Published online May 28, 2015. doi: 10.3748/wjg.v21.i20.6180
Peer-review started: October 13, 2014
First decision: November 14, 2014
Revised: December 12, 2014
Accepted: February 12, 2015
Article in press: February 13, 2015
Published online: May 28, 2015
Processing time: 230 Days and 2.7 Hours
AIM: To investigate the role of serum-and-glucocorticoid-inducible-kinase-1 (SGK1) in colitis and its potential pathological mechanisms.
METHODS: SGK1 expression in mucosal biopsies from patients with active Crohn’s disease (CD) and normal controls was detected by immunohistochemistry. We established an acute colitis model in mice induced by 2,4,6-trinitrobenzene sulfonicacid, and demonstrated the presence of colitis using the disease activity index, the histologic activity index and hematoxylin and eosin staining. The cellular events and potential mechanisms were implemented with small interference RNA and an inhibitor of signaling molecule (i.e., U0126) in intestinal epithelial cells (IECs). The interaction between SGK1 and the signaling molecule was assessed by co-immunoprecipitation.
RESULTS: SGK1 expression was significantly increased in the inflamed epithelia of patients with active CD and TNBS-induced colitis model (0.58 ± 0.055 vs 0.85 ± 0.06, P < 0.01). At the cellular level, silencing of SGK1 by small interference RNA (siSGK1) significantly inhibited the phosphorylation of mitogen-activated protein kinase kinase 1 (MEK1) and the downstream molecule extracellular signal regulated protein kinase (ERK) 1/2, which induced the upregulation of p53 and Bcl-2-associated X protein, mediating the subsequent cellular apoptosis and proliferation in IECs. Cells treated with MEK1 inhibitor (i.e., U0126) before siSGK1 transfection showed a reversal of the siSGK1-induced cellular apoptosis.
CONCLUSION: Our data suggested that SGK1 may protect IECs in colitis from tumor necrosis factor-α-induced apoptosis partly by triggering MEK/ERK activation.
Core tip: This study showed that serum-and-glucocorticoid-inducible-kinase-1 (SGK1) expression was significantly increased in the inflamed epithelia of patients with active Crohn’s disease (CD) in a TNBS-induced colitis model. At the cellular level, silencing of SGK1 inhibited the phosphorylation of mitogen-activated protein kinase kinase 1 (MEK1) and the downstream molecule ERK1/2, which induced the upregulation of p53 and Bcl-2-associated X protein, triggering subsequent cellular apoptosis and inhibition of proliferation in intestinal epithelial cells. A MEK1 inhibitor (i.e., U0126) was used to show that this was a MEK/ERK-dependent process. Co-immunoprecipitation analysis uncovered the mechanism of the interaction between SGK1 and MEK1. Our results provide a new therapeutic approach to CD therapy.
-
Citation: Bai JA, Xu GF, Yan LJ, Zeng WW, Ji QQ, Wu JD, Tang QY. SGK1 inhibits cellular apoptosis and promotes proliferation
via the MEK/ERK/p53 pathway in colitis. World J Gastroenterol 2015; 21(20): 6180-6193 - URL: https://www.wjgnet.com/1007-9327/full/v21/i20/6180.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i20.6180
Crohn’s disease (CD) is a chronic, relapsing and debilitating colitis. It is characterized as transmural inflammation with the clinical features of bowel obstruction, stricture, and diarrhea with blood or mucus, or both[1]. In the last decade, substantial advances aimed at uncovering the molecular pathogenesis of CD have been made, and 71 distinct loci for CD on 17 chromosomes have been identified in genome-wide association studies (GWAS)[2]. Nevertheless, the precise pathogenesis of CD remains poorly elucidated. Various components are involved in CD, such as intestinal epithelial cells (IECs), environmental and microbial factors, and innate and adaptive immunity[3]. IECs are the most important component of the epithelial barrier and play a pivotal role in intestinal immune homeostasis[4]. Accordingly, a sequence of events, such as apoptosis due to the stimulation of inflammation, disrupts homeostasis and mucosal integrity. Meanwhile, IEC proliferation and differentiation repair the barrier and sustain homeostasis. In some cases, the balance of disruption and repair is disrupted, followed by chronic gut inflammation, also known as inflammatory bowel disease (IBD), which occurs in CD and ulcerative colitis (UC)[5]. Further investigation of targeted therapies aimed at alleviating apoptosis and boosting IEC proliferation may lead to new developments in CD treatment.
Serum-and-glucocorticoid-inducible-kinase-1 (SGK1) was first named according to its gene upregulation by serum and glucocorticoids in rat mammary tumor cells[6]. The human SGK1 gene is located on chromosome 6q23. It is ubiquitously expressed in almost all tissues of the digestive tract, such as the esophagus, stomach, liver, intestine, and pancreas. SGK1 is also a gene that can encode serine/threonine protein kinase and may be involved in cell signaling pathways related to cellular survival[7]. Human SGK1 expression is stimulated by cell shrinkage and cytokines[8]; intestinal SGK1 is also regulated by saline ingestion[9]. SGK1 disorder contributes to the regulation of cell apoptosis, migration, proliferation, and epithelial transport. In addition, several studies have indicated a fatal role of SGK1 in the pathophysiology of various diseases, including autoimmune disease, inflammation, and tumor growth[10]. Therefore, we deduced that SGK1 inhibition may be a potential therapeutic option in the treatment of these disorders, especially CD.
The mitogen-activated protein kinase (MAPK) cascade is well known as a vital regulator of diverse cellular functions, such as cell proliferation, apoptosis, migration and differentiation[11]. The MAPK cascade acts as a critical mediator that can transduce cellular signaling from the cell surface to the cytosol and nucleus, and can even regulate cellular responses to endogenous or exogenous stimuli[12]. Tumor necrosis factor (TNF)-α has the capacity to trigger many elements of the inflammatory response in the gastrointestinal mucosa, and is an efficient stimulus of the extracellular signal regulated protein kinase αα(ERK)1/2pathway[13]. Three MAPK cascades have been elucidated in mammals: mitogen-activated protein kinase kinase kinase (Raf or MAP3K), mitogen-activated protein kinase kinase (MEK or MAP2K), and extracellular signal regulated protein kinase (ERK or MAPK). The MEK/ERK signaling cascade possesses serine/threonine kinase activities and critically mediates cellular proliferation, apoptosis, cycle progression, and migration in various cell types[14]. One of the critical mechanisms of MEK/ERK cascade-induced apoptosis is p53-related Bax dysregulation. Previous studies have reported that the MEK/ERK cascade mediates gene expression by the phosphorylation of several transcription factors, including p53[15]. In addition, the MEK/ERK pathway participates in cellular apoptosis by regulating gene expression of the pro-survival B-cell lymphoma-2 (Bcl-2) family proteins, such as Bax (a conserved key regulator of apoptosis), and by inducing anti-apoptotic proteins for proteasomal degradation[16]. P53, which has been referred to as the “guardian of the genome”, is a tumor suppressor[17]. Numerous studies have indicated that p53-induced apoptosis activates the multi-domain pro-apoptotic protein Bax, thereby triggering caspase activation and cellular apoptosis[18].
It has remained poorly understood whether and how SGK1 is involved in the initiation and development of colitis. In our paper, for the first time, we validated that SGK1 plays a critical role in the protection of IECs from apoptosis in a colitis model and even in CD via the interaction with MEK1, which activates the MEK/ERK pathway. Our results provide new potential therapeutic targets for CD therapy.
The animal protocol was designed to minimize pain or discomfort in the animals. The animals were acclimatized to laboratory conditions (23 °C, 12 h/12 h light/dark, 50% humidity, ad libitum access to food and water) for 2 wk prior to experimentation. Intragastric gavage was performed on conscious animals, using straight gavage needles appropriate for the animal size (15-17 g body weight: 22 gauge, 1 inch length, 1.25 mm ball diameter). All animals were euthanazed by barbiturate overdose (150 mg/kg pentobarbital sodium iv) and tissue samples subsequently collected.
