Published online Dec 14, 2014. doi: 10.3748/wjg.v20.i46.17330
Revised: July 25, 2014
Accepted: September 12, 2014
Published online: December 14, 2014
Processing time: 293 Days and 22.2 Hours
Video capsule endoscopy (VCE) was launched in 2000 and has revolutionized direct endoscopic imaging of the gut. VCE is now a first-line procedure for exploring the small bowel in cases of obscure digestive bleeding and is also indicated in some patients with Crohn’s disease, celiac disease, and polyposis syndrome. A video capsule has also been designed for visualizing the esophagus in order to detect Barrett’s esophagus or esophageal varices. Different capsules are now available and differ with regard to dimensions, image acquisition rate, battery life, field of view, and possible optical enhancements. More recently, the use of VCE has been extended to exploring the colon. Within the last 5 years, tremendous developments have been made toward increasing the capabilities of the colon capsule. Although colon capsule cannot be proposed as a first-line colorectal cancer screening procedure, colon capsule may be used in patients with incomplete colonoscopy or in patients who are unwilling to undergo colonoscopy. In the near future, new technological developments will improve the diagnostic yield of VCE and broaden its therapeutic capabilities.
Core tip: The authors review the indications and developments of the video capsule endoscopy (VCE) both for exploring the esophagus, the small bowel and the colon. Interestingly, the use of VCE in case of obscure digestive bleeding is defined in a decisional algorithm. Actual and potential indications for colon capsule endoscopy are described enlighten technical developments.
- Citation: Bouchard S, Ibrahim M, Gossum AV. Video capsule endoscopy: Perspectives of a revolutionary technique. World J Gastroenterol 2014; 20(46): 17330-17344
- URL: https://www.wjgnet.com/1007-9327/full/v20/i46/17330.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i46.17330
Video capsule endoscopy (VCE) has been available in clinical practice for evaluation of the small bowel since 2001. The first capsule that was approved in Europe and in the United States was the M2A® (Given Imaging; Yoqneam, Israel). VCE has revolutionized direct endoscopic imaging of the small-bowel and is now widely used in clinical practice worldwide[1]. VCE has been an important subject of research with close to 1500 publications in the past fifteen years and continues to generate great scientific interest. VCE technology is in constant evolution as new indications and new capsule functions are being developed in order to improve the diagnostic and possible therapeutic utility of VCE[2]. Initially used only for small-bowel evaluation, VCE was recently adapted for evaluation of the esophagus and the colon. In this review, we have mainly focused on the use of VCE for evaluation of the small-bowel and the colon and we discuss the latest advancements and future plans in VCE technology.
Several studies haven shown that the Pillcam esophageal video capsule (ECE) has a good sensitivity and specificity for detecting esophageal abnormalities or Barrett’s esophagus[3-5].
Domingos et al[6] evaluated ECE with methylene blue chromoendoscopy for detecting esophageal lesions in which there was a suspicion of cancer, the length and pattern of Barrett esophagus and the presence of hiatal hernia.
ECE sensitivity, negative predictive value and accuracy were 100%, 100% and 79%, respectively for the detection of esophageal lesions suspected of cancer. ECE accuracy in assessing Barrett’s esophagus length was 89%. ECE was not so good for detecting hiatal hernia (sensitivity was 43%). Chang et al[7] also showed that esophageal VCE was acceptable, feasible and safe to screen Barrett’s esophagus in the community.
There were some encouraging reports about the use of a string esophageal capsule endoscopy for detecting Barrett’s esophagus[8]. Chen et al[9] recently describe the use of a string esophageal capsule endoscopy with real-time viewing that improved the visualization of the distal esophageal Z-line.
In conclusion, esophageal capsule endoscopy seems to be a convenient and sensitive method for detecting Barrett’s esophagus and esophagitis but is not yet widely used in comparison with standard endoscopy that allows chromoendoscopy and biopsy.
Various studies have evaluated the potential of esophageal capsule endoscopy for diagnosing esophageal varices. A recent meta-analysis including 9 studies with 631 patients showed a sensitivity of 83% and a specificity of 85% respectively for detecting esophageal varices[10]. It is accepted that esophageal capsule endoscopy could be used in some situations but could not be recommended to replace standard optical endoscopy.
The small bowel videocapsule endoscopy (SBVCE) system consists of a wireless capsule containing a video camera, a sensing system (including either a sensing belt or pad, a data recorder unit, a battery pack, and a real-time viewer) and a personal computer workstation. The workstation is equipped with manufacturer software that offers various functions (localization system, color enhancement system, blood detector, quick viewer, scoring system, image atlas) that can assist the examiner in reviewing and interpreting the SBVCE images[11].
Different capsule endoscopy systems are currently available: PillCam (Given Imaging®; Yoqneam, Israel), EndoCapsule (Olympus; Center Valley, PA, United States), Mirocam (IntroMedic; Seoul, South Korea), OMOM capsule (Jinshan Science and Technology; Chongqing, China) and CapsoCam (CapsoVision Saratoga; United States). The various capsules differ with regard to dimensions, image acquisition rate, battery life, field of view, and possible optical enhancements (Table 1). PillCam has been the most studied capsule endoscopy system.
| Pillcam | EndoCapsule | MiroCam | OMOM | Capso-vision | ||||
| Colon2 | ESO2 | SB2 | SB3 | |||||
| Length | 31 | 26 | 26 | 26 | 26 | 24 | 27.9 | 31 |
| Weight, g | 3.4 | 3.4 | 3.4 | 3 | 3.8 | 3.4 | 6 | - |
| Number of imaging heads | 2 | 2 | 1 | 1 | 1 | 1 | 1 | 4 |
| Frames, s | 4-35 | 14 | 2 | 2-6 | 2 | 3 | 2 | 12-20 |
| Image sensor | CMOS | CMOS | CMOS | CCD | CCD | CCD | White LEDs | |
| Battery life, h | 10 | 30 min | 8 | ≥ 11 | 9 | 11 | 8 | 15 |
| Antennas | 8 | NA | 8 | 8 | 8 | 9 | 14 | Wire-free |
PillCam and MiroCam capture images using a complementary metal oxide silicon sensor while endocapsule and the OMOM capsule use a charge-coupled device[12].
In August 2013, the new generation of PillCam (SB3) received FDA clearance. This capsule system has an adaptative frame rate technology (2 to 6 frames per second) that adjusts to capsule speed and generates small-bowel images with improved resolution.
The CapsoCam SV-1 has four cameras that together provide a 360 degree view of the small-bowel and uses Smart Motion Sense Technology that allows the cameras to capture images only when the capsule is moving. CapsoCam SV-1 is a wire-free capsule system. There is no need for a recording device. The capsule must be collected by the patient in a special waste collector.
SBVCE is contraindicated in patients with known or suspected gastrointestinal obstruction, strictures, or fistulas, due to the risk of capsule impaction and retention. The overall rate of capsule retention has been reported to be 1.4%[1], but can be higher in patients at higher risk, such as in patients with known Crohn’s disease. In the event of capsule retention, endoscopic or surgical removal may be necessary.
The patency capsule (Agile; Given Imaging) is a dissolvable capsule that allows physicians to verify the patency of the gastrointestinal (GI) tract before performing a SBVCE examination in patients at risk for capsule retention (Crohn’s disease, small-bowel tumor, suspected or known strictures). The patency capsule has a similar shape and size to PillCam SB2. It consists of a dissolvable body composed of lactose and barium sulfate surrounded by cellophane walls. Inside the patency capsule, a radiofrequency identification tag allows detection by radiography or a portable radiofrequency scanner.
In a fluid-filled environment, the patency capsule begins to dissolve after 30 h. After dissolution of the capsule body, the non-degraded outer membrane is able to pass through strictures. It is determined to be safe to perform an SBVCE examination if the patency capsule has been excreted intact or if it is not detected by the radiofrequency scanner 30 h after its ingestion[13,14].
In patients with cardiac pacemakers or implanted electromedical devices, there is a theoretical risk of electromagnetic interference with SBVCE. Many capsule manufacturers do not recommend the use of SBVCE in these patients. A recent study suggests that although interference can occur between SBVCE and cardiac devices, causing loss of images or impaired quality of video, SBVCE in these patients is safe and is not associated with cardiac events or cardiac device dysfunction[15]. In such situations, it may be preferable to use CapsoCam technology.
Pregnancy is a contraindication to SBVCE due to a lack of safety data. Patients with swallowing disorders are at risk of tracheal aspiration of the capsule. In these patients, the capsule should be placed endoscopically[13].
Before SBVCE, a clear liquid diet and an 8-hour fast is suggested by manufacturers of VCE systems. Numerous studies have suggested the benefit of various bowel preparation schedules before SBVCE, yet there is still no consensus on the optimal preparation regimen (Table 2).
| Preparation |
| No preparation |
| Fasting since the day before |
| Clear liquid diet/a 8-h fast |
| Ingestion of PEG (1 or 2 L) |
| Simethicone |
| Indications |
| Obscure gastrointestinal bleeding |
| Crohn’s disease |
| Polyposis (FAP, Peutz-Jeghers…) |
| Coeliac disease |
| Intestinal tumors |
| SB mucosal breaks |
| Unsolved abdominal pain/diarrhea |
| Factors associated with a higher detection rate of positive findings |
| Early CE within the first days of admission for overt OGIB |
| Advanced age |
| Male sex |
| Inpatient status |
| Use of anticoagulation |
| Hepatic comorbidity |
| Occurrence of more than one episode of bleeding |
| Ongoing overt OGIB |
| Hemoglobin level of less than 10 g/dL |
| Coexistence of connective tissue disease |
| Need for a high number of transfusions before SBVCE |
According to two recent meta-analyses, small-bowel purgative preparation [with polyethylene glycol (PEG) solution or sodium phosphate] improves small-bowel mucosa visualization but does not influence small bowel transit time or SBVCE completion rate[16,17]. One of these meta-analyses also suggested that purgative preparation improves the diagnostic yield of the examination[16]. No clinically significant adverse event was related to small-bowel preparation[16].
A recent meta-analysis from Kotwal et al[18], also concluded that PEG solution improves visualization of the mucosa and that purgative preparation improves the diagnostic yield. Again, there were no effects on small-bowel transit time or completion rate. The use of simethicone also seems to improve visualization by reducing air bubbles. Prokinetics did not improve SBVCE completion rate.
Preparations with PEG solutions have been the most widely studied and there is no difference regarding the quality of bowel preparation, diagnostic yield, or completion rate between patients receiving two or four liters[19].
Different subjective scoring systems exist to assess the quality of SB preparation. Recently developed, the computer-assisted cleansing score is directly derived from SBVCE images and is based on the ratio of color intensities on a tissue color bar as a measure of small bowel contamination[20].
Around 80% of patients undergoing SBVCE have a complete examination of the small bowel[16]. Factors associated with incomplete examinations include inpatient status, delayed gastric emptying, prior abdominal surgery, and, possibly, older age and diabetes mellitus[12].
The most common applications of SBVCE include investigation of obscure GI bleeding, suspected Crohn’s disease, suspected or refractory celiac disease, suspected small-intestinal tumors, and surveillance of patients with hereditary polyposis syndromes[13] (Table 2).
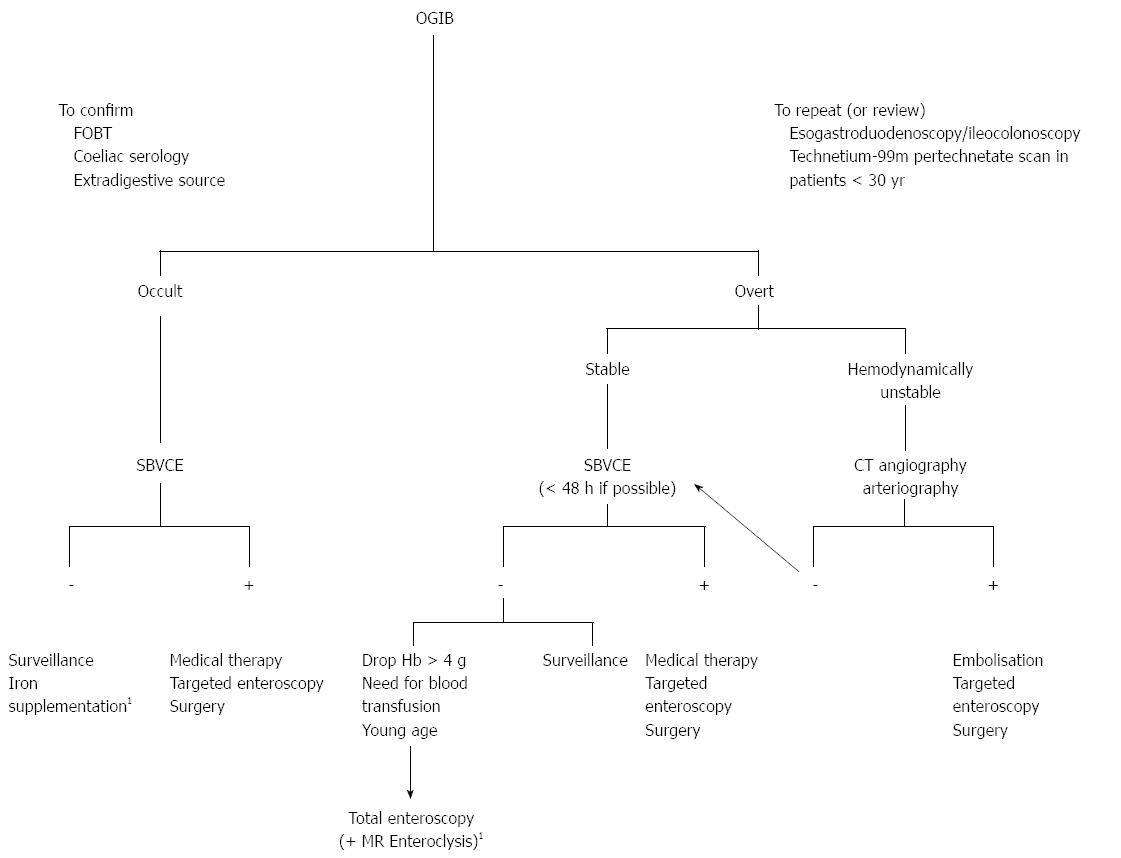
Obscure gastrointestinal bleeding (OGIB) is defined by recurrent or persistent bleeding of unknown origin after a negative initial investigation with esophagogastroduoden- oscopy (EGD) and colonoscopy. OGIB can be further categorized as either overt (visible GI bleeding) or occult (recurrent iron deficiency anemia and/or recurrent positive fecal occult blood test results). This represents approximately 5% of all cases of GI bleeding[13].
For patients with active overt OGIB or with occult OGIB, the 2010 American Society of Gastrointestinal Endoscopy guidelines for endoscopic management of OGIB recommend repeating an EGD if the clinical presentation suggests upper GI bleeding or repeating a colonoscopy if there is a clinical suspicion of lower GI bleeding. If this is not the case, small-bowel evaluation is recommended and, in most patients, SBVCE is a reasonable first choice[21] (Figure 1).
The diagnostic yield (DY) of SBVCE in OGIB ranges from 35%-77%[13]. Many factors are associated with a higher detection rate of positive findings[22-28] (Table 2).
To assess the DY of SBCE in IDA data from relevant studies were pooled[29]. The pooled DY of SBCE in IDA, evaluated by a random-effects model, was 47% (95%CI: 42%-52%), but there was statistically significant heterogeneity among the included studies (I2 = 78.8%, P < 0.0001). The pooled DY of SBCE in studies focused solely on patients with IDA (subset 1, 4 studies) was 66.6% (95%CI: 61.0%-72.3%, I2 = 44.3%); conversely, that of studies not focusing only on IDA patients (subset 2, 20 studies) was 44% (95%CI: 39%-48%, I2 = 64.9%). In patients younger than 40 years with IDA, SBVCE may reveal a more serious pathology (small-bowel malignancy, significant inflammation, strictures, celiac disease) in 25% of patients[30].
As many as 21% of patients with OGIB might actually have a missed or underestimated lesion at upper or lower endoscopy[31,32]. The most common small bowel (SB) cause of OGIB, especially in elderly patients, is arteriovenous malformations or angiectasis (20%-55% of cases of OGIB). Small bowel tumors represent 10%-20% of cases of OGIB and are the leading cause of OGIB in patients less than 50 years old. Other SB causes are: Crohn’s disease, celiac disease, Meckel’s diverticulum, drug-induced enteropathy, Dieulafoy lesion, and ectopic varices[31].
The therapeutic effect of SBVCE in OGIB has been evaluated in many studies. In a retrospective study from Redondo-Cerezo et al[33], 50.7% of patients with positive findings received a specific treatment [including discontinuation of non-steroidal anti-inflammatory drugs (NSAIDs)] and bleeding stopped or stabilized in a majority of patients. In a recent nationwide analysis in South Korea, interventional treatment was performed in 22.9% of patients with significant findings at SBVCE but did not influence the rebleeding rate[34].
At long-term follow-up, the rebleeding rate after a negative SBVCE varies from 4% to 27%[35-37]. The long-term rebleeding rate after a positive CE has been reported to be higher compared to patients with a negative SBVCE[37], although some studies have demonstrated comparable rebleeding rates. Specific treatment after a positive SBVCE does seem to lower the risk of rebleeding[38]. There are several reported risk factors for rebleeding[34,39] (Table 3).
| Hemoglobin level of less than 8 g/dL |
| Age over 70 yr |
| Presence of a significant lesion at SBVCE |
| Presence of angiectasis |
| Duration of OGIB for more than 3 mo |
| Continuation of anticoagulation |
In OGIB, SBVCE has a better diagnostic yield compared to radiographic barium studies[40]. Many studies have also demonstrated that SBVCE is superior to computed tomodensitometry enterography (CTE) and magnetic resonance enterography (MRE)[41,42]. In a recent prospective study, patients with overt OGIB were randomized to early CE or angiography. Early SBVCE had a higher diagnostic yield than angiography (53.3% vs 20.0%) and long-term outcomes were comparable[43].
SBVCE has also been compared to other SB endoscopic procedures in OGIB. A meta-analysis of fourteen studies[32] demonstrated that the diagnostic yield for clinically significant lesions is higher for SBVCE than push-enteroscopy (DY 56% vs 26%). In comparison to double-balloon enteroscopy (DBE), three meta-analyses have shown comparable diagnostic yields[44-46]. SBVCE is a less invasive procedure than DBE, but DBE has the advantage of allowing biopsies and treatment of endoscopic findings.
After a negative SBVCE, stable patients with OGIB can be managed conservatively with iron therapy[21]. However, patients with persistent or recurrent bleeding can benefit from a second-look SBVCE, as positive findings have been found in 41.6% of patients in a recent study. This is particularly true if the bleeding presentation changes from occult to overt or if hemoglobin values drop by ≥ 4 g/dL[47].
Crohn’s disease is a chronic inflammatory bowel disease that can affect the entire length of the GI tract. As many as half of patients have disease involving both the small bowel and the colon, and up to one-third of patients have only small-bowel involvement[48]. There is no single gold-standard for the diagnosis of CD. Diagnosis is based on a combination of clinical (chronic diarrhea, abdominal pain, weight loss) and physical examination features, as well as laboratory, radiographic, endoscopic, and histopathologic findings[49].
In most patients, endoscopic and histopathologic confirmation of CD is done by ileocolonoscopy. In patients with CD involving only parts of the small-bowel out of the reach of standard endoscopic examinations, SBVCE may be used as an initial diagnostic modality. However, in the presence of known strictures or obstructive symptoms, initial evaluation by SBVCE is contraindicated. In this situation, evaluation of the small bowel by CTE or MRE is recommended[50]. SBVCE has a high negative predictive value for CD. Thus, a diagnosis of small bowel CD is improbable if SBVCE evaluation is normal[51].
In patients with established CD, CTE or MRE is often preferable, because of the possibility to evaluate for strictures, disease distribution, and extraluminal disease[50]. In the absence of strictures or if a test with a patency capsule has been done, SBVCE may be used for different indications: assessment of mucosal healing under treatment[52], anemia, or atypical symptoms. SBVCE findings may influence a change of management in a majority of patients[53].
Other clinical situations where SBVCE can be used in established CD patients include evaluation of disease recurrence after surgery[54] and clarification of diagnosis in patients with inflammatory bowel disease unclassified[55].
SBVCE findings in CD may include erosions, ulcerations, mucosal erythema or edema, loss of villi, strictures, mucosal fissures, and fistula scarring. However, there are no precise validated endoscopic criteria to confirm the diagnosis of CD[56]. Endoscopic findings such as mucosal ulcerations are not specific to CD and are associated with diverse conditions such as Behçet’s disease, vasculitis, radiation enteritis, and drug-induced enteropathy[50]. Most importantly, mucosal anomalies have been reported in 71% of chronic users of non-steroidal anti-inflammatory drugs[57]. Because NSAID-induced mucosal lesions may mimic CD, it is recommended that intake of NSAIDs be stopped at least four weeks before performing a SBVCE in patients with suspected CD[50]. Interestingly, small-bowel lesions are not only seen in pathologic settings, as SBVCE may detect minor mucosal breaks or erosions in 10% of healthy individuals[58].
In order to improve the specificity of SBVCE findings in patients with suspected CD, different predictive markers of CD have been studied such as weight loss[59], perianal disease[60], and levels of fecal calprotectin. A recent retrospective study suggests that in patients with suspected CD and negative initial bi-directional endoscopic investigations, a fecal calprotectin level higher than 100 μg/g is a good predictor of positive SBVCE findings and a level higher than 200 μg/g is associated with confirmed cases of CD in 50% of patients[61]. In the 2013 European evidence-based consensus for endoscopy in inflammatory bowel disease, it was suggested that the pre-test probability of detecting CD may be improved by selecting patients with extra-intestinal manifestations, inflammatory markers, and/or elevated fecal calprotectin levels[50].
Endoscopic scores have been developed for the evaluation of CD by SBVCE. The Lewis score[62] was validated by Rosa et al[63] and has been incorporated into the PillCam software (RAPID, Given Imaging) to improve the standardization of CD activity. The score incorporates three endoscopic variables (villous edema, ulcer, and stenosis) used to measure disease activity in the proximal, middle, and distal third of the small bowel. The small-bowel tertile with the most points determines the final score. The Lewis score may also be a useful diagnostic tool when performing SBVCE in patients with suspected CD. More recently, a new score called the capsule endoscopy crohn’s disease activity index (CECDAI or Niv Score) has been validated in a multicenter prospective study[64]. The score incorporates three endoscopic parameters: inflammation, extent of disease, and stricture. The final score is calculated by adding the scores of both the proximal and distal small bowel segments.
The diagnostic yield of SBVCE in patients with suspected or established CD has been compared to small-bowel radiology [small-bowel follow-through (SBFT) or enteroclysis] in many studies[65-68]. These studies suggest that the diagnostic yield of CE is higher and that many mucosal lesions are missed with SBFT or enteroclysis.
Studies have also compared the diagnostic yield of SBVCE with cross-sectional imaging (CTE or MRE). Although a prospective blinded comparison trial did not show an improved sensitivity of SBVCE (83%) vs CTE (82%) for detection of CD[69], many studies have reported an advantage in DY for SBVCE (DY 61%-77%) when compared to the diagnostic yield of CT enteroclysis (DY 29%)[70] or CT enterography (DY 50%-53%)[66,71].
A prospective study in patients with suspected or newly diagnosed CD has compared the performance of SBVCE for detecting CD vs other modalities. SBVCE identified proximal CD in more patients than CTE or MRE. Sensitivity and specificity for detecting distal small-bowel CD was also better for SBVCE (100% and 91%) than for CTE (76% and 85%) or MRE (81% and 86%)[72].
A recent meta-analysis compared the diagnostic yield of SBVCE for detecting small-bowel CD with other modalities such as small-bowel radiology (SBR), CTE, MRE, push-enteroscopy (PE), and ileocolonoscopy in patients with suspected or established CD[73]. A total of thirty trials were included in this meta-analysis. For patients with suspected CD, the diagnostic yield of SBVCE was higher than SBR, CTE, and ileocolonoscopy. For patients with established small-bowel CD, SBVCE was better than SBR, CTE, and PE. This meta-analysis was not able to demonstrate an incremental yield when comparing SBVCE with MRE.
The evaluation of the severity and extent of active ulcerative colitis (UC) using Pillcam colon capsule endoscopy (PCCE) is new domain for VCE. The extent of mucosal damage and inflammatory lesions was identified in a comparative study between PCCE-2 and conventional colonoscopy on 26 patients with significant correlation in the severity and extent of UC between the PCCE-2 and conventional colonoscopy[74]. Another recent series of forty patients with histological confirmed diagnosis of UC confirmed the strong correlation between PCCE and conventional colonoscopy in scoring UC severity using Matts endoscopic scores as judged by PCCE-2 and conventional colonoscopy[75].
This data was confirmed by larger multicenter study with 100 patients with sensitivity of PCCE-2 to detect active colonic inflammation was 89% and specificity was 75%[76].
PCCE is a safe procedure to monitor mucosal healing in ulcerative colitis. Oliva et al[77] recently reported an observational study assessing the diagnostic accuracy of PCCE-2 in pediatric patients with ulcerative colitis. The sensitivity of PCCE-2 for disease activity was 96% and specificity was 100%. However, at this stage, CCE cannot be recommended to replace conventional colonoscopy in the management of this condition.
In a meta-analysis of 6 studies including a total of 166 patients with biopsy-proven celiac disease, the sensitivity and specificity of SBVCE for diagnosing celiac disease were, respectively, 89% (95%CI: 82%-94%) and 95% (95%CI: 89%-98%)[78]. However, the role of SBVCE in this setting is rather limited because of the inability to perform small-bowel biopsies that are necessary to confirm the diagnosis.
SBVCE has the advantage of being non-invasive and allows complete visualization of the small-bowel mucosa. In patients with suspected celiac disease who are either unwilling or unable to undergo an EGD, SBVCE could be an alternative[79]. In patients with positive celiac serology despite normal duodenal biopsies, a recent study suggests that SBVCE is unlikely to detect endoscopic findings of celiac disease[80].
SBVCE findings in celiac disease include scalloping of duodenal folds, loss or reduction of duodenal folds, mosaic mucosal pattern, and micronodular pattern of the mucosa[78].
SBVCE seems to have a role in patients with complicated or refractory celiac disease. Visualization of the entire length of the small bowel may help identify complications such as ulcerative jejunoileitis and small bowel malignancy[81]. In a prospective study of 47 patients with complicated celiac disease, SBVCE revealed findings consistent with celiac disease in 87% and unexpected findings in over 50% of patients (ulcerations, nodularity, cancer, stricture)[82].
In patients undergoing examination with SBVCE for various indications, the prevalence of SB tumors ranges from 2%-12%[83]. The most common indication for SBVCE in patients diagnosed with a small-bowel tumor is OGIB[12]. SB tumors are found in the jejunum in 40%-60%, in the ileum in 25%-40%, and in the duodenum in 15%-20%. The majority of patients diagnosed with an SB tumor by SBVCE have already been investigated by multiple previous negative procedures[84].
In a large multicenter European study of 5129 patients undergoing SBVCE, 2.4% of patients had small-bowel tumors. Ninety percent of the tumors were primary and 10% were metastatic. The most common primary tumors were gastrointestinal stromal tumors (GIST) (32%), adenocarcinomas (20%), and carcinoid tumors (15%). Most metastatic tumors are secondary to melanoma[85]. Other primary malignant tumors that can be found in the small-bowel are lymphomas, sarcomas, and hamartomas. The most common benign SB tumors are GIST, hemangiomas, hamartomas, adenomas, and granulation tissue polyps[84].
In patients with familial adenomatous polyposis (FAP), duodenal adenomas occur in 60%-90% of patients and have a tendency to involve the periampullary region. Four to twelve percent of patients will develop duodenal cancer in their life. The occurrence of jejunal and ileal adenomas is, respectively, 40% and 20%, with a lower risk of malignant transformation[48]. FAP patients with duodenal adenomas seem to be at higher risk of having more distal SB adenomas[86]. For this reason, SBVCE should be considered in FAP patients with duodenal polyps[12]. Since SBVCE detects the periampullary region in a low proportion of patients[86], EGD with a side-viewing endoscope starting at age 25-30 with screening intervals based on Spigelman stages is still recommended in all patients with FAP[12,48].
Patients with Peutz-Jeghers syndrome have GI polyps and mucocutaneous pigmentation. Most gastrointestinal polyps occur in the small bowel[87]. SBVCE should be considered as a first-line screening modality for surveillance in patients with Peutz-Jeghers syndrome[12,87]. This screening should begin at age 8 and, if no polyps are found, it should be continued every 3 years starting at age 18[87].
For surveillance of the SB in patients with hereditary polyposis syndromes, SBVCE is superior to barium contrast studies[88]. When compared to MRE, SBVCE seems better for detecting polyps less than 5 mm, but the detection rate for larger polyps seems similar[89,90]. However, MRE may be more accurate for the description of size and location of polyps and has the advantage of giving extraintestinal information[13].
Fuji intelligent color enhancement (FICE) technology processes ordinary endoscopic images into spectral images of given wavelengths. This image reconstruction results in fine high-contrast images. FICE software has been incorporated into the Given imaging workstation (Given Imaging Ltd; Yokneam, Israel) and the examiner can simply switch from conventional white light images to three different settings of FICE reconstructed images by clicking an icon in the Rapid Reader software. Blue mode is an additional setting that is incorporated into the Rapid Software and represents a color coefficient shift of light in the short wavelength range (490-430 nm) superimposed onto a white light image[91].
Data on the role of FICE in capsule endoscopy and its impact on the visualization of small-bowel lesions are still limited and some of the results have been discordant.
In a study by Gupta et al[92], sixty SBVCE examinations for OGIB were retrospectively analyzed with and without FICE enhancement by two observers. Randomization was such that an observer did not evaluate the same examination with and without FICE enhancement. FICE did not improve the diagnostic yield of the examination and significantly more non-pathological lesions were observed with FICE. In patients with vascular lesions, some were better characterized by FICE. Another study by Matsumura et al[93] also found that although FICE detected significantly more small bowel lesions than conventional imaging (2.5 ± 2.1 vs 1.8 ± 1.7 respectively, P = 0.001), there was no improvement in diagnostic yield.
Use of FICE and Blue-mode enhancement in capsule endoscopy images of six different types of small bowel lesion was also evaluated by Krystallis et al[91]. FICE was ineffective in improving endoscopic images except in a small proportion of certain lesions (ulcer/aphthae, lesions of indeterminate clinical significance or blood in lumen) that were improved with FICE setting 1 [RGB wavelength, (595, 540, 535 nm)]. Most importantly, Blue-mode enhancement was associated with an improved image when compared to white light images in 83% of patients for all types of lesions.
These conclusions differ from those of a study by Imagawa et al[94] suggesting that the use of FICE enhances image quality of angiectasis, erosions/ulcerations, and tumors. Blue-mode enhancement was not evaluated in this study.
Another technique that has been recently studied is three-dimensional reconstruction of capsule endoscopy images using software algorithms (shape-from-shading) from two-dimensional images. The use of this software leads to improved visualization of a significant proportion of vascular lesions[95].
Rondonotti et al[96] evaluated whether “coupling” 3D reconstructed video clips with the standard 2-dimensional (2D) counterparts helps in distinguishing masses from bulges. The adjunction of a 3D reconstruction to the 2D video reading platform does not improve the performance of expert SBVCE although it significantly increases the performance of novices in distinguishing masses from bulging.
A case-control study was conducted to evaluate whether the use of an external real-time viewer could improve the completion rate of SBVCE and if it influenced the rate of positive findings. For patients followed with a real-time viewer, water and/or metoclopramide was administered if capsule transit was delayed. Use of an external real-time viewer was associated with a higher completion rate and a higher diagnostic yield[97].
For intraoperative identification of a bleeding lesion in the small bowel, Yamashita et al[98] have reported the use of real-time capsule endoscopy in two patients. The patients had a long double-lumen tube inserted nasally 3 or 4 d before the surgery. This tube migrates to the anus by the day of surgery. During surgery, a capsule is attached to the distal end of the tube and the site of the bleeding lesion is identified precisely by pulling on the tube.
Several prototypes of self-propelled capsules (using fins, legs, or a self-contained propeller) have recently been evaluated in an attempt to allow capsules to navigate and stabilize in areas of pathology, which could improve their diagnostic and possibly therapeutic capabilities[2]. Magnetically-guided capsule endoscopy (MGCE) has been studied mostly for examination of the stomach because visualization of the gastric mucosa is incomplete when the capsule is propelled only by gastric motility. A recent blinded nonrandomized comparative study of gastric evaluation with MGCE vs conventional gastroscopy showed less missed endoscopic findings with MGCE but similar diagnostic yields[99].
Pasricha et al[100] recently reported of a novel method of controlled colonic insufflation (CO2) via an untethered capsule in vivo. This technological innovation that was assessed in porcing colons addresses a critical need in colon capsule endoscopy.
Capsules that can perform mucosal tagging, biopsies or therapeutic interventions are in development. For example, a magnetically-maneuvered capsule that can release a nitinol clip to treat an iatrogenic bleed has been evaluated in a pig model. Non-video capsules that can deliver drugs with a pH-activated or temperature-activated release mechanism have also been evaluated. However, improvement in capsule maneuvering capabilities are necessary before these capsules can be further developed[2,101].
Colon capsule endoscopy (CCE) is recently developed to image the whole colon in a noninvasive way. Only few studies are available in the literature. Reported results are still controversial and conflicting. There is still a high degree of uncertainty regarding clinical indications of CCE. Colorectal cancer screening still remains a challenge because most patients decline to participate in screening programs due to anxiety directly related to the endoscopic procedure. In this context, the colon capsule has a potential future but more data in needed to answer many pending questions such as the best preparation method, the best scoring method, the best booster and mainly the best indications.
In this part we are going to demonstrate the available literature regarding indication, efficiency of CCE and future hopes.
The first generation PillCam Colon (Given Imaging; Ltd., Israel) has been extensively described in previous studies[102,103]. It is a 31 mm × 11 mm ingestible capsule, specifically designed for colon exploration, It has dual cameras, enabling it to acquire images from both ends. The angle of view from each imager is 156°. CCE is equipped with an advanced light control in order to ensure an optimal visualization of the colonic mucosa. It acquires images at a rate of 4 frames per second.
Recently, a second-generation colon capsule system has been developed to increase capsule sensitivity for detection of colonic findings and to simplify the procedure[104]. This new system comprises new developments of the colon capsule (PCCE-2), data recorder and software for video processing and viewing. The new CCE-2 is 11.6 mm × 31.5 mm in size, slightly bigger than the previous capsule. It has 2 imagers with wider angle of view that has been increased to 172° for each imager, allowing nearly 360° coverage of the colon. PCCE-2 captures 35 images per second when in motion and 4 images per second when it is virtually stationary. The new data recorder (DR3) also assists and guides the physician and the patient through the procedure. In fact, it buzzes and vibrates and displays instructions on its liquid crystal diode screen to alert the patient to continue the preparation protocol.
The new DR3 by Given Imaging Ltd; Yoqneam, Israel not only stores the capsule’s incoming images but also analyzes them in real time to control the capsule capture rate of images at an adaptive frame rate. When DR3 recognizes that the capsule is virtually stationary, it sets the image capture rate to 4 frames per second. When the DR3 recognizes that the capsule is in motion, it sets the image capture rate to 35 frames per second. A recent study tested the reliability of the automatic detection of the small bowel (SB) mucosa and the subsequent alert for booster ingestion by the data recorder 3 (DR3) of the second-generation colon capsule endoscopy (PCCE-2). A series of 120 consecutive cases of PCCE-2 was analyzed for proper DR3 automatic detection of the capsule entering the SB. The DR3 correctly identified the proper time for ingestion of the laxative (booster) in 118 of 120 cases, corresponding to a sensitivity of 98.3% (95%CI: 97%-100%). The median time difference between DR3 automatic SB detection to the observed entrance of the capsule into the SB was 210 s (interquartile range: 95-357 s)[105].
One of the major limitation PCCE examination is the time-consuming video reading but there is normal view examination is very important especially in polyp detection, A recent study assessed the theoretical time-saving potential of “QuickView” which may reduce the frames to be analyzed but the risk of less identification of candidates for therapeutic colonoscopy[106].
The colon preparation has been adapted for meeting several objectives: first, to clean the colon as well as possible, second, to provoke the progression of the capsule throughout the small bowel up to the colon and third, to provide residual clear liquid in order to facilitate the visualization of the colonic mucosa using the so-called “submarine view”. Patients have to follow a clear liquid diet the day before the procedure. On the evening before colon ingestion, patients orally ingested 3 L of polyethylene glycol solution (ColoPEG®) and an additional liter between 6.00 and 7.00 on the day of the procedure. Then the patient ingest the capsule after having received two 10 mg Domperidone. Two hours after the capsule ingestion, patients orally ingested 45 mL of sodium phosphate solution (Fleet Phosphosoda) that serves as a booth for facilitating the capsule progression. The progression of the capsule is assessed with the real time viewing system performed with rapid access real time tablet DC (Given Imaging). The quality of the colon cleansing is determined according to a four-point grading scale (excellent, good, fair, poor) as previously described[103,107].
The standard preparation was recently challenged in many studies aiming to have better patient comfort as well higher cleansing levels. A recent study examined the idea of decreasing the time needed for preparation i.e., one day instead of two days in a randomize fashion, the one day group received a fiber-free diet and 3 L of PEG on day 0; while two-day group received a liquid diet and 3 L of PEG in the evening of day-1, and 1 L of PEG in the early morning of day 0. The authors were not able to show statistically significant difference between both groups but with better cleanliness tendency in the group of one day preparation[108].
A recent multicenter prospective series compared again the “reduced volume method” (group A) with the “conventional volume method” (group B) preparation regimens, where Group A did not drink polyethylene glycol electrolyte lavage solution (PEG-ELS) the day before the capsule procedure, while group B drank 2 L. During the procedure day, groups A and B drank 2 L and 1 L of PEG-ELS, respectively, two hours later the first booster of 100 g magnesium citrate mixed with 900 mL water was administered to both groups, and the second booster was administered six hours post capsule ingestion as long as the capsule had not been excreted by that time. Sixty-four subjects were enrolled, with results from 60 analyzed with no significant difference between the 2 groups in the term of colon cleanliness[109].
Sodium phosphate (NaP) is needed as booster for PCCE although many potential risks are documented with NaP. A comparative study assessed the feasibility of substituting sodium phosphate with PEG-boosters in the protocol preparation for PCCE, The excretion rate was higher in NaP group but without significant difference in terms of colon cleansing[110].
Polyp detection: The prospective, multicentre European study comparing CCE to optical colonoscopy was performed in 328 adults (mean age: 58.6, range: 22-84 years) with known or suspected lesions for the detection of colorectal polyps or cancer[72]. Optical colonoscopy (OC) was considered as the gold standard against which PCCE-1 was compared. At 1:45 am post-ingestion (upon “wake-up”) the capsule remained proximal to the cecum in 97.5% of the patients. Sixty-nine percent of the patients excreted the capsule within 6 h post-ingestion and 92.8% within 10 h. Colon cleanliness was considered to be good-to-excellent in 72% of patients for PCCE and in 87% for conventional optical colonoscopy. In this cohort of 320 patients who were included in the accuracy analysis, 68% of the patients had at least one polyp, the majority being less 6 mm in size. Fifty patients (16%) had at least one polyp equal or greater than 10 mm. OC detected 23 invasive carcinomas in 19 patients. Twenty two of the 23 invasive carcinomas measured greater than 10 mm. The sensitivity and specificity of PCCE for detecting polyps that were 6 mm in size or bigger were 64% and 84%, respectively, and for detecting advanced adenoma, the sensitivity and specificity were 73% and 79%, respectively. Interestingly, the sensitivity was significantly higher in the patients with good-to-excellent cleanliness (72% of the global population) as compared with the patients with poor or fair cleanliness, with a limited effect on specificity[107].
A second-generation colon capsule endoscopy system (PCCE-2) was developed to increase sensitivity for colorectal polyp detection compared with the first-generation system. A prospective, multicenter trial including 8 European sites involved 117 patients PCCE-2 sensitivity for polyps ≥ 6 mm and ≥ 10 mm was 84% and 88%, with specificities of 64% and 95%, respectively[111] (Table 4).
The role of CCE in incomplete colonoscopy could be of great impact mainly due the reverse direction of examination, i.e., an incomplete colonoscopy misses the examination of caecum and right colon while CCE starts the examination by examination such areas. A recent prospective study studied 75 patients with incomplete colonoscopy confirmed that CCE reached or went beyond the colon segment at which colonoscopy stopped in 91%[112], another smaller series examining same concept but in known or suspected Crohn’s disease patients who refused colonoscopy or underwent incomplete colonoscopy confirmed the effectiveness of CCE in this indication[113].
The efficacy of CCE in helping physicians make decisions about patients with incomplete conventional colonoscopies was assessed in another recent prospective work, where CCE findings allowed formulation of a specific medical plan (polypectomy or surgery for advanced colorectal neoplasia) for 58% of patients[114]. Spada et al[115] performed a prospective, comparative trial comparing colon capsule vs CT colonography (CTC) in patients with incomplete colonoscopy. One hundred consecutive patients were enrolled. PCCE-2 and CTC were able to achieve complete colonic evaluation in 98% of the cases. The relative sensitivity of PCCE-2 compared to CTC was 2.0, indicating a significant increase in sensitivity for lesions ≥ 6 mm. Positive predictive values for polyps ≥ 6 mm and ≥ 10 mm were 96% and 85.7%, and 83.3% and 100% for PCCE-2 and CTC, respectively; the overall diagnostic yield of colon capsule was superior to CTC. In early February 2014, Given Imaging received FDA clearance for Pillcam Colon in patients following incomplete colonoscopy.
The accuracy of CCE in high risk patients who are unable or unwilling to do colonscopy was recently tested in a series of 70 patients, CCE showed positive findings in 34% of patients, six patients were diagnosed with tumors: 4 with colon cancers, 1 with gastric cancer and 1 with a small bowel cancer. The capsule findings were confirmed after surgery in all these patients. This may be another new indication of CCE as an adequate alternative diagnostic tool in patients unable or unwilling to undergo colonoscopy[116]. A Prospective multicenter study confirmed this data by assessing the CCE diagnostic yield in patients with colonoscopy failure or anesthesia contraindication
CCE showed positive findings in 33.6% of patients whom subsequently underwent therapeutic intervention[117].
This confirms the usefulness of CCE in the situation of colonoscopy failure or contraindication.
Up to now, classical colorectal imaging procedures may not be performed on an out-of-clinic basis. This represents a major drawback compared with fecal tests. Because PCCE-2 automatically detects small bowel mucosa, it has the potential to become the first colorectal imaging test to be performed out-of-clinic. A recent study performed on 41 patients with the help of preregistered date recorder DR3 alerts, patients were able to know when to have first booster as well as the need for second booster, the patient compliance to DR3 alerts was 100% which may open a new prospective for home based PCCE-2[118].
SBVCE has become a routinely-used method for exploring the small bowel. SBCE is considered as the first-line procedure in case of obscure gastrointestinal bleeding and has some room in case of Crohn’s disease, coeliac disease, small-bowel polyposis, unexplained abdominal pain and/or diarrhea.
Esophageal VCE is feasible but has a limited room in clinical practice.
Recent studies have shown that the second generation of the colon capsule (PCCE-2) has an excellent sensitivity and specificity for detecting colonic polyp or cancer. PCCE-2 is now recommended in case of incomplete colonoscopy (FDA approval) but also in patients who are unwilling or contraindicated for standard colonoscopy. Further studies are ongoing to determine the accuracy of PCCE-2 as a first-line screening procedure for colorectal cancer and maybe for the follow-up of mucosal healing in patients with inflammatory bowel diseases.
There are now several SB capsules that are available on the market with continuous technological developments. Researches are done for performing biopsy sample and therapeutic maneuvers and motion control.
P- Reviewer: Andus T, Aytac E, Gurvits GE, Kato M, Ogata H, Stanciu C, Sieg A, Triantafyllou K S- Editor: Gou SX L- Editor: A E- Editor: Ma S
| 1. | Liao Z, Gao R, Xu C, Li ZS. Indications and detection, completion, and retention rates of small-bowel capsule endoscopy: a systematic review. Gastrointest Endosc. 2010;71:280-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 561] [Cited by in RCA: 483] [Article Influence: 30.2] [Reference Citation Analysis (1)] |
| 2. | Fisher LR, Hasler WL. New vision in video capsule endoscopy: current status and future directions. Nat Rev Gastroenterol Hepatol. 2012;9:392-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 3. | Eliakim R, Sharma VK, Yassin K, Adler SN, Jacob H, Cave DR, Sachdev R, Mitty RD, Hartmann D, Schilling D. A prospective study of the diagnostic accuracy of PillCam ESO esophageal capsule endoscopy versus conventional upper endoscopy in patients with chronic gastroesophageal reflux diseases. J Clin Gastroenterol. 2005;39:572-578. [PubMed] |
| 4. | Gralnek IM, Adler SN, Yassin K, Koslowsky B, Metzger Y, Eliakim R. Detecting esophageal disease with second-generation capsule endoscopy: initial evaluation of the PillCam ESO 2. Endoscopy. 2008;40:275-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 44] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Heresbach D, Leray E, d’Halluin PN, Cholet F, Lapalus MG, Gaudric M, Ben Soussan E, Gaudin JL, Vahedi K, Quentin V. Diagnostic accuracy of esophageal capsule endoscopy versus conventional upper digestive endoscopy for suspected esophageal squamous cell carcinoma. Endoscopy. 2010;42:93-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Domingos TA, Moura EG, Mendes DC, Martins BC, Sallum RA, Nasi A, Sakai P, Cecconello I. Comparative evaluation of esophageal Barrett’s epithelium through esophageal capsule endoscopy and methylene blue chromoendoscopy. Rev Gastroenterol Mex. 2013;78:57-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 7. | Chang JY, Talley NJ, Locke GR, Katzka DA, Schleck CD, Zinsmeister AR, Dunagan KT, Wu TT, Wang KK, Prasad GA. Population screening for barrett esophagus: a prospective randomized pilot study. Mayo Clin Proc. 2011;86:1174-1180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Ramirez FC, Shaukat MS, Young MA, Johnson DA, Akins R. Feasibility and safety of string, wireless capsule endoscopy in the diagnosis of Barrett’s esophagus. Gastrointest Endosc. 2005;61:741-746. [PubMed] |
| 9. | Chen WS, Zhu LH, Li DZ, Chen L, Wu YL, Wang W. String esophageal capsule endoscopy with real-time viewing improves visualization of the distal esophageal Z-line: a prospective, comparative study. Eur J Gastroenterol Hepatol. 2014;26:309-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Guturu P, Sagi SV, Ahn D, Jaganmohan S, Kuo YF, Sood GK. Capsule endoscopy with PILLCAM ESO for detecting esophageal varices: a meta-analysis. Minerva Gastroenterol Dietol. 2011;57:1-11. [PubMed] |
| 11. | Kopylov U, Seidman EG. Clinical applications of small bowel capsule endoscopy. Clin Exp Gastroenterol. 2013;6:129-137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 12. | Ladas SD, Triantafyllou K, Spada C, Riccioni ME, Rey JF, Niv Y, Delvaux M, de Franchis R, Costamagna G. European Society of Gastrointestinal Endoscopy (ESGE): recommendations (2009) on clinical use of video capsule endoscopy to investigate small-bowel, esophageal and colonic diseases. Endoscopy. 2010;42:220-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 173] [Article Influence: 10.8] [Reference Citation Analysis (1)] |
| 13. | ASGE Technology Committee; Wang A, Banerjee S, Barth BA, Bhat YM, Chauhan S, Gottlieb KT, Konda V, Maple JT, Murad F, Pfau PR, Pleskow DK, Siddiqui UD, Tokar JL, Rodriguez SA. Wireless capsule endoscopy. Gastrointest Endosc. 2013;78:805-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 196] [Article Influence: 15.1] [Reference Citation Analysis (2)] |
| 14. | Caunedo-Alvarez A, Romero-Vazquez J, Herrerias-Gutierrez JM. Patency and Agile capsules. World J Gastroenterol. 2008;14:5269-5273. [PubMed] |
| 15. | Bandorski D, Lotterer E, Hartmann D, Jakobs R, Brück M, Hoeltgen R, Wieczorek M, Brock A, de Rossi T, Keuchel M. Capsule endoscopy in patients with cardiac pacemakers and implantable cardioverter-defibrillators - a retrospective multicenter investigation. J Gastrointestin Liver Dis. 2011;20:33-37. [PubMed] |
| 16. | Rokkas T, Papaxoinis K, Triantafyllou K, Pistiolas D, Ladas SD. Does purgative preparation influence the diagnostic yield of small bowel video capsule endoscopy?: A meta-analysis. Am J Gastroenterol. 2009;104:219-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 130] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 17. | Niv Y. Efficiency of bowel preparation for capsule endoscopy examination: a meta-analysis. World J Gastroenterol. 2008;14:1313-1317. [PubMed] |
| 18. | Kotwal VS, Attar BM, Gupta S, Agarwal R. Should bowel preparation, antifoaming agents, or prokinetics be used before video capsule endoscopy? A systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2014;26:137-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 90] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 19. | Song HJ, Moon JS, Do JH, Cha IH, Yang CH, Choi MG, Jeen YT, Kim HJ. Guidelines for Bowel Preparation before Video Capsule Endoscopy. Clin Endosc. 2013;46:147-154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (1)] |
| 20. | Van Weyenberg SJ, De Leest HT, Mulder CJ. Description of a novel grading system to assess the quality of bowel preparation in video capsule endoscopy. Endoscopy. 2011;43:406-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 21. | ASGE Standards of Practice Committee; Fisher L, Lee Krinsky M, Anderson MA, Appalaneni V, Banerjee S, Ben-Menachem T, Cash BD, Decker GA, Fanelli RD, Friis C, Fukami N, Harrison ME, Ikenberry SO, Jain R, Jue T, Khan K, Maple JT, Strohmeyer L, Sharaf R, Dominitz JA. The role of endoscopy in the management of obscure GI bleeding. Gastrointest Endosc. 2010;72:471-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 167] [Article Influence: 10.4] [Reference Citation Analysis (1)] |
| 22. | Singh A, Marshall C, Chaudhuri B, Okoli C, Foley A, Person SD, Bhattacharya K, Cave DR. Timing of video capsule endoscopy relative to overt obscure GI bleeding: implications from a retrospective study. Gastrointest Endosc. 2013;77:761-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 94] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 23. | Yamada A, Watabe H, Kobayashi Y, Yamaji Y, Yoshida H, Koike K. Timing of capsule endoscopy influences the diagnosis and outcome in obscure-overt gastrointestinal bleeding. Hepatogastroenterology. 2012;59:676-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 24. | Scaglione G, Russo F, Franco MR, Sarracco P, Pietrini L, Sorrentini I. Age and video capsule endoscopy in obscure gastrointestinal bleeding: a prospective study on hospitalized patients. Dig Dis Sci. 2011;56:1188-1193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Lepileur L, Dray X, Antonietti M, Iwanicki-Caron I, Grigioni S, Chaput U, Di-Fiore A, Alhameedi R, Marteau P, Ducrotté P. Factors associated with diagnosis of obscure gastrointestinal bleeding by video capsule enteroscopy. Clin Gastroenterol Hepatol. 2012;10:1376-1380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 26. | Sidhu R, Sanders DS, Kapur K, Leeds JS, McAlindon ME. Factors predicting the diagnostic yield and intervention in obscure gastrointestinal bleeding investigated using capsule endoscopy. J Gastrointestin Liver Dis. 2009;18:273-278. [PubMed] |
| 27. | Van Gossum A, Ibrahim M. Video capsule endoscopy: what is the future? Gastroenterol Clin North Am. 2010;39:807-826. [PubMed] |
| 28. | Shahidi NC, Ou G, Svarta S, Law JK, Kwok R, Tong J, Lam EC, Enns R. Factors associated with positive findings from capsule endoscopy in patients with obscure gastrointestinal bleeding. Clin Gastroenterol Hepatol. 2012;10:1381-1385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 29. | Koulaouzidis A, Rondonotti E, Giannakou A, Plevris JN. Diagnostic yield of small-bowel capsule endoscopy in patients with iron-deficiency anemia: a systematic review. Gastrointest Endosc. 2012;76:983-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 91] [Article Influence: 6.5] [Reference Citation Analysis (1)] |
| 30. | Koulaouzidis A, Yung DE, Lam JH, Smirnidis A, Douglas S, Plevris JN. The use of small-bowel capsule endoscopy in iron-deficiency anemia alone; be aware of the young anemic patient. Scand J Gastroenterol. 2012;47:1094-1100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 31. | Liu K, Kaffes AJ. Review article: the diagnosis and investigation of obscure gastrointestinal bleeding. Aliment Pharmacol Ther. 2011;34:416-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 32. | Tacheci I, Devière J, Kopacova M, Douda T, Bures J, Van Gossum A. The importance of upper gastrointestinal lesions detected with capsule endoscopy in patients with obscure digestive bleeding. Acta Gastroenterol Belg. 2011;74:395-399. [PubMed] |
| 33. | Redondo-Cerezo E, Pérez-Vigara G, Pérez-Sola A, Gómez-Ruiz CJ, Chicano MV, Sánchez-Manjavacas N, Morillas J, Pérez-García JI, García-Cano J. Diagnostic yield and impact of capsule endoscopy on management of patients with gastrointestinal bleeding of obscure origin. Dig Dis Sci. 2007;52:1376-1381. [PubMed] |
| 34. | Min YW, Kim JS, Jeon SW, Jeen YT, Im JP, Cheung DY, Choi MG, Kim JO, Lee KJ, Ye BD. Long-term outcome of capsule endoscopy in obscure gastrointestinal bleeding: a nationwide analysis. Endoscopy. 2014;46:59-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 35. | Lai LH, Wong GL, Chow DK, Lau JY, Sung JJ, Leung WK. Long-term follow-up of patients with obscure gastrointestinal bleeding after negative capsule endoscopy. Am J Gastroenterol. 2006;101:1224-1228. [PubMed] |
| 36. | Kim JB, Ye BD, Song Y, Yang DH, Jung KW, Kim KJ, Byeon JS, Myung SJ, Yang SK, Kim JH. Frequency of rebleeding events in obscure gastrointestinal bleeding with negative capsule endoscopy. J Gastroenterol Hepatol. 2013;28:834-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 37. | Iwamoto J, Mizokami Y, Shimokobe K, Yara S, Murakami M, Kido K, Ito M, Hirayama T, Saito Y, Honda A. The clinical outcome of capsule endoscopy in patients with obscure gastrointestinal bleeding. Hepatogastroenterology. 2011;58:301-305. [PubMed] |
| 38. | Park JJ, Cheon JH, Kim HM, Park HS, Moon CM, Lee JH, Hong SP, Kim TI, Kim WH. Negative capsule endoscopy without subsequent enteroscopy does not predict lower long-term rebleeding rates in patients with obscure GI bleeding. Gastrointest Endosc. 2010;71:990-997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 39. | Cañas-Ventura A, Márquez L, Bessa X, Dedeu JM, Puigvehí M, Delgado-Aros S, Ibáñez IA, Seoane A, Barranco L, Bory F. Outcome in obscure gastrointestinal bleeding after capsule endoscopy. World J Gastrointest Endosc. 2013;5:551-558. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 40. | Triester SL, Leighton JA, Leontiadis GI, Fleischer DE, Hara AK, Heigh RI, Shiff AD, Sharma VK. A meta-analysis of the yield of capsule endoscopy compared to other diagnostic modalities in patients with obscure gastrointestinal bleeding. Am J Gastroenterol. 2005;100:2407-2418. [PubMed] |
| 41. | Milano A, Balatsinou C, Filippone A, Caldarella MP, Laterza F, Lapenna D, Pierdomenico SD, Pace F, Cuccurullo F, Neri M. A prospective evaluation of iron deficiency anemia in the GI endoscopy setting: role of standard endoscopy, videocapsule endoscopy, and CT-enteroclysis. Gastrointest Endosc. 2011;73:1002-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 42. | Wiarda BM, Heine DG, Mensink P, Stolk M, Dees J, Hazenberg HJ, Stoker J, Kuipers EJ. Comparison of magnetic resonance enteroclysis and capsule endoscopy with balloon-assisted enteroscopy in patients with obscure gastrointestinal bleeding. Endoscopy. 2012;44:668-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 43. | Leung WK, Ho SS, Suen BY, Lai LH, Yu S, Ng EK, Ng SS, Chiu PW, Sung JJ, Chan FK. Capsule endoscopy or angiography in patients with acute overt obscure gastrointestinal bleeding: a prospective randomized study with long-term follow-up. Am J Gastroenterol. 2012;107:1370-1376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 44. | Chen X, Ran ZH, Tong JL. A meta-analysis of the yield of capsule endoscopy compared to double-balloon enteroscopy in patients with small bowel diseases. World J Gastroenterol. 2007;13:4372-4378. [PubMed] |
| 45. | Pasha SF, Leighton JA, Das A, Harrison ME, Decker GA, Fleischer DE, Sharma VK. Double-balloon enteroscopy and capsule endoscopy have comparable diagnostic yield in small-bowel disease: a meta-analysis. Clin Gastroenterol Hepatol. 2008;6:671-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 274] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 46. | Teshima CW, Kuipers EJ, van Zanten SV, Mensink PB. Double balloon enteroscopy and capsule endoscopy for obscure gastrointestinal bleeding: an updated meta-analysis. J Gastroenterol Hepatol. 2011;26:796-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 211] [Article Influence: 14.1] [Reference Citation Analysis (1)] |
| 47. | Viazis N, Papaxoinis K, Vlachogiannakos J, Efthymiou A, Theodoropoulos I, Karamanolis DG. Is there a role for second-look capsule endoscopy in patients with obscure GI bleeding after a nondiagnostic first test? Gastrointest Endosc. 2009;69:850-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 73] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 48. | Feldman M, Friedman LS, Brandt LJ. Sleisenger and Fordtran’s gastrointestinal and liver Disease. 9th ed. Philadelphia, Pa: Saunders Elsevier 2010; P.1949. |
| 49. | Van Assche G, Dignass A, Panes J, Beaugerie L, Karagiannis J, Allez M, Ochsenkühn T, Orchard T, Rogler G, Louis E. The second European evidence-based Consensus on the diagnosis and management of Crohn’s disease: Definitions and diagnosis. J Crohns Colitis. 2010;4:7-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 818] [Cited by in RCA: 798] [Article Influence: 49.9] [Reference Citation Analysis (0)] |
| 50. | Annese V, Daperno M, Rutter MD, Amiot A, Bossuyt P, East J, Ferrante M, Götz M, Katsanos KH, Kießlich R. European evidence based consensus for endoscopy in inflammatory bowel disease. J Crohns Colitis. 2013;7:982-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 640] [Cited by in RCA: 600] [Article Influence: 46.2] [Reference Citation Analysis (1)] |
| 51. | Leighton JA. The role of endoscopic imaging of the small bowel in clinical practice. Am J Gastroenterol. 2011;106:27-36; quiz 37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 52. | Efthymiou A, Viazis N, Mantzaris G, Papadimitriou N, Tzourmakliotis D, Raptis S, Karamanolis DG. Does clinical response correlate with mucosal healing in patients with Crohn’s disease of the small bowel? A prospective, case-series study using wireless capsule endoscopy. Inflamm Bowel Dis. 2008;14:1542-1547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 81] [Article Influence: 4.5] [Reference Citation Analysis (36)] |
| 53. | Dussault C, Gower-Rousseau C, Salleron J, Vernier-Massouille G, Branche J, Colombel JF, Maunoury V. Small bowel capsule endoscopy for management of Crohn’s disease: a retrospective tertiary care centre experience. Dig Liver Dis. 2013;45:558-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 54. | Pons Beltrán V, Nos P, Bastida G, Beltrán B, Argüello L, Aguas M, Rubín A, Pertejo V, Sala T. Evaluation of postsurgical recurrence in Crohn’s disease: a new indication for capsule endoscopy? Gastrointest Endosc. 2007;66:533-540. [PubMed] |
| 55. | Kalla R, McAlindon ME, Drew K, Sidhu R. Clinical utility of capsule endoscopy in patients with Crohn’s disease and inflammatory bowel disease unclassified. Eur J Gastroenterol Hepatol. 2013;25:706-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (1)] |
| 56. | Bourreille A, Ignjatovic A, Aabakken L, Loftus EV, Eliakim R, Pennazio M, Bouhnik Y, Seidman E, Keuchel M, Albert JG. Role of small-bowel endoscopy in the management of patients with inflammatory bowel disease: an international OMED-ECCO consensus. Endoscopy. 2009;41:618-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 240] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 57. | Graham DY, Opekun AR, Willingham FF, Qureshi WA. Visible small-intestinal mucosal injury in chronic NSAID users. Clin Gastroenterol Hepatol. 2005;3:55-59. [PubMed] |
| 58. | Lewis JR, Pashinsky Y, Tinsley A, Lewis BS. Capsule endoscopy in healthy individuals. Gastroenterology. 2012;142:S52-S53. |
| 59. | Shim KN, Kim YS, Kim KJ, Kim YH, Kim TI, Do JH, Ryu JK, Moon JS, Park SH, Hee Park C. Abdominal pain accompanied by weight loss may increase the diagnostic yield of capsule endoscopy: a Korean multicenter study. Scand J Gastroenterol. 2006;41:983-988. [PubMed] |
| 60. | Adler SN, Yoav M, Eitan S, Yehuda C, Eliakim R. Does capsule endoscopy have an added value in patients with perianal disease and a negative work up for Crohn’s disease? World J Gastrointest Endosc. 2012;4:185-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 28] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 61. | Koulaouzidis A, Douglas S, Rogers MA, Arnott ID, Plevris JN. Fecal calprotectin: a selection tool for small bowel capsule endoscopy in suspected IBD with prior negative bi-directional endoscopy. Scand J Gastroenterol. 2011;46:561-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 73] [Article Influence: 4.9] [Reference Citation Analysis (1)] |
| 62. | Gralnek IM, Defranchis R, Seidman E, Leighton JA, Legnani P, Lewis BS. Development of a capsule endoscopy scoring index for small bowel mucosal inflammatory change. Aliment Pharmacol Ther. 2008;27:146-154. [PubMed] |
| 63. | Rosa B, Moreira MJ, Rebelo A, Cotter J. Lewis Score: a useful clinical tool for patients with suspected Crohn’s Disease submitted to capsule endoscopy. J Crohns Colitis. 2012;6:692-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 82] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 64. | Niv Y, Ilani S, Levi Z, Hershkowitz M, Niv E, Fireman Z, O’Donnel S, O’Morain C, Eliakim R, Scapa E. Validation of the Capsule Endoscopy Crohn’s Disease Activity Index (CECDAI or Niv score): a multicenter prospective study. Endoscopy. 2012;44:21-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 136] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 65. | Park CH, Kim JO, Choi MG, Kim KJ, Kim YH, Kim YS, Kim TI, Do JH, Ryu JK, Moon JS. Utility of capsule endoscopy for the classification of Crohn’s disease: a multicenter study in Korea. Dig Dis Sci. 2007;52:1405-1409. [PubMed] |
| 66. | Eliakim R, Suissa A, Yassin K, Katz D, Fischer D. Wireless capsule video endoscopy compared to barium follow-through and computerised tomography in patients with suspected Crohn’s disease--final report. Dig Liver Dis. 2004;36:519-522. [PubMed] |
| 67. | Costamagna G, Shah SK, Riccioni ME, Foschia F, Mutignani M, Perri V, Vecchioli A, Brizi MG, Picciocchi A, Marano P. A prospective trial comparing small bowel radiographs and video capsule endoscopy for suspected small bowel disease. Gastroenterology. 2002;123:999-1005. [PubMed] |
| 68. | Dubcenco E, Jeejeebhoy KN, Petroniene R, Tang SJ, Zalev AH, Gardiner GW, Baker JP. Capsule endoscopy findings in patients with established and suspected small-bowel Crohn’s disease: correlation with radiologic, endoscopic, and histologic findings. Gastrointest Endosc. 2005;62:538-544. [PubMed] |
| 69. | Solem CA, Loftus EV, Fletcher JG, Baron TH, Gostout CJ, Petersen BT, Tremaine WJ, Egan LJ, Faubion WA, Schroeder KW. Small-bowel imaging in Crohn’s disease: a prospective, blinded, 4-way comparison trial. Gastrointest Endosc. 2008;68:255-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 234] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 70. | Voderholzer WA, Beinhoelzl J, Rogalla P, Murrer S, Schachschal G, Lochs H, Ortner MA. Small bowel involvement in Crohn’s disease: a prospective comparison of wireless capsule endoscopy and computed tomography enteroclysis. Gut. 2005;54:369-373. [PubMed] |
| 71. | Hara AK, Leighton JA, Heigh RI, Sharma VK, Silva AC, De Petris G, Hentz JG, Fleischer DE. Crohn disease of the small bowel: preliminary comparison among CT enterography, capsule endoscopy, small-bowel follow-through, and ileoscopy. Radiology. 2006;238:128-134. [PubMed] |
| 72. | Jensen MD, Nathan T, Rafaelsen SR, Kjeldsen J. Diagnostic accuracy of capsule endoscopy for small bowel Crohn’s disease is superior to that of MR enterography or CT enterography. Clin Gastroenterol Hepatol. 2011;9:124-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 200] [Article Influence: 13.3] [Reference Citation Analysis (36)] |
| 73. | Dionisio PM, Gurudu SR, Leighton JA, Leontiadis GI, Fleischer DE, Hara AK, Heigh RI, Shiff AD, Sharma VK. Capsule endoscopy has a significantly higher diagnostic yield in patients with suspected and established small-bowel Crohn’s disease: a meta-analysis. Am J Gastroenterol. 2010;105:1240-1248; quiz 1249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 287] [Article Influence: 17.9] [Reference Citation Analysis (36)] |
| 74. | Ye CA, Gao YJ, Ge ZZ, Dai J, Li XB, Xue HB, Ran ZH, Zhao YJ. PillCam colon capsule endoscopy versus conventional colonoscopy for the detection of severity and extent of ulcerative colitis. J Dig Dis. 2013;14:117-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 75. | Hosoe N, Matsuoka K, Naganuma M, Ida Y, Ishibashi Y, Kimura K, Yoneno K, Usui S, Kashiwagi K, Hisamatsu T. Applicability of second-generation colon capsule endoscope to ulcerative colitis: a clinical feasibility study. J Gastroenterol Hepatol. 2013;28:1174-1179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 76. | Sung J, Ho KY, Chiu HM, Ching J, Travis S, Peled R. The use of Pillcam Colon in assessing mucosal inflammation in ulcerative colitis: a multicenter study. Endoscopy. 2012;44:754-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 70] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 77. | Oliva S, Di Nardo G, Hassan C, Spada C, Aloi M, Ferrari F, Redler A, Costamagna G, Cucchiara S. Second-generation colon capsule endoscopy vs. colonoscopy in pediatric ulcerative colitis: a pilot study. Endoscopy. 2014;46:485-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 78. | Rokkas T, Niv Y. The role of video capsule endoscopy in the diagnosis of celiac disease: a meta-analysis. Eur J Gastroenterol Hepatol. 2012;24:303-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 84] [Article Influence: 6.0] [Reference Citation Analysis (1)] |
| 79. | Chang MS, Rubin M, Lewis SK, Green PH. Diagnosing celiac disease by video capsule endoscopy (VCE) when esophagogastroduodenoscopy (EGD) and biopsy is unable to provide a diagnosis: a case series. BMC Gastroenterol. 2012;12:90. [PubMed] |
| 80. | Lidums I, Cummins AG, Teo E. The role of capsule endoscopy in suspected celiac disease patients with positive celiac serology. Dig Dis Sci. 2011;56:499-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 81. | Tennyson CA, Green PH. The role of capsule endoscopy in patients with nonresponsive celiac disease. Gastrointest Endosc. 2011;74:1323-1324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 82. | Culliford A, Daly J, Diamond B, Rubin M, Green PH. The value of wireless capsule endoscopy in patients with complicated celiac disease. Gastrointest Endosc. 2005;62:55-61. [PubMed] |
| 83. | Achour J, Serraj I, Amrani L, Amrani N. Small bowel tumors: what is the contribution of video capsule endoscopy? Clin Res Hepatol Gastroenterol. 2012;36:222-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 84. | Schwartz GD, Barkin JS. Small-bowel tumors detected by wireless capsule endoscopy. Dig Dis Sci. 2007;52:1026-1030. [PubMed] |
| 85. | Rondonotti E, Pennazio M, Toth E, Menchen P, Riccioni ME, De Palma GD, Scotto F, De Looze D, Pachofsky T, Tacheci I. Small-bowel neoplasms in patients undergoing video capsule endoscopy: a multicenter European study. Endoscopy. 2008;40:488-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 172] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 86. | Yamada A, Watabe H, Iwama T, Obi S, Omata M, Koike K. The prevalence of small intestinal polyps in patients with familial adenomatous polyposis: a prospective capsule endoscopy study. Fam Cancer. 2014;13:23-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 87. | Beggs AD, Latchford AR, Vasen HF, Moslein G, Alonso A, Aretz S, Bertario L, Blanco I, Bülow S, Burn J. Peutz-Jeghers syndrome: a systematic review and recommendations for management. Gut. 2010;59:975-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 516] [Cited by in RCA: 469] [Article Influence: 29.3] [Reference Citation Analysis (1)] |
| 88. | Mata A, Llach J, Castells A, Rovira JM, Pellisé M, Ginès A, Fernández-Esparrach G, Andreu M, Bordas JM, Piqué JM. A prospective trial comparing wireless capsule endoscopy and barium contrast series for small-bowel surveillance in hereditary GI polyposis syndromes. Gastrointest Endosc. 2005;61:721-725. [PubMed] |
| 89. | Caspari R, von Falkenhausen M, Krautmacher C, Schild H, Heller J, Sauerbruch T. Comparison of capsule endoscopy and magnetic resonance imaging for the detection of polyps of the small intestine in patients with familial adenomatous polyposis or with Peutz-Jeghers’ syndrome. Endoscopy. 2004;36:1054-1059. [PubMed] |
| 90. | Gupta A, Postgate AJ, Burling D, Ilangovan R, Marshall M, Phillips RK, Clark SK, Fraser CH. A prospective study of MR enterography versus capsule endoscopy for the surveillance of adult patients with Peutz-Jeghers syndrome. AJR Am J Roentgenol. 2010;195:108-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 86] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 91. | Krystallis C, Koulaouzidis A, Douglas S, Plevris JN. Chromoendoscopy in small bowel capsule endoscopy: Blue mode or Fuji Intelligent Colour Enhancement? Dig Liver Dis. 2011;43:953-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 92. | Gupta T, Ibrahim M, Deviere J, Van Gossum A. Evaluation of Fujinon intelligent chromo endoscopy-assisted capsule endoscopy in patients with obscure gastroenterology bleeding. World J Gastroenterol. 2011;17:4590-4595. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 93. | Matsumura T, Arai M, Sato T, Nakagawa T, Maruoka D, Tsuboi M, Hata S, Arai E, Katsuno T, Imazeki F. Efficacy of computed image modification of capsule endoscopy in patients with obscure gastrointestinal bleeding. World J Gastrointest Endosc. 2012;4:421-428. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 94. | Imagawa H, Oka S, Tanaka S, Noda I, Higashiyama M, Sanomura Y, Shishido T, Yoshida S, Chayama K. Improved detectability of small-bowel lesions via capsule endoscopy with computed virtual chromoendoscopy: a pilot study. Scand J Gastroenterol. 2011;46:1133-1137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (1)] |
| 95. | Koulaouzidis A, Karargyris A, Rondonotti E, Noble CL, Douglas S, Alexandridis E, Zahid AM, Bathgate AJ, Trimble KC, Plevris JN. Three-dimensional representation software as image enhancement tool in small-bowel capsule endoscopy: a feasibility study. Dig Liver Dis. 2013;45:909-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 96. | Rondonotti E, Koulaouzidis A, Karargyris A, Giannakou A, Fini L, Soncini M, Pennazio M, Douglas S, Shams A, Lachlan N. Utility of 3-dimensional image reconstruction in the diagnosis of small-bowel masses in capsule endoscopy (with video). Gastrointest Endosc. 2014;80:642-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 97. | Shiotani A, Honda K, Kawakami M, Nishi R, Murao T, Ishii M, Matsumoto H, Kusunoki H, Hata J, Haruma K. Use of an external real-time image viewer coupled with prespecified actions enhanced the complete examinations for capsule endoscopy. J Gastroenterol Hepatol. 2011;26:1270-1274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 98. | Yamashita K, Okumura H, Oka Y, Urakami A, Shiotani A, Nakashima H, Matsumoto H, Hirai T, Nakamura M. Minimally invasive surgery using intraoperative real-time capsule endoscopy for small bowel lesions. Surg Endosc. 2013;27:2337-2341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 99. | Rey JF, Ogata H, Hosoe N, Ohtsuka K, Ogata N, Ikeda K, Aihara H, Pangtay I, Hibi T, Kudo SE. Blinded nonrandomized comparative study of gastric examination with a magnetically guided capsule endoscope and standard videoendoscope. Gastrointest Endosc. 2012;75:373-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 103] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 100. | Pasricha T, Smith BF, Mitchell VR, Fang B, Brooks ER, Gerding JS, Washington MK, Valdastri P, Obstein KL. Controlled colonic insufflation by a remotely triggered capsule for improved mucosal visualization. Endoscopy. 2014;46:614-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 101. | Valdastri P, Quaglia C, Susilo E, Menciassi A, Dario P, Ho CN, Anhoeck G, Schurr MO. Wireless therapeutic endoscopic capsule: in vivo experiment. Endoscopy. 2008;40:979-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 62] [Article Influence: 3.4] [Reference Citation Analysis (1)] |
| 102. | Eliakim R, Fireman Z, Gralnek IM, Yassin K, Waterman M, Kopelman Y, Lachter J, Koslowsky B, Adler SN. Evaluation of the PillCam Colon capsule in the detection of colonic pathology: results of the first multicenter, prospective, comparative study. Endoscopy. 2006;38:963-970. [PubMed] |
| 103. | Schoofs N, Devière J, Van Gossum A. PillCam colon capsule endoscopy compared with colonoscopy for colorectal tumor diagnosis: a prospective pilot study. Endoscopy. 2006;38:971-977. [PubMed] |
| 104. | Eliakim R, Yassin K, Niv Y, Metzger Y, Lachter J, Gal E, Sapoznikov B, Konikoff F, Leichtmann G, Fireman Z. Prospective multicenter performance evaluation of the second-generation colon capsule compared with colonoscopy. Endoscopy. 2009;41:1026-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 212] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 105. | Adler S, Hassan C, Metzger Y, Sompolinsky Y, Spada C. Accuracy of automatic detection of small-bowel mucosa by second-generation colon capsule endoscopy. Gastrointest Endosc. 2012;76:1170-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 106. | Farnbacher MJ, Krause HH, Hagel AF, Raithel M, Neurath MF, Schneider T. QuickView video preview software of colon capsule endoscopy: reliability in presenting colorectal polyps as compared to normal mode reading. Scand J Gastroenterol. 2014;49:339-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 107. | Van Gossum A, Munoz-Navas M, Fernandez-Urien I, Carretero C, Gay G, Delvaux M, Lapalus MG, Ponchon T, Neuhaus H, Philipper M. Capsule endoscopy versus colonoