorac Dis. 2014;6:1681-1689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 15. | Stallion A, Kou TD, Latifi SQ, Miller KA, Dahms BB, Dudgeon DL, Levine AD. Ischemia/reperfusion: a clinically relevant model of intestinal injury yielding systemic inflammation. J Pediatr Surg. 2005;40:470-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 72] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 16. | Merry HE, Phelan P, Doak MR, Zhao M, Hwang B, Mulligan MS. Role of toll-like receptor-4 in lung ischemia-reperfusion injury. Ann Thorac Surg. 2015;99:1193-1199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 17. | Yapca OE, Borekci B, Turan MI, Gulapoglu M. The effect of agomelatine on oxidative stress induced with ischemia/reperfusion in rat ovaries. Adv Clin Exp Med. 2014;23:715-721. [PubMed] |
| 18. | Wu K, Li H, Tian J, Lei W. Protective effect of baicalein on renal ischemia/reperfusion injury in the rat. Ren Fail. 2015;37:285-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Quesnelle KM, Bystrom PV, Toledo-Pereyra LH. Molecular responses to ischemia and reperfusion in the liver. Arch Toxicol. 2015;89:651-657. [PubMed] |
| 20. | He GZ, Zhou KG, Zhang R, Wang YK, Chen XF. Impact of intestinal ischemia/reperfusion and lymph drainage on distant organs in rats. World J Gastroenterol. 2012;18:7271-7278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 21. | Zhang YX, Zhang JR, Wang ZG. Mycophenolate mofetil affects monocyte Toll-like receptor 4 signaling during mouse renal ischemia/reperfusion injury. Chin Med J (Engl). 2013;126:1224-1229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 22. | Zhang J, Wu Y, Weng Z, Zhou T, Feng T, Lin Y. Glycyrrhizin protects brain against ischemia-reperfusion injury in mice through HMGB1-TLR4-IL-17A signaling pathway. Brain Res. 2014;1582:176-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 83] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 23. | Hu X, Zhang K, Xu C, Chen Z, Jiang H. Anti-inflammatory effect of sodium butyrate preconditioning during myocardial ischemia/reperfusion. Exp Ther Med. 2014;8:229-232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 24. | Li XQ, Lv HW, Tan WF, Fang B, Wang H, Ma H. Role of the TLR4 pathway in blood-spinal cord barrier dysfunction during the bimodal stage after ischemia/reperfusion injury in rats. J Neuroinflammation. 2014;11:62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 90] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 25. | Cotroneo TM, Nemzek-Hamlin JA, Bayliss J, Su GL. Lipopolysaccharide binding protein inhibitory peptide alters hepatic inflammatory response post-hemorrhagic shock. Innate Immun. 2012;18:866-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Szatmary Z. Molecular biology of toll-like receptors. Gen Physiol Biophys. 2012;31:357-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 27. | Fan J, Li Y, Levy RM, Fan JJ, Hackam DJ, Vodovotz Y, Yang H, Tracey KJ, Billiar TR, Wilson MA. Hemorrhagic shock induces NAD(P)H oxidase activation in neutrophils: role of HMGB1-TLR4 signaling. J Immunol. 2007;178:6573-6580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 239] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 28. | Tsung A, Tohme S, Billiar TR. High-mobility group box-1 in sterile inflammation. J Intern Med. 2014;276:425-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 161] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 29. | Levy RM, Mollen KP, Prince JM, Kaczorowski DJ, Vallabhaneni R, Liu S, Tracey KJ, Lotze MT, Hackam DJ, Fink MP. Systemic inflammation and remote organ injury following trauma require HMGB1. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1538-R1544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 175] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 30. | Doi K, Ishizu T, Tsukamoto-Sumida M, Hiruma T, Yamashita T, Ogasawara E, Hamasaki Y, Yahagi N, Nangaku M, Noiri E. The high-mobility group protein B1-Toll-like receptor 4 pathway contributes to the acute lung injury induced by bilateral nephrectomy. Kidney Int. 2014;86:316-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 31. | Ding HS, Yang J, Gong FL, Yang J, Ding JW, Li S, Jiang YR. High mobility group [corrected] box 1 mediates neutrophil recruitment in myocardial ischemia-reperfusion injury through toll like receptor 4-related pathway. Gene. 2012;509:149-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 32. | Zhao H, Perez JS, Lu K, George AJ, Ma D. Role of Toll-like receptor-4 in renal graft ischemia-reperfusion injury. Am J Physiol Renal Physiol. 2014;306:F801-F811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 97] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 33. | Lu YC, Yeh WC, Ohashi PS. LPS/TLR4 signal transduction pathway. Cytokine. 2008;42:145-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1860] [Cited by in RCA: 2535] [Article Influence: 140.8] [Reference Citation Analysis (0)] |
| 34. | Hosmane S, Tegenge MA, Rajbhandari L, Uapinyoying P, Kumar NG, Thakor N, Venkatesan A. Toll/interleukin-1 receptor domain-containing adapter inducing interferon-β mediates microglial phagocytosis of degenerating axons. J Neurosci. 2012;32:7745-7757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 82] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 35. | Kawamoto T, Ii M, Kitazaki T, Iizawa Y, Kimura H. TAK-242 selectively suppresses Toll-like receptor 4-signaling mediated by the intracellular domain. Eur J Pharmacol. 2008;584:40-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 261] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 36. | Shi Z, Lian A, Zhang F. Nuclear factor-κB activation inhibitor attenuates ischemia reperfusion injury and inhibits Hmgb1 expression. Inflamm Res. 2014;63:919-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Hei ZQ, Liu QL, Senturk GE, Yu LCH S- Editor: Yu J L- Editor: AmEditor E- Editor: Liu XM