Anti-SGK1, anti-MEK1, and anti-phosphorylated-MEK1 (p-MEK1) antibodies were purchased from SAB, United States. The anti-ERK1/2, anti-phosphorylated-ERK1/2 (p-ERK1/2), anti-Bax, anti-p53, and anti-GAPDH antibodies were purchased from CST, United States. The anti-IgG antibodies, the immunofluorescence staining kit, cell counting Kit-8 (CCK-8), terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) kit and inhibitors of MEK1 (U0126) were purchased from Beyotime Institute of Biotechnology. The immunostaining streptavidin-peroxidase (SP) kit was purchased from the Maixin Institute of Biotechnology, China. TNBS was purchased from Sigma Chemical Co., St. Louis, MO, United States. Dulbecco’s modified Eagle’s medium (DMEM), RPMI 1640 medium, and fetal bovine serum (FBS) were purchased from Gibco, United States. Female BALB/c mice (6-8-wk-old, 19-22 g body weight) were purchased from the Animal Center of Nanjing Medical College, China. The human colorectal cancer cell line HCT-116 and normal rabbit intestinal endothelial cells 6 (IEC-6) were obtained from Nanjing Medical University, China. All the experiments were approved by Nanjing Medical College Animal Care and Use Committee.
Biopsy specimens were obtained from inflamed mucosal areas of patients with active CD (n = 8) who were diagnosed according to clinical and macroscopic criteria. Control samples were collected from healthy subjects (n = 8). Tissue samples were immediately fixed in formalin and embedded in paraffin for an immunohistochemistry assay. The study was approved by the Institutional Review Board of Nanjing Medical University.
All mice were randomly distributed into 2 groups and housed at room temperature of 22-24 °C with a 12-h light/dark cycle, and access to unrestricted tap water and standard rodent food. Mice were weighed and an acute CD model was induced by intra-rectal instillation of a solution consisting of 2.5% TNBS in 50% absolute ethanol (v/v) via a 3.5F catheter under deep anesthesia induced by 3% pentobarbital i.p., as previously described[19]. The mice were held in a vertical position for 1 min to ensure sufficient contact of the TNBS with the entire colon wall and were then returned to their cages. Intra-rectal administration of 100 L 50% ethanol in a similar manner served as the control treatment. Mice were killed by cervical dislocation on days 1, 2, 3, 5, 7, and 10 (n = 4 per day). To evaluate the degree of colitis, the mice were weighed daily, and fecal consistency and presence of bloody stool were recorded for 10 d after TNBS treatment. The colon length was recorded as a parameter of inflammation. The disease activity index (DAI) consisted of weight loss, stool consistency, and the degree of occult blood, as previously described[20]. The histologic activity index (HAI) was determined based on previous criteria[21]. Two personnel blinded to the source of the samples independently performed all evaluations.
All the tissues that remained were sliced as frozen samples of 5 μm thickness via a microtome. Antigen retrieval was performed by incubating the samples in sodium citrate buffer (0.01 mol/L, pH 6.0) for 3 min with heat applied. To block endogenous peroxidase, the samples were incubated with liquid A (endogenous peroxidase blockers) for 10 min, washed in PBS 3 times, then blocked with liquid B for 10 min. Samples were incubated with the primary antibody at room temperature for 2 h. The samples were then washed in PBS 3 times and incubated with liquid C as the secondary antibody for 10 min at room temperature. After washing 3 times with PBS, the samples were incubated with liquid D (streptavidin-peroxidase) for 10 min at room temperature. Finally, all samples were dyed with 0.05% freshly prepared diaminobenzedine (Beyotime Institute of Biotechnology, China) solution for 5 min, monitored under a microscope, rinsed with tap water, counterstained with hematoxylin for 1 min, dehydrated with graded concentrations of ethanol, hyalinized with dimethylbenzene, and covered with neutral gum. The ethanol enema samples were prepared as negative controls. These slices were visualized with a microscope (IX71; Olympus Corp., Tokyo, Japan). The images were analyzed with Image Pro Plus (version 6.0).
The HCT-116 cells were cultured in RPMI 1640 medium, and IEC-6 cells were cultured in DMEM (4.5 g/L glucose). All media were supplemented with 10% FBS. The cells were maintained in a humidified atmosphere of 5% CO2 at 37 °C. At a density of 80%-85%, cells were cultured with TNF-α (5 ng/mL, respectively) for 0, 1, 6, 12, 18 or 24 h as time-dependent groups. The other three 60-mm cell culture dishes were supplemented with TNF-α at different concentrations of 5, 10, or 20 ng as density-dependent groups. In transfection experiments, 5 μL of Lipofectamine 2000 (Invitrogen, California, United States) was mixed with 95 μL of Opti-MEM (Invitrogen, California, United States), and 2 μL of 50 nmol/L SGK1 siRNA (Genepharma, Shanghai, China) was mixed with 98 μL of Opti-MEM medium. Subsequently, all solutions were mixed and incubated for 20 min at room temperature. After incubation for 6 h with Opti-MEM, each dish was changed to complete medium for 48 h. An equal volume of Lipofectamine 2000 was used as the negative control. The cells were harvested for immunoblotting.
Colonic tissues and cells were split, and equal protein amounts were collected for SDS-polyacrylamide gel electrophoresis. The proteins were then transferred onto a polyvinylidene fluoride membrane (Millipore, Bedford, MA) using a transblot. The membranes were then kept in a solution of PBST and 5% non-fat milk for 2 h to terminate the nonspecific sites and were subsequently incubated with prepared primary antibodies at 4 °C overnight. After incubation with horseradish peroxidase-conjugated anti-rabbit immunoglobulin G (IgG, 1:10000) for 1 h at room temperature, the bands were developed using a chemiluminescence kit (ECL; Pierce Supersignal, United States). GAPDH was used as an internal control. For quantitative analysis, the bands were analyzed using Quantity One software (Bio-Rad Laboratories, CA, United States).The difference between the control and treatment groups was recorded and analyzed using ImageJ software.
Co-immunoprecipitation (Co-IP) was performed according to the manufacturer’s protocol. Briefly, we washed the adherent cells with ice-cold PBS, added RIPA buffer, scraped the cells and centrifuged the lysate. The supernatant was pre-cleared with the addition of protein A/G-agarose beads (Abmart, Shanghai, China) for 10 min at 4 °C. After a brief spin, the pre-cleared supernatant was incubated with primary antibodies (1:100) for 2 h at 4 °C on a rotator, and the cell lysate-protein-antibody complexes were recovered using recombinant protein A/G-Agarose beads. Empty vector (Input) and purified rabbit IgG-transfected cells were used as negative and positive controls, respectively. Samples were detected with immunoblotting as described above.
A one-step TUNEL kit was used to perform TUNEL assays according to the manufacturer’s instructions. Briefly, the tissue samples and pre-treated cells were immobilized with 4% paraformaldehyde for 30 min and permeabilized with 0.1% Triton X-100 for 2 min at 4 °C, followed by TUNEL for 1 h at 37 °C. The fluorescein isothiocyanate (FITC)-labeled TUNEL-positive IECs were examined using a microscope. The IECs with green fluorescence were defined as apoptotic cells.
A CCK-8 assay was performed following the manufacturer’s protocol to evaluate cell proliferation. Pre-treated cells were seeded into a 96-well plate at an approximate density of 2 × 103 cells per well with 100 μL medium. The blank control wells contained medium alone (i.e., no cells or drugs). After culturing for 0, 6, 12, 24 and 48 h, each well was supplemented with 10 μL tetrazolium substrate and incubated at 37 °C for 1 h. The optical density (OD) was then read at 450 nm in a Synergy HT microtiter plate reader (Bio-Tek, United States). This experiment was repeated 3 times independently.
All data are expressed as mean ± SD, and the statistical significance of differences between 2 groups was analyzed using the Student two-tailed t test. The significance levels were set at either P < 0.05 or P < 0.01. All statistical analyses were performed using SPSS software (version 14.0; SPSS Inc., Chicago, IL, United States).
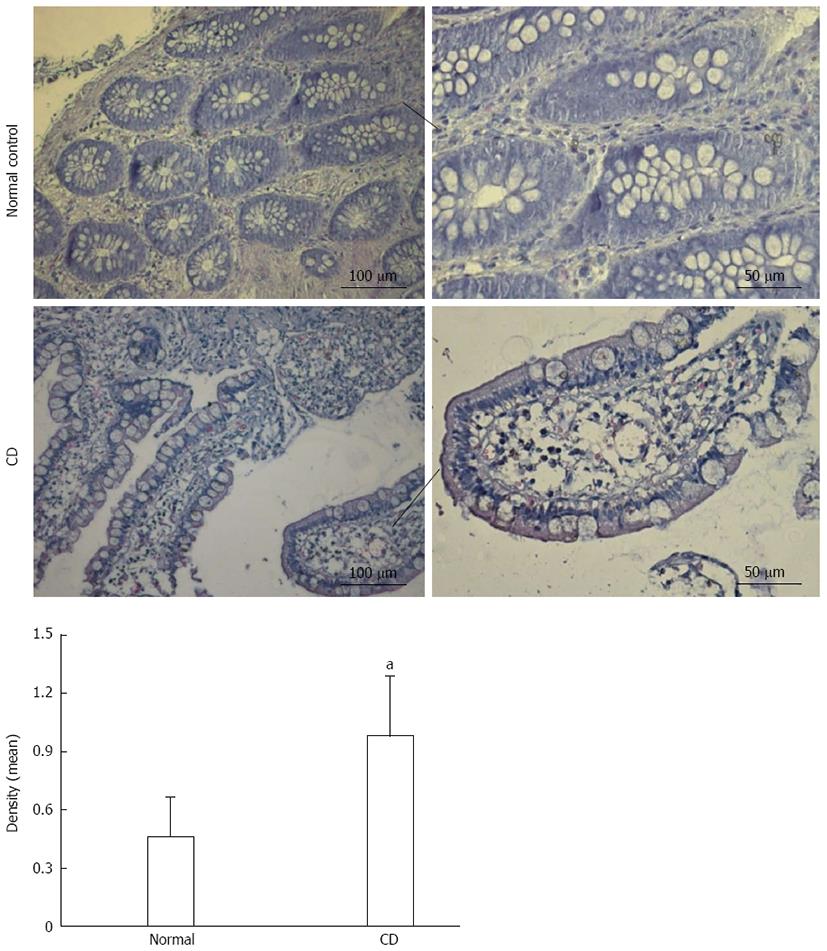
To evaluate the aberrant expression and localization of SGK1 in CD, we performed immunohistochemistry assays on samples from patients with active CD and normal controls. Weak SGK1 staining was observed in the normal control group, while strong SGK1 staining was observed in the samples from patients with active CD and was mainly located in the cytoplasm of IECs (Figure 1).
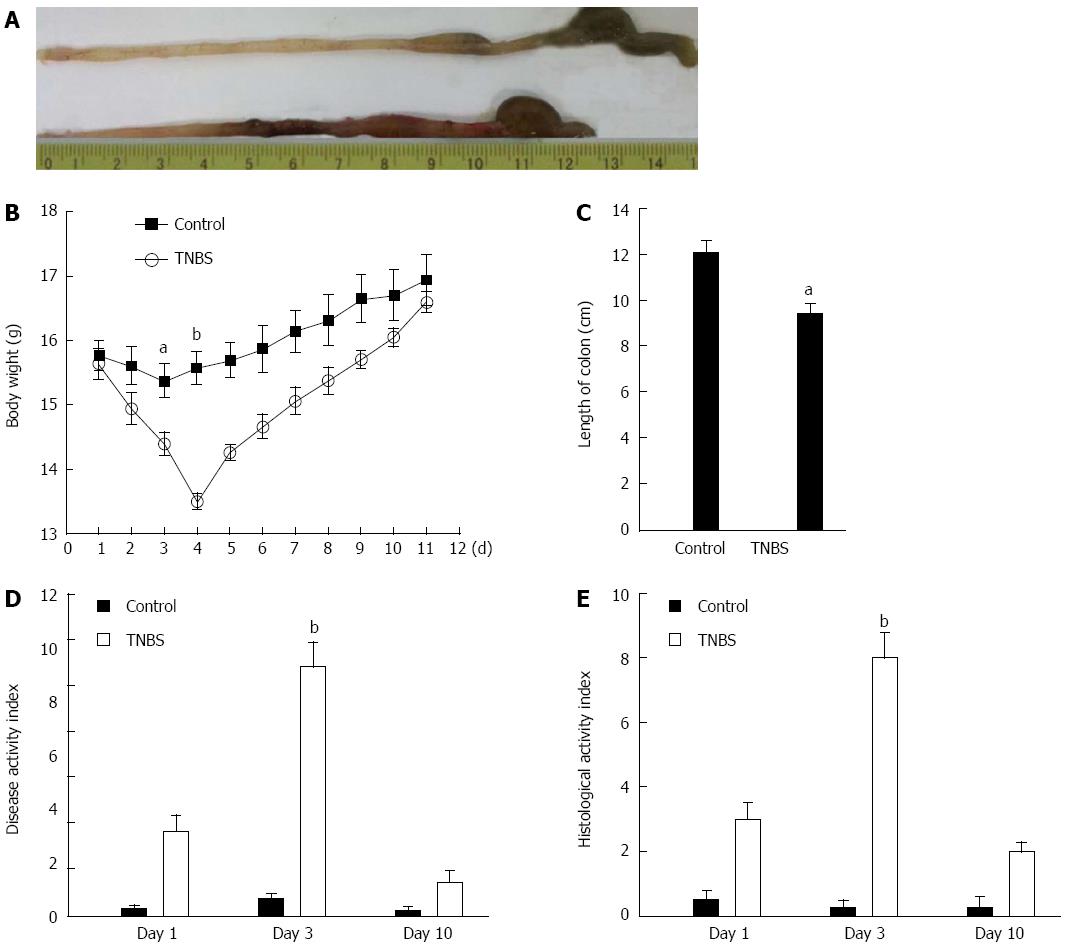
To evaluate the severity of TNBS-induced colitis in mice, we recorded the appearance of the colon, weight loss, length of the colon, stool character, and presence of blood in the stool. The TNBS-induced colitis group showed more severe damage (Figure 2A, upper line) in the colon compared with controls (50% ethanol, Figure 2A, lower line). In addition, the TNBS-induced colitis group showed more obvious weight loss. The mean body weight loss was 6.8% over 24 h and reached a peak of 13.9% at 72 h compared with the baseline body weight at the time of TNBS administration (Figure 2B). In addition, there were more loose stools and apparently bloody stools in the TNBS-induced mice than in the ethanol-treated mice. The length of the colon was significantly reduced in the TNBS group compared with controls (Figure 2C). The DAI and HAI demonstrated maxima of 9.2 and 8.0, respectively, at 72 h (Figure 2D and E). The pathology assay also showed the most severe colitis at day 3 (Figure 3A).
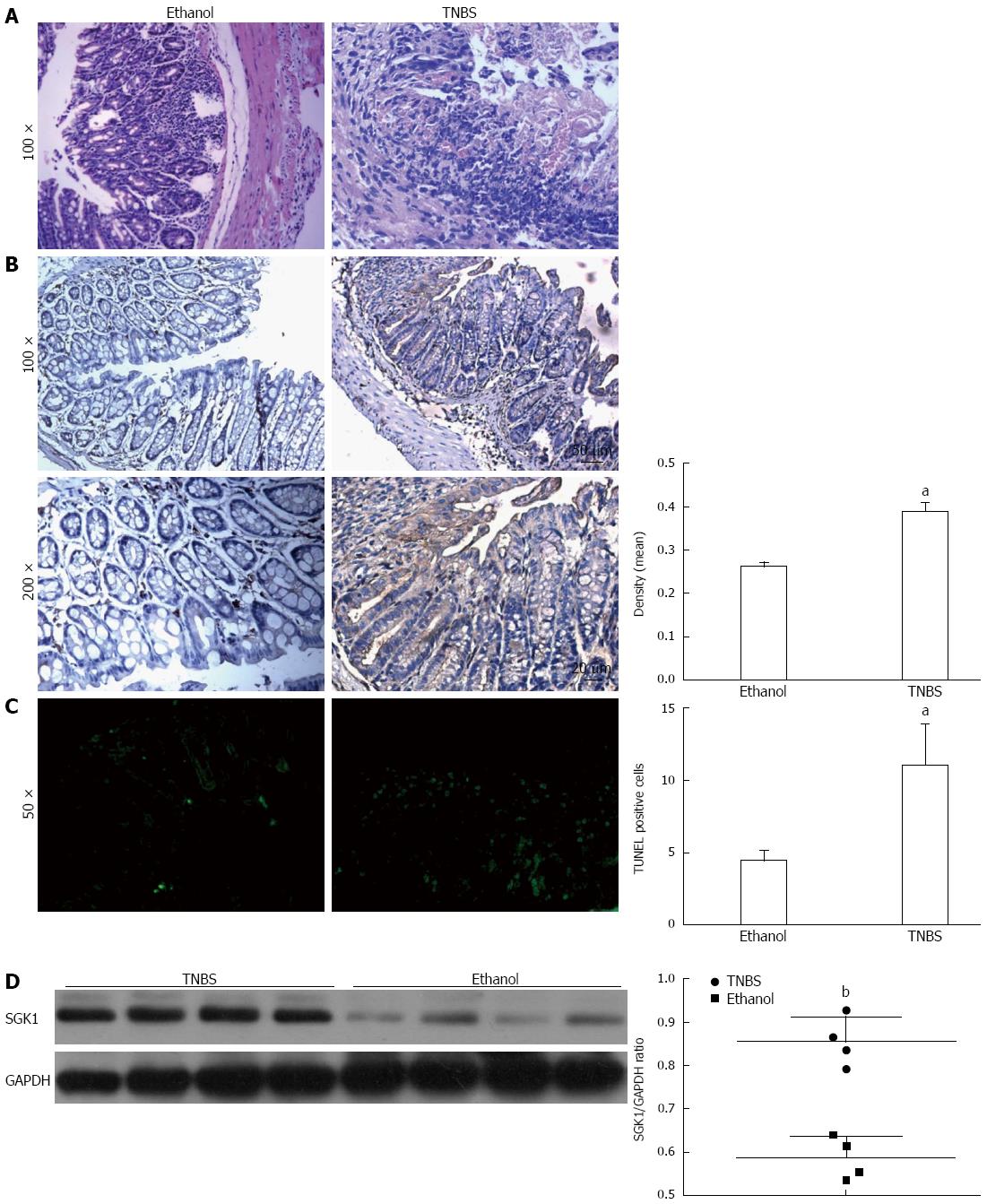
Immunohistochemical staining showed that SGK1 was expressed mainly in the cytoplasm of IECs and at higher levels in TNBS-induced colon tissue at day 3 compared with control (Figure 3B). To further confirm the apoptosis of IECs induced by TNBS, we performed a TUNEL assay on the tissue samples and noted a significant increase in IEC apoptosis in TNBS mice (Figure 3C). Western blotting showed that SGK1 expression was markedly increased in mice with TNBS-induced colitis (Figure 3D).
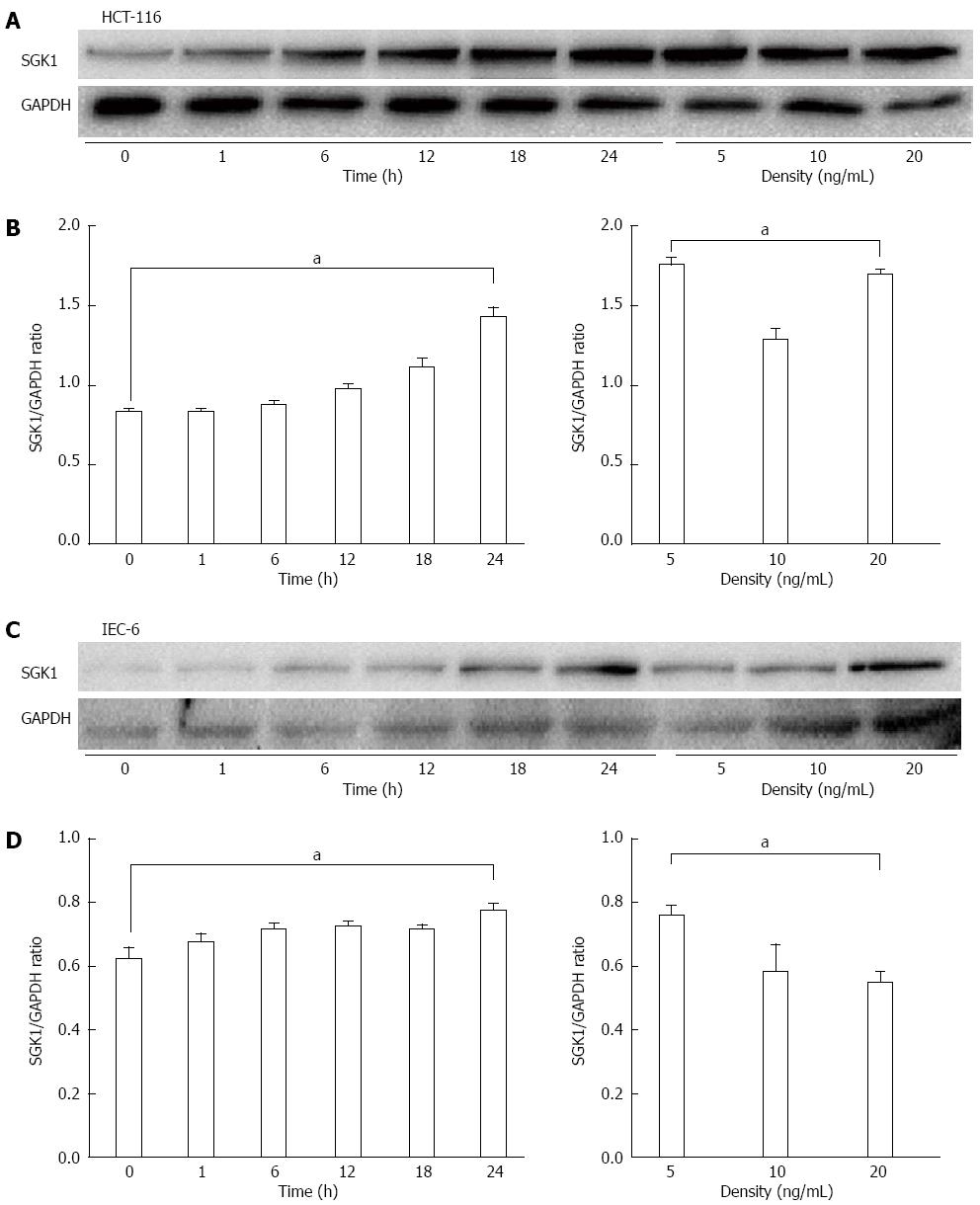
We next examined the biological behavior of SGK1 in vitro in HCT-116 cells and IEC-6 cells to elucidate the mechanism. We determined SGK1 expression by western blotting after TNF-α treatment and found that the TNF-induced SGK1 expression occurred in a time- and dose-dependent manner. Specifically, the peak of SGK1 expression after TNF-α treatment appeared at 24 h in HCT-116 cells (Figure 4A and B) and IEC-6 cells (Figure 4C and D) at a dose of 5 ng/mL (Figure 4).
