Published online Mar 14, 2015. doi: 10.3748/wjg.v21.i10.3100
Peer-review started: August 14, 2014
First decision: September 15, 2014
Revised: September 18, 2014
Accepted: October 14, 2014
Article in press: October 15, 2014
Published online: March 14, 2015
Processing time: 214 Days and 19 Hours
AIM: To explore effects of nonselective beta-blockers (NSBBs) in cirrhotic patients with no or small varices.
METHODS: The PubMed, EMBASE, Science Direct, and Cochrane library databases were searched for relevant papers. A meta-analysis was performed using ORs with 95%CI as the effect sizes. Subgroup analysis was conducted according to the studies including patients without varices and those with small varices.
RESULTS: Overall, 784 papers were initially retrieved from the database searches, of which six randomized controlled trials were included in the meta-analysis. The incidences of large varices development (OR = 1.05, 95%CI: 0.25-4.36; P = 0.95), first upper gastrointestinal bleeding (OR = 0.59, 95%CI: 0.24-1.47; P = 0.26), and death (OR = 0.70, 95%CI: 0.45-1.10; P = 0.12) were similar between NSBB and placebo groups. However, the incidence of adverse events was significantly higher in the NSBB group compared with the placebo group (OR = 3.47, 95%CI: 1.45-8.33; P = 0.005). The results of subgroup analyses were similar to those of overall analyses.
CONCLUSION: The results of this meta-analysis indicate that NSBBs should not be recommended for cirrhotic patients with no or small varices.
Core tip: Nonselective beta-blockers have been recommended for the primary and secondary prophylaxis of variceal bleeding in cirrhotic patients with high-risk varices and those with previous bleeding. However, their role remains uncertain in cirrhotic patients with no or small varices. Our meta-analysis demonstrates that the use of nonselective beta-blockers should not be recommended for cirrhotic patients with no or small varices.
- Citation: Qi XS, Bao YX, Bai M, Xu WD, Dai JN, Guo XZ. Nonselective beta-blockers in cirrhotic patients with no or small varices: A meta-analysis. World J Gastroenterol 2015; 21(10): 3100-3108
- URL: https://www.wjgnet.com/1007-9327/full/v21/i10/3100.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i10.3100
Variceal bleeding is the most common lethal complication of liver cirrhosis[1]. The first variceal bleeding can lead to a six-week mortality of 15%-20%. Based on the results of meta-analyses and numerous randomized controlled trials (RCTs)[2-7], the current practice guidelines and consensus have clearly recommended the use of nonselective beta-blockers (NSBBs) for the primary prophylaxis of variceal bleeding in cirrhotic patients with medium or large varices without any previous bleeding and for the secondary prophylaxis in those with a history of variceal bleeding[8-10]. However, the recommendations of NSBBs in cirrhotic patients with no or small varices remain obscure. Herein, we collected all available data from RCTs to explore whether the use of NSBBs could prevent the development of large varices and first variceal bleeding, improve the survival, and increase the incidence of adverse events in such patients.
The PubMed, EMBASE, ScienceDirect, and Cochrane library databases were searched for relevant papers. The last search was performed on May 3, 2014. Eligibility criteria were as follows: (1) the study design should be RCT; (2) the outcomes should include the change in the diameter of varices and/or development of variceal bleeding; (3) the participants should include the cirrhotic patients with no varices and those with small or low-risk varices, but without any previous bleeding; (4) the intervention should be NSBBs; and (5) the comparator should be placebo or no active treatment. Because the detailed information regarding small varices was different among studies, we did not arbitrarily employ any sole definition. However, small varices should be identified according to the pre-existing criteria. Notably, the data concerning medium to large varices were excluded from our studies.
The primary items extracted were as follows: the study design, enrollment period, target population, definition of small varices, number of patients, age, sex, underlying etiology of liver diseases, follow-up information, number of patients with no and small varices, incidence of development of large varices, incidence of first upper-gastrointestinal bleeding, mortality, and incidence of adverse events.
The Cochrane Collaboration’s tool for assessing the risk of bias was employed. It included six entries: the random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data addressed, and selective reporting. If one study had more than two “high-risk” entries, it was considered to be of low quality; otherwise, it was considered to be of high quality.
Odds ratios (ORs) with 95% confidence intervals (CIs) were calculated to assess the effect of dichotomous data. I2 and P values were calculated to assess the heterogeneity among studies (I2 > 50% and/or P < 0.1 were considered statistically significant). The ORs were pooled using only a random effects model to calculate a more conservative result. Publication bias was evaluated by Egger’s test. Subgroup analyses were performed according to the patients with small and no varices. The difference of subgroup results was also tested. Sensitivity analyses were performed in the high-quality studies. P < 0.05 was considered to have a statistically significant difference in the outcomes between NSBBs and placebo groups. Review Manager version 5.1.6 software (The Nordic Cochrane Centre, Copenhagen, Denmark) and StatsDirect version 3.0.113 software (StatsDirect Ltd., Cheshire, United Kingdom) were employed for the statistical analyses.
