INTRODUCTION
Gastrointestinal cancers, comprised of esophageal, gastric and colorectal cancers, are some of the most common and fatal cancers worldwide. However, mortality rates have declined due to improved efforts in cancer prevention, earlier detection and a growing number of treatment options, especially in the development of therapies targeted to specific signaling pathways. These therapies are founded upon the body of basic scientific research that has implicated various signaling pathways in different gastrointestinal (GI) malignancies. Recent literature suggests that the nuclear factor kappa B (NF-κB) signaling cascade in particular may serve as a central mediator of carcinogenesis. The NF-κB signaling pathway has numerous physiological roles, including regulation of innate immunity and inflammation, and therefore may be involved in carcinogenesis in multiple ways[1,2]. The primary function of this signaling cascade is to protect the cell from harm, but if it becomes aberrant, then the transition from inflammation to cancerous growth can be elicited. In our previous study, we explored gastrointestinal epithelial response to injury and by extension intend to scrutinize NF-κB‘s effects on the gut in this review[3].
Recent epidemiological investigations have shown that chronic inflammation, including infection, is associated with 15%-20% of all human malignancies[4]. Numerous genetic studies have also exposed NF-κB’s potential role in inflammation-related cancers[4-7]. For example, NF-κB’s constitutive activation has been demonstrated in various human solid tumors, including those of ovarian, lung, breast, melanoma, hepatocellular, thyroid, pancreatic, prostate, colon, esophageal, gastric, laryngeal, parathyroid, bladder, endometrial, retinoblastoma, astrocytoma and squamous cell carcinoma of the head and neck[8-17]. Moreover, aberrant NF-κB regulation has been observed in hematopoietic malignancies, such as multiple myeloma, mantle cell lymphoma, MALT lymphoma, diffuse large B-cell lymphoma, Hodgkin’s lymphoma, myelodysplastic syndrome, adult T - cell leukemia, chronic lymphocytic leukemia, acute myeloid leukemia, and chronic myeloid leukemia[8,11,18,19].
NF-κB is a pleiotropic transcriptional factor that is encoded by a family of five main genes[5,20]. These genes contain the homologous domain Rel homology domain (RHD), which includes RelA (p65), RelB, CRel (REI), p50 and p52. NF-κB1 (p100) and NF-κB2 (p102) are processed to produce p52 and p50 respectively[21]. The RHD allows these proteins to homo- and hetero-dimerize, localize to the nucleus, bind to DNA and interact with the inhibitor of NF-κB (IκB)[22]. NF-κB homodimers or heterodimers remain inhibited in the cytoplasm bound to ankyrin rich regions of IκB proteins, which prevent NF-κB translocation to the nucleus and thus transcription[22]. The IκB family consists of IκBα, IκBβ, IκBγ, IκBε, BCL-3 and p100 and p105. It is through IκB kinase dependent (IKK-dependent) phosphorylation, polyubiquitination and proteasomal degradation of IκB proteins that the NF-κB proteins are liberated[22]. The IKK family consists of two catalytic subunits, IKKα and IKKβ, complexed to the regulatory subunit IKKγ/NEMO (NF-κB essential modulator)[22].
The tight regulation and activation of NF-κB is controlled by two principal signaling pathways, modulated by divergent but specific stimuli: the classical or canonical pathway and the alternative pathway[1,22]. With regards to the former, inflammatory stimuli, including TNF-α, IL-1β, TLRs and viruses, promote phosphorylation at two serine sites of IκB through the activation of IKKα, IKKβ and NEMO[21]. This leads to polyubiquitination at adjacent lysine residues and eventually proteolysis, releasing NF-κB dimers-most commonly p50/p65 heterodimers-and degradation of IκB[5]. The alternative pathway is characterized by its independence from IKKβ and NEMO and reliance on IKKα[22]. Following stimulation by TNF-α receptor superfamily members (lymphotoxin (LT) βR, B-cell activating factor receptor (BAFFR), receptor activator of NF-κB (RANK), and CD40), IKK mediates the phosphorylation of NF-κB2/p100:RelB complexed with an IκB[22]. The complex is subsequently polyubiquinated, leading to the proteasome-dependent processing of RelB’s inhibitor NF-κB2 p100, which results in the liberation of the ReIB-p50 heterodimer[1,21]. In both pathways, the dimers proceed to translocate to the nucleus, bind to NF-κB DNA sites and activate transcription. It should be noted that one of the first proteins transcribed in this process is IκBα, which then translocates to the nucleus and binds to the dimer to inhibit further transcription[5]. The classical pathway is implicated in preventing apoptosis and in innate immunity and inflammation[1,21]. The alternative pathway is involved in secondary lymphoid organogenesis and B cell maturation and survival[21]. Thus, both are responsible for cell survival and ultimately play a role in carcinogenesis.
