Published online Apr 7, 2015. doi: 10.3748/wjg.v21.i13.3813
Peer-review started: October 15, 2014
First decision: November 14, 2014
Revised: December 11, 2014
Accepted: February 12, 2015
Article in press: February 13, 2015
Published online: April 7, 2015
Processing time: 174 Days and 14.7 Hours
The liver is the largest internal organ of the body, which may suffer acute or chronic injury induced by many factors, leading to cirrhosis and hepatocarcinoma. Cirrhosis is the irreversible end result of fibrous scarring and hepatocellular regeneration, characterized by diffuse disorganization of the normal hepatic structure, regenerative nodules and fibrotic tissue. Cirrhosis is associated with a high co-morbidity and mortality without effective treatment, and much research has been aimed at developing new therapeutic strategies to guarantee recovery. Liver-based gene therapy has been used to downregulate specific genes, to block the expression of deleterious genes, to delivery therapeutic genes, to prevent allograft rejection and to augment liver regeneration. Viral and non-viral vectors have been used, with viral vectors proving to be more efficient. This review provides an overview of the main strategies used in liver-gene therapy represented by non-viral vectors, viral vectors, novel administration methods like hydrodynamic injection, hybrids of two viral vectors and blocking molecules, with the hope of translating findings from the laboratory to the patient´s bed-side.
Core tip: Cirrhosis is the irreversible end result of fibrous scarring and hepatocellular regeneration. Cirrhosis is a disease without effective treatment and new therapeutic strategies to accomplish healing are continuously being sought. Liver-based gene therapy has been used to improve liver function using viral and non-viral vectors. This review provides an overview of the main strategies used in liver-gene therapy, with the hope of finding a niche application in a given clinical scenario.
- Citation: Salazar-Montes AM, Hernández-Ortega LD, Lucano-Landeros MS, Armendariz-Borunda J. New gene therapy strategies for hepatic fibrosis. World J Gastroenterol 2015; 21(13): 3813-3825
- URL: https://www.wjgnet.com/1007-9327/full/v21/i13/3813.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i13.3813
The liver is the largest internal organ in the body. The main function of the liver is to take up nutrients, to store them and to provide nutrients to other organs. Cirrhosis is associated with high morbidity and mortality, and is induced by many factors, including chronic hepatitis, virus infections, alcohol and drug abuse[1].
During acute injury, the changes in liver architecture are transient and reversible. With chronic injury, there is progressive substitution of the liver parenchyma by scar tissue[2]. Despite ongoing injury, the liver has a remarkable regenerative capacity, and, as a result, patients often progress slowly to cirrhosis over decades. Substantial improvements in the treatment of chronic liver disease have accelerated interest in uncovering the mechanisms underlying hepatic fibrosis and its resolution[3].
In this setting, the present review deals with targeted gene delivery using viral and non-viral “shuttle” vectors, as a relatively novel technology that has the potential to treat both genetic and acquired disorders. The mammalian liver is an organ that can be targeted for gene transfer applications because its blood-supply can be accessed reliably using current technology. In addition, hepatocytes are long-lived cells that can sustain gene expression from episomal vectors[4]. The potential application of gene therapy protocols to human cirrhosis will depend on the successful and tissue-specific delivery of therapeutic genes to livers affected by extensive fibrosis.
In this context, experimental protocols of gene therapy directed to treat extensive
liver fibrosis have been designed to deliver specific genes to fibrotic organs. These protocols are mainly based in the use of non-viral and viral shuttle vectors. Here we describe the most important protocols published to date.
These delivery methods do not involve the use of viruses. Among these methods are plasmids, liposomes, conjugation with inert polymers of high molecular weight, such as diethylaminoethyl dextran, polyethylene glycol for the folding of DNA.
Plasmids are attractive vectors for direct injection into organs and tissues. Despite the relatively low expression achieved after a single plasmid administration, this expression is enough to reach physiological and therapeutics levels of the desired protein. Additionally, improvements in techniques and plasmid formulations have been performed to increase the transfection rate [5].
Augmentation of liver regeneration (ALR) is a novel cytokine, which stimulates hepatic cell proliferation and is able to block acute liver failure by inhibition of hepatic natural killer cell activity in acute liver injury[6]. ALR is an important regulator of liver regeneration, with trophic effects on the regenerating liver and potent anti-hepatitis effects. In this context, Li et al[7] investigated the effect of an ALR recombinant plasmid on rat hepatic fibrosis. Histological examination revealed less hepatic fibrosis in the ALR group with respect to the control group. There were also reductions in serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), and expression of Col-I, Col III and tissue inhibitor of metalloproteinase-1 (TIMP-1), suggesting that ALR recombinant plasmid enhanced hepatic regeneration of injured liver cells.
Transforming growth factor-beta 1 (TGF-β1) is the most prominent cytokine implicated in hepatic fibrosis. TGF-β1 stimulates production of extracellular matrix in hepatic stellate cells in the liver. It has been reported that blockage of TGF-β1 signaling prevents hepatic fibrosis. In this context, Nakamuta et al[8] evaluated the effect of transfection of a plasmid containing the soluble receptor type II TGF-β1 cDNA into skeletal muscle in an experimental model of dimethylnitrosamine (DMN) induced fibrosis in rats. This treatment decreased significantly DMN-induced hepatic fibrosis, hydroxyproline content, collagen and alpha-smooth muscle actin (α-SMA) expression. The authors suggested that this strategy may be useful for gene therapy of hepatic fibrosis.
Activation of metalloproteinases has also been evaluated. The delivery of an antisense molecule for the TIMP-1 into a plasmid to rats with hepatic fibrosis induced by pig serum injection, resulted in an increased activity of interstitial collagenase, which increased the degradation of collagen[9].
A promising method of gene transfer in large animals is hydrodynamic gene transfer (HGT), which consists of the application of controlled hydrodynamic pressure in capillaries to enhance endothelial and parenchymal cell permeability. It was used for first time in the late 1990s by Budker et al[10], who demonstrated a successful gene transfer into rat skeletal muscle by a rapid injection of plasmid DNA solution into the femoral artery.
The first clinical trial to test HGT in humans was reported at the 9th Annual meeting of the American Society of Gene Therapy[11]. The most successful application of hydrodynamic delivery was observed in hepatocytes in rodents. This procedure involves a tail vein injection in few seconds of 8%-10% vol/body weight of physiological solution. The high DNA solution in the tail vein enters directly into the inferior vena cava and drives the injected solution into the liver in a retrograde fashion[12,13].
Huang et al[14] investigated the effect of recombinant interleukin-10 (rIL-10) gene by HGT on liver fibrosis progression induced by intraperitoneal administration of porcine serum (PS) in rats. Plasmid expressing rIL-10 was transferred into rats by HGT and the major organ expressing rIL-10 was evaluated by immunohistochemistry and reverse transcription (RT)-PCR. The results showed the major expression of rIL-10 in the liver after HGT. The rIL-10 gene treatment attenuated liver inflammation and fibrosis in PS-induced fibrotic rats, decreasing collagen deposition and expression of α-SMA.
To test the efficiency between plasmid vs foamy virus (FV) for liver gene delivery, Zacharoulis et al[15] applied HGT in four juvenile pigs, to deliver the same plasmid backbone than naked FV vector particle to compare both vectors. Gene transfer efficiency and persistence of expression was assayed by PCR at 1 wk and 1 mo, respectively, after the infusions. HGT was well tolerated and no adverse reactions were observed. Plasmid injections resulted in no detectable DNA sequences at 1 wk. After 1 mo, 13% of liver sections analyzed were positive for plasmid DNA. When FV vectors were infused under identical conditions, 64.3% of liver samples were positive for vector sequences. These results indicated that the relative mild pressure obtained by hydrodynamic injection and the flooding of the liver was adequate for the entry of plasmids in hepatocytes and medium-term therapeutic levels of gene expression can be obtained with FV vectors. This effect could be attributed to the potential of HGT procedure and to the FV vector’s natural affinity for hepatocytes[15].
