Published online Dec 14, 2014. doi: 10.3748/wjg.v20.i46.17324
Revised: July 26, 2014
Accepted: September 18, 2014
Published online: December 14, 2014
Processing time: 195 Days and 1 Hours
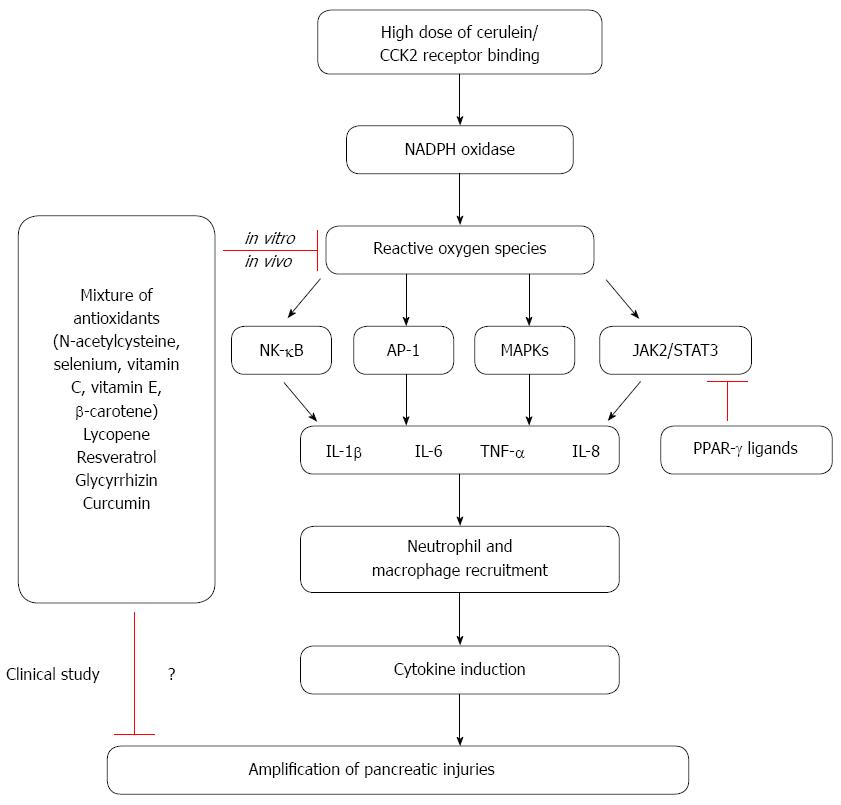
Oxidative stress is considered to be an important regulator of the pathogenesis of acute pancreatitis. Reactive oxygen species (ROS) regulate the activation of inflammatory cascades, the recruitment of inflammatory cells and tissue damage in acute pancreatitis. A hallmark of the inflammatory response in pancreatitis is the induction of cytokine expression, which is regulated by a number of signaling molecules including oxidant-sensitive transcription factors such as nuclear factor-κB (NF-κB) and activator protein-1 (AP-1), signal transducer and activator of transcription 3 (STAT3), and mitogen-activated protein kinases (MAPKs). Cross-talk between ROS and pro-inflammatory cytokines is mediated by NF-κB, AP-1, STAT3, and MAPKs; this crosstalk amplifies the inflammatory cascade in acute pancreatitis. Therapeutic studies have shown that antioxidants and natural compounds can have beneficial effects for patients with pancreatitis and can also influence the expression of proinflammatory cytokines in cerulein-induced pancreatitis. Since oxidative stress may activate inflammatory signaling pathways and contribute to the development of pancreatitis, antioxidant therapy may alleviate the symptoms or prevent the development of pancreatitis. Since chronic administration of high doses of antioxidants may have deleterious effects, dosage levels and duration of antioxidant treatment should be carefully determined.
Core tip: The pathogenesis of acute pancreatitis is not completely elucidated. Oxidative stress may contribute to the development of acute pancreatitis. Evidence supporting the role of reactive oxygen species and cytokines as a risk for pancreatitis and the concept of antioxidant supplementation as a preventive approach for pancreatitis has been proposed. Here we review the literature on oxidative stress, cytokine expression, inflammatory signaling, and natural antioxidant supplementation using an experimental model of cerulein-induced acute pancreatitis.