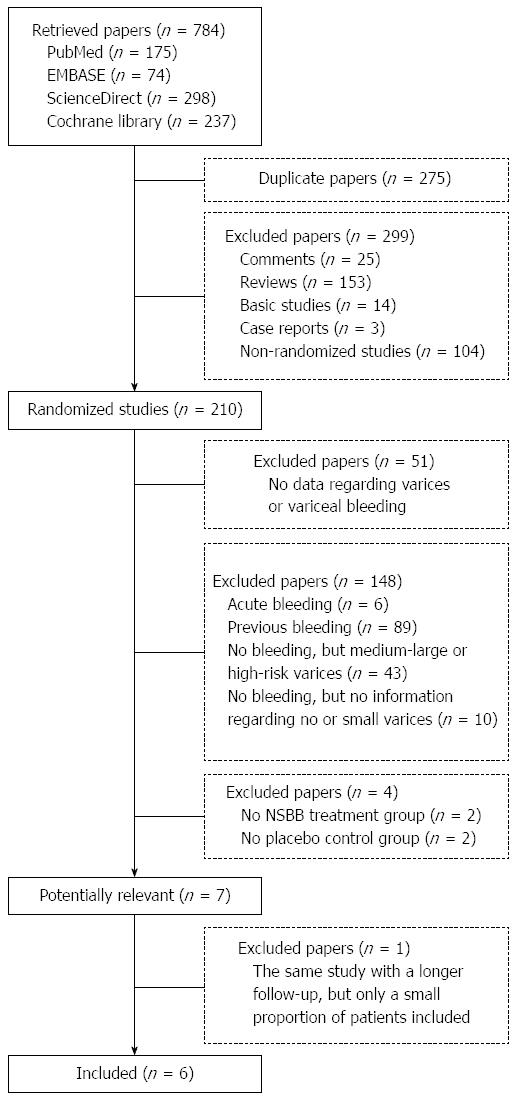
A total of 784 papers were retrieved from the four databases, among which seven papers were considered potentially relevant[11-17]. Notably, one of them was excluded because it included only a smaller proportion of patients than another paper by the same team[16,17]. Finally, six papers were included in the meta-analysis[11-16] (Figure 1). Study and patient characteristics are summarized in Tables 1 and 2, respectively.
| Ref. | Year | Study design and regions | Period of enrollment | Target population | Groups | Definitions of small varices | n |
| Andreani et al[11] | 1990 | Multi-center RCT from two centers in Paris | Nov. 1985 to Feb. 1988 | LC without previous bleeding, but with esophageal varices (small or large) | Propranolol vs placebo | Non-confluent esophageal varices flattened by insufflation | 84 |
| Conn et al[16] | 1991 | Multi-center double-blinded RCT from three centers in the United States and Spain | Oct. 1982 to Aug. 1986 | LC without previous bleeding, but with esophageal varices (small or large) | Propranolol vs placebo | Diameter: 1-3 mm with Valsalva | 102 |
| Calés et al[12] | 1999 | Multi-center double-blinded RCT from 14 centers in France | April 1991 to June 1993 | LC without varices or small esophageal varices | Propranolol vs placebo | Diameter: < 5 mm | 206 |
| Merkel et al[14] | 2004 | Multi-center single-blinded RCT from seven hospitals in Italy | Dec. 1996 to April 2000 | LC with small varices | Nadolol vs placebo | F1 without red signs according to Beppu et al[27] (small straight varices, minimally elevated on the esophageal mucosal surface) | 161 |
| Groszmann et al[13] | 2005 | Multi-center double-blinded RCT from four hospitals in the United States, Spain, and United Kingdom | Aug. 1993 to March 1999 | LC with an HVPG of ³ 6 mmHg, and without gastroesophageal varices | Timolol vs placebo | NA | 213 |
| Sarin et al[15] | 2013 | Single-center single-blinded RCT in India | Oct. 2004 to June 2007 | LC with small varices, without any history of variceal bleed | Propranolol vs placebo | Grade 1 or 2 according to the classification of Conn[28] or small according to de Franchis et al[29] | 150 |
| Ref. | Groups | n | Age (yr) | Sex (M/F) | Etiology (alcohol/viral/other) | Child-Pugh score or class A/B/C | Follow-up (mo) | Lost to follow-up | Small varices, n | No varices, n |
| Andreani et al[11] | Propranolol | 43 | 55.0 ± 1.3 | 27/16 | 33/-/10 | 10/19/13 | NA | 6 | 15 | 0 |
| Placebo | 41 | 55.6 ± 1.7 | 23/18 | 33/-/8 | 10/21/10 | NA | 2 | 17 | 0 | |
| Conn et al[16] | Propranolol | 51 | 54 ± 9 | 38/13 | 39/-/12 | Mean: 8.0 | 17.1 ± 10.9 | NA | 26 | 0 |
| Placebo | 51 | 54 ± 11 | 35/16 | 41/-/10 | Mean: 8.3 | 16.3 ± 12 | NA | 29 | 0 | |
| Calés et al[12] | Propranolol | 102 | 52.7 ± 10.4 | 69/33 | 88/-/24 | 6.8 ± 2.1 | NA | 41 | 60 | 42 |
| Placebo | 104 | 52.7 ± 11.4 | 68/36 | 81/-/23 | 6.8 ± 2.0 | NA | 32 | 67 | 37 | |
| Merkel et al[14] | Nadolol | 83 | 56 ± 9 | 45/38 | 47/34/2 | 6.8 ± 1.6 | 36 ± 18 | 11 | 83 | 0 |
| Placebo | 78 | 57 ± 9 | 38/40 | 45/28/5 | 7.1 ± 1.9 | 35 ± 15 | 10 | 78 | 0 | |
| Groszmann et al[13] | Timolol | 108 | 46 ± 11 | 70/38 | 26/73/9 | 5.4 ± 0.7 | Median: 52.7 | 0 | 0 | 108 |
| Placebo | 105 | 44 ± 11 | 56/49 | 25/69/11 | 5.4 ± 0.8 | Median: 57.9 | 0 | 0 | 105 | |
| Sarin et al[15] | Propranolol | 77 | 42 ± 13 | 63/14 | 27/42/8 | 7.4 ± 1.9 | 25 ± 12.6 | 0 | 77 | 0 |
| Placebo | 73 | 44 ± 13 | 57/16 | 26/38/9 | 7.7 ± 2.3 | 0 | 73 | 0 |