NF-κB AND CANCER
Virchow first postulated and proposed the causal relationship between chronic inflammation and cancer. Chronic inflammation due to irritants is thought to lead to increased cell multiplication, predisposing cells to neoplastic DNA changes. An important mechanistic link may include NF-κB, as recent studies have suggested that NF-κB promotes tumor growth by inducing downstream proteins that have oncogenic effects[22]. Initial evidence of NF-κB’s link to cancer was the identification of the p50 subunit and RelA and its close homology with the oncoprotein v-REL of the avian REL retrovirus[23]. The onco-virus constitutively activates the v-REL oncoprotein, which is an important catalyst in the progression of lymphomas[23]. Similarly, the human T-cell leukemia virus’s oncoprotein TAX has been shown to constitutively activate the IKK complex, stimulating both pathways and again leading to upregulation of NF-κB’s downstream proteins[24].
Hanahan and Weinberg’s postulation of each step of tumorigenesis includes self-sufficiency in growth signals, insensitivity to growth inhibitor signals, evasion of apoptosis, limitless replicative potential, sustained angiogenesis, and tissue invasion and metastasis[25-28]. NF-κB can exert its effects on each of these aspects of tumorigenesis through the induction of downstream protein expression, and thus might be the basis of the transition from inflammation to cancer growth. Essentially, NF-κB controls cell proliferation by activating growth factors, including IL-2, granulocyte monocyte colony stimulator factor and CD40L[25,26]. It also serves as a positive regulator of cell cycle progression as it can activate c-myc and cyclin D1[26]. NF-κB inhibition of programmed cell death, through its regulation of the anti-apoptotic proteins ciAPS, c-FLP and members of the bcl-2 family, is also well documented[25,26]. These proteins are vital in sustaining genetically altered precancerous cells, and their effects therefore increase the probability of malignant changes. NF-κB activity can also lead to increased angiogenesis and metastasis through the upregulation of chemokines, such as IL-8 (migration), VEGF (angiogenesis) and MMP (spread). The altered expression of these genes, with NF-κB as a key mediator, is associated with tumor growth and spread[25,26]. Finally, the heavily scrutinized pro-inflammatory gene COX-2, which is under direct control of NF-κB, has been demonstrated to be closely involved in the process of carcinogenesis[25-30].
This review article will explore NF-κB’s role in cancers of the gastrointestinal tract, including esophageal, gastric and colonic. Discussion of each cancer will begin with a presentation of inflammatory stimuli, including infection and other inciting factors that affect NF-κB’s expression. Evidence will then be provided in support of the fact that NF-κB activity increases through progression of inflammation to cancer on a continuum that culminates in constitutive expression. The documented upregulation of NF-κB’s downstream proteins in each specific GI malignancy will be discussed next, with emphasis of COX-2 expression. This will be followed by an illustration of the importance of targeting the NF-κB pathway for greater therapeutic efficacy, including addressing chemoresistance. In essence, the amalgamation of the data presented in this review will uncover NF-κB as the mechanistic link between inflammation and tumor growth, specifically in gastrointestinal cancers. Finally, common themes will be clarified in emphasizing the connection between the two entities.
NF-κB’S ROLE IN ESOPHAGEAL CARCINOGENESIS
Esophageal cancer is responsible for roughly 16000 deaths in the United States per year; mortality depends on cell type (adenocarcinoma vs squamous), risk factors and location of pathology[31]. There is increasing evidence that the NF-κB pathway mediates the progression of inflamed esophageal epithelium through carcinogenesis[30,31]. This begins when the upper GI tract is exposed to noxious stimuli that promote inflammation and foster a propensity for cancerous growth[30,31]. Examples of such stimuli include tobacco smoke, alcohol, reflux, corrosive injury, nitrosamines, HPV and thermal injury[31]. Moreover, multiple studies link exposure to upregulation of NF-κB. One study conducted by Abdel-Latif et al[32] showed that bile acids and low pH induced expression of NF-κB in esophageal cell lines. Similarly, another study illustrated that physiological levels of bile acid not only activated NF-κB, but also led to increased expression of the downstream protein IL-8[33]. It is suggested that after epithelial cells are exposed to inflammatory stimuli, they defensively resort to activating the NF-κB pathway in order to prevent apoptosis and sustain existence by DNA repair[34]. This persistence predisposes the cells to malignancy as they continue to proliferate due to genetic alterations[35].