- Citation: Yu JH, Kim H. Oxidative stress and inflammatory signaling in cerulein pancreatitis. World J Gastroenterol 2014; 20(46): 17324-17329
- URL: https://www.wjgnet.com/1007-9327/full/v20/i46/17324.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i46.17324
Acute pancreatitis is a disease characterized by the activation of digestive proteases, inflammatory infiltration of macrophages and neutrophils, and necrosis of the pancreatic tissue. High doses of a cholecystokinin (CCK) analogue, cerulein, have been shown to stimulate the maximum secretion of pancreatic amylase and lipase[1]. This increased secretion results in pancreatitis, which is characterized by cytoplasmic vacuolization, the death of acinar cells, edema formation, and infiltration of inflammatory cells into the pancreas[2]. Interestingly, neutrophils obtained from patients with acute pancreatitis have been shown to exert enhanced production of reactive oxygen species (ROS)[3]. ROS have been proposed to play a critical role in the pathogenesis and development of acute pancreatitis. The major source of ROS in acute inflammation appears to be the NADPH oxidases; on the other hand, the major target of ROS and redox signaling in acute pancreatitis is nuclear factor-κB (NF-κB)[4,5]. Both the activation of NF-κB and the NF-κB-regulated expression of interleukin-1β (IL-1β), IL-6, and TNF-α have been shown to be involved in initiation and aggravation of acute pancreatitis. Studies focusing on natural compounds, have shown that caffeine-free extract from green tea reduces the degree of acute pancreatitis, the activation of NF-κB, and reduces the expression of pro-inflammatory cytokines[6]. Other antioxidants such as ascorbic acid and N-acetyl cysteine (NAC) have also been shown to exert beneficial effects against acinar cell degeneration, pancreatic edema, intracellular vacuolization and inflammatory infiltration in cerulein-induced pancreatitis[7]. This review will focus on the involvement of ROS in inflammatory signaling pathways in the context of the cerulein-induced acute pancreatitis model. In addition, natural compounds that may alleviate the symptoms or prevent the development of pancreatitis will also be discussed.
Depletion of pancreatic glutathione (GSH) has been shown to be involved in the early phase of acute pancreatitis[8] and also to influence the extent of disease severity[9]. The activities of multiple antioxidant enzymes, including glutathione peroxidase, superoxide dismutase (SOD), and catalase, decrease in the course of pancreatitis; the levels of antioxidant vitamins have also been shown to decrease[10,11]. Moreover, the level of pancreatic glutathione peroxidase is reduced both in cerulein-induced acute pancreatitis models[7] and in patients with acute pancreatitis[12]. The serum level of thioredoxin-1, an antioxidant, has been shown to increase in patients with severe acute pancreatitis[13]. However, overexpression of thioredoxin-1 has been shown to attenuate the inflammatory response in acute pancreatitis[14]. Interestingly, cerulein-induced pancreatitis induces expression of metallothionein-1, and overexpression of metallothionein-1 has been shown to protect against pancreatic damage after induction of pancreatitis in mice[15]. Thus, oxidative stress appears to regulate the early phase of acute pancreatitis, since an improved antioxidant status is associated with improved clinical outcomes in patients with acute pancreatitis. The major source of ROS in inflammation has been reported to be NADPH oxidases[4,5,16]. Deficient production of NADPH oxidase was shown to reduce trypsin activation in mice with cerulein-induced pancreatitis[17]; moreover, the NADPH oxidase NOX1 has been demonstrated to play a critical role in the induction of IL-6 expression and apoptosis in pancreatic AR42J acinar cells stimulated with cerulein[18].
