Published online May 14, 2015. doi: 10.3748/wjg.v21.i18.5735
Peer-review started: November 18, 2014
First decision: December 26, 2014
Revised: January 25, 2015
Accepted: February 11, 2015
Article in press: February 11, 2015
Published online: May 14, 2015
Processing time: 182 Days and 8.1 Hours
A 41-year-old man with a continuous-flow left ventricular assist device presented for evaluation of dysphagia and dark urine. He was found to have a significantly elevated L-lactate dehydrogenase and an elevated plasma free hemoglobin consistent with intravascular hemolysis. After the hemolysis ceased, both the black urine and dysphagia resolved spontaneously. Transient esophageal dysfunction, as a manifestation of gastrointestinal dysmotility, is known to occur in the setting of hemolysis. Paroxysmal nocturnal hemoglobinuria is another recognized cause of massive hemolysis with gastrointestinal dysmotility occurring in 25%-35% of patients during a paroxysm. Intravascular hemolysis increases plasma free hemoglobin, which scavenges nitric oxide (NO), an important second messenger for smooth muscle cell relaxation. The decrease in NO can lead to esophageal spasm and resultant dysphagia. In our patient the resolution of hemolysis resulted in resolution of dysphagia.
Core tip: Transient esophageal dysfunction, as a manifestation of gastrointestinal dysmotility, is known to occur in the setting of hemolysis. We present a case of dysphagia secondary to hemolysis caused by a continuous-flow left ventricular assist device. Our case report aims to bring this etiology of dysphagia to the attention of gastroenterologists and cardiologists to limit invasive investigations in these patients and highlight the possibility that hemolysis may serve as an early indicator of pump thrombosis and adverse outcomes.
- Citation: Wuschek A, Iqbal S, Estep J, Quigley E, Richards D. Left ventricular assist device hemolysis leading to dysphagia. World J Gastroenterol 2015; 21(18): 5735-5738
- URL: https://www.wjgnet.com/1007-9327/full/v21/i18/5735.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i18.5735
Hemolysis is a known complication in patients with left ventricular assist devices (LVADs)[1]. Hemoglobin-mediated nitric oxide scavenging, a well described circumstance in paroxysmal nocturnal hemoglobinuria[2], results in several clinical sequelae. Nitric oxide is an important second messenger which among other actions, mediates esophageal smooth muscle relaxation during the act of swallowing[3]. Attributed to a the abundance of free hemoglobin and subsequent lack of nitric oxide, esophageal spasm, and associated dysphagia, are commonly seen in patients with paroxysmal nocturnal hemoglobinuria and occur in 25%-35% of patients during paroxysms. Similar symptoms and motility patterns have been elicited in healthy volunteers who received solutions of plasma free hemoglobin[4,5]. The overall clinical scenario and the elimination of other causes of dysphagia in our patient supports the hypothesis that the same phenomenon was at work in our patient with LVAD hemolysis and dysphagia. This has only been reported once before in the literature and is an under-recognized cause of dysphagia in a growing LVAD population[6].
A 41-year-old obese African-American man with end stage heart failure treated with a continuous-flow LVAD was transferred to our institution with a three-day history of dark urine and dysphagia. He had never had similar complaints before and could clearly differentiate the new symptom of dysphagia from any of the symptoms related to long-standing gastro-esophageal reflux disease. He had a several year history of mild typical epigastric burning discomfort attributed to acid reflux that was relieved with use of pantoprazole 40 mg po daily, which he was taking on admission. He had not had an upper endoscopy prior to admission. Additional medications present on admission included warfarin, hydralazine, furosemide, magnesium oxide, fluticasone nasal spray, tiotropium, levetiracetam, alprazolam, carvedilol, isosorbide dinitrate, digoxin, gabapentin, aspirin, and clonidine. His primary difficulty related to swallowing solids. He had attempted drinking liquids to help get the food down but this had been unsuccessful and resulted in epigastric pain. Whenever he tried to drink liquids on their own, they were immediately regurgitated.