The high efficiency and simplicity of hydrodynamic injection have raised interest among medical community towards its possible application in patients. The major focus has centered on injection volume reduction while maintaining an adequate pressure for gene transfer. A proposed strategy to reduce the injection volume is to inject directly into the vasculature of the target tissues. Zhang et al[16] reduced the volume to < 1.5% of body weight in rats by targeting the liver through the hepatic vein, with successful results. These results point out the possibility of liver gene delivery to hepatocytes in a human weighing 70 kg at a volume of 500-700 mL, a volume that might be clinically acceptable.
Many investigators have focused on the production of effective non-viral vectors gene therapeutic systems. These synthetic systems are largely based on polycationic structures, because of their ability to interact with negatively charged nucleic acids[17].
Within this group, liposomes are considered as a novel strategy for delivery of drugs and genes to cells. Use of liposome formulations for gene delivery in vivo is valuable for gene therapy and would avoid several problems associated with viral delivery[18]. Ueki et al[19] transfected the human hepatocyte growth factor (HGF) gene, incorporated in liposomes, into skeletal muscles in rats with cirrhosis induced by dimethylnitrosamine. HGF is a potent mitogen for hepatocytes with anti-apoptotic activity and is essential for hepatic regeneration. The vector comprised liposomes containing the hemagglutinating virus of Japan with mixed liposomes (HVJ liposomes). This strategy induced a high plasma level of HGF, which binds and induces tyrosine phosphorylation of the HGF/c-Met receptor and suppression of TGF-β1 inhibiting fibrogenesis and hepatocyte apoptosis, resulting in fibrosis resolution.
Viral vectors are the most frequently used method to deliver genes into living organisms. Delivery of genes by a virus is termed transduction and the infected cells are described as transduced. Comparing the different vectors for gene therapy, viral vectors can ensure that nearly 100% of cells are infected, without severely affecting cell viability. The first modified virus for gene therapy was constructed in 1970 by Paul Berg. He modified the simian virus-40 (SV40) virus by addition of the bacteriophage lambda DNA and infected monkey kidney cells successfully in vitro[20]. Since then, the use of viral vectors has been reported by several authors in experimental and clinical protocols. To date, there has been no clinical protocol for liver fibrosis. However, several important strategies of gene therapy in animal models have shed light on this issue, which promise to lead to the use of viral vectors in humans in the not too distant future. Here we describe the most significant progress in this area.
Adenoviruses have been shown to be the most efficient vector in fibrotic liver models, overpassing technological hurdles and showing high hepatic tropism. In this context, several authors have used specific strains of adenovirus to deliver therapeutic genes, with promising results. Arias et al[21] cloned the adenoviral construct Ad5- cytomegalovirus (CMV)-AS-TGF-β1 expressing an antisense complementary to the 3’-portion of rat TGF-β1 mRNA and a control virus expressing the reporter gene green fluorescent protein (GFP). Both transgenes were driven by the human CMV promoter and were fused to the SV40 early mRNA polyadenylation signal. The authors found that transduction with Ad5-CMV-AS-TGF-β1 induced significantly more mRNA production than the endogenous gene. In cirrhotic rats with ligature of the common bile duct (BDL), the adenoviral vector abrogated production of collagen and α-smooth muscle actin, but had no significant impact on serum levels of AST, ALT, or bilirubin. The authors concluded that transfer of the TGF-β1 antisense was sufficient to abolish ongoing liver fibrogenesis, but did not interfere with the injury per se[21].
A different strategy was used by Salgado et al[22], where an adenoviral vector carrying a modified cDNA coding for a non-secreted form of human urokinase plasminogen activator (Ad-ΔhuPA) was administered to rats with liver fibrosis. The non-secreted uPA was chosen to diminish the risk of bleeding, which is an important problem in cirrhotic animals that may have preexisting coagulopathy, and because huPA is known as a potent activator of latent hepatic collagenases, which in turn would promote the degradation of extracellular matrix deposited in cirrhotic livers. Salgado et al[22] demonstrated that a single application of Ad-ΔhuPA through the iliac vein of severely cirrhotic rats induced profound beneficial changes, such as a significant reversion in carbon tetrachloride (CCl4)-induced hepatic fibrosis. Salgado et al[22] showed that Ad-ΔhuPA treated rats had an enormous improvement at day 10 via reduction of α-SMA, increase of MMP-2 and stimulation of liver regeneration with 40% more presence of PCNA. HGF expression was increased, which correlated with its cognate receptor c-Met. Furthermore, an improvement in functional hepatic tests was indicted by reduced ALT, AST and ALP levels.
Metalloproteinases (MMPs) play a crucial role in the pathogenesis of liver fibrosis and may represent an important therapeutic target in the design of anti-fibrotic strategies for chronic liver diseases. In this context, several types of MMPs can digest fibrillar collagens. The most potent MMPs against these kinds of collagens are: metalloproteinase-1 (MMP1) metalloproteinase-8 (MMP8) and metalloproteinase-13 (MMP13).
Delivery of collagenases has been reported in experimental models of cirrhosis by several authors.
Iimuro et al[23] delivered a cDNA encoding human pro-MMP-1 in an adenoviral vector into established liver fibrosis in rats, supposing that the manipulation of the imbalance between collagenases and their inhibitors (TIMPs) might attenuate liver fibrosis. After Ad-MMP-1 transduction, they found that liver fibrosis was significantly attenuated, as indicated by Masson’s trichrome staining. Notably, the area of α-SMA positive cells (a marker of activated HSC) dramatically decreased. Active MMP1 protein was detected by western blotting, indicating that expressed pro-MMP-1 protein was activated in vivo. Hepatocytes proliferation was also induced by MMP-1 expression in the liver. Degradation of fibrillar collagens could affect the interaction between ECM and hepatocytes. This modification possibly stimulates several growth factors bound to extracellular matrix, thereby improving liver function.
Meanwhile, in cirrhotic mice induced by CCl4 intoxication, Kim et al[24] delivered a plasmid containing an internal ribosome entry site, the gene of the green fluorescent reporter protein and MMP-13 gene into a vector cationic polymer, which has a relatively high transfection efficiencies and prolonged gene expression. Intravenous injection of pMMP3 in the vector cationic polymer increased the level of theMMP-13 mRNA by 25 times in liver tissue, slowing liver fibrosis, reducing collagen I deposition and restoring plasma AST levels compared with the control group mice treated with empty vector.
Considering all the experimental data reported by several authors, gene delivery of collagenases seems promising for the treatment of advanced cirrhosis in humans.
However, persistent overexpression of collagenases in the liver might digest normal architectures in addition to pathologically deposited ECM. Therefore, precise, controlled delivery of active interstitial MMPs may be necessary to develop a treatment for clinical use. In this context, our group cloned MMP-8, a neutrophil collagenase, which degrades type I collagen preferentially, under the transcriptional control of a phosphoenol pyruvate carboxykinase (PEPCK)-gene promoter, which contains the regulatory sites for hormonal regulation of expression in the liver. Experiments were conducted in HepG2 to demonstrate that addition of glucagon resulted in MMP-8 overexpression compared with the control using a plasmid without PEPCK gene promoter. These results showed that expression of a therapeutic gene like MMP-8 could be controlled at will, allowing modulation of the quantity of the extracellular matrix according to the body’s needs[25].
In a different approach, Siller-López et al[26] showed that adenoviral administration containing the MMP-8 gene promoted in situ degradation of extracellular matrix proteins in liver fibrosis induced by CCL4 intoxication and bile duct ligation in rats, releasing hepatic growth factors, and freeing up space for hepatic cell proliferation. Furthermore, they used a single application of 3 × 1011 VP/kg of AdMMP8 via the iliac vein in severely cirrhotic rats, obtaining in situ production of the cognate protein. AdMMP8-treated rats had a variable, yet remarkable, degree of hepatic fibrosis resolution by day 14 after adenovirus vector administration, the authors proposed that degradation of fibrotic tissue could also be taking place via activation of latent tissue gelatinases.