In two studies, the target populations were cirrhotic patients with endoscopically documented varices, irrespective of sizes[11,16]. Only the data regarding small varices were employed for meta-analyses. In one study, the target populations included cirrhotic patients with no varices and those with small varices[12]. In one study, the target populations were cirrhotic patients without any varices[13]. In two studies, the target populations were cirrhotic patients with small varices[14,15].
NSBBs included propranolol in four studies[11,12,15,16], timolol in one study[13], and nadolol in one study[14]. Placebo included vitamin K in one study[11] and a tablet that was identical to NSBBs in appearance in two studies[13,16]. The detailed information regarding placebo was not available in three studies[12,14,15].
The baseline characteristics regarding age, sex, etiology of liver cirrhosis, and Child-Pugh score of patients were comparable between NSBBs and placebo groups. Notably, only the characteristics of all included patients, but not those of patients with small varices, could be extracted in two studies[11,16].
Risk of bias assessment for each study is summarized in Table 3. Two studies were of low quality[11,14], and four were of high quality[12,13,15,16].
| Entry | Judgment | Support for judgment |
| Andreani (1990) | ||
| Random sequence generation (selection bias) | Low risk | Quote: “the patients in each center were randomly assigned” |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) | High risk | Quote: “these treatments were not administered blindly” |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described |
| Incomplete outcome data addressed (attrition bias) | High risk | Quote: “Fourteen patients were lost to follow-up after a period of 4.9 ± 1.9 mo (propranolol=six, sclerosis=six, placebo=two)” |
| Selective reporting (reporting bias) | Low risk | Both potential efficacy and complications were reported. Review authors do not believe that bias will be introduced. |
| Conn (1991) | ||
| Random sequence generation (selection bias) | Low risk | Quote: “the patients were randomly selected” |
| Allocation concealment (selection bias) | Low risk | Quote: “using a sealed envelope technique and computer-generated randomization” |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: “double-blinded”, “The placebo and the propranolol tablets were identical in appearance” |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: “double-blinded”, “the patients were examined on each visit by a nurse and the postdoctoral fellow assigned to the study” |
| Incomplete outcome data addressed (attrition bias) | Unclear risk | Not described |
| Selective reporting (reporting bias) | Low risk | Both potential efficacy and complications were reported. Review authors do not believe that bias will be introduced |
| Cales (1999) | ||
| Random sequence generation (selection bias) | Low risk | Quote: “patients were randomized” |
| Allocation concealment (selection bias) | Low risk | Quote: “by the opaque sealed envelope method” |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: “double-blinded”, “Patients and physicians were unaware of the treatment” |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: “double-blinded”, “Patients and physicians were unaware of the treatment” |
| Incomplete outcome data addressed (attrition bias) | High risk | Quote: “In the propranolol group, 41 patients were lost to follow-up, compared with 32 in the placebo group” |
| Selective reporting (reporting bias) | Low risk | Both potential efficacy and complications were reported. Review authors do not believe that bias will be introduced |
| Merkel (2004) | ||
| Random sequence generation (selection bias) | Low risk | Quote: “A total of 83 patients were randomized to” |