Hyper-stimulation of the CCK receptor, using supramaximal doses of the CCK analogue cerulein, has been shown to lead to NF-κB activation in pancreatic acinar cells[19]. Cerulein also produces ROS by activating the NADPH oxidase, NOX1, in pancreatic acinar cells[18]. Cerulein-mediated induction of acute pancreatitis is known to trigger NF-κB activation; this effect can be attenuated by pretreatment with NAC[19]. Pro-inflammatory cytokines, such as IL-1β, IL-6, and tumor necrosis factor-α (TNF-α), play a major role in the inflammatory response associated with acute pancreatitis[20-23]. Antioxidants inhibit the expression of these inflammatory cytokines by suppressing NF-κB activation[21]. Clinical studies have revealed the presence of inflammatory cytokines such as IL-1β, IL-6, and TNF-α in the sera of patients with acute pancreatitis. Patients with pancreatitis have been shown to have enhanced NF-κB activity; moreover, inhibiting NF-κB has been shown to reduce the inflammatory effects of pancreatitis[24]. Both experimental and clinical studies have implicated a role for NF-κB in the pathogenesis of acute pancreatitis[24,25]. Conditional overexpression of IκB kinase, a molecule which helps activate NF-κB by phosphorylating its inhibitory protein IκBα, has been shown to induce an inflammatory response in mice with acute pancreatitis[26]. Moreover, genetic silencing of NF-κB was shown to reduce the extent of pancreatic damage and to down-regulate the expression of TNF-α in cerulein-induced acute pancreatitis[27]. TNF-α and IL-1β are considered to be the primary cytokines in acute pancreatitis, since these cytokines initiate and propagate most of the consequences of the systemic inflammatory response[28]. These two cytokines also amplify the inflammatory cascade by activating mitogen-activated protein kinases (MAPKs) and NF-κB, which in turn induces the release of chemokines and other cytokines. The induction of chemokines occurs via a positive feedback loop, in which each chemokine also up-regulates its own expression[29]. Serum IL-6 levels have also been shown to be increased in patients with acute pancreatitis, these levels also correlate with disease severity[30]. In an IL-6 transgenic mouse model, cerulein-induced acute pancreatitis was shown to be more severe than in wild-type mice[31]. Cerulein-induced expression of IL-8 has also been reported to be regulated by NF-κB, AP-1, and MAPKs in pancreatic acinar cells[32]. A recent study showed that the janus kinase 2 (JAK2)/signal transducer and activator of transcription 3 (STAT3) pathway is activated by the CCK2 receptor in pancreatic AR42J cells[33]. High doses of cerulein have also been shown to trigger phosphorylation of JAK2 and STAT3 in pancreatic acinar cells[34]. Moreover, inhibition of JAK2 and STAT3 via the anti-inflammatory properties of peroxisome proliferator activated receptor-γ (PPAR-γ) ligands, such as 15-deoxy-Delta-(12,14)-prostaglandin J2 (15dPG-J2) and troglitazone, has been shown to reduce the expression of IL-6[34]. Recent study shows that cerulein induces oxidative injury, inflammatory cytokines, and nucleosome release in pancreatic tissues and acinar cells of mice with pancreas-specific disruption in HMGB1 (high mobility group box 1)[35]. Treatment of NAC attenuates cerulein-induced pancreatic injury in these mice[35], suggesting that intracellular HMGB1 may prevent oxidative injury of pancreas and limit acute pancreatitis.
An antioxidant mixture was shown to reduce the level of malondialdehyde, and increase the activities of catalase and glutathione peroxidase in L-arginine-induced pancreatitis rats. Moreover, supplementation with an antioxidant mixture containing NAC, selenium, and vitamin C was shown to reduce pancreatic injury in rats[36]. Furthermore, treatment with NAC alone was sufficient to attenuate sodium taurocholate-induced pancreatitis in rats[37]. A combination treatment of ebselen [2-phenyl-1,2-benzisoselenazol-3(2H)-one], which is a mimic of GSH peroxidase, and ethylhydroxyethyl cellulose (EHEC) was also shown to attenuate severe acute pancreatitis in rats[38]. Resveratrol, a plant-derived polyphenolic phytoalexin, has also be shown to reduce the expression of TNF-α and IL-8 by inhibiting NF-κB signaling in acute pancreatitis[39]. In the early stage of acute pancreatitis, NF-κB is activated in macrophages, which then produce cytokines. During acute pancreatitis, treatment with resveratrol reduces the expression of IL-1β and TNF-α in macrophages via NF-κB signaling pathways[40]. In cerulein-induced acute pancreatitis, treatment with resveratrol has been shown to prevent tissue damage, reduce the expression of IL-1β, and induce the expression of IL-10, an anti-inflammatory cytokine[41]. The effect of resveratrol may be due to its antioxidant effect with induction of catalase and MnSOD[42]. Moreover, cerulein-induced upregulation of IL-1β and TNF-α and depletion of GSH are rescued by treatment with lycopene, a natural carotenoid[43]. Glycyrrhizin treatment of acute pancreatitis has also been shown to suppress the production of proinflammatory cytokines (IL-6, IL-1β and TNF-α) and to stimulate recovery from histological changes such as acinar cell necrosis, hemorrhage, and edema[44]. Glycyrrhizin treatment has been shown to not only decrease the serum levels of MCP-1 and MIP-2 in cerulein-induced acute pancreatitis, but also to reduce the number of infiltrated granulocytes and monocytes in pancreatic tissues[45]. Glycyrrhizin exerted antioxidant effects and reduced activation of NF-κB, c-Jun N-terminal kinase (JNK), and p38, redox-sensitive signaling events known to be relevant for influenza A virus replication[46]. Glycyrrhizin treatment decreased the incidence of free radical-induced lipid peroxidation and improved immunity activities in the blood and nasal mucosa of allergic rhinitis mice[47]. In addition, bioflavonoid curcumin, the pigment in turmeric (Curcuma longa), inhibited the activation of NF-κB and the expression of TNF-α and thus ameliorated cerulein pancreatitis in mice[48].
Antioxidant therapy is believed to have great potential, since its therapeutic efficacy has already been demonstrated in experimental acute pancreatitis. Patients admitted within 72 h of onset of pain were randomized to receive either placebo or antioxidants (vitamin C 500 mg, NAC 200 mg 8 hourly and antoxyl forte 1 capsule hourly with standard medical treatment) daily[49]. Treatment with vitamin C and NAC was shown to decrease oxidative stress and to improve the antioxidant status of 23 patients with acute pancreatitis[49]. Moreover, antioxidant therapy with selenium and D-α-tocopherol in 99 patients showed beneficial effects against necrotizing or mild acute pancreatitis[50]. A combination therapy of daily doses of antioxidants including 600 μg organic selenium, 9000 IU β-carotene, 0.54 g vitamin C, 270 IU vitamin E, and 2 g methionine was studied in three controlled clinical trials[51-53]. After treatment with a combination of antioxidants to 28 patients with idiopathic chronic, alcoholic chronic, or idiopathic acute pancreatitis, recurrent attacks and pancreatic pain were significantly attenuated[51]. Another study with 36 chronic pancreatitis patients, pain was reduced after the combination therapy and quality of life, physical, social functioning and health perception were enhanced[52]. In clinical trial with 147 patients, the antioxidants were administered for 6 mo, and pain and hospitalization were reduced[53]. Twenty patients with chronic pancreatitis received 500 mg curcumin with 5 mg of piperine or placebo for 6 wk[54]. Treatment of curcumin reduced erythrocyte malondialdehyde levels compared to placebo[54]. Bolus intravenous administration of vitamin C (10 g/d) for 5 d has been shown to alleviate pancreatitis symptoms, enhance the cure rate, reduce the complications, and decrease the length of hospital stays in 84 patients with acute pancreatitis[55].
In contrast, a multidrug approach was investigated in a randomized control trial using intravenous NAC, selenium and vitamin C in 43 patients with severe acute pancreatitis for 7 d. While makers for oxidative stress were lower in the treatment group, there was no significant difference in patient outcomes[56]. The study shows a lack of benefit from antioxidant therapy in severe acute pancreatitis. In another randomized study with multiple antioxidants, 39 patients with severe acute pancreatitis were randomized to standard treatment or standard treatment and vitamin C (1000 mg in 100 mL normal saline), vitamin E (200 mg oral), and vitamin A (10000 IU intramuscularly) for 14 d. No significant difference was demonstrated in the two treatment groups regarding, length of hospital stay and organ dysfunction[57]. Also, antioxidants therapy does not seem to confer protection in patients with post endoscopic retrograde cholangiopancreatography (ERCP)-induced pancreatitis. In a double-blinded trial, patients were given a single dose (2 g) of β-carotene 12 hours prior to an ERCP. There was no difference in the incidence of acute pancreatitis between the patients who received antioxidant (9.4%) with those who had received placebo and developed (10%)[58]. Even though there has been no report for adverse effect directly attributable to antioxidant therapy, we could not exclude possible deleterious effects of chronic administration of high doses of antioxidants. Therefore, dosage levels and duration of antioxidant treatment should be carefully determined. The role of oxidative stress on inflammatory signaling and anti-inflammatory effects of natural compounds in cerulein-induced pancreatitis are summarized in Figure 1.
Oxidative stress is well established to increase throughout the course of pancreatitis. Furthermore, ROS are known to mediate the activation of NF-κB, AP-1, MAPKs, and STAT3 in pancreatic acinar cells stimulated with cerulein. Crosstalk between ROS and pro-inflammatory cytokines, which is mediated by NF-κB, STAT3, and MAPKs, is believed to contribute to the inflammatory process in pancreas. Antioxidant combinations of NAC, organic selenium, β-carotene, vitamin C, and vitamin E may inhibit cytokine expression and thus reduce the severity of pancreatitis. Natural compounds with antioxidant effects, such as lycopene, resveratrol, and glycyrrhizin, also reduce the expression of inflammatory cytokines (TNF-α, IL-6, and IL-8) by suppressing NF-κB signaling in acute pancreatitis. Cumulatively, these studies demonstrate that oxidative stress plays an important role in the activation of inflammatory signaling pathways and in the pathogenesis of pancreatitis. Therefore, reducing the levels of ROS by antioxidant therapy may be clinically valuable for the treatment and/or prevention of pancreatitis. However, dosage levels and duration of antioxidant treatment should be carefully determined to prevent possible side effects to the patients with acute and chronic pancreatitis.
| 1. | Sato S, Stark HA, Martinez J, Beaven MA, Jensen RT, Gardner JD. Receptor occupation, calcium mobilization, and amylase release in pancreatic acini: effect of CCK-JMV-180. Am J Physiol. 1989;257:G202-G209. [PubMed] |
| 2. | Lerch MM, Adler G. Experimental animal models of acute pancreatitis. Int J Pancreatol. 1994;15:159-170. [PubMed] |
| 3. | Tsuji N, Watanabe N, Okamoto T, Niitsu Y. Specific interaction of pancreatic elastase and leucocytes to produce oxygen radicals and its implication in pancreatitis. Gut. 1994;35:1659-1664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Yu JH, Lim JW, Kim H, Kim KH. NADPH oxidase mediates interleukin-6 expression in cerulein-stimulated pancreatic acinar cells. Int J Biochem Cell Biol. 2005;37:1458-1469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 65] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 5. | Kim H. Inhibitory mechanism of lycopene on cytokine expression in experimental pancreatitis. Ann N Y Acad Sci. 2011;1229:99-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Babu BI, Malleo G, Genovese T, Mazzon E, Di Paola R, Crisafulli C, Caminiti R, Siriwardena AK, Cuzzocrea S. Green tea polyphenols ameliorate pancreatic injury in cerulein-induced murine acute pancreatitis. Pancreas. 2009;38:954-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Eşrefoğlu M, Gül M, Ates B, Batçioğlu K, Selimoğlu MA. Antioxidative effect of melatonin, ascorbic acid and N-acetylcysteine on caerulein-induced pancreatitis and associated liver injury in rats. World J Gastroenterol. 2006;12:259-264. [PubMed] |
| 8. | Gómez-Cambronero L, Camps B, de La Asunción JG, Cerdá M, Pellín A, Pallardó FV, Calvete J, Sweiry JH, Mann GE, Viña J. Pentoxifylline ameliorates cerulein-induced pancreatitis in rats: role of glutathione and nitric oxide. J Pharmacol Exp Ther. 2000;293:670-676. [PubMed] |
| 9. | Alsfasser G, Gock M, Herzog L, Gebhard MM, Herfarth C, Klar E, Schmidt J. Glutathione depletion with L-buthionine-(S,R)-sulfoximine demonstrates deleterious effects in acute pancreatitis of the rat. Dig Dis Sci. 2002;47:1793-1799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Curran FJ, Sattar N, Talwar D, Baxter JN, Imrie CW. Relationship of carotenoid and vitamins A and E with the acute inflammatory response in acute pancreatitis. Br J Surg. 2000;87:301-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 11. | Cullen JJ, Mitros FA, Oberley LW. Expression of antioxidant enzymes in diseases of the human pancreas: another link between chronic pancreatitis and pancreatic cancer. Pancreas. 2003;26:23-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 96] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 12. | Szuster-Ciesielska A, Daniluk J, Kandefer-Szerszeń M. Oxidative stress in blood of patients with alcohol-related pancreatitis. Pancreas. 2001;22:261-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Ohashi S, Nishio A, Nakamura H, Kido M, Kiriya K, Asada M, Tamaki H, Fukui T, Kawasaki K, Watanabe N. Clinical significance of serum thioredoxin 1 levels in patients with acute pancreatitis. Pancreas. 2006;32:264-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Ohashi S, Nishio A, Nakamura H, Kido M, Ueno S, Uza N, Inoue S, Kitamura H, Kiriya K, Asada M. Protective roles of redox-active protein thioredoxin-1 for severe acute pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2006;290:G772-G781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 59] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Fu K, Tomita T, Sarras MP, De Lisle RC, Andrews GK. Metallothionein protects against cerulein-induced acute pancreatitis: analysis using transgenic mice. Pancreas. 1998;17:238-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Ushio-Fukai M. Compartmentalization of redox signaling through NADPH oxidase-derived ROS. Antioxid Redox Signal. 2009;11:1289-1299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 295] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 17. | Gukovskaya AS, Vaquero E, Zaninovic V, Gorelick FS, Lusis AJ, Brennan ML, Holland S, Pandol SJ. Neutrophils and NADPH oxidase mediate intrapancreatic trypsin activation in murine experimental acute pancreatitis. Gastroenterology. 2002;122:974-984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 212] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 18. | Yu JH, Lim JW, Kim KH, Morio T, Kim H. NADPH oxidase and apoptosis in cerulein-stimulated pancreatic acinar AR42J cells. Free Radic Biol Med. 2005;39:590-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 60] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 19. | Steinle AU, Weidenbach H, Wagner M, Adler G, Schmid RM. NF-kappaB/Rel activation in cerulein pancreatitis. Gastroenterology. 1999;116:420-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 196] [Article Influence: 7.3] [Reference Citation Analysis (2)] |
| 20. | Norman J. The role of cytokines in the pathogenesis of acute pancreatitis. Am J Surg. 1998;175:76-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 492] [Cited by in RCA: 512] [Article Influence: 18.3] [Reference Citation Analysis (2)] |
| 21. | Yu JH, Lim JW, Namkung W, Kim H, Kim KH. Suppression of cerulein-induced cytokine expression by antioxidants in pancreatic acinar cells. Lab Invest. 2002;82:1359-1368. [PubMed] |
| 22. | Baron TH, Morgan DE. Acute necrotizing pancreatitis. N Engl J Med. 1999;340:1412-1417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 299] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 23. | Mercurio F, Manning AM. NF-kappaB as a primary regulator of the stress response. Oncogene. 1999;18:6163-6171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 328] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 24. | Vaquero E, Gukovsky I, Zaninovic V, Gukovskaya AS, Pandol SJ. Localized pancreatic NF-kappaB activation and inflammatory response in taurocholate-induced pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2001;280:G1197-G1208. [PubMed] |
| 25. | Satoh A, Masamune A, Kimura K, Kaneko K, Sakai Y, Yamagiwa T, Satoh M, Kikuta K, Asakura T, Shimosegawa T. Nuclear factor kappa B expression in peripheral blood mononuclear cells of patients with acute pancreatitis. Pancreas. 2003;26:350-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 26. | Aleksic T, Baumann B, Wagner M, Adler G, Wirth T, Weber CK. Cellular immune reaction in the pancreas is induced by constitutively active IkappaB kinase-2. Gut. 2007;56:227-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 27. | Altavilla D, Famulari C, Passaniti M, Galeano M, Macrì A, Seminara P, Minutoli L, Marini H, Calò M, Venuti FS. Attenuated cerulein-induced pancreatitis in nuclear factor-kappaB-deficient mice. Lab Invest. 2003;83:1723-1732. [PubMed] |
| 28. | Escobar J, Pereda J, Arduini A, Sandoval J, Sabater L, Aparisi L, López-Rodas G, Sastre J. Cross-talk between oxidative stress and pro-inflammatory cytokines in acute pancreatitis: a key role for protein phosphatases. Curr Pharm Des. 2009;15:3027-3042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 80] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 29. | Rahman I, MacNee W. Regulation of redox glutathione levels and gene transcription in lung inflammation: therapeutic approaches. Free Radic Biol Med. 2000;28:1405-1420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 373] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 30. | Inagaki T, Hoshino M, Hayakawa T, Ohara H, Yamada T, Yamada H, Iida M, Nakazawa T, Ogasawara T, Uchida A. Interleukin-6 is a useful marker for early prediction of the severity of acute pancreatitis. Pancreas. 1997;14:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 87] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 31. | Suzuki S, Miyasaka K, Jimi A, Funakoshi A. Induction of acute pancreatitis by cerulein in human IL-6 gene transgenic mice. Pancreas. 2000;21:86-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 32. | Ju KD, Yu JH, Kim H, Kim KH. Role of mitogen-activated protein kinases, NF-kappaB, and AP-1 on cerulein-induced IL-8 expression in pancreatic acinar cells. Ann N Y Acad Sci. 2006;1090:368-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 33. | Ferrand A, Kowalski-Chauvel A, Bertrand C, Escrieut C, Mathieu A, Portolan G, Pradayrol L, Fourmy D, Dufresne M, Seva C. A novel mechanism for JAK2 activation by a G protein-coupled receptor, the CCK2R: implication of this signaling pathway in pancreatic tumor models. J Biol Chem. 2005;280:10710-10715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 59] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 34. | Yu JH, Kim KH, Kim H. SOCS 3 and PPAR-gamma ligands inhibit the expression of IL-6 and TGF-beta1 by regulating JAK2/STAT3 signaling in pancreas. Int J Biochem Cell Biol. 2008;40:677-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 86] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 35. | Kang R, Zhang Q, Hou W, Yan Z, Chen R, Bonaroti J, Bansal P, Billiar TR, Tsung A, Wang Q. Intracellular Hmgb1 inhibits inflammatory nucleosome release and limits acute pancreatitis in mice. Gastroenterology. 2014;146:1097-1107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 272] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 36. | Hardman J, Shields C, Schofield D, McMahon R, Redmond HP, Siriwardena AK. Intravenous antioxidant modulation of end-organ damage in L-arginine-induced experimental acute pancreatitis. Pancreatology. 2005;5:380-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 37. | Yagci G, Gul H, Simsek A, Buyukdogan V, Onguru O, Zeybek N, Aydin A, Balkan M, Yildiz O, Sen D. Beneficial effects of N-acetylcysteine on sodium taurocholate-induced pancreatitis in rats. J Gastroenterol. 2004;39:268-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 38. | Zhao H, Lu HG, Shi YB, Zhao LM, Bai C, Wang X. Role of enteral nutrition supplemented with ebselen and EHEC in pancreatitis-associated multiple organ dysfunction in rats. Inflamm Res. 2006;55:423-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 39. | Meng Y, Ma QY, Kou XP, Xu J. Effect of resveratrol on activation of nuclear factor kappa-B and inflammatory factors in rat model of acute pancreatitis. World J Gastroenterol. 2005;11:525-528. [PubMed] |
| 40. | Ma ZH, Ma QY, Wang LC, Sha HC, Wu SL, Zhang M. Effect of resveratrol on peritoneal macrophages in rats with severe acute pancreatitis. Inflamm Res. 2005;54:522-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 58] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 41. | Carrasco C, Holguín-Arévalo MS, Martín-Partido G, Rodríguez AB, Pariente JA. Chemopreventive effects of resveratrol in a rat model of cerulein-induced acute pancreatitis. Mol Cell Biochem. 2014;387:217-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 42. | Inglés M, Gambini J, Miguel MG, Bonet-Costa V, Abdelaziz KM, El Alami M, Viña J, Borrás C. PTEN mediates the antioxidant effect of resveratrol at nutritionally relevant concentrations. Biomed Res Int. 2014;2014:580852. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 43. | Ozkan E, Akyüz C, Dulundu E, Topaloğlu U, Sehirli AÖ, Ercan F, Sener G. Protective effects of lycopene on cerulein-induced experimental acute pancreatitis in rats. J Surg Res. 2012;176:232-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 44. | Yildirim AO, Ince M, Eyi YE, Tuncer SK, Kaldirim U, Eroglu M, Oztas E, Cayci T, Kilic A, Inal V. The effects of glycyrrhizin on experimental acute pancreatitis in rats. Eur Rev Med Pharmacol Sci. 2013;17:2981-2987. [PubMed] |
| 45. | Fakhari S, Abdolmohammadi K, Panahi Y, Nikkhoo B, Peirmohammadi H, Rahmani MR, Moghadam AS, Jalili A. Glycyrrhizin attenuates tissue injury and reduces neutrophil accumulation in experimental acute pancreatitis. Int J Clin Exp Pathol. 2014;7:101-109. [PubMed] |
| 46. | Michaelis M, Geiler J, Naczk P, Sithisarn P, Leutz A, Doerr HW, Cinatl J. Glycyrrhizin exerts antioxidative effects in H5N1 influenza A virus-infected cells and inhibits virus replication and pro-inflammatory gene expression. PLoS One. 2011;6:e19705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 121] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 47. | Li XL, Zhou AG, Zhang L, Chen WJ. Antioxidant status and immune activity of glycyrrhizin in allergic rhinitis mice. Int J Mol Sci. 2011;12:905-916. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 65] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 48. | Yu WG, Xu G, Ren GJ, Xu X, Yuan HQ, Qi XL, Tian KL. Preventive action of curcumin in experimental acute pancreatitis in mouse. Indian J Med Res. 2011;134:717-724. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 49. | Sateesh J, Bhardwaj P, Singh N, Saraya A. Effect of antioxidant therapy on hospital stay and complications in patients with early acute pancreatitis: a randomised controlled trial. Trop Gastroenterol. 2009;30:201-206. [PubMed] |
| 50. | Kuklinski B, Buchner M, Müller T, Schweder R. [Anti-oxidative therapy of pancreatitis--an 18-month interim evaluation]. Z Gesamte Inn Med. 1992;47:239-245. [PubMed] |
| 51. | Uden S, Bilton D, Nathan L, Hunt LP, Main C, Braganza JM. Antioxidant therapy for recurrent pancreatitis: placebo-controlled trial. Aliment Pharmacol Ther. 1990;4:357-371. [PubMed] |
| 52. | Kirk GR, White JS, McKie L, Stevenson M, Young I, Clements WD, Rowlands BJ. Combined antioxidant therapy reduces pain and improves quality of life in chronic pancreatitis. J Gastrointest Surg. 2006;10:499-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 89] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 53. | Bhardwaj P, Garg PK, Maulik SK, Saraya A, Tandon RK, Acharya SK. A randomized controlled trial of antioxidant supplementation for pain relief in patients with chronic pancreatitis. Gastroenterology. 2009;136:149-159.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 161] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 54. | Durgaprasad S, Pai CG, Vasanthkumar JF, Namitha S. A pilot study of the antioxidant effect of curcumin in tropical pancreatitis. Indian J Med Res. 2005;122:315-318. [PubMed] |
| 55. | Du WD, Yuan ZR, Sun J, Tang JX, Cheng AQ, Shen DM, Huang CJ, Song XH, Yu XF, Zheng SB. Therapeutic efficacy of high-dose vitamin C on acute pancreatitis and its potential mechanisms. World J Gastroenterol. 2003;9:2565-2569. [PubMed] |
| 56. | Siriwardena AK, Mason JM, Balachandra S, Bagul A, Galloway S, Formela L, Hardman JG, Jamdar S. Randomised, double blind, placebo controlled trial of intravenous antioxidant (n-acetylcysteine, selenium, vitamin C) therapy in severe acute pancreatitis. Gut. 2007;56:1439-1444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 127] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 57. | Bansal D, Bhalla A, Bhasin DK, Pandhi P, Sharma N, Rana S, Malhotra S. Safety and efficacy of vitamin-based antioxidant therapy in patients with severe acute pancreatitis: a randomized controlled trial. Saudi J Gastroenterol. 2011;17:174-179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
P- Reviewer: Acuna-Castroviejo D, Antonelli A, Dimcevski G, Gao RP, Morini S S- Editor: Qi Y L- Editor: A E- Editor: Ma S