The systemic administration of adenovirus allows at least some of them to be introduced in different organs to the liver; therefore, Liu et al[27] cloned a cDNA of the truncated active MMP-8 in a hepatitis B virus vector. This vector was fused with an adenovirus to create a chimeric vector, with the aim of increasing liver tropism and transduction efficiency simultaneously. Rats with thioacetamide-induced liver cirrhosis were injected with this vector to evaluate therapeutic efficacy. They observed beneficial effects of this vector on hepatic fibrosis and hepatocyte regeneration.
The imbalance between MMPs and TIMPs is considered a crucial parameter of deposition and breakdown of the extracellular matrix. TIMP-1, the most important endogenous inhibitor of MMPs, plays a crucial role in the pathogenesis of liver fibrosis, and may represent an important therapeutic target in the design of anti-fibrotic strategies for chronic liver disease. TIMP-1 expression is upregulated in cirrhotic rats compared with normal liver. TIMP-1 binds to MMPs and inhibits their activity. Roderfeld et al[28] had already shown in vitro that an inactive MMP-9 (MMP-9-H401A) inactivated TIMP-1 by binding to it. Thus, they investigated the potential anti-fibrotic effect of WT-MMP-9 and MMP-9 mutants delivered by adenovirus vector to cirrhotic mice in a CCl4 model. They showed that inactive MMP-9 mutants delivered by adenovirus inhibited hepatic fibrogenesis, collagen-1 gene expression and hepatic stellate cells activation associated with decreased TIMP-1. This was the first work using an inactivated enzyme acting as a TIMP-1 scavenger as a therapeutic agent against fibrosis. The authors concluded that application of MMP-9 mutants as TIMP-1 scavengers opens up a new avenue for the treatment of hepatic fibrosis.
Several authors have reported that liver fibrogenesis involves a disturbance in mineral physiological concentrations, in particular zinc. The availability of zinc affects the activities of the zinc-dependent enzymes like MMPs. In this context, several studies have shown the beneficial effect of zinc supplementation on liver fibrosis. Metallothionein is a protein involved in the regulation of zinc homeostasis. For this reason, Jiang et al[29] delivered adenovirus containing the human MT-II gene (Ad-MT2A) through intravenous injection, to study the effect on liver fibrosis induced by CCl4 in mice. Ad-MT2A reversed fibrosis along with increased hepatocyte regeneration. MT was associated with increased activities of liver collagenases. This study indicated that MT makes an important contribution in the resolution of chemical-induced hepatic fibrosis and could be a therapeutical outcome in patients with liver fibrosis of certain etiologies.
Otherwise, Marquez-Aguirre et al[30] constructed a recombinant adenovirus containing the truncated receptor for TGFβ1 (Ad-TβRII∆cyt). They administrated a single injection of Ad-TβRII∆cyt (5 × 1011 vp/kg) via the iliac vein in rats with TAA-induced cirrhosis. This single injection diminished significantly hepatic fibrosis and the expressions of fibrogenic genes, such as collagen α1, TGF-β1, PAI-1, and MMP-2. Ad-TβRII∆cyt also increased the expression of anti-fibrotic transcriptional factor SnoN in sinusoidal cells. There was also a significant difference in serum levels of AST and total bilirubin between cirrhotic rats and cirrhotic rats transduced with TβRII∆cyt. The results suggested that delivery of TβRII∆cyt in an adenovirus is effective to express this therapeutic gene. Blocking of TGF-β1 signal with Ad-TβRII∆cyt could upregulate the transcriptional repressor SnoN, which antagonizes TGF-β1 signaling (TGF-β/Smad-pathway inhibitor) and downregulated profibrogenic genes expression[30].
Increased intrahepatic vascular tone in cirrhosis has been attributed to a decrease of hepatic nitric oxide (NO), secondary to alterations in the post-translational regulation of the enzyme eNOS[31]. Low activity of superoxide dismutase contributes to a reduction of NO bioavailability in cirrhotic livers. Thus, Laviña et al[32] investigated whether the removal of NO by a superoxide dismutase could improve endothelial dysfunction and reduce portal pressure in cirrhotic rats. To achieve this, they delivered an adenoviral vector expressing extracellular superoxide dismutase or beta-galactosidase (Ad-βgal) via the tail vein to CCl4-induced cirrhotic rats. This transduction to fibrotic livers reduced O2- levels significantly, increasing cGMP and decreasing liver nitrotyrosinated proteins, which are associated with a significant improvement in vasodilatation. Portal pressure was also significantly decreased in comparison with control rats. The authors suggested that scavenging of O2- might be a good therapeutic strategy in the management of portal hypertension in cirrhosis[32].
On the other hand, the bone morphogenic protein 7, a member of the TGF-β1 superfamily, has been reported to counteract the profibrogenic actions of TGF-β1 Kinoshita et al[33] examined if adenovirus-mediated overexpression of bone morphogenetic protein-7 (BMP-7), administered via the tail vain, could antagonize the effect of TGFβ1 in an experimental model of fibrosis induced by thioacetamide in rats. They found that hydroxyproline content and Sirius red stained areas were significantly reduced compared with the control.
Qiu et al[34] used adenovirus for dual gene transfer, human IL-10 and human hepatocyte growth factor, to rats with liver fibrosis induced by CCl4. This strategy protected hepatocytes from damage by reducing hepatocyte degeneration, hepatic fibrosis, and intra-hepatic inflammatory cell infiltration, thereby preserving liver function. The authors concluded that this liver protection could be the consequence of the regulation of the immune response caused by IL-10 and that this dual gene expression vector constitutes one of the most promising current strategies for liver gene therapy.
Meanwhile, Lin et al[35] used a combinatorial delivery of urokinase-type plasminogen activator (uPA) and HGF genes to investigate the effect of these two genes on hepatic fibrosis. Ad vectors expressing uPA (Ad-uPA), HGF (Ad-HGF) or uPA + HGF (Ad-uPA + HGF) were generated and injected into rats with hepatic fibrosis. Extracellular matrix and collagen type I and type III expression in the fibrotic liver decreased significantly more in the dual-gene transduction group compared with the individual AdHGF and AduPA groups, indicating that combinatorial gene delivery had a larger effect on reversion of hepatic fibrosis than mono-gene therapy, probably by a synergistic effect of these two genes on hepatic fibrosis resolution.
A different strategy was devised by Ozawa et al[36], who used the combination of truncated type II TGF-β1 receptor (TβTR) gene and HGF in an adenoviral vector (AdTβTR + AdHGF) to analyze the effect on liver fibrosis induced by chronic administration of dimethylnitrosamine in rats. The body and rats-liver weight treated with the combination and hepatocyte proliferation increased, while the grading of fibrosis was significantly less compared with an irrelevant vector AdLacZ or the single administration of either AdTβTR or AdHGF, supporting the premise that the combination of two therapeutics genes for liver fibrosis treatment is more effective than individual delivery.
Similar to adenovirus, adeno-associated viral (AAV) vectors have been shown to be efficient in experimental cirrhosis models. They have high cellular tropism, can achieve long-term gene expression and are now feasible for use in human gene therapy, because they do not awaken an exacerbated cellular immune response. For these reasons, AAV has emerged as an attractive vector for gene therapy. Production and purification of AAV has been improved recently, and it is now possible to produce high yields of vector, free from contaminating cellular and helper virus proteins. Eventually, tissue specific vectors to evade the immune response will be manufactured[37].