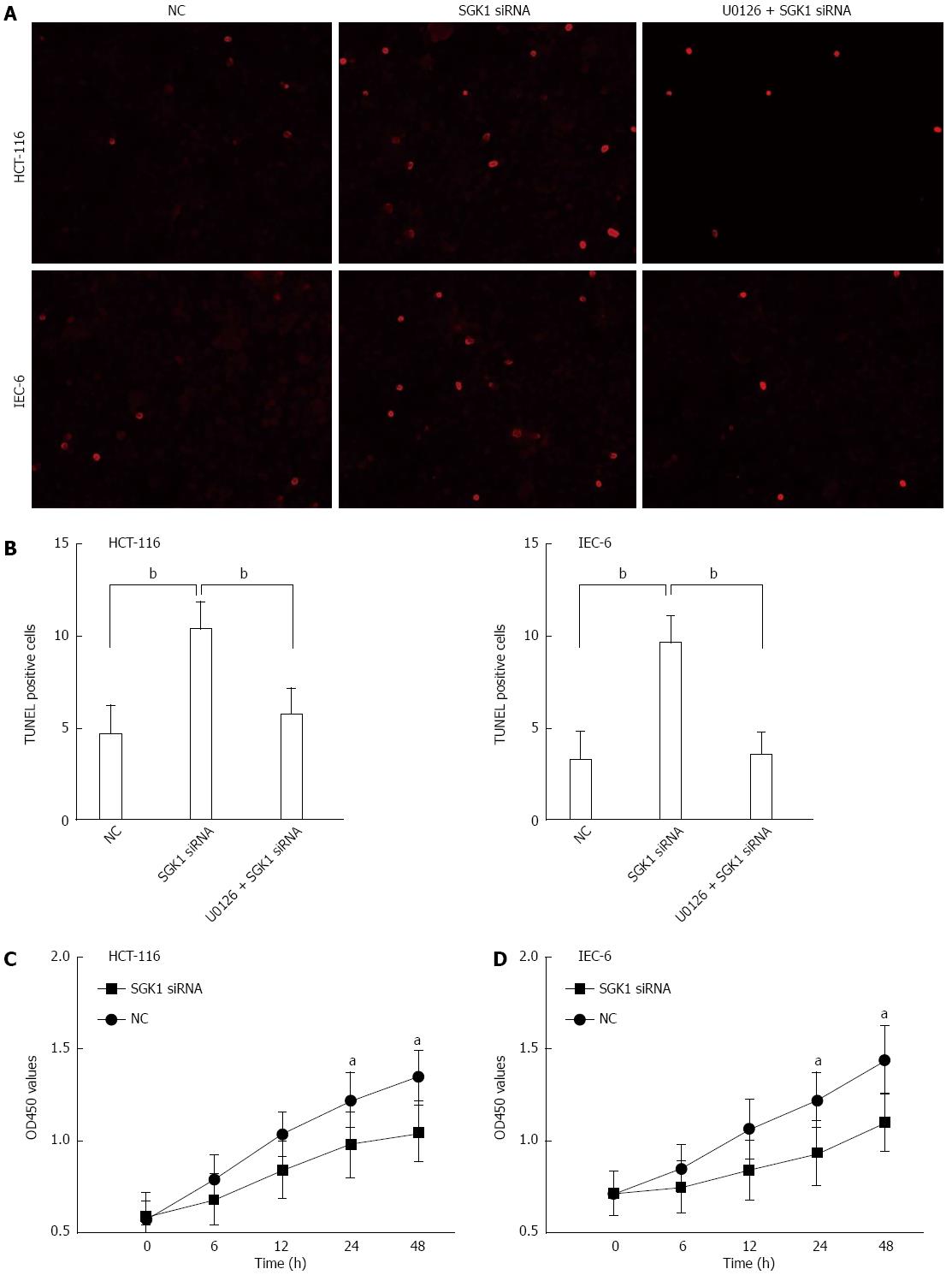
Next, we determined whether knockdown of SGK1 by SGK1 siRNA could affect cellular events in IECs. TUNEL assays were used to determine the effect of SGK1 siRNA on apoptosis by DNA fragmentation in IECs. As shown in Figure 5A and B, SGK1 siRNA treatment caused a significant increase in the number of TUNEL-positive cells (n = 6, P < 0.01 vs the control group). However, pre-treatment with an inhibitor of the MEK1 pathway (U0126, 10 μmol/L) before SGK1 siRNA transfection abolished the increase in TUNEL-positive cells.
Further investigation of cellular proliferation (via theCCK8 assay) was performed following the above protocol. The effect of SGK1 silencing on HCT-116 and IEC-6 cell proliferation is shown in Figure 5C and D, respectively. We found that the SGK1 siRNA-transfected HCT-116 and IEC-6 cells showed significantly lower proliferation potential compared with the negative control (NC) siRNA-transfected cells at 0-48 h post-transfection.
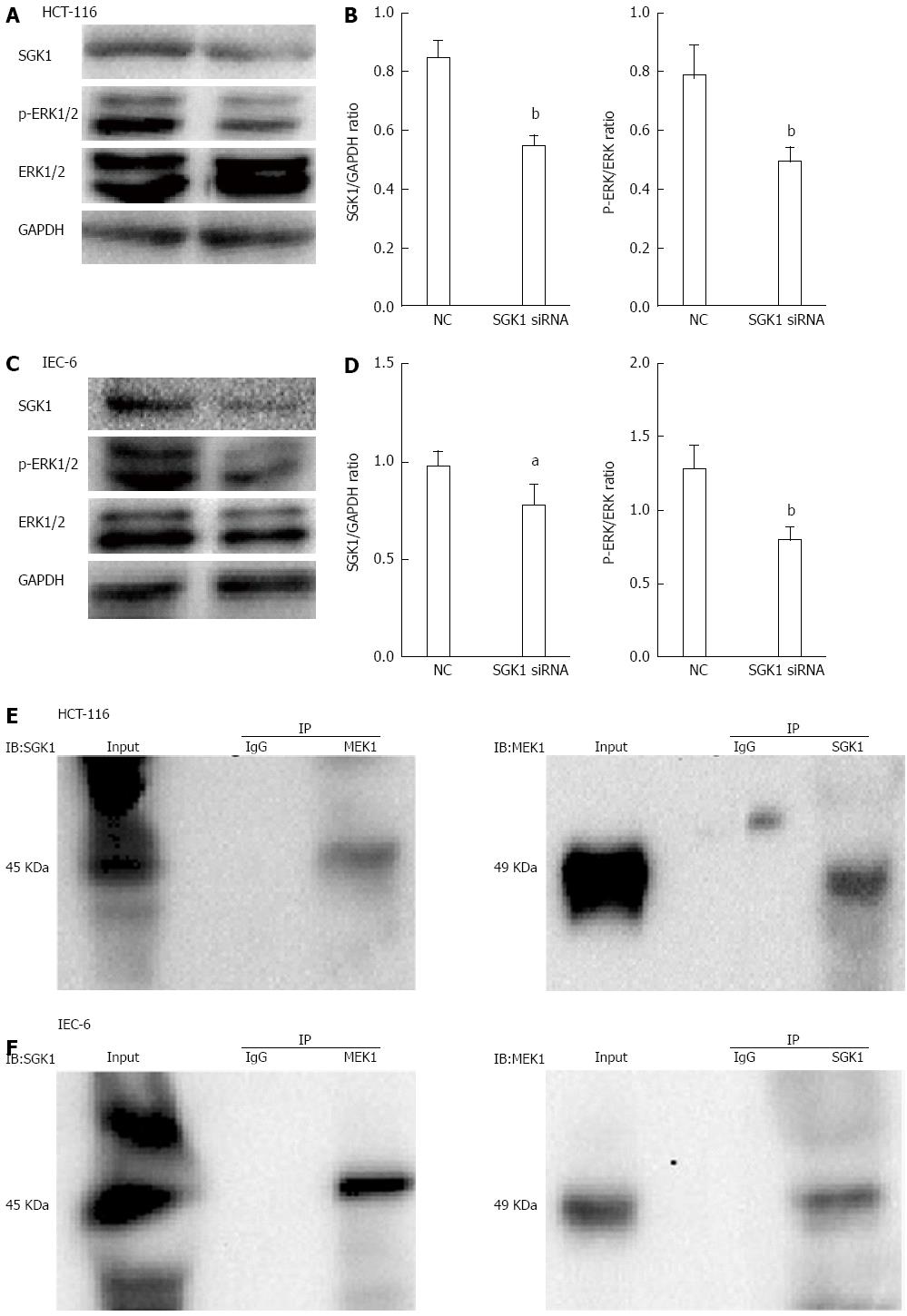
We further investigated the potential mechanisms involved in SGK1 siRNA-induced cellular events. The efficiency of siRNA knockdown on IECs (assessed by SGK1/GAPDH) was detected by western blotting. As shown in Figure 6A and C, SGK1 expression was significantly downregulated compared with cells transfected with NC siRNA after 48 h. In addition, the ERK1/2 phosphorylation level (assessed by p-ERK/ERK1/2) was significantly decreased after SGK1 knockdown. The quantities were measured with ImageJ and are shown in Figure 6B and D. To identify and characterize the possible interacting proteins of SGK1, co-IP was performed to investigate any potential association (direct or indirect) with IECs. SGK1 antibody co-immunoprecipitated with MEK1 but failed to co-immunoprecipitate with purified rabbit IgG (Figure 5E). In addition, MEK1 antibody co-immunoprecipitated with SGK1 but failed to co-immunoprecipitate with purified rabbit IgG (Figure 5F).