| Allocation concealment (selection bias) | Low risk | Quote: “Randomization was generated by tables of random numbers, stratified by participating centers, prepared at the University of Padua, and administered by opaque sealed and consecutively numbered envelopes containing randomization” |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: “The single-blind study design was chosen” |
| Blinding of outcome assessment (detection bias) | High risk | Quote: “The single-blind study design was chosen” |
| Incomplete outcome data addressed (attrition bias) | High risk | Quote: “11 patients randomized to nadolol and 10 patients randomized to placebo were lost to follow-up” |
| Selective reporting (reporting bias) | Low risk | Both potential efficacy and complications were reported. Review authors do not believe that bias will be introduced |
| Groszmann (2005) | ||
| Random sequence generation (selection bias) | Low risk | Quote: “patients were randomly assigned” |
| Allocation concealment (selection bias) | Low risk | Quote: “The randomization code was generated by computer for each participating center” |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: “The study was an investigator-initiated, randomized, double-blind, placebo-controlled, clinical trial conducted at four sites” |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: “double-blinded”, “To maintain study blinding, the patient’s heart rate was measured by the study nurse and not by the investigators” |
| Incomplete outcome data addressed (attrition bias) | Low risk | Quote: “The remaining 277 were excluded for the following reasons: …6 were lost to follow-up…” Patients who were lost to follow-up were excluded from the final analysis |
| Selective reporting (reporting bias) | Low risk | Both potential efficacy and complications were reported. Review authors do not believe that bias will be introduced |
| Sarin (2012) | ||
| Random sequence generation (selection bias) | Low risk | Quote: “Patients were randomly assigned” |
| Allocation concealment (selection bias) | Low risk | Quote: “All randomizations were done by computer-generated random numbers” |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: “single-blind” |
| Blinding of outcome assessment (detection bias) | High risk | Quote: “single-blind” |
| Incomplete outcome data addressed (attrition bias) | Low risk | Quote: “Another 14 patients were excluded because they dropped out before the completion of 6 months of study” Patients who were lost to follow-up were excluded from the final analysis |
| Selective reporting (reporting bias) | Low risk | Both potential efficacy and complications were reported. Review authors do not believe that bias will be introduced |
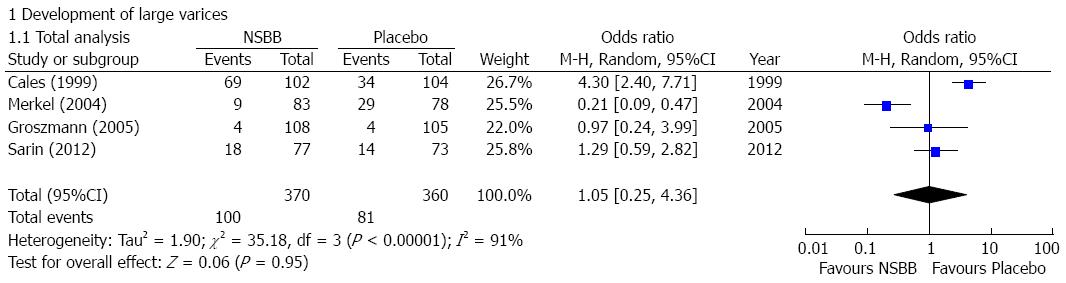
Development of large varices: Four studies reported the data regarding the development of large varices in cirrhotic patients with no or small varices[12-15]. The incidence of development of large varices was similar between NSBBs and placebo groups (OR = 1.05, 95%CI: 0.25-4.36; P = 0.95) (Figure 2). Heterogeneity among studies was significant (I2 = 91%; P < 0.01). Publication bias was not significant (Egger’s bias = -5.64, 95%CI: -32.848-21.565; P = 0.47).
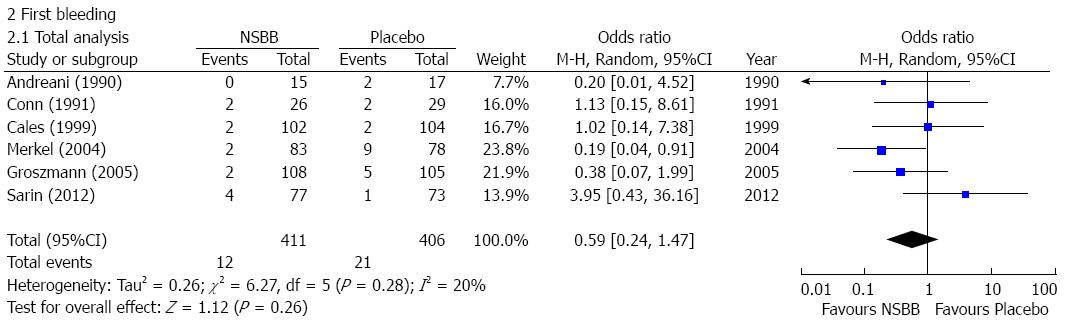
First upper-gastrointestinal bleeding: Six studies reported the occurrence of first upper-gastrointestinal bleeding in cirrhotic patients with no or small varices[11-16]. The incidence of first upper-gastrointestinal bleeding was not significantly different between NSBBs and placebo group (OR = 0.59, 95%CI: 0.24-1.47; P = 0.26) (Figure 3). Heterogeneity among studies was not significant (I2 = 20%; P = 0.28). Publication bias was not significant (Egger’s bias = 1.55, 95%CI: -4.995-8.086; P = 0.55).
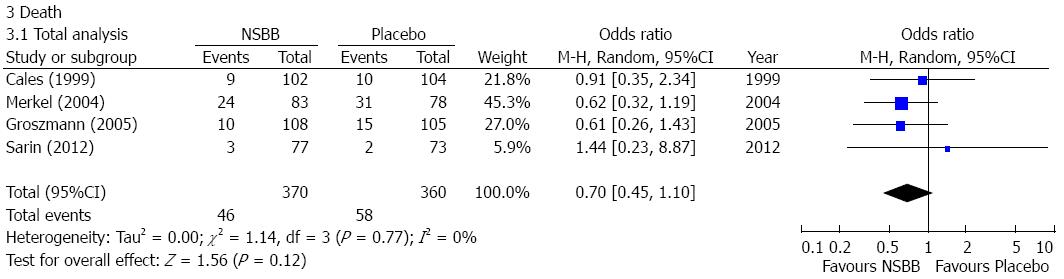
Death: Four studies reported the data regarding the death in cirrhotic patients with no or small varices[12-15]. The incidence of death was lower in the NSBBs group than the placebo group, but the difference was not statistically significant (OR = 0.70, 95%CI: 0.45-1.10; P = 0.12) (Figure 4). Heterogeneity among studies was not significant (I2 = 0%; P = 0.77). Publication bias was not significant (Egger’s bias = 1.53, 95%CI: -0.939-3.993; P = 0.12).
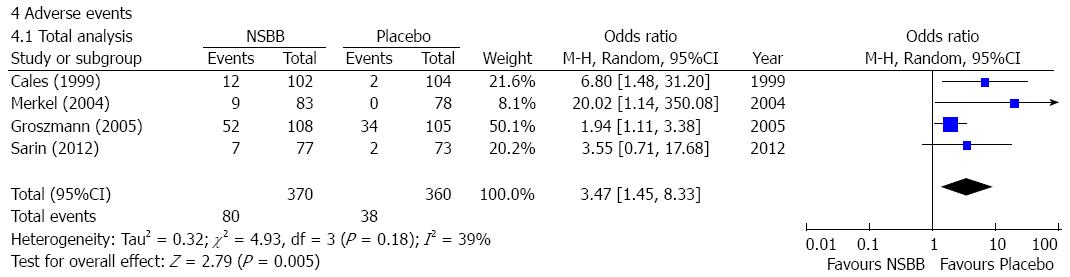
Adverse events: Four studies reported adverse events in cirrhotic patients with no or small varices[12-15]. The incidence of adverse events was significantly higher in the NSBBs group than the placebo group (OR = 3.47, 95%CI: 1.45-8.33; P < 0.01) (Figure 5). Heterogeneity among studies was not significant (I2 = 39%; P = 0.18). Publication bias was significant (Egger’s bias = 1.89, 95%CI: 0.075-3.701; P < 0.05).
The results of the subgroup analyses were similar to those of the overall analysis, with no significant differences between patients with no varices or small varices (Table 4).
| Category | Studies, | Patients, | OR (95%CI) | Heterogeneity | Subgroup difference |
| n | n | ||||
| Development of large varices | |||||
| No varices | 2 | 292 | 2.43 (0.44-13.55) | I² = 71% | I² = 0% |
| P = 0.31 | P = 0.06 | P = 0.51 | |||
| Small varices | 3 | 444 | 1.07 (0.19-6.18) | I² = 93% | |
| P = 0.94 | P < 0.01 | ||||
| First bleeding | |||||
| No varices | 1 | 213 | 0.38 (0.07-1.99) | NA | I² = 0% |
| P = 0.25 | P = 0.64 | ||||
| Small varices | 4 | 398 | 0.64 (0.15-2.79) | I² = 47% | |
| P = 0.55 | P = 0.13 | ||||
| Death | |||||
| No varices | 1 | 213 | 0.61 (0.26-1.43) | NA | I² = 0 |
| P = 0.26 | P = 0.84 | ||||
| Small varices | 2 | 311 | 0.68 (0.37-1.26) | I² = 0% | |
| P = 0.22 | P = 0.39 | ||||
| Adverse events | |||||
| No varices | 1 | 213 | 1.94 (1.11-3.38) | NA | I² = 37.1% |
| P = 0.02 | P = 0.21 | ||||
| Small varices | 2 | 311 | 5.75 (1.17-28.29) | I² = 15% | |
| P = 0.03 | P = 0.28 | ||||
The results of the sensitivity analysis were similar to those of the overall analysis (Table 5).
| Outcomes | Studies, | Patients, | OR (95%CI) | Heterogeneity |
| n | n | |||
| Development of large varices | 3 | 569 | 1.95 (0.73-5.24) | I² = 74% |
| P = 0.18 | P = 0.02 | |||
| First bleeding | 4 | 624 | 0.96 (0.37-2.54) | I² = 0% |
| P = 0.94 | P = 0.42 | |||
| Death | 3 | 569 | 0.79 (0.43-1.43) | I² = 0% |
| P = 0.43 | P = 0.65 | |||
| Adverse events | 3 | 569 | 2.68 (1.33-5.43) | I² = 24% |
| P = 0.01 | P = 0.27 |
At the first diagnosis of liver cirrhosis, the prevalence of gastroesophageal varices is diagnosed in about 50% of patients[9]. In cirrhotic patients without any pre-existing varices, the incidence of esophageal varices is 5 and 28% at one and three years, respectively. In cirrhotic patients with small varices, the incidence of variceal progression is 12% and 31% at one and three years, respectively[18]. Once large varices develop, the risk of bleeding is significantly increased[19]. Accordingly, pre- and early-primary prophylaxis of variceal bleeding has been proposed in patients with no and small varices, respectively. The former therapeutic objective is to prevent the formation of varices in patients without any pre-existing varices, and the latter aims to inhibit the progression from small to large varices in cirrhotic patients[8-10].
The major mechanisms of NSBBs for the management of portal hypertension in liver cirrhosis include the reduction of cardiac output and splanchnic vasoconstriction, which potentially decrease the portal pressure and blood flow. Currently, the role of NSBBs for delaying and avoiding the occurrence and enlargement of varices has been debated. However, the results of RCTs were not consistent. The present meta-analysis evaluated the efficacy and safety of NSBBs in cirrhotic patients with no and small varices by collecting all available high-level evidence. Unfortunately, we did not find any significant benefits of NSBBs in preventing the development of large varices, decreasing the incidence of first bleeding, or improving the survival. In contrast, we found a significantly higher incidence of adverse events in the NSBBs group compared to the placebo group. These findings do not support the use of NSBBs in cirrhotic patients with no and small varices.
Considering that the risk of first bleeding was significantly higher in patients with small varices compared to those without[18], subgroup analyses were conducted to explore the treatment effect of NSBBs in both patient groups. Sensitivity analyses were also performed to avoid the potential influence of study quality on the results of our meta-analysis. However, the results of both subgroup and sensitivity analyses were similar to those of the overall analysis.
In spite of a negative result in the overall analysis, we did not readily exclude any slight benefits of NSBBs in pre- and early-primary prophylaxis of variceal bleeding. Undoubtedly, a proportion of cirrhotic patients responded to NSBBs, thereby reducing the hepatic venous pressure gradient that was associated with the reduction of hepatocellular carcinoma and hepatic decompensation[20,21]. On the other hand, we observed a trend towards a lower mortality in the NSBBs group. It is possible that a statistical significance might be achieved if the sample size was increased. Notably, NSBBs might improve the non-hemodynamic outcomes of cirrhotic patients, independently of its hemodynamic benefits (i.e., the prevention of variceal bleeding). A meta-analysis by Senzolo et al[22] indicated that NSBBs may protect against the development of bacterial translocation in cirrhotic patients, thereby decreasing the incidence of spontaneous bacterial infection. A recent review by Thiele et al[23] also suggested that NSBBs decrease the development of hepatocellular carcinoma. Certainly, the potential deleterious effects of NSBBs on liver cirrhosis should never be neglected, such as a decreased survival in patients with refractory ascites via development of paracentesis-induced circulatory dysfunction[24,25] and an increased risk of portal vein thrombosis in cirrhotic patients via reduced portal flow[26].
This study had several limitations. First, a small number of included studies limited us to perform more comprehensive subgroup analyses. Second, the small sample sizes of the included studies may produce bias. Third, only three of six included studies were published after 2000, and only one of them was published within the last three years. Thus, the definition of small varices varied greatly among studies. Fourth, a significant heterogeneity among studies was observed in the meta-analysis regarding NSBBs for the development of large varices. But it should be noted that only a random effects model was employed. Fifth, two of six RCTs were considered to be of low quality. However, a sensitivity analysis of high-quality studies was employed to avoid the potential risk of bias. Sixth, a proportion of patients were lost to follow-up in three studies[11,12,14], which might influence the actual results.
In conclusion, based on the current evidence from a meta-analysis of RCTs, the use of NSBBs might not be recommended for cirrhotic patients with no or small varices. Certainly, further studies with larger sample sizes are warranted to confirm the association of NSBBs with survival in such patients.
Nonselective beta-blockers (NSBBs) should be recommended for the primary prophylaxis of variceal bleeding in cirrhotic patients with medium or large varices without any previous bleeding and for the secondary prophylaxis in those with a history of variceal bleeding.
Although NSBBs decreases the hepatic venous pressure gradient, their efficacy and safety remain controversial in cirrhotic patients with no or small varices.
The present meta-analysis evaluated the efficacy and safety of NSBBs in cirrhotic patients with no and small varices by collecting all available high-level evidence. Unfortunately, the authors did not find any significant benefits of NSBBs in preventing the development of large varices, decreasing the incidence of first bleeding, or improving the survival of patients.
The use of NSBBs might not be recommended for cirrhotic patients with no or small varices.
Nonselective beta-blockers are oral drugs that can reduce the cardiac output via inhibiting β1 receptor and contract the splanchnic vessels via inhibiting β2 receptor, such as propranolol, timolol, and nadolol, etc. Portal hypertension is defined as portal venous pressure gradient exceeds 5 mmHg. Varices will develop as portal venous pressure gradient exceeds 10 mmHg, and will bleed as the pressure exceeds 12 mmHg.
Prevention of the development of complications of portal hypertension is an important area of research, and the role of NSBBs remains uncertain in cirrhotic patients with no or small varices. Although this meta-analysis has several limitations, it provides evidence supporting the recommendation of the guidelines and the manuscript is well written.
| 1. | Garcia-Tsao G, Bosch J. Management of varices and variceal hemorrhage in cirrhosis. N Engl J Med. 2010;362:823-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 639] [Cited by in RCA: 654] [Article Influence: 40.9] [Reference Citation Analysis (0)] |
| 2. | D’Amico G, Pagliaro L, Bosch J. Pharmacological treatment of portal hypertension: an evidence-based approach. Semin Liver Dis. 1999;19:475-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 600] [Cited by in RCA: 485] [Article Influence: 18.0] [Reference Citation Analysis (1)] |
| 3. | Pagliaro L, D’Amico G, Sörensen TI, Lebrec D, Burroughs AK, Morabito A, Tiné F, Politi F, Traina M. Prevention of first bleeding in cirrhosis. A meta-analysis of randomized trials of nonsurgical treatment. Ann Intern Med. 1992;117:59-70. [PubMed] |
| 4. | Pascal JP, Cales P. Propranolol in the prevention of first upper gastrointestinal tract hemorrhage in patients with cirrhosis of the liver and esophageal varices. N Engl J Med. 1987;317:856-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 220] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 5. | Poynard T, Calès P, Pasta L, Ideo G, Pascal JP, Pagliaro L, Lebrec D. Beta-adrenergic-antagonist drugs in the prevention of gastrointestinal bleeding in patients with cirrhosis and esophageal varices. An analysis of data and prognostic factors in 589 patients from four randomized clinical trials. Franco-Italian Multicenter Study Group. N Engl J Med. 1991;324:1532-1538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 313] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 6. | Idéo G, Bellati G, Fesce E, Grimoldi D. Nadolol can prevent the first gastrointestinal bleeding in cirrhotics: a prospective, randomized study. Hepatology. 1988;8:6-9. [PubMed] |
| 7. | Lebrec D, Poynard T, Hillon P, Benhamou JP. Propranolol for prevention of recurrent gastrointestinal bleeding in patients with cirrhosis: a controlled study. N Engl J Med. 1981;305:1371-1374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 347] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 8. | de Franchis R. Revising consensus in portal hypertension: report of the Baveno V consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol. 2010;53:762-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1066] [Cited by in RCA: 1047] [Article Influence: 65.4] [Reference Citation Analysis (0)] |
| 9. | Garcia-Tsao G, Sanyal AJ, Grace ND, Carey W. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology. 2007;46:922-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1229] [Cited by in RCA: 1222] [Article Influence: 64.3] [Reference Citation Analysis (2)] |
| 10. | Sarin SK, Kumar A, Angus PW, Baijal SS, Chawla YK, Dhiman RK, Janaka de Silva H, Hamid S, Hirota S, Hou MC. Primary prophylaxis of gastroesophageal variceal bleeding: consensus recommendations of the Asian Pacific Association for the Study of the Liver. Hepatol Int. 2008;2:429-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Andreani T, Poupon RE, Balkau BJ, Trinchet JC, Grange JD, Peigney N, Beaugrand M, Poupon R. Preventive therapy of first gastrointestinal bleeding in patients with cirrhosis: results of a controlled trial comparing propranolol, endoscopic sclerotherapy and placebo. Hepatology. 1990;12:1413-1419. [PubMed] |
| 12. | Calés P, Oberti F, Payen JL, Naveau S, Guyader D, Blanc P, Abergel A, Bichard P, Raymond JM, Canva-Delcambre V. Lack of effect of propranolol in the prevention of large oesophageal varices in patients with cirrhosis: a randomized trial. French-Speaking Club for the Study of Portal Hypertension. Eur J Gastroenterol Hepatol. 1999;11:741-745. [PubMed] |
| 13. | Groszmann RJ, Garcia-Tsao G, Bosch J, Grace ND, Burroughs AK, Planas R, Escorsell A, Garcia-Pagan JC, Patch D, Matloff DS. Beta-blockers to prevent gastroesophageal varices in patients with cirrhosis. N Engl J Med. 2005;353:2254-2261. [PubMed] |
| 14. | Merkel C, Marin R, Angeli P, Zanella P, Felder M, Bernardinello E, Cavallarin G, Bolognesi M, Donada C, Bellini B. A placebo-controlled clinical trial of nadolol in the prophylaxis of growth of small esophageal varices in cirrhosis. Gastroenterology. 2004;127:476-484. |