Some experiments have focused on demonstrating that AAV can efficiently transduce livers with fibrosis. Sobrevals et al[38] compared the ability of AAV to transduce normal and cirrhotic rat livers. They injected AAV serotype-1 (AAV1) encoding the reporter luciferase gene (AAV1Luc) through the hepatic artery, portal vein, into the biliary tree of normal and cirrhotic rats. They found that AAV1Luc allowed long-term and constant luciferase expression in rat livers. Interestingly, intra-portal administration led to higher expression levels in healthy livers compared with cirrhotic livers, whereas the opposite occurred when using intra-arterial injection. Intra-hepatic administration led to similar transgene expression in both animal groups, whereas intra-biliary infusion was the least effective route. After 70% partial hepatectomy, luciferase expression decreased in the regenerating liver, suggesting a lack of efficient integration of AAV1 DNA into the host genome. Transgene expression was found mainly in hepatocytes.
Different protocols using AAV have been developed for the treatment of hepatic fibrosis. In 2005, Chen et al[39] constructed a recombinant AAV vector encoding human IFN-gamma (rAAV-IFN-γ), and took the primary rat hepatic stellate cells and carbon tetrachloride-injury induced rats as the experimental hepatic fibrosis model in vitro and in vivo. Histological examination revealed that rAAV-IFN-γ could inhibit the progression of hepatic fibrosis, hydroxyproline content, and serum AST and ALT levels were decreased compared with the fibrosis control group. mRNA expressions of TIMP-1, TGF-β1 and MMP-13 were decreased.
In the same year, Tsui et al[40], demonstrated that rAAV exhibit high efficiency in transduction of a homeostatic gene, heme oxygenase-1 (HO-1), to activated stellate cells, where the binding of rAAVs to HSCs increased significantly after serum-stimulated activation compared with the quiescent state. Portal injection of rAAVs to normal or CCl4-induced liver fibrosis showed a distinct distribution of rAAV binding. The majority of injected rAAVs bound to the cells in fibrotic areas that were associated with higher expression levels of fibroblast growth factor receptor-1alpha at 2 h after administration. Isolation of different types of cells from CCl4-induced fibrotic livers showed predominant expression of the transgene in stellate cells after rAAV/HO-1 administration on day 3 and remained stable for 12 wk. In addition, HO-1-transduced stellate cells showed reduced transcript levels of type 1 collagen and impaired proliferative ability compared with controls.
In 2007, Suzumura et al[41] constructed an AAV vector expressing HGF (AAV5-HGF) and examined its effect in two mouse hepatic fibrosis models: CCl4 administration and BDL in Balb/c mice. Mice that received AAV5-HGF achieved stable HGF expression both in the serum and liver for at least 12 wk. In both models, significant improvement of liver fibrosis was observed in all mice receiving AAV5-HGF, based on Azan-Mallory staining. Suppression of HSCs was confirmed by immunohistochemistry. Expressions of TGF-β1, collagen I and α-SMA mRNAs were significantly suppressed in the liver of AAV5-HGF transduced mice damaged with CCl4 or BDL. Expression of the inhibitor of matrix metalloproteinases, TIMP-1, was significantly suppressed in livers of AAV5-HGF-transduced mice in both animal models[41].
In 2009, with the aim of investigating the effects of TGFβ3 on rat hepatic fibrosis, Liu et al[42] cloned the TGFβ3 cDNA into the rAAV2 vector. TGFβ3 is an anti-fibrotic cytokine that inhibits collagen production. Rats were randomly divided into four groups: normal control group, model group, negative control group and TGFβ3 group. Hepatic fibrosis was induced by hypodermic injection of 40% CCl4. Recombinant AAV2-TGFβ3 viral particles were injected via caudal vein one week before CCl4 treatment. Rats were sacrificed 8 wk after CCl4 treatment, and global histological changes were observed after HE staining, indicating that collagen fibers were reduced in the TGFβ3 group. Masson staining showed that collagen fibers deposited around the blood vessels, portal area and the perisinusoidal space in liver tissues of TGFβ3 group were significantly decreased.
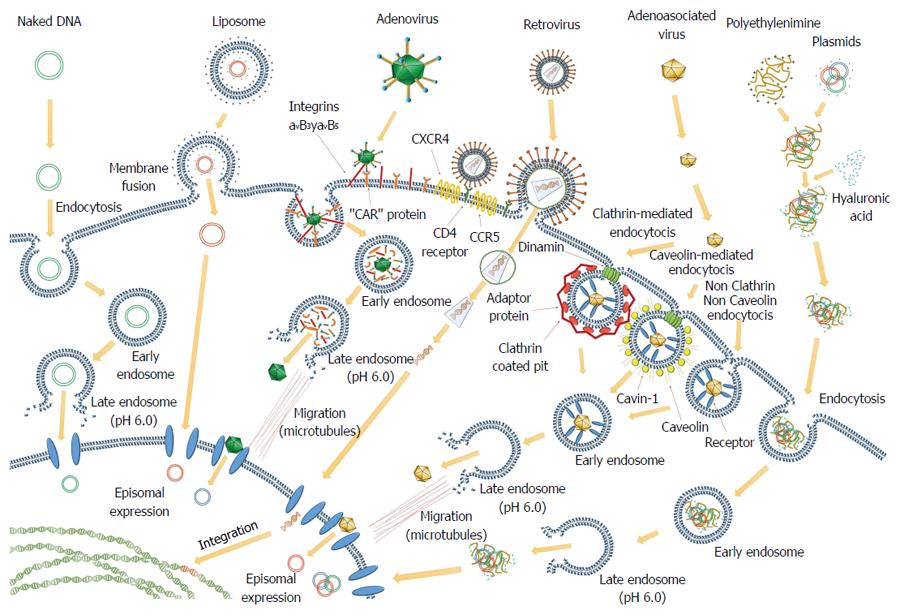
New approaches have been tested with novel administration methods that are less invasive and easier to perform. For example, Hao et al[43] used the BMP-7, a potent antagonist of TGF-β1 and an antifibrotic factor. In that study, they generated a recombinant AAV carrying BMP-7 (AAV-BMP-7) and tested its ability to suppress CCl4-induced hepatic fibrosis in mice. The results showed that ectopic expression of BMP-7 in gastrointestinal (GI) mucosa caused by AAV-BMP-7 administration led to long-term elevation of serum BMP-7 concentrations and resulted in drastic amelioration of CCl4-induced hepatic fibrosis in BALB/c mice. Immunostaining for α-SMA and desmin demonstrated that AAV-BMP-7 inhibited HSCs activation and promoted hepatocyte proliferation. The authors suggested that oral AAV-BMP-7 could be developed into a safe, simple, and effective therapy for hepatic fibrosis (Figure 1).
Even though some serotypes of Ads and AAVs, such as Ad-5 or AAV-5/8, have high hepatic tropism, scientists continue the search for the best vector. Several investigations involved the usage of other recombinant viruses. In 2006, Merle et al[44], proved the principle of a lentiviral gene transfer in the Long-Evans cinnamon (LEC) rat, an animal model of Wilson disease. Rats were treated either by systemic application of lentiviral vectors or by intrasplenic transplantation of LEC-rat hepatocytes lentivirally transduced with ATP7B gene. ATP7B gene encodes a copper transport protein that plays a key role in incorporating copper into ceruloplasmin and moving excess copper out of the liver. ATP7B gene expression was analyzed by RT-PCR and its hepatic expression was detected at different time-points post-treatment and lasted for up to 24 wk (end of experiment). Liver copper levels were lowered in all treatment groups compared with untreated LEC rats. Twenty-four weeks after treatment, the area of the examined liver-tissue sections occupied by fibrosis was significantly minor, only with small fibrous septa in rats treated with cell therapy compared with untreated rats.
In the same manner Hamada et al[45], assessed the usefulness of oncostatin M (OSM) gene therapy in liver regeneration. They examined whether the introduction of OSM cDNA could enhance regeneration of livers damaged by DMN in rats. They enclosed the cDNA of OSM in hemagglutinating virus of Japan envelope into the spleen, resulting in the exclusive expression of OSM protein in Kupffer cells of the liver, which was accompanied by increases in body weight, liver weight, and serum albumin levels and reduction of serum liver injury parameters (bilirubin, AST and ALT) and a serum fibrosis parameter (hyaluronic acid). Histological examination showed that OSM gene therapy reduced centrilobular necrosis and inflammatory cell infiltration, and augmented hepatocyte proliferation. Apoptosis of hepatocytes and fibrosis were suppressed by OSM gene therapy.