Involvement of NF-κB in promoting inflammatory changes to metaplasia and the progression to esophageal cancer has been demonstrated in the literature[36]. Current evidence supports constitutive NF-κB activity in cancerous growth of both adenocarcinoma and squamous cell carcinoma in in vivo and in vitro studies[11,36-38]. In one study, nuclear immunologic staining revealed increased nuclear expression of p50, p52 and ReI in patient biopsies of esophageal cancer cells compared to normal squamous cells[25]. Abdel-Latif et al[38] showed that overexpression of NF-κB in tumor tissues correlated with higher expression of Rel-A and lower expression of IκB when compared to expression levels in non-tumor cells. This was further supported by the finding that NF-κB expression was increased in patients of Barrett’s epithelium, however there was virtually no expression in patients with normal esophageal epithelium[38,39]. In another study, 40% NF-κB expression was noted in Barrett’s tissues, but markedly increased to 76% in adenocarcinomas[36]. In addition to demonstrating NF-κB’s presence in esophageal malignancy, studies have also correlated NF-κB activity with metastasis[40].
It is the downstream proteins that are responsible for exerting oncogenic effects, however, and these are vital in inflammation-related tumorigenesis. A number of studies have examined this increase in inflammatory proteins specific to esophageal malignancy. O’Riordan et al[37] demonstrated the elevation of proinflammatory cytokines IL-8 and IL-1B in esophagitis, Barrett’s epithelium and adenocarcinoma in human histological specimens. These cytokines contribute to tumor progression by regulating angiogenesis, sustaining cancer cell growth and promoting tumor cell migration. Investigators further found a significant association of NF-κB activation and cytokine upregulation in adenocarcinoma. Similar results have been found in other studies with regard to increased pro-inflammatory cytokines in esophageal cancerous growth[11,38]. Recent evidence also supports NF-κB’s role in invasion and metastasis in esophageal carcinomas as it leads to the upregulation of MMP-9 and reduction of E cadherin, two proteins involved in cell migration[41].
Another example illustrating NF-κB’s involvement in neoplastic progression is the overexpression of COX-2, an enzyme important in regulating prostaglandin synthesis[42]. In the context of cancer, increased COX-2 activity has been shown to be associated with key pathways that control cell proliferation, migration, apoptosis, and angiogenesis[11]. Previous studies have revealed increased simultaneous expression of NF-κB and COX-2 in cells exposed to inflammation and cells of malignant growth when compared to normal esophageal mucosa[29]. Studies exploring the effect of COX-2 inhibitors have also shed light upon the relationship between COX-2 and progression to malignancy. Liu et al[43] studied the effects of aspirin at different concentrations and different times in esophageal squamous cell carcinoma cell lines and found that aspirin significantly reduced COX-2 mRNA and protein expression and prostaglandin synthesis. The study’s results demonstrate a dose-dependent response to aspirin in reducing cell proliferation and inducing apoptosis. In a recent meta-analysis, use of aspirin and other COX inhibitors was shown to be associated with reduced risk of esophageal adenocarcinoma among patients. They noted that the use of COX inhibitors decreased the risk of transition from Barrett’s esophagus to esophageal cancer. Many in vivo and in vitro studies have reported similar results and advocate for aspirin use in both preventing and treating abnormal esophageal changes.