The patient’s past medical history is significant for non-ischemic cardiomyopathy, felt to be secondary to remote history of cocaine abuse, which resulted in severe systolic heart failure and a left ventricular ejection fraction of 20%. A HeartMate II (Thoratec Corp, Pleasanton, CA) LVAD had been implanted 18 mo prior to admission as a bridge to heart transplantation. Physical examination was notable for marked obesity with a BMI of 34. Neither jugular venous distension nor a hepatojugular reflux could be appreciated. On examination of the abdomen neither a fluid wave nor shifting dullness were detected. There was no lower extremity edema. On auscultation, a constant LVAD hum, superimposed on a faint S1 and S2, could be heard. No other murmurs or carotid bruits were auscultated.
On admission, free plasma hemoglobin was 125.1 mg/dL [reference interval (RI): 0.5-5 mg/dL] and Haptoglobin was less than 10 mg/dL (RI: 30-200 mg/dL). L-lactate dehydrogenase (LDH) was elevated to 1607 IU/L (RI: 87-225 U/L). In our patient population an LDH greater than 1050 IU/L is associated with a sensitivity and specificity of 80% and 86%, respectively, for the detection of device thrombosis and malfunction and is similar to the LDH cutoff (1103) reported by Estep et al[7] and Uriel et al[8] as an indicator for device thrombosis. Platelet count was 126 (RI: 150 × 109/L-400 × 109/L), his INR calculated to 1.3, and plasma fibrinogen was 486 (150-400 mg/dL). Blood creatinine was found to be elevated to 4.4 mg/dL, which was elevated from a baseline of 1.5 mg/dL. The patient had black tea colored urine with a positive urine test strip for hemoglobin but red blood cells were absent on microscopy.
Controller device interrogation of the patient’s LVAD had shown a speed of 9200 revolutions per minute, an estimated flow of 4.5 L/min, pulsatility index of 5.5 and a power of 6.4 watts. Renal ultrasound and chest X-ray did not reveal any significant findings. A transthoracic echocardiogram found no evidence of intracardiac inflow cannula, or outflow cannula thrombus. Continuous wave Doppler peak inflow velocity was not increased. Other cardiac dimensions were unchanged compared to a prior study.
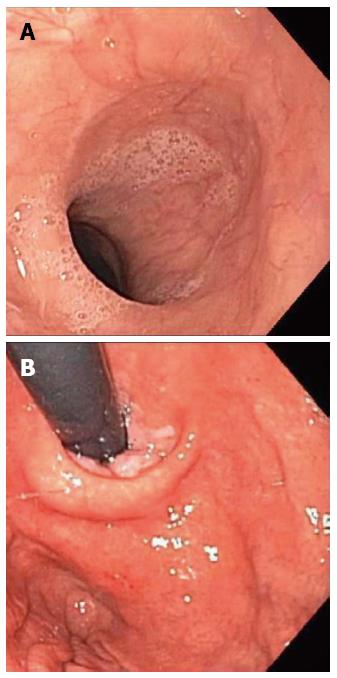
In order to rule out immediately treatable causes like a food bolus impaction, the patient underwent esophagogastroduodenoscopy, which did not show any macroscopic abnormalities (Figure 1) and pathological examination of esophageal biopsy specimens revealed normal esophageal epithelium.
The patient was initially started on a heparin infusion, initially because his INR was subtherapeutic despite warfarin therapy. Thrombolytic therapy with tissue plasminogen activator was not initiated, per our institutional protocol because of concern for the development of intracranial hemorrhage. Over the course of two days, the patient’s dysphagia resolved with simultaneous return of his urine color to normal. Plasma hemoglobin, haptoglobin and LDH trended toward normal values. Creatinine kept on rising over the following week and he was diagnosed with heme pigment nephropathy. This acute kidney injury slowly resolved over the course of the subsequent weeks. He has not experienced any further dysphagia.
To our knowledge, one case has been previously described of dysphagia in a patient with LVAD pump thrombosis[6]. In comparison, our case is unique in terms of its presentation and the detail of its evaluation with the latter strongly supporting our contention that intravascular hemolysis was the reason for dysphagia. Furthermore, endoscopy excluded food impaction, neoplasm, stricture, eosinophilic esophagitis, and candida esophagitis as possible causes of this individual’s difficulty with swallowing. Moreover, this is the first publication to describe the occurrence of dysphagia in the setting of LVAD associated hemolysis in the absence of definitive evidence of pump thrombus. Thus, the patient’s echocardiogram did not show any abnormalities in the inflow or outflow cannulas, inflow peak velocities were within normal limits, and the expected paradoxical increase in LVAD power was absent[9,10]. Moreover, with pump speed ramp up echocardiography testing, the patients LVAD was noted to be functioning appropriately.
While, in the absence of serial esophageal imaging and manometric studies, it is difficult to conclusively prove our hypothesis that dysphagia, in this instance, resulted from NO scavenging by free heme, a convergence of several highly suggestive pieces of circumstantial evidence renders this extremely unlikely. Most convincingly, both the dysphagia and the discoloration of the patient’s urine (proven to be due to hemoglobinuria) developed and resolved simultaneously.
The proposal that gastrointestinal dysmotility could be induced by large concentrations of circulation free heme was previously proposed based on observations among subjects with paroxysmal nocturnal hemoglobinuria (PNH). In PNH paroxysms of intravascular hemolysis lead to the massive release of free heme; similar values of plasma free hemoglobin were recorded in the patient reported here. Smooth muscle dysfunction, leading to dysmotility, characterized as esophageal spasm is a common clinical manifestation in PNH, occurring in 25%-35% of patients during a paroxysm[5,11]. Hemoglobinuria is also frequently observed[2]. More direct evidence of the impact of intravascular hemolysis was provided in a trial where recombinant human hemoglobin was administered intravenously to healthy volunteers. Spontaneous, simultaneous high-pressure contractions were induced in 8 of 9 subjects, lower esophageal sphincter relaxation was inhibited, and several subjects developed lower sub-sternal discomfort during swallowing[5].
Our presentation of this case aims to accomplish several goals. Firstly, for the gastroenterologist, it draws attention to intravascular hemolysis as a potential cause of dysphagia among patients with LVADs, thereby obviating the need of invasive investigations, such as endoscopy. Secondly, for the cardiologist, it emphasizes that hemolysis, in patients with LVADs, may serve as an early indicator of pump thrombosis and adverse outcomes[12,13]. Larger studies may be needed to fully establish the cause and effect relationship between pump failure, hemolysis, and dysphagia but could prove valuable should they establish that dysphagia is an early marker of LVAD dysfunction.
The patient presented with dysphagia to solids and liquids secondary to hemolysis caused by a continuous-flow left ventricular assist device (LVAD).
The patient’s physical exam was notable for auscultation of a continuous hum from his LVAD and esophagogastroduodenoscopy was grossly normal.
The differential diagnosis included esophageal obstruction from benign causes like food impaction and malignant causes, eosinophilic esophagitis, and esophageal motility disorders.
Elevated plasma free hemoglobin 125.1 mg/dL, haptoglobin less than 10 mg/dL LDH elevated to 1607 IU/L, platelet count 126, INR 1.3, creatinine elevated to 4.4 mg/dL from a baseline of 1.5 mg/dL, black tea colored urine with a positive urine test strip for hemoglobin but absent red blood cells on microscopy.
Renal ultrasound and chest X-ray did not reveal any significant findings, transthoracic echocardiogram found no evidence of intracardiac inflow cannula, or outflow cannula thrombus and continuous wave Doppler peak inflow velocity was not increased.
Histopathologic examination of endoscopic biopsies showed normal esophageal epithelium.
The patient was placed on a heparin infusion and thrombolytic therapy with tissue plasminogen activator was not initiated per our institutional protocol because of concern for the development of intracranial hemorrhage.
One case had been reported before or LVAD associated related hemolysis and associated dysphagia that resolved with treatment with tissue plasminogen activator and cessation of hemolysis. A similar mechanism of gastrointestinal dysmotility has been described in patients with paroxysmal nocturnal hemoglobinuria and hemolysis.
Left ventricular assist devices are implantable pumps designed to unload and assist the left ventricle and are approved for both bridge to transplantation and destination therapy.
Intravascular hemolysis is a potential cause of dysphagia among patients with LVADs and recognition of this pathophysiologic mechanism may obviate the need for invasive investigations like endoscopy. Recognition of hemolysis in patients with LVADs may serve as an early indicator of pump thrombosis and adverse outcomes.
The authors have described a case of dysphagia secondary to LVAD induced hemolysis. The article highlights gastrointestinal dysmotility secondary to hemolysis-induced release of free plasma hemoglobin and the subsequent scavenging and reduction of nitric oxide.
| 1. | Pagani FD, Miller LW, Russell SD, Aaronson KD, John R, Boyle AJ, Conte JV, Bogaev RC, MacGillivray TE, Naka Y. Extended mechanical circulatory support with a continuous-flow rotary left ventricular assist device. J Am Coll Cardiol. 2009;54:312-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 716] [Cited by in RCA: 717] [Article Influence: 42.2] [Reference Citation Analysis (0)] |
| 2. | Rother RP, Bell L, Hillmen P, Gladwin MT. The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin: a novel mechanism of human disease. JAMA. 2005;293:1653-1662. [PubMed] |
| 3. | Yamato S, Saha JK, Goyal RK. Role of nitric oxide in lower esophageal sphincter relaxation to swallowing. Life Sci. 1992;50:1263-1272. [PubMed] |
| 4. | Brodsky RA. Narrative review: paroxysmal nocturnal hemoglobinuria: the physiology of complement-related hemolytic anemia. Ann Intern Med. 2008;148:587-595. [PubMed] |
| 5. | Murray JA, Ledlow A, Launspach J, Evans D, Loveday M, Conklin JL. The effects of recombinant human hemoglobin on esophageal motor functions in humans. Gastroenterology. 1995;109:1241-1248. [PubMed] |
| 6. | Mentz RJ, Schlendorf K, Hernandez AF, Milano CA, Felker GM, Blue LJ, Schroder JN, Rogers JG, Patel CB. Dysphagia in the setting of left ventricular assist device hemolysis. ASAIO J. 2013;59:322-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Estep JD, Vivo RP, Cordero-Reyes AM, Bhimaraj A, Trachtenberg BH, Torre-Amione G, Chang SM, Elias B, Bruckner BA, Suarez EE. A simplified echocardiographic technique for detecting continuous-flow left ventricular assist device malfunction due to pump thrombosis. J Heart Lung Transplant. 2014;33:575-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 8. | Uriel N, Morrison KA, Garan AR, Kato TS, Yuzefpolskaya M, Latif F, Restaino SW, Mancini DM, Flannery M, Takayama H. Development of a novel echocardiography ramp test for speed optimization and diagnosis of device thrombosis in continuous-flow left ventricular assist devices: the Columbia ramp study. J Am Coll Cardiol. 2012;60:1764-1775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 270] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 9. | Estep JD, Stainback RF, Little SH, Torre G, Zoghbi WA. The role of echocardiography and other imaging modalities in patients with left ventricular assist devices. JACC Cardiovasc Imaging. 2010;3:1049-1064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 121] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 10. | Slaughter MS, Pagani FD, Rogers JG, Miller LW, Sun B, Russell SD, Starling RC, Chen L, Boyle AJ, Chillcott S. Clinical management of continuous-flow left ventricular assist devices in advanced heart failure. J Heart Lung Transplant. 2010;29:S1-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 686] [Cited by in RCA: 640] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 11. | Hill A, Rother RP, Hillmen P. Improvement in the symptoms of smooth muscle dystonia during eculizumab therapy in paroxysmal nocturnal hemoglobinuria. Haematologica. 2005;90:ECR40. [PubMed] |
| 12. | Bartoli CR, Ghotra AS, Pachika AR, Birks EJ, McCants KC. Hematologic markers better predict left ventricular assist device thrombosis than echocardiographic or pump parameters. Thorac Cardiovasc Surg. 2014;62:414-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Cowger JA, Romano MA, Shah P, Shah N, Mehta V, Haft JW, Aaronson KD, Pagani FD. Hemolysis: a harbinger of adverse outcome after left ventricular assist device implant. J Heart Lung Transplant. 2014;33:35-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 123] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Kakushima N S- Editor: Ma YJ L- Editor: A E- Editor: Ma S