| 15. | Sarin SK, Mishra SR, Sharma P, Sharma BC, Kumar A. Early primary prophylaxis with beta-blockers does not prevent the growth of small esophageal varices in cirrhosis: A randomized controlled trial. Hepatol Int. 2013;7:248-256. |
| 16. | Conn HO, Grace ND, Bosch J, Groszmann RJ, Rodés J, Wright SC, Matloff DS, Garcia-Tsao G, Fisher RL, Navasa M. Propranolol in the prevention of the first hemorrhage from esophagogastric varices: A multicenter, randomized clinical trial. The Boston-New Haven-Barcelona Portal Hypertension Study Group. Hepatology. 1991;13:902-912. [PubMed] |
| 17. | Abraczinskas DR, Ookubo R, Grace ND, Groszmann RJ, Bosch J, Garcia-Tsao G, Richardson CR, Matloff DS, Rodés J, Conn HO. Propranolol for the prevention of first esophageal variceal hemorrhage: a lifetime commitment? Hepatology. 2001;34:1096-1102. [PubMed] |
| 18. | Merli M, Nicolini G, Angeloni S, Rinaldi V, De Santis A, Merkel C, Attili AF, Riggio O. Incidence and natural history of small esophageal varices in cirrhotic patients. J Hepatol. 2003;38:266-272. [PubMed] |
| 19. | North Italian Endoscopic Club for the Study and Treatment of Esophageal Varices. Prediction of the first variceal hemorrhage in patients with cirrhosis of the liver and esophageal varices. A prospective multicenter study. N Engl J Med. 1988;319:983-989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 996] [Cited by in RCA: 845] [Article Influence: 22.2] [Reference Citation Analysis (2)] |
| 20. | Ripoll C, Groszmann R, Garcia-Tsao G, Grace N, Burroughs A, Planas R, Escorsell A, Garcia-Pagan JC, Makuch R, Patch D. Hepatic venous pressure gradient predicts clinical decompensation in patients with compensated cirrhosis. Gastroenterology. 2007;133:481-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 747] [Cited by in RCA: 849] [Article Influence: 44.7] [Reference Citation Analysis (1)] |
| 21. | Ripoll C, Groszmann RJ, Garcia-Tsao G, Bosch J, Grace N, Burroughs A, Planas R, Escorsell A, Garcia-Pagan JC, Makuch R. Hepatic venous pressure gradient predicts development of hepatocellular carcinoma independently of severity of cirrhosis. J Hepatol. 2009;50:923-928. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 336] [Cited by in RCA: 300] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 22. | Senzolo M, Cholongitas E, Burra P, Leandro G, Thalheimer U, Patch D, Burroughs AK. beta-Blockers protect against spontaneous bacterial peritonitis in cirrhotic patients: a meta-analysis. Liver Int. 2009;29:1189-1193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 174] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 23. | Thiele M, Wiest R, Gluud LL, Albillos A, Krag A. Can non-selective beta-blockers prevent hepatocellular carcinoma in patients with cirrhosis? Med Hypotheses. 2013;81:871-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Sersté T, Melot C, Francoz C, Durand F, Rautou PE, Valla D, Moreau R, Lebrec D. Deleterious effects of beta-blockers on survival in patients with cirrhosis and refractory ascites. Hepatology. 2010;52:1017-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 380] [Article Influence: 23.8] [Reference Citation Analysis (1)] |
| 25. | Sersté T, Francoz C, Durand F, Rautou PE, Melot C, Valla D, Moreau R, Lebrec D. Beta-blockers cause paracentesis-induced circulatory dysfunction in patients with cirrhosis and refractory ascites: a cross-over study. J Hepatol. 2011;55:794-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 138] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 26. | Qi XS, Bai M, Fan DM. Nonselective β-blockers may induce development of portal vein thrombosis in cirrhosis. World J Gastroenterol. 2014;20:11463-11466. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (1)] |
| 27. | Beppu K, Inokuchi K, Koyanagi N, Nakayama S, Sakata H, Kitano S, Kobayashi M. Prediction of variceal hemorrhage by esophageal endoscopy. Gastrointest Endosc. 1981;27:213-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 608] [Cited by in RCA: 558] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 28. | Conn HO. Ammonia tolerance in the diagnosis of esophageal varices. A comparison of endoscopic, radiologic, and biochemical techniques. J Lab Clin Med. 1967;70:442-451. [PubMed] |
| 29. | de Franchis R, Pascal JP, Ancona E, Burroughs AK, Henderson M, Fleig W, Groszmann R, Bosch J, Sauerbruch T, Soederlund C. Definitions, methodology and therapeutic strategies in portal hypertension. A Consensus Development Workshop, Baveno, Lake Maggiore, Italy, April 5 and 6, 1990. J Hepatol. 1992;15:256-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 194] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Benedetto N, Saito M, Wu B S- Editor: Qi Y L- Editor: AmEditor E- Editor: Liu XM