Different sorts of vectors have been tested, such as SV40, an icosahedral papovavirus, which has recently been modified to serve as a gene delivery vector. Recombinant SV40 vectors (rSV40) are good candidates for gene transfer, as they display some unique features: they are non-replicative vectors, easy-to-make, and can be produced in high titers. They also efficiently transduce both resting and dividing cells, deliver persistent transgene expression to a wide range of cell types, and are non-immunogenic.
In 2007, Vera et al[46], analyzed the efficacy of a rSV40 encoding IGF-I (rSVIGF-I) to prevent cirrhosis progression. The transgenic expression of luciferase was evaluated in mice. The results showed long-term hepatic expression of the transgene, with luciferase expression increased significantly in CCl4-damaged livers and upon IGF-I administration. Thus, liver injury and IGF-I expression from rSVIGF-I should favor transgene expression. rSVIGF-I therapeutic efficacy was studied in rats where cirrhosis was induced by CCl4 inhalation during 36 wk. At the end of the study, hepatic levels of IGF-I and IGF-binding protein 3 were higher in rSVIGF-I-treated rats than in control cirrhotic animals.
This vector was also used in experiments performed by Sobrevals et al[47], where they found that injection through the hepatic artery with an SV40 vector encoding insulin growth factor (SVIGF-I) in cirrhotic rats increased hepatic levels of IGF-I, improved liver function tests, and reduced fibrosis associated with diminished α-SMA expression, upregulation of MMPs and decreased expression of tissue inhibitors of MMPs, TIMP-1 and TIMP-2. SVIGF-I therapy induced downregulation of TGF-β1, amphiregulin, platelet-derived growth factor (PDGF), connective tissue growth factor (CTGF), vascular endothelium growth factor (VEGF) and induction of the antifibrogenic and cytoprotective protein, HGF.
Some virus have a high natural tropism for the liver, one of them is the hepatitis B virus (HBV). However, HBV vectors have a limited insertion capacity and are replication-defective. Conversely, in an HBV infected cell, vector replication may be rescued in trans by the resident virus, allowing conditional vector amplification and spread. Capitalizing on a resident pathogen to help in its elimination and/or in treating its pathological consequences would represent a novel strategy. However, resident HBV may also reduce susceptibility to HBV vector super-infection. Thus, a size-compatible truncated MMP-8 (tMMP8) gene was cloned into an HBV vector, which was then used to generate a chimeric Ad-HBV shuttle vector that was not subject to super-infection exclusion. Rats with TAA-induced extensive liver fibrosis were injected with this chimera to evaluate therapeutic efficacy. The data demonstrated that infectious HBV vector particles could be obtained via trans-complementation by wild-type virus, and that tMMP8 HBV vector could efficiently be shuttled by an Ad vector into cirrhotic rat livers. In the liver, it exerted a comparable beneficial effect on fibrosis and hepatocyte proliferation markers as a conventional full-length MMP-8 Ad vector[27].
HBV also contain numerous overlapping open reading frames and regulatory cis-elements, which have hampered early attempts to harness HBV into a gene-transfer vector by simple insertion of foreign sequences. HBV vectors obtained in this way selectively accumulate in the liver after inoculation into peripheral vessels, efficiently infected quiescent hepatocytes, and successfully transduced genes for GFP and type I interferon (IFN-γ) These data suggested that HBV-based vectors may become useful against other liver diseases[27].
HSCs play a central role in hepatic fibrosis and their elimination is a crucial step towards the resolution and reversion of liver fibrosis. Arabpour et al[48], investigated the potential application of a fused protein of an anti-epidermal growth factor receptor scFv antibody-TNF-α (scFv425- sTRAIL) delivery by an Ad vector on the targeted elimination of activated HSCs in cell culture. Treatment with Ad-scFv425-sTRAIL induced a reduction of around 100% in HSC viability, a 60% reduction in ECM production, and decreased caspase inhibition, where no effect was observed on hepatic parenchymal cells. The authors suggested that this strategy may represent a new therapeutic strategy against liver fibrosis.
In a different study, other authors developed a CCl4-induced micronodular cirrhosis model to study the effect of rAAV/HO-1 administration, where expression of HO-1 by rAAV/HO-1 significantly increased the HO enzymatic activities in a stable manner. The development of micronodular cirrhosis was significantly inhibited in rAAV/HO-1-transduced animals. Portal hypertension was markedly diminished in rAAV/HO-1-transduced animals compared with controls, and no significant changes in systolic blood pressure were noted. These findings were accompanied with improved liver biochemistry, fewer infiltrating macrophages and fewer activated HSCs in rAAV/HO-1-transduced livers[49].
In a different study, Reetz et al[50] described a novel method to deliver genes for HSC in fibrotic liver, ablating the native tropism to liver. This paper was based on the concept that the expression of P75 neutrophin receptor (P75NTR) is increased in HSC in liver fibrosis, compared with low expression in quiescence HSCs and no expression in hepatocytes. Nerve growth factor (NGF) is a neutrophin that binds P75NTR. According to this premise, NGF was conjugated to the Ad surface using an adapter derived from a single chain antibody via polientilenglicol. The Ads carried the reporter gene GFP. This vector was injected systemically in mice and GFP expression was evaluated. The authors showed that the GFP expression was detected in the liver, but not in other organs like the lung and brain. Liver expression was selective and increased in HSCs of liver fibrosis compared to normal cells. There was no expression in hepatocytes. The authors concluded that this strategy might provide an effective mechanism for direct therapeutic gene delivery into activated HCSs without affecting hepatocytes[50].
To reduce liver fibrosis, Narmada et al[51] delivered HGF specifically to activated HSCs in fibrotic livers using vitamin A-coupled liposomes by retrograde intrabiliary infusion to bypass capillarized hepatic sinusoids. The HSC-targeted transgene enhanced the antifibrotic effect by reduction of α-SMA and collagen genes.
Technology based in the delivery of short DNAs or RNAs is a revolutionary tool employed to silence the expression of specific genes in cells with no toxic response. These molecules are delivered to the cells or produced by them using expression cassettes, which are introduced into cells through viral vectors, plasmids or DNA constructs[52]. In this context, several molecules, such as decoys, antisense oligonucleotides, short interfering RNAS (siRNAs) and mircoRNAs (miRNA), have been investigated to evaluate their effect on expression inhibition of important genes for cirrhosis development.
The usage of decoy technology has been recently reported. A synthetic double-stranded oligodeoxynucleotide (ODN) containing the consensus binding sequence of the transcription factor Sp1, which regulates the inflammation-repair process and suppresses expression of several genes including TGF-β1, collagen type I, VEGF, to block its activity, was used by Park et al[53] in a CCl4-liver fibrosis model. They injected 10 μg of this ODN through tail vein in mice. The decoy molecule for Sp1 reduced gene expression of TNF-α, IL-1β, IL-6, VEGF and MCP-1 and also decreased the production of pro-fibrogenic proteins like fibronectin, α-SMA, TGFβ-1 and TIMP-1.
In 2005, Cheng et al[54], developed an anti-gene approach using a type alpha1 (I) promoter specific triplex-forming oligonucleotide (TFO) to inhibit collagen gene expression. In this report, biodistribution and hepatic cellular and subcellular localization of the 25-mer antiparallel phosphorothioate TFO were determined after intravenous injection into rats. TFOs distributed to all the major organs, with higher uptake in the liver, kidney and spleen. Competition studies with polyinosinic acid and dextran sulfate suggested the involvement of scavenger receptors in the hepatic uptake of TFO. Intrahepatic cellular distribution by Kupffer, endothelial and HSCs accounted for almost 70% of the liver uptake of P-TFO, while only 30% was associated with hepatocytes. The level of liver nuclei-associated TFO was much lower relative to that found in the cytoplasm at 2 and 4 h post-injection. However, the TFO inhibited collagen expression, as evidenced by sirius red staining of the liver section of fibrotic rats. In conclusion, systemic delivery of the TFO against type alpha1 (I) collagen gene promoter may be used for the treatment of liver fibrosis.