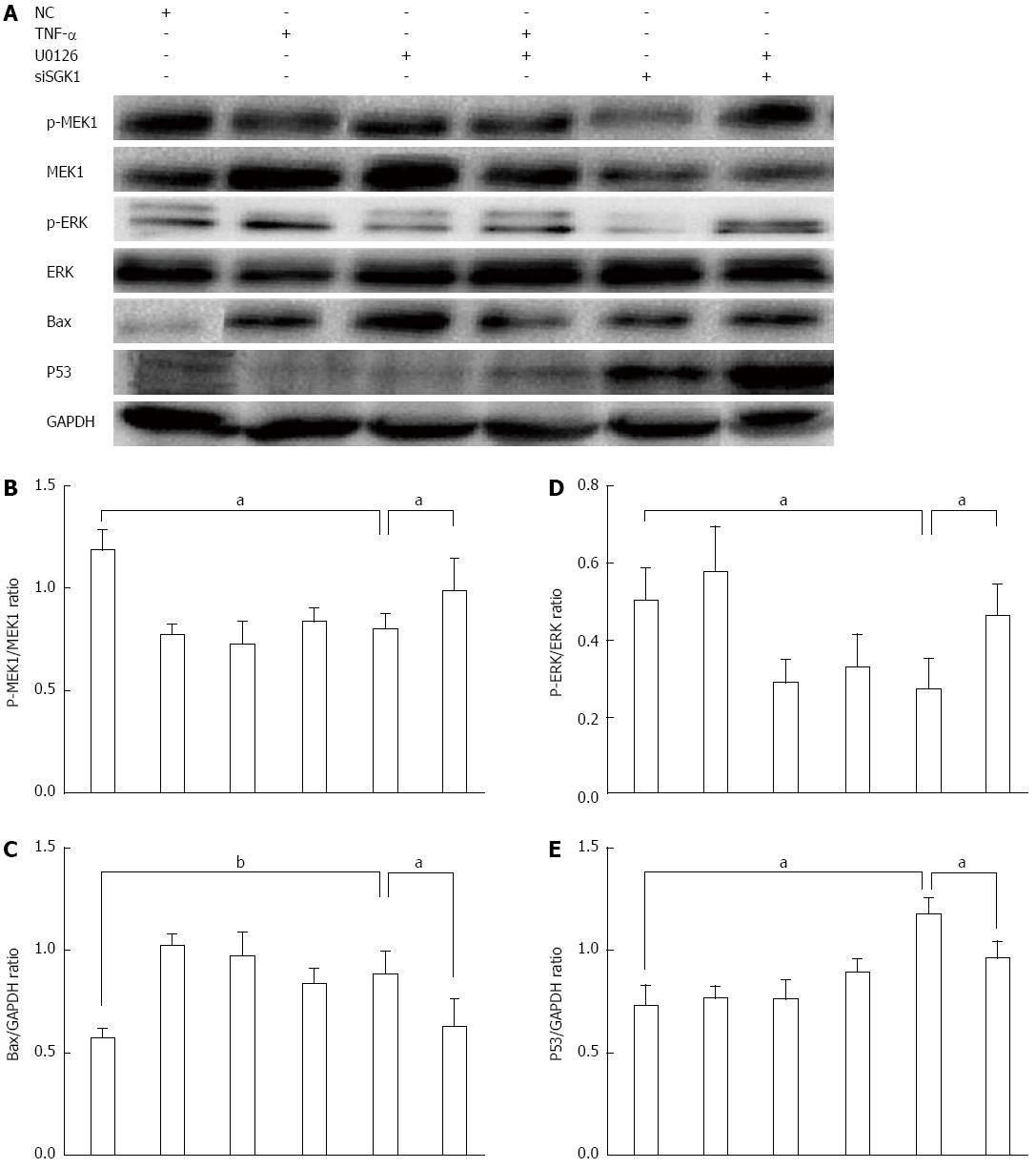
To further explore whether SGK1 silencing-induced apoptosis occurred mainly via the MEK1/ERK pathway, we determined the MEK1 phosphorylation level (assessed by p-MEK1/MEK1) in IEC-6 cells and found a significant decrease after SGK1 silencing (Figure 7A and B). In addition, we found a significant inhibition of ERK1/2 phosphorylation after SGK1 silencing, assessed by p-ERK/ERK (Figure 7D). We also found that SGK1 silencing triggered p53 and Bax upregulation, which indicates activation of apoptosis (Figure 7C and E). Furthermore, we treated IECs with U0126 (10 μmol/L) for 1 h before TNF-α (5 ng/mL) treatment in the absence or presence of SGK1 knockdown. U0126 obviously and significantly inhibited MEK1 and ERK1/2 phosphorylation. In addition, inhibition of the MEK/ERK pathway attenuated the upregulation of p53 and Bax induced by SGK1 silencing (Figure 7A, C and E).
CD is characterized by inflammation of the gastrointestinal tract, resulting from a range of factors, including immunogens that mediate colitis onset. Numerous studies have focused on effective therapeutic targets aiming to attenuate or even cure CD. SGK1 is well known as a serine-threonine kinase involved in intracellular signal transduction pathways and is induced by serum and glucocorticoids. SGK1 is involved in several cellular functions, including activation of ion channels (e.g., epithelial Na+ channel, Ca2+ channel, and K+ channel), and the regulation of several enzymes (e.g., glycogen synthase kinase-3, phosphatidylinositol-3-kinase, and Akt) and transcription factors (e.g., β-catenin and nuclear factor-κB)[10,22,23]. Moreover, excessive SGK1 expression is involved in the pathophysiology of several disorders, including inflammatory disease, fibrosing disease, tumor growth, and neurodegeneration[24]. Our study demonstrated for the first time that SGK1 expression was upregulated in both CD patients and in a TNBS-induced mouse model of colitis.
Previous studies have shown that SGK1 can regulate the interaction of membrane receptors that mediate cellular apoptosis in prostate and colon cancers[25,26]. Three pathways are involved in SGK1-related cellular apoptosis. In the first pathway, SGK1 phosphorylates and inhibits pro-apoptotic transcription factors, such as Forkheadbox O3a (FOXO3a) and GSK3β[27]. In the second pathway, SGK1 facilitates cell survival by phosphorylating mouse double minute 2 (MDM2) by enhanced p53 ubiquitylation and degradation[28]. Intracellular MDM2 is critical in regulating the expression of p53. In the third pathway, SGK1 regulates apoptosis by regulating ion channels and transporters, such as Ca2+and several K+ channels[29,30]. In consideration of the roles of SGK1 in the inflammatory process and immunoreaction, together with CD pathogenesis, we suggest that SGK1 may mediate CD initiation by triggering aberrant cellular events, including proliferation and apoptosis, in parallel with the clinical manifestations of hyperplasia and ulcers. Consistent with previous studies, knockdown of SGK1 in our study induced cellular apoptosis and inhibited IEC proliferation.
Recent reports have shown that SGK1 can downregulate or upregulate ERK2 activity and MEK/ERK complex formation. Additionally, SGK1 has been shown to inhibit ERK1/2 activity in liver HepG2 cells and db/db mice[31,32]. We observed that activation of the ERK1/2 pathway was significantly inhibited after SGK1 silencing. In addition, knockdown of SGK1 induced p53 and Bax upregulation. An increasing number of therapeutic strategies targeting the MEK/ERK pathway have been evaluated in clinical trials. MEK acts as a choke point in the initiation of the MEK/ERK pathway and related oncogenesis[33]. ERK1/2 is a potential kinase that reacts with numerous substrates in both the nucleus and the cytoplasm and plays a pivotal role in cell growth and differentiation. MEK1/2, which exhibits sole substrate specificity for ERK1/2, regulates the MEK/ERK cascade. Dysregulation of the MEK/ERK cascade by cytokines or other stimuli has been shown to be involved in numerous human malignancies, contributing to the initiation of oncogenesis. MEK phosphorylates both serine/threonine and tyrosine residues of ERK1/2, triggering its activation and the subsequent cytosolic and nuclear activity. In addition, this pathway participates in cellular events, such as abnormal proliferation, apoptosis and mobility[34-36]. It has been well known for years that ERK1/2 activation can lead to cellular events through p53 accumulation[37]. Accordingly, wild-type p53 is well known as a critical tumor suppressor and has been called the “guardian of the genome” in mammalian cells. Rubbi and Milner have reported that ultraviolet-induced DNA damage can result in p53 activation and cell cycle arrest[38]. The primary mechanism of DNA damage-related p53 dysregulation is associated with MDM2, which negatively regulates p53 by ubiquitin-mediated proteasomal degradation. To date, numerous p53-inducible gene products have been identified, including pro-apoptotic Bax and p53-upregulated modulator of apoptosis[39]. A diverse range of studies has indicated the role of ERK1/2 in the regulation of anti-apoptotic responses. The MEK/ERK pathway also plays a vital role in the regulation of apoptosis by triggering the expression of Bcl-2 family proteins[16]. The anti-apoptotic effect of ERK is mediated mainly via members of the Bcl-2 family, such as Bcl-2 and Bcl-xL. In addition, activated ERK has been reported to interfere with apoptotic factor caspase-8 cleavage and Bax translocation[40]. Our study indicated, via co-IP analysis, that SGK1 interacts with MEK1, which promotes MEK1 and ERK1/2 phosphorylation. A MEK1 inhibitor (U0126, 10 μmol/L) showed that SGK acts in a MEK/ERK-dependent manner. Furthermore, U0126 significantly inhibited MEK1 and ERK1/2 activation, which attenuated the upregulation of p53 and Bax, and cellular apoptosis induced by SGK1 silencing.
Taken together, our research showed that SGK1 expression plays a critical role in the initiation and development of TNF-α-induced apoptosis of IECs in colitis via modulation of the ERK1/2 cascade. We uncovered its mechanism for the first time by elucidating an interaction between SGK1 and MEK1, which activated the MEK/ERK pathway and mediated cellular apoptosis and proliferation via p53 and Bax dysregulation. Further studies are needed to determine the interactions and the potential clinical value. Our study indicates that SGK1 may protect IECs from apoptosis and provide a previously unrecognized therapeutic approach for the treatment of colitis and even CD.
Crohn’s disease (CD) is a chronic, relapsing and debilitating colitis. Until now, many therapies have been tested in clinical patients with active CD but have shown no marked effects. Serum-and-glucocorticoid-inducible-kinase-1 (SGK1), a potential immunomodulatory factor, is involved in cell signaling pathways and mediates cellular apoptosis, migration, proliferation, and epithelial transport. It is well recognized that impairment of the epithelial barrier is one of the most important factors in the origin of CD. Intestinal epithelial cells (IECs) are the most important component of the epithelial barrier. IEC apoptosis and proliferation regulate the homeostasis of the intestinal mucosa.
Many studies have shown that SGK1 plays a vital role in the pathophysiology of autoimmune disease and inflammatory disease. In liver HepG2 cells and db/db mice, SGK1 inhibits ERK1/2 activity and mediates cell survival. However, the function of SGK1 in CD remains unclear.
In this study, for the first time, the authors found that SGK1 expression was upregulated in both patients with active CD and in a TNBS-induced mouse model. Silencing of SGK1 inhibited the phosphorylation of mitogen-activated protein kinase kinase 1 (MEK1) and a downstream molecule, extracellular signal regulated protein kinase 1/2 (ERK1/2), which triggered the upregulation of p53 and Bcl-2-associated X protein (Bax), accompanied by cellular apoptosis and IEC proliferation.
This research demonstrated a function for SGK1 in the protection of IECs from apoptosis via activation of the MEK/ERK pathway and may provide a previously unappreciated approach to CD therapy.
SGK1 is a ubiquitously expressed gene that encodes a serine/threonine protein kinase and is involved in cell signaling pathways related to cell apoptosis, migration, proliferation, epithelial transport and inflammation-immune functions.
The authors discovered for the first time that SGK1 expression was increased in the tissues of patients with active CD. They established a mouse model of TBNS-induced CD and investigated its relationship with SGK1. SGK1 expression was up-regulated in the TNBS-induced mouse model. Silencing of SGK1 inhibited the phosphorylation of MEK1 and the downstream molecule ERK1/2, which triggered p53 and Bax up-regulation, which, in turn, mediated cellular apoptosis and IEC-6 and HCT-116 cell proliferation. Taken together, this manuscript is highly relevant and interesting.
| 1. | Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med. 2009;361:2066-2078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1967] [Cited by in RCA: 2254] [Article Influence: 132.6] [Reference Citation Analysis (10)] |
| 2. | Franke A, McGovern DP, Barrett JC, Wang K, Radford-Smith GL, Ahmad T, Lees CW, Balschun T, Lee J, Roberts R. Genome-wide meta-analysis increases to 71 the number of confirmed Crohn’s disease susceptibility loci. Nat Genet. 2010;42:1118-1125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2110] [Cited by in RCA: 2033] [Article Influence: 127.1] [Reference Citation Analysis (0)] |
| 3. | Corridoni D, Arseneau KO, Cominelli F. Inflammatory bowel disease. Immunol Lett. 2014;161:231-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 93] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 4. | Pastorelli L, De Salvo C, Mercado JR, Vecchi M, Pizarro TT. Central role of the gut epithelial barrier in the pathogenesis of chronic intestinal inflammation: lessons learned from animal models and human genetics. Front Immunol. 2013;4:280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 283] [Cited by in RCA: 351] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 5. | Parlato M, Yeretssian G. NOD-like receptors in intestinal homeostasis and epithelial tissue repair. Int J Mol Sci. 2014;15:9594-9627. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 6. | Firestone GL, Giampaolo JR, O’Keeffe BA. Stimulus-dependent regulation of serum and glucocorticoid inducible protein kinase (SGK) transcription, subcellular localization and enzymatic activity. Cell Physiol Biochem. 2003;13:1-12. [PubMed] |
| 7. | Brunet A, Park J, Tran H, Hu LS, Hemmings BA, Greenberg ME. Protein kinase SGK mediates survival signals by phosphorylating the forkhead transcription factor FKHRL1 (FOXO3a). Mol Cell Biol. 2001;21:952-965. [PubMed] |
| 8. | Lang F, Böhmer C, Palmada M, Seebohm G, Strutz-Seebohm N, Vallon V. (Patho)physiological significance of the serum- and glucocorticoid-inducible kinase isoforms. Physiol Rev. 2006;86:1151-1178. [PubMed] |
| 9. | Pasham V, Rotte A, Gu S, Yang W, Bhandaru M, Rexhepaj R, Pathare G, Lang F. Upregulation of intestinal NHE3 following saline ingestion. Kidney Blood Press Res. 2013;37:48-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Lang F, Artunc F, Vallon V. The physiological impact of the serum and glucocorticoid-inducible kinase SGK1. Curr Opin Nephrol Hypertens. 2009;18:439-448. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 117] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 11. | Chung E, Kondo M. Role of Ras/Raf/MEK/ERK signaling in physiological hematopoiesis and leukemia development. Immunol Res. 2011;49:248-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 77] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 12. | Runchel C, Matsuzawa A, Ichijo H. Mitogen-activated protein kinases in mammalian oxidative stress responses. Antioxid Redox Signal. 2011;15:205-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 144] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 13. | Ruemmele FM, Dionne S, Levy E, Seidman EG. TNFalpha-induced IEC-6 cell apoptosis requires activation of ICE caspases whereas complete inhibition of the caspase cascade leads to necrotic cell death. Biochem Biophys Res Commun. 1999;260:159-166. [PubMed] |
| 14. | Shaul YD, Seger R. The MEK/ERK cascade: from signaling specificity to diverse functions. Biochim Biophys Acta. 2007;1773:1213-1226. [PubMed] |
| 15. | Tanaka T, Huang X, Halicka HD, Zhao H, Traganos F, Albino AP, Dai W, Darzynkiewicz Z. Cytometry of ATM activation and histone H2AX phosphorylation to estimate extent of DNA damage induced by exogenous agents. Cytometry A. 2007;71:648-661. [PubMed] |
| 16. | Balmanno K, Cook SJ. Tumour cell survival signalling by the ERK1/2 pathway. Cell Death Differ. 2009;16:368-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 400] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 17. | Krifka S, Petzel C, Bolay C, Hiller KA, Spagnuolo G, Schmalz G, Schweikl H. Activation of stress-regulated transcription factors by triethylene glycol dimethacrylate monomer. Biomaterials. 2011;32:1787-1795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | Ming L, Wang P, Bank A, Yu J, Zhang L. PUMA Dissociates Bax and Bcl-X(L) to induce apoptosis in colon cancer cells. J Biol Chem. 2006;281:16034-16042. [PubMed] |
| 19. | Hollenbach E, Vieth M, Roessner A, Neumann M, Malfertheiner P, Naumann M. Inhibition of RICK/nuclear factor-kappaB and p38 signaling attenuates the inflammatory response in a murine model of Crohn disease. J Biol Chem. 2005;280:14981-14988. [PubMed] |
| 20. | Naito Y, Katada K, Takagi T, Tsuboi H, Isozaki Y, Handa O, Kokura S, Yoshida N, Ichikawa H, Yoshikawa T. Rosuvastatin, a new HMG-CoA reductase inhibitor, reduces the colonic inflammatory response in dextran sulfate sodium-induced colitis in mice. Int J Mol Med. 2006;17:997-1004. [PubMed] |
| 21. | Scheiffele F, Fuss IJ. Induction of TNBS colitis in mice. Curr Protoc Immunol. 2002;Chapter 15:Unit 15.19. [PubMed] |
| 22. | Park J, Leong ML, Buse P, Maiyar AC, Firestone GL, Hemmings BA. Serum and glucocorticoid-inducible kinase (SGK) is a target of the PI 3-kinase-stimulated signaling pathway. EMBO J. 1999;18:3024-3033. [PubMed] |
| 23. | Miyata S, Koyama Y, Takemoto K, Yoshikawa K, Ishikawa T, Taniguchi M, Inoue K, Aoki M, Hori O, Katayama T. Plasma corticosterone activates SGK1 and induces morphological changes in oligodendrocytes in corpus callosum. PLoS One. 2011;6:e19859. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 99] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 24. | Pessôa BS, Peixoto EB, Papadimitriou A, Lopes de Faria JM, Lopes de Faria JB. Spironolactone improves nephropathy by enhancing glucose-6-phosphate dehydrogenase activity and reducing oxidative stress in diabetic hypertensive rat. J Renin Angiotensin Aldosterone Syst. 2012;13:56-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 25. | Gu S, Papadopoulou N, Gehring EM, Nasir O, Dimas K, Bhavsar SK, Föller M, Alevizopoulos K, Lang F, Stournaras C. Functional membrane androgen receptors in colon tumors trigger pro-apoptotic responses in vitro and reduce drastically tumor incidence in vivo. Mol Cancer. 2009;8:114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 63] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 26. | Papadopoulou N, Charalampopoulos I, Anagnostopoulou V, Konstantinidis G, Föller M, Gravanis A, Alevizopoulos K, Lang F, Stournaras C. Membrane androgen receptor activation triggers down-regulation of PI-3K/Akt/NF-kappaB activity and induces apoptotic responses via Bad, FasL and caspase-3 in DU145 prostate cancer cells. Mol Cancer. 2008;7:88. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 27. | Dehner M, Hadjihannas M, Weiske J, Huber O, Behrens J. Wnt signaling inhibits Forkhead box O3a-induced transcription and apoptosis through up-regulation of serum- and glucocorticoid-inducible kinase 1. J Biol Chem. 2008;283:19201-19210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 98] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 28. | Amato R, D’Antona L, Porciatti G, Agosti V, Menniti M, Rinaldo C, Costa N, Bellacchio E, Mattarocci S, Fuiano G. Sgk1 activates MDM2-dependent p53 degradation and affects cell proliferation, survival, and differentiation. J Mol Med (Berl). 2009;87:1221-1239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 80] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 29. | Eylenstein A, Schmidt S, Gu S, Yang W, Schmid E, Schmidt EM, Alesutan I, Szteyn K, Regel I, Shumilina E. Transcription factor NF-κB regulates expression of pore-forming Ca2+ channel unit, Orai1, and its activator, STIM1, to control Ca2+ entry and affect cellular functions. J Biol Chem. 2012;287:2719-2730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 65] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 30. | Eylenstein A, Gehring EM, Heise N, Shumilina E, Schmidt S, Szteyn K, Münzer P, Nurbaeva MK, Eichenmüller M, Tyan L. Stimulation of Ca2+-channel Orai1/STIM1 by serum- and glucocorticoid-inducible kinase 1 (SGK1). FASEB J. 2011;25:2012-2021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 73] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 31. | Won M, Park KA, Byun HS, Kim YR, Choi BL, Hong JH, Park J, Seok JH, Lee YH, Cho CH. Protein kinase SGK1 enhances MEK/ERK complex formation through the phosphorylation of ERK2: implication for the positive regulatory role of SGK1 on the ERK function during liver regeneration. J Hepatol. 2009;51:67-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 32. | Liu H, Yu J, Xia T, Xiao Y, Zhang Q, Liu B, Guo Y, Deng J, Deng Y, Chen S. Hepatic serum- and glucocorticoid-regulated protein kinase 1 (SGK1) regulates insulin sensitivity in mice via extracellular-signal-regulated kinase 1/2 (ERK1/2). Biochem J. 2014;464:281-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 33. | Friday BB, Adjei AA. Advances in targeting the Ras/Raf/MEK/Erk mitogen-activated protein kinase cascade with MEK inhibitors for cancer therapy. Clin Cancer Res. 2008;14:342-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 473] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 34. | Akinleye A, Furqan M, Mukhi N, Ravella P, Liu D. MEK and the inhibitors: from bench to bedside. J Hematol Oncol. 2013;6:27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 202] [Cited by in RCA: 234] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 35. | Kohno M, Tanimura S, Ozaki K. Targeting the extracellular signal-regulated kinase pathway in cancer therapy. Biol Pharm Bull. 2011;34:1781-1784. [PubMed] |
| 36. | McCubrey JA, Steelman LS, Chappell WH, Abrams SL, Franklin RA, Montalto G, Cervello M, Libra M, Candido S, Malaponte G. Ras/Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR cascade inhibitors: how mutations can result in therapy resistance and how to overcome resistance. Oncotarget. 2012;3:1068-1111. [PubMed] |
| 37. | Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell. 1997;88:593-602. [PubMed] |
| 38. | Rubbi CP, Milner J. Disruption of the nucleolus mediates stabilization of p53 in response to DNA damage and other stresses. EMBO J. 2003;22:6068-6077. [PubMed] |
| 39. | Oda E, Ohki R, Murasawa H, Nemoto J, Shibue T, Yamashita T, Tokino T, Taniguchi T, Tanaka N. Noxa, a BH3-only member of the Bcl-2 family and candidate mediator of p53-induced apoptosis. Science. 2000;288:1053-1058. [PubMed] |
| 40. | Mori M, Uchida M, Watanabe T, Kirito K, Hatake K, Ozawa K, Komatsu N. Activation of extracellular signal-regulated kinases ERK1 and ERK2 induces Bcl-xL up-regulation via inhibition of caspase activities in erythropoietin signaling. J Cell Physiol. 2003;195:290-297. [PubMed] |
Date sharing: No additional data are available.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Koch TR, Tan GH S- Editor: Qi Y L- Editor: Cant MR E- Editor: Zhang DN