Patients with esophageal cancer often have a poor prognosis due to late presentation of the disease. Therefore, it is important to explore new treatments, such as those that target NF-κB[44,45]. In esophageal adenocarcinoma, elevated NF-κB expression is associated with advanced stages and inversely correlated with response to neoadjuvant chemotherapy and radiation[46,47]. NF-κB expression in esophageal cancers is indicative of poor prognosis and may be implicated in multidrug resistance and treatment failure. In a recent study, NF-κB activity in patients with esophageal squamous and adenocarcinoma correlated with TMN staging, increased susceptibility to spread, chemoresistance and overall poor survival rate[48]. Interestingly, their data also demonstrated patients who were established to have NF-κB negative cancer prior to treatment became positive after receiving chemotherapy, suggesting chemotherapy as a potential initiator of tumor resistance through upregulation of NF-κB[48]. Treatment with chemotherapy may induce resistance to the therapy, and therefore, result in poorer prognosis. In addition, Izzo et al[48] showed that after constitutive NF-κB activity was established, exposure to NF-κB inhibitors, such as Bay11-7082 and sulfasalazine, reduced cancer cell proliferation and induced apoptosis. They also found that treatment with these inhibitors in combination with chemotherapeutic drugs 5-fluorouracil and cisplatin led to a synergistic effect on inhibiting cell growth, decreasing chemoresistance[11]. This investigation also illustrated that Bay 11 was associated with decreased tumor growth and angiogenesis in animal studies[11]. Tian et al[49] found similar results with siRNA molecules of p65. They first found p65 expression was decreased in ESCC cell lines when exposed to siRNA p65 after establishing constitutive activity of the NF-κB signaling pathway. Moreover, they too found a synergistic effect of siRNA with 5FU as ESCC cells became more sensitive to 5-FU after exposure to siRNA p65. Finally, utilization of neoadjuvant treatment with NF-κB inhibitors in the management of esophageal carcinomas was shown to help in a specific cohort of refractory treatment cases[46,47].
NF-κB’S ROLE IN GASTRIC CARCINOGENESIS
Gastric cancer further elucidates the role of NF-κB as a central mediator of carcinogenesis and as an important link between inflammation and cancer[50,51]. Gastric cancer, especially the intestinal type, entails the progression from chronic gastritis to chronic atrophic gastritis to intestinal metaplasia, then dysplasia and finally adenocarcinoma[52]. The initiation of chronic inflammation typically begins with the presence of such risk factors as Helicobacter pylori (H. pylori) infection, pernicious anemia, smoking or high salt diet. This leads to longstanding chronic superficial gastritis, which eventually progresses to intestinal metaplasia followed by adenocarcinoma[53,54]. H. pylori’s detrimental effect on gastric mucosa has been well established in the literature through clinical data, animal studies and cell cultures[52,53]. However, only certain H. pylori strains have been shown to be involved in malignancy and these have the pathogenic island, CagA[54]. Current studies have established that CagA-positive H. pylori significantly contributes to gastric cancer cell tissue invasion[55,56]. Wu et al[57] visualized the link between CagA-positive H. pylori and NF-κB by using NF-κB blockers. Exposing gastric cancer cells infected by CagA-positive strains to NF-κB blockers significantly reduced tissue invasiveness. This not only supported CagA’s effects as a pathogenic factor, but demonstrated the significant role of NF-κB. These data support the idea that H. pylori becomes a potent NF-κB activator if it carries CagA pathogenicity. The exact molecular pathway between CagA and NF-κB cannot be addressed due to a paucity of evidence. Some studies speculate the connection lies in the protein transforming growth factor-beta-activated kinase 1 (TAK1)[54].
Constitutive activation of the NF-κB pathway and its increased production in gastric cancer cells of human tissue and cell lines has been demonstrated through various laboratory techniques, including IHC, EMSA and Western blotting[5,57,58]. Constitutive NF-κB activity has also been documented in H. pylori gastritis and diffuse gastric cancer[11,59]. Although chronic irritants initiate mucosal inflammation, it is the NF-κB pathway which is vital in sustaining the gastritis-metaplasia-carcinoma sequence. Studies have shown that NF-κB is notably higher in patients with H. pylori gastritis, and this is significantly correlated with histological scores of gastritis[60]. In intestinal type gastric cancer, increased NF-κB expression is strongly associated with CagA strain-induced metaplasia, dysplasia and gastric cancer itself[56]. This is currently under debate for diffuse type gastric cancer. Yamanaka et al[61] identified a major function of NF-κB in carcinogenesis when they found that patients with higher NF-κB activity had a shorter survival rate and concluded that NF-κB was a prognostic indicator in gastric cancer. Interestingly, NF-κB expression is also associated with stage, depth of invasion, WHO classification and Lauren’s histological classification[11,51,52,58,62]. Levidou et al[11] further concluded through multivariate survival analysis that NF-κB1 expression was an independent predictor of gastric cancer prognosis.