In the same year, Jiang et al[9], constructed a rat antisense TIMP-1 recombinant plasmid that could be expressed in eukaryotic cells. The recombinant plasmids were encapsulated with glycosyl-poly-L-lysine and injected into rats suffering from pig serum-induced liver fibrosis. The expression of the exogenous transfected plasmid was assessed by northern blotting, RT-PCR and western blotting. The antisense construct was successfully expressed in vivo and could block the gene and protein expression of TIMP-1. Active and latent hepatic interstitial collagenase activities were elevated. The hepatic hydroxyproline content and the accumulation of collagen types I and III were lowered, and liver fibrosis was alleviated in the antisense TIMP-1 group compared with the model group.
The use of these kinds of molecules has increased in recent years and a large number of molecules have been tested. Lu et al[55] constructed a recombinant plasmid for a rat antisense RNA for CTGF, which could be expressed in eukaryotic cells. The recombinant plasmids were encapsulated with lipofectamine and then transduced into a CCl4-induced rat liver fibrosis model. The gene and protein expression of CTGF were significantly decreased in the fibrotic liver transfected with antisense-CTGF compared with the control group. Index fibrosis and collagens type I and type III were also significantly minimized in this group.
SiRNAs are a recent powerful tool for post-transcriptional gene silencing and have opened up new avenues in gene therapy. The problems of lack of cell specificity in vivo and the subsequent occurrence of side effects, has hampered their development for hepatic fibrosis treatment. To overcome these shortcomings, several targeted strategies using siRNA for cirrhosis treatment have been developed. For example, in 2007 George et al[56] used a CTGF siRNA to prevent the progression of NDMA-induced hepatic fibrosis. The serial administration of NDMA resulted in activation of HSCs, upregulation of CTGF and TGF-β1 at both the mRNA and protein levels, and well-developed hepatic fibrosis. Immunostaining, western blotting and semiquantitative real-time RT-PCR studies showed downregulation of CTGF and TGF-β1 after treatment with the CTGF siRNA. These results demonstrated that CTGF gene silencing through siRNA reduced the activation of hepatic stellate cells, prevented upregulation of CTGF and TGF-β1 gene expression, and inhibited accumulation of liver connective tissue proteins.
Three years later, another working group also tested the anti-fibrogenesis properties of a single intra-portal vein injection CTGF siRNA in a rat model of liver fibrosis. The authors observed that in CTGF siRNA-treated cirrhotic rats, protein expression of CTGF and α-SMA, and the number of active HSC, decreased compared with the model group. Attenuation of liver fibrosis was also observed[57].
In 2008, Chen et al[58], constructed a PDGFR-β siRNA expression plasmid and investigated its effect on the activation of HSCs. A hydrodynamics-based transfection method was used to deliver PDGFR-β subunit-siRNA to rats with hepatic fibrosis. PDGFR-β-siRNA significantly downregulated PDGFR-β expression, and suppressed HSCs activation and proliferation in vitro. The progression of fibrosis in the liver was significantly suppressed by PDGFR-β siRNA in two animal models of fibrosis: DMN intoxication and BDL. The authors suggested that the plasmid could be delivered into activated HSCs by the hydrodynamics-based transfection method, and remarkably improved liver function in cirrhotic rats.
In the same year, Cheng et al[59], designed a siRNA and short hairpin RNA (shRNA) targeting different regions of TGF-β1 mRNA, and measured the silencing effect after transfection into immortalized rat liver HSC (HSC-T6). There was not only a significant decrease in TGF-β1, TIMP-1, α-SMA and type I collagen after transfection with TGF-β1 siRNAs, but also synergism in gene silencing when siRNAs targeting two different start sites were used as a pool for transfection. The two siRNA sequences, which efficiently inhibited TGF-β1 gene expression, were converted to shRNAs via cloning into the pSilencer1.0. In conclusion, both siRNA and shRNA showed sequence-specific and dose dependent TGF-β1 gene silencing and have the potential to treat liver fibrosis.
MiRNAs are short, endogenous, noncoding RNA molecules that regulate gene expression at a post-translational level. MiRNAs have been recognized in the regulation of physiological conditions. Moreover, awareness of the association between dysregulated miRNAs and human diseases is increasing, which consequently brings miRNAs into the frontline of novel therapeutic strategies.
This technology is effective, as demonstrated by the work of Yang et al[60]. They investigated the antifibrotic effects of an artificial miRNA targeting CTGF, using the ultrasound-targeted cationic liposome-bearing microbubble destruction gene delivery system. Plasmids carrying the most effective artificial miRNA sequences were delivered by this method to rats with hepatic fibrosis. The results showed that this method of gene delivery effectively transported the plasmids into the rat liver. The artificial miRNA reduced hepatic fibrosis, pathological alterations, and the protein and mRNA expressions of CTGF and TGF-β1. Furthermore, CTGF gene silencing decreased the levels of type I collagen and α-SMA. These data suggested that delivery of an artificial miRNA targeted against CTGF using ultrasound-targeted cationic liposome-bearing microbubble destruction may be an effective therapeutic method to ameliorate hepatic fibrosis.
Gene therapy represents a novel alternative for the treatment of those diseases that currently have no satisfactory cure. In recent years, gene therapy has been directed to the treatment of mortal chronic degenerative diseases to offer the patient a better quality of life. For those diseases caused by lack of control in the expression of certain genes, such hepatic fibrosis, delivery of genes that counteract this overexpression should be an excellent strategy to control it. Thus, numerous articles have been published in the experimental field of gene therapy, addressing innovative strategies that demonstrate that hepatic fibrosis could be treated with gene therapy, supporting its use in a near future in cirrhotic patients.
A large number of vectors used in gene therapy have been implemented, each with its advantages and disadvantages. The use of gene therapy in humans has been controversial because the delivered genes could potentially be integrated into the cell genome, causing an insertional mutation that might result in cancer, especially if the vector used is a virus. As mentioned earlier, adenoviruses are the most commonly used viral vectors for therapeutic gene delivery to the liver[61], with the advantage that they recognize the CAR receptor present in hepatocytes, do not integrate into the cell genome, and the risk of insertional mutation is zero[62]. Other vectors, like liposomes, plasmids and adeno-associated viral vectors, have been proved; however, the ideal vector to deliver genes to a diseased liver remains to be established. Some clinical trials using gene therapy against hepatocarcinoma and hepatitis C infection are being implemented[61]; however, there are only experimental models directed to liver fibrosis at this moment. These experimental approaches have been demonstrated as effective to decrease and prevent experimental fibrosis. We await their implementation in humans in the near future.
| 1. | Ramadori G, Moriconi F, Malik I, Dudas J. Physiology and pathophysiology of liver inflammation, damage and repair. J Physiol Pharmacol. 2008;59 Suppl 1:107-117. [PubMed] |
| 2. | Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655-1669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2139] [Cited by in RCA: 2191] [Article Influence: 121.7] [Reference Citation Analysis (1)] |
| 3. | Longo CR, Patel VI, Shrikhande GV, Scali ST, Csizmadia E, Daniel S, Sun DW, Grey ST, Arvelo MB, Ferran C. A20 protects mice from lethal radical hepatectomy by promoting hepatocyte proliferation via a p21waf1-dependent mechanism. Hepatology. 2005;42:156-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 49] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 4. | Guha C, Roy-Chowdhury N, Jauregui H, Roy-Chowdhury J. Hepatocyte-based gene therapy. J Hepatobiliary Pancreat Surg. 2001;8:51-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Costa D, Valente AJ, Miguel MG, Queiroz J. Plasmid DNA hydrogels for biomedical applications. Adv Colloid Interface Sci. 2014;205:257-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Tanigawa K, Sakaida I, Masuhara M, Hagiya M, Okita K. Augmenter of liver regeneration (ALR) may promote liver regeneration by reducing natural killer (NK) cell activity in human liver diseases. J Gastroenterol. 2000;35:112-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 64] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 7. | Li Q, Liu DW, Zhang LM, Zhu B, He YT, Xiao YH. Effects of augmentation of liver regeneration recombinant plasmid on rat hepatic fibrosis. World J Gastroenterol. 2005;11:2438-2443. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Nakamuta M, Morizono S, Tsuruta S, Kohjima M, Kotoh K, Enjoji M. Remote delivery and expression of soluble type II TGF-beta receptor in muscle prevents hepatic fibrosis in rats. Int J Mol Med. 2005;16:59-64. [PubMed] |
| 9. | Jiang W, Wang JY, Yang CQ, Liu WB, Wang YQ, He BM. Effects of a plasmid expressing antisense tissue inhibitor of metalloproteinase-1 on liver fibrosis in rats. Chin Med J (Engl). 2005;118:192-197. [PubMed] |
| 10. | Budker V, Zhang G, Danko I, Williams P, Wolff J. The efficient expression of intravascularly delivered DNA in rat muscle. Gene Ther. 1998;5:272-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 115] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 11. | Tan PH. 9th American Society of Gene Therapy annual meeting. Expert Opin Biol Ther. 2006;6:839-842. [PubMed] |
| 12. | Crespo A, Peydró A, Dasí F, Benet M, Calvete JJ, Revert F, Aliño SF. Hydrodynamic liver gene transfer mechanism involves transient sinusoidal blood stasis and massive hepatocyte endocytic vesicles. Gene Ther. 2005;12:927-935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 64] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 13. | Suda T, Gao X, Stolz DB, Liu D. Structural impact of hydrodynamic injection on mouse liver. Gene Ther. 2007;14:129-137. [PubMed] |
| 14. | Huang YH, Chen YX, Zhang LJ, Chen ZX, Wang XZ. Hydrodynamics-based transfection of rat interleukin-10 gene attenuates porcine serum-induced liver fibrosis in rats by inhibiting the activation of hepatic stellate cells. Int J Mol Med. 2014;34:677-686. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Zacharoulis D, Rountas C, Katsimpoulas M, Morianos J, Chatziandreou I, Vassilopoulos G. Efficient liver gene transfer with foamy virus vectors. Med Sci Monit Basic Res. 2013;19:214-220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Zhang X, Dong X, Sawyer GJ, Collins L, Fabre JW. Regional hydrodynamic gene delivery to the rat liver with physiological volumes of DNA solution. J Gene Med. 2004;6:693-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 17. | Novo L, Mastrobattista E, van Nostrum CF, Lammers T, Hennink WE. Decationized polyplexes for gene delivery. Expert Opin Drug Deliv. 2015;12:507-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Smith TN. Optimization of Nonviral Gene Therapeutics. 2th Edition. Gene and Cell Therapy, Therapeutic Mechanisms and Strategies. USA: Editorial Marcel Dekker Inc 2004; . |
| 19. | Ueki T, Kaneda Y, Tsutsui H, Nakanishi K, Sawa Y, Morishita R, Matsumoto K, Nakamura T, Takahashi H, Okamoto E. Hepatocyte growth factor gene therapy of liver cirrhosis in rats. Nat Med. 1999;5:226-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 456] [Cited by in RCA: 473] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 20. | Goff SP, Berg P. Construction of hybrid viruses containing SV40 and lambda phage DNA segments and their propagation in cultured monkey cells. Cell. 1976;9:695-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 96] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 21. | Arias M, Sauer-Lehnen S, Treptau J, Janoschek N, Theuerkauf I, Buettner R, Gressner AM, Weiskirchen R. Adenoviral expression of a transforming growth factor-beta1 antisense mRNA is effective in preventing liver fibrosis in bile-duct ligated rats. BMC Gastroenterol. 2003;3:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 76] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 22. | Salgado S, Garcia J, Vera J, Siller F, Bueno M, Miranda A, Segura A, Grijalva G, Segura J, Orozco H. Liver cirrhosis is reverted by urokinase-type plasminogen activator gene therapy. Mol Ther. 2000;2:545-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 92] [Article Influence: 3.5] [Reference Citation Analysis (4)] |
| 23. | Iimuro Y, Nishio T, Morimoto T, Nitta T, Stefanovic B, Choi SK, Brenner DA, Yamaoka Y. Delivery of matrix metalloproteinase-1 attenuates established liver fibrosis in the rat. Gastroenterology. 2003;124:445-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 193] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 24. | Kim EJ, Cho HJ, Park D, Kim JY, Kim YB, Park TG, Shim CK, Oh YK. Antifibrotic effect of MMP13-encoding plasmid DNA delivered using polyethylenimine shielded with hyaluronic acid. Mol Ther. 2011;19:355-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 25. | Siller-López F, García-Bañuelos J, Hasty KA, Segura J, Ramos-Márquez M, Qoronfleh MW, Aguilar-Cordova E, Armendáriz-Borunda J. Truncated active matrix metalloproteinase-8 gene expression in HepG2 cells is active against native type I collagen. J Hepatol. 2000;33:758-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Siller-López F, Sandoval A, Salgado S, Salazar A, Bueno M, Garcia J, Vera J, Gálvez J, Hernández I, Ramos M. Treatment with human metalloproteinase-8 gene delivery ameliorates experimental rat liver cirrhosis. Gastroenterology. 2004;126:1122-1133; discussion 949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 134] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 27. | Liu J, Cheng X, Guo Z, Wang Z, Li D, Kang F, Li H, Li B, Cao Z, Nassal M. Truncated active human matrix metalloproteinase-8 delivered by a chimeric adenovirus-hepatitis B virus vector ameliorates rat liver cirrhosis. PLoS One. 2013;8:e53392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 28. | Roderfeld M, Weiskirchen R, Wagner S, Berres ML, Henkel C, Grötzinger J, Gressner AM, Matern S, Roeb E. Inhibition of hepatic fibrogenesis by matrix metalloproteinase-9 mutants in mice. FASEB J. 2006;20:444-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 114] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 29. | Jiang Y, Kang YJ. Metallothionein gene therapy for chemical-induced liver fibrosis in mice. Mol Ther. 2004;10:1130-1139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 30. | Marquez-Aguirre A, Sandoval-Rodriguez A, Gonzalez-Cuevas J, Bueno-Topete M, Navarro-Partida J, Arellano-Olivera I, Lucano-Landeros S, Armendariz-Borunda J. Adenoviral delivery of dominant-negative transforming growth factor beta type II receptor up-regulates transcriptional repressor SKI-like oncogene, decreases matrix metalloproteinase 2 in hepatic stellate cell and prevents liver fibrosis in rats. J Gene Med. 2009;11:207-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 31. | Bosch J, García-Pagán JC. Complications of cirrhosis. I. Portal hypertension. J Hepatol. 2000;32:141-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 339] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 32. | Laviña B, Gracia-Sancho J, Rodríguez-Vilarrupla A, Chu Y, Heistad DD, Bosch J, García-Pagán JC. Superoxide dismutase gene transfer reduces portal pressure in CCl4 cirrhotic rats with portal hypertension. Gut. 2009;58:118-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 65] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 33. | Kinoshita K, Iimuro Y, Otogawa K, Saika S, Inagaki Y, Nakajima Y, Kawada N, Fujimoto J, Friedman SL, Ikeda K. Adenovirus-mediated expression of BMP-7 suppresses the development of liver fibrosis in rats. Gut. 2007;56:706-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 130] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 34. | Qiu H, Yan Y, Xing J, Zhu Y, Fang L, Cao X, Su C. Adenovirus-mediated dual gene expression of human interleukin-10 and hepatic growth factor exerts protective effect against CCl4-induced hepatocyte injury in rats. Dig Dis Sci. 2012;57:1857-1865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 35. | Lin Y, Xie WF, Chen YX, Zhang X, Zeng X, Qiang H, Chen WZ, Yang XJ, Han ZG, Zhang ZB. Treatment of experimental hepatic fibrosis by combinational delivery of urokinase-type plasminogen activator and hepatocyte growth factor genes. Liver Int. 2005;25:796-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 36. | Ozawa S, Uchiyama K, Nakamori M, Ueda K, Iwahashi M, Ueno H, Muragaki Y, Ooshima A, Yamaue H. Combination gene therapy of HGF and truncated type II TGF-beta receptor for rat liver cirrhosis after partial hepatectomy. Surgery. 2006;139:563-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 37. | Grieger JC, Samulski RJ. Adeno-associated virus as a gene therapy vector: vector development, production and clinical applications. Adv Biochem Eng Biotechnol. 2005;99:119-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 38. | Sobrevals L, Enguita M, Rodriguez C, Gonzalez-Rojas J, Alzaguren P, Razquin N, Prieto J, Fortes P. AAV vectors transduce hepatocytes in vivo as efficiently in cirrhotic as in healthy rat livers. Gene Ther. 2012;19:411-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 39. | Chen M, Wang GJ, Diao Y, Xu RA, Xie HT, Li XY, Sun JG. Adeno-associated virus mediated interferon-gamma inhibits the progression of hepatic fibrosis in vitro and in vivo. World J Gastroenterol. 2005;11:4045-4051. [PubMed] |
| 40. | Tsui TY, Lau CK, Ma J, Glockzin G, Obed A, Schlitt HJ, Fan ST. Adeno-associated virus-mediated heme oxygenase-1 gene transfer suppresses the progression of micronodular cirrhosis in rats. World J Gastroenterol. 2006;12:2016-2023. [PubMed] |
| 41. | Suzumura K, Hirano T, Son G, Iimuro Y, Mizukami H, Ozawa K, Fujimoto J. Adeno-associated virus vector-mediated production of hepatocyte growth factor attenuates liver fibrosis in mice. Hepatol Int. 2008;2:80-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 42. | Liu P, Gao XL, Yu J, Qian W, Xu KS. [Effects of transforming growth factor beta 3 on the histopathology and expression of collagen I in experimental hepatic fibrotic rats]. Zhonghua Gan Zang Bing Za Zhi. 2009;17:446-450. [PubMed] |
| 43. | Hao ZM, Cai M, Lv YF, Huang YH, Li HH. Oral administration of recombinant adeno-associated virus-mediated bone morphogenetic protein-7 suppresses CCl(4)-induced hepatic fibrosis in mice. Mol Ther. 2012;20:2043-2051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 44. | Merle U, Encke J, Tuma S, Volkmann M, Naldini L, Stremmel W. Lentiviral gene transfer ameliorates disease progression in Long-Evans cinnamon rats: an animal model for Wilson disease. Scand J Gastroenterol. 2006;41:974-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 39] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 45. | Hamada T, Sato A, Hirano T, Yamamoto T, Son G, Onodera M, Torii I, Nishigami T, Tanaka M, Miyajima A. Oncostatin M gene therapy attenuates liver damage induced by dimethylnitrosamine in rats. Am J Pathol. 2007;171:872-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 46. | Vera M, Sobrevals L, Zaratiegui M, Martinez L, Palencia B, Rodríguez CM, Prieto J, Fortes P. Liver transduction with a simian virus 40 vector encoding insulin-like growth factor I reduces hepatic damage and the development of liver cirrhosis. Gene Ther. 2007;14:203-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 47. | Sobrevals L, Rodriguez C, Romero-Trevejo JL, Gondi G, Monreal I, Pañeda A, Juanarena N, Arcelus S, Razquin N, Guembe L. Insulin-like growth factor I gene transfer to cirrhotic liver induces fibrolysis and reduces fibrogenesis leading to cirrhosis reversion in rats. Hepatology. 2010;51:912-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 48. | Arabpour M, Poelstra K, Helfrich W, Bremer E, Haisma HJ. Targeted elimination of activated hepatic stellate cells by an anti-epidermal growth factor-receptor single chain fragment variable antibody-tumor necrosis factor-related apoptosis-inducing ligand (scFv425-sTRAIL). J Gene Med. 2014;16:281-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 49. | Tsui TY, Lau CK, Ma J, Wu X, Wang YQ, Farkas S, Xu R, Schlitt HJ, Fan ST. rAAV-mediated stable expression of heme oxygenase-1 in stellate cells: a new approach to attenuate liver fibrosis in rats. Hepatology. 2005;42:335-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 49] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 50. | Reetz J, Genz B, Meier C, Kowtharapu BS, Timm F, Vollmar B, Herchenröder O, Abshagen K, Pützer BM. Development of Adenoviral Delivery Systems to Target Hepatic Stellate Cells In Vivo. PLoS One. 2013;8:e67091. [PubMed] |
| 51. | Narmada BC, Kang Y, Venkatraman L, Peng Q, Sakban RB, Nugraha B, Jiang X, Bunte RM, So PT, Tucker-Kellogg L. Hepatic stellate cell-targeted delivery of hepatocyte growth factor transgene via bile duct infusion enhances its expression at fibrotic foci to regress dimethylnitrosamine-induced liver fibrosis. Hum Gene Ther. 2013;24:508-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 52. | Xiang S, Fruehauf J, Li CJ. Short hairpin RNA-expressing bacteria elicit RNA interference in mammals. Nat Biotechnol. 2006;24:697-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 192] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 53. | Park JH, Jo JH, Kim KH, Kim SJ, Lee WR, Park KK, Park JB. Antifibrotic effect through the regulation of transcription factor using ring type-Sp1 decoy oligodeoxynucleotide in carbon tetrachloride-induced liver fibrosis. J Gene Med. 2009;11:824-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 54. | Cheng K, Ye Z, Guntaka RV, Mahato RI. Biodistribution and hepatic uptake of triplex-forming oligonucleotides against type alpha1(I) collagen gene promoter in normal and fibrotic rats. Mol Pharm. 2005;2:206-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 55. | Lu CH, Lu JX, Hua GP, Zhu J, Wang H, Huang JF, Gu MZ, Zhou Q, Ni RZ. [Effects of antisense RNA of connective tissue growth factor expressing plasmid on rat liver fibrosis]. Zhonghua Gan Zang Bing Za Zhi. 2007;15:118-121. [PubMed] |
| 56. | George J, Tsutsumi M. siRNA-mediated knockdown of connective tissue growth factor prevents N-nitrosodimethylamine-induced hepatic fibrosis in rats. Gene Ther. 2007;14:790-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 110] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 57. | Li GM, Li DG, Fan JG, Xie Q. [Effect of silencing connective tissue growth factor on the liver fibrosis in rats]. Zhonghua Gan Zang Bing Za Zhi. 2010;18:822-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 58. | Chen SW, Zhang XR, Wang CZ, Chen WZ, Xie WF, Chen YX. RNA interference targeting the platelet-derived growth factor receptor beta subunit ameliorates experimental hepatic fibrosis in rats. Liver Int. 2008;28:1446-1457. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 59. | Cheng K, Yang N, Mahato RI. TGF-beta1 gene silencing for treating liver fibrosis. Mol Pharm. 2009;6:772-779. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 100] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 60. | Yang D, Gao YH, Tan KB, Zuo ZX, Yang WX, Hua X, Li PJ, Zhang Y, Wang G. Inhibition of hepatic fibrosis with artificial microRNA using ultrasound and cationic liposome-bearing microbubbles. Gene Ther. 2013;20:1140-1148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 61. | Database of clinical trials, Reviewed December 08, 2014. Cited December 2014. Available from: https://www.ClinicalTrial.gov. |
| 62. | Arnberg N. Adenovirus receptors: implications for tropism, treatment and targeting. Rev Med Virol. 2009;19:165-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 101] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Tsolaki E S- Editor: Yu J L- Editor: Stewart G E- Editor: Wang CH