For the gastritis-to-carcinoma sequence to be successful, each step requires a pattern of oncoprotein expression to promote carcinogenesis. As in esophageal cancer, NF-κB regulates these oncoproteins in gastric cancer as well in terms of endorsing tumor growth, preventing apoptosis, and initiating tumor angiogenesis and migration. Keates et al[63] demonstrated an increased activation of NF-κB and its downstream proteins by infecting gastric epithelial cells with H. pylori and then observing increases in p50/p65 heterodimers and p50 dimers, followed by increased IL-8 mRNA and protein synthesis. Yin et al[64] found that IL-6 and VEGF were significantly increased in NF-κB positive gastric cancer cells compared to their levels in normal mucosa. Like IL-8, IL-6 is also associated with malignancy-both facilitate cancer cell apoptosis and stimulation of angiogenesis. VEGF is a well-known growth factor implicated in angiogenesis and therefore is crucial in carcinogenesis[65]. Other studies have shown that gastric cell lines exposed to H. pylori have displayed upregulation of important tumorigenesis markers including MMP-9, VEGF and COX-2[50].
NF-κB is also an important regulator of COX-2 in gastric cancer as it is in esophageal cancer. Expression of NF-κB and COX-2 in the same neoplastic mucosa has been established in previous studies[66,67]. Studies exploring COX-2 inhibitors also shed light upon the specific actions of COX-2 and its potential applications[58,68]. In one study, p50 positive cells treated with COX inhibitors showed a dose-dependent suppression of cell growth in gastric cells[66]. A large population-based case-control study conducted by Farrow et al[69] revealed that patients who took aspirin or NSAIDs had a decreased risk of gastric and esophageal cancer compared to never users. Evidence provides support that COX-2 inhibitors may prevent gastric carcinogenesis and can aid in treating gastric malignancy[69]. COX-2 expression is, in part, facilitated by NF-κB expression; if the central regulator can be inhibited, then all downstream proteins, including COX-2, may be suppressed, thereby resulting in reduced oncogenesis[50].
Despite advances in current therapeutic approaches, gastric cancer is still one the most prevalent cancers and the second leading cause of death due to cancer, worldwide. Therefore, new treatment options should be explored by scrutinizing the molecular pathway of the gastritis- adenocarcinoma sequence. The NF-κB pathway provides a link between inflammation and cancer and can explain many of the hallmarks of cancer[50,52]. Moreover, literature has illustrated the dysregulation of the NF-κB signaling pathway in gastric cancer, with its presence indicating poor prognosis[61]. Studies have shown in CagA-mediated gastric cancer that cell migration is attenuated by NF-κB inhibitors. These results were expanded upon in a study conducted by Keates et al[63], which found that pretreatment with PDTC, a potent NF-κB inhibitor, reduced H. pylori-activated p65 and IL-8. In one study, parthenolide, another NF-κB inhibitor, was used on three gastric cancer cell lines and significantly induced apoptosis in all three[70]. They also found a synergistic effect when NF-κB inhibitors were combined with chemotherapeutic drugs[71]. This finding is analogous to that of combining NF-κB inhibitors and chemotherapy in addressing esophageal cancers.
NF-κB’S ROLE IN COLORECTAL CARCINOGENESIS
The development of colorectal cancer (CRC) is a multistep process and there is growing evidence in favor of the connection between inflammation and carcinogenesis. NF-κB has become the main focus of this neoplastic transformation. CRC can be divided into sporadic CRC, hereditary CRC and colitis-associated carcinoma (CAC). CAC primarily stems from the two major forms of inflammatory bowel disease (IBD): ulcerative colitis and Crohn’s disease. NF-κB’s role as the mechanistic link between inflammation and cancer has been intensively investigated regarding constitutive activity of NF-κB in both IBDs and CAC[71-74]. CAC is one of the most well-known examples of an inflammation-dysplasia-carcinoma sequence[73,75]. IBD prompts a chronic inflammatory state leading to the constant production of noxious compounds including reactive oxygen species (ROS) and other cytokines (TNF-α, IL-6, and IL-1). In the long term, ROS are detrimental as they repress regulators of DNA damage and induce mutagenic enzymes, such as activation-induced cytidine deaminase[74]. Furthermore, studies indicate that both ROS and cytokines activate the NF-κB pathway[74]. These cytokines activate signal transducer and activator of transcription 3 (STAT3) in intestinal epithelial cells. This, in turn, contributes to further inflammation, perpetuating noxious stimuli and ultimately creating a positive feedback loop. The combination of DNA damaging agents and inflammatory cytokines results in constant activation of the NF-κB pathway, setting up the progression to carcinogenesis.
Overexpression of NF-κB has been demonstrated in colon cancer cell lines and human tumor specimens, including those of sporadic CRC and CAC[74,76]. This is synonymous with genetic syndromes including familial adenomatous polyposis (FAP) and hereditary non-polyposis cancer (HNPCC)[72]. The expression of NF-κB was determined to be significantly higher in the adenocarcinoma tissue compared to expression levels in tissue specimens earlier in the inflammation-adenocarcinoma sequence[77,78]. Furthermore, studies have shown that NF-κB activity increases in association with histological tumor progression[74,79]. This indicates that NF-κB activity correspondingly increases from the initiation of inflammation to malignant proliferation. Notably, one study concluded that NF-κB activation in CRC served as a poor prognostic indicator[78].
As in esophageal and gastric cancer, NF-κB regulates expression of various oncoproteins in colorectal cancer as well to sustain inflammation and support cell proliferation, inhibition of apoptosis, angiogenesis, invasion and metastasis. Overexpression of NF-κB and its downstream effectors are well recognized in CRC[56,79]. For example, IL-8 upregulation was also described in CRC and was identified in various ways including microarray and protein array analyses[28]. In addition, another cytokine, IL-6, has been found to be weakly correlated with poor prognosis in CRC. In a well-known study, Greten et al[24] investigated the role of NF-κB in inflammation-associated tumor growth using a mouse model and found that IKKβ deletion led to a direct decrease in tumor incidence. They attributed these results to decreased expression of anti-apoptotic proteins. CRC also has a significant association between NF-κB overexpression and VEGF[24].
Another emerging theme is the relationship between NF-κB and its downstream protein COX-2. Studies of CRC have shown an upregulation of both NF-κB and COX-2 and the literature also supports their individual and coexistent overexpression[23,24,27,75,78-82]. Maihöfner et al[83] studied surgically resected tissues of CRC patients and found a high expression of p65, IL-6 and COX-2 compared to controls[81,82]. It is well established that regular aspirin use is associated with a significant reduction in the risk of COX-2-positive CRC[80]. The relative risk of CRC is reduced by 40%-50% when patients consume aspirin or NSAIDs over a period of 10-15 years[24]. Moreover, epidemiologic evidence shows that NSAID usage protects against CRC to a greater extent than against other GI malignancies. In one study, aspirin exposure resulted in apoptosis of colorectal cancer cell lines in a concentration-dependent manner[83]. Specifically, given that CAC is the result of inflammatory processes, it follows that anti-inflammatory medication would reduce the risk of cancer.
Inhibition of the master regulator NF-κB will shut off the different steps of carcinogenesis. NF-κB inhibitors, such as sulfasalazine, mesalamine, and glucocorticoids, have already shown promise in treating IBD[23]. Gan et al[84] explored the effects of sulfasalazine on the NF-κB signaling pathway. After establishing that patients with ulcerative colitis (UC) had higher NF-κB and downstream proteins including IL-6 and IL-8, those who were exposed to sulfasalazine demonstrated a decrease in all three proteins[84]. Corticosteroids strongly inhibit NF-κB activation in vivo and in vitro, and clinical trials have found dexamethasone to be useful in inhibiting CRC metastasis. One study illustrated this by using NF-κB inhibitors and displaying that blocking the action of NF-κB led to inhibition of angiogenesis in CRC[79]. Another study reported these same inhibitors prompted apoptosis, as NF-κB could not overcome the anti-apoptotic machinery to exert pro-survival activity[72]. After establishing constitutive activity of NF-κB in CRC, Lind et al[72] further showed that NF-κB binding was increased in CRC cell lines after exposure to the chemotherapeutic agent. In contrast, pretreating cells with NF-κB inhibitors prior to administration of gemcitabine showed a decrease in tumor size. The common theme of chemoresistance resurfaces and NF-κB may be involved in its development. With regards to CRC, NF-κB inhibitors are already showing clinical applications. Further research should be employed, as this pathway is involved in many innate processes and therefore may have a large range of systemic side effects.
CONCLUSION
This review showed support for NF-κB’s role in inflammation-associated cancers. NF-κB activation is elicited by various inflammatory stimuli (Figure 1). Chronic irritation induces constitutive NF-κB activity, promoting carcinogenesis (Figure 1). NF-κB’s mechanistic link to cancer is best displayed through its pleiotropic effects, as it leads to upregulation of important proteins that promote tumor progression (Figure 1). Upregulation of COX-2 by NF-κB in GI malignancies is consistently supported in the literature and the efficacy of COX-2 inhibitors is still being scrutinized. This review also confirmed the presence of NF-κB in advanced gastrointestinal malignancies. Additionally, this review noted that NF-κB may play a role in developing chemo-resistance in gastrointestinal malignancies. Recent investigations by Vyas et al[85] postulate that chemotherapies may pose therapy-induced resistance by stimulating various signaling pathways including the NF-κB cascade. The authors clearly delineated literature supporting that first-line chemotherapy agents such as doxorubicin, 5-FU, cisplatin and paclitaxel commonly induce NF-κB production. They showed that this led to increased expression of downstream proteins that promote proliferation, anti-apoptosis and angiogenesis to sustain tumor growth[85]. These findings demonstrated the need for further research in discovering common themes among the gastrointestinal malignancies. Interestingly, Kim et al[86] recently reported a significant increase in the protein caspase-associated recruitment domain 6 (CARD6), a NF-κB activator in esophageal, gastric and colorectal tissues[87]. Finally, targeted therapy focusing upon NF-κB inhibition and its outcomes for the individual cancers was also discussed. The amalgamation of all the literature presented in this review emphasizes the importance of exploring molecular targeted therapy to hone in on NF-κB. In addition, although NF-κB is the central mediator, targeting the regulators of the actual pathway itself could also prove fruitful. For instance, the use of histone deacetylases (HDACs) has gained popularity as an emerging strategy in inhibiting the NF-κB pathway. Histone acetyltransferases and deacetylases modulate NF-κB activity through their actions on the Re1A/p65 subunit[87]. Yun et al[88] showed acetylation of Re1A/p65 subsequently increased NF-κB activation and deacetylation lead to diminished levels of Re1A/p65 and thus NF-κB. This was further explored when Yeung et al[89] demonstrated that the use of sirtuins, a group of nicotinamide adenosine dinucleotide-dependent HDACs, led to increased apoptosis as these compounds decreased NF-κB levels; this is yet another potential strategy for intervention in the future.
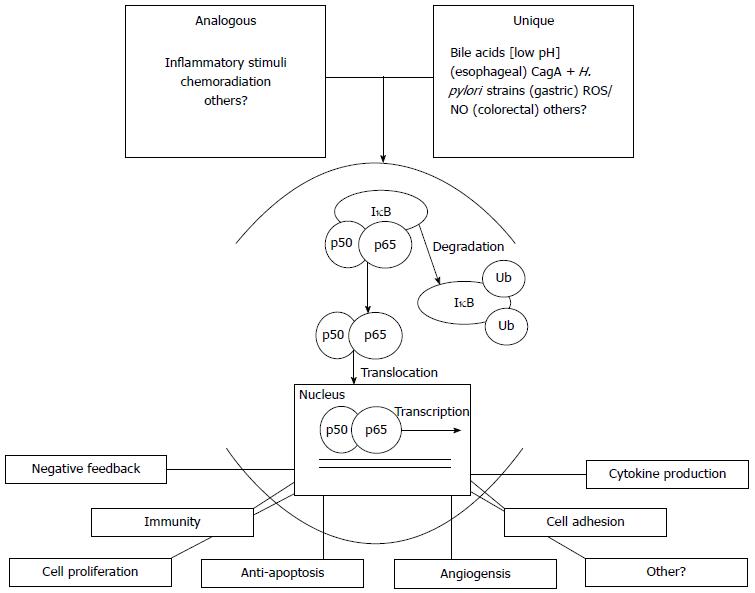
Figure 1 Summary model representing stimulation of the NF-κB pathway leading to the classical pathway, followed by expression of NF-κB dependent proteins.
Analogous inflammatory stimuli are common amongst esophageal, gastric and colorectal tissue. The unique stimuli commonly induce inflammation. The classic pathway is displayed and this model would be consistent with the alternate pathway. An array of genes are transcribed and translated contributing to the carcinogenesis. ROS: Reactive oxygen species.
NF-κB may induce inflammatory-associated gastrointestinal carcinomas which are often refractory to current treatments. We, therefore, urge greater exploration and development of therapies specifically targeting the NF-κB pathway as these may prove more successful for patients than existing therapeutic options.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Bashashati M, Passi A S- Editor: Qi Y L- Editor: A E- Editor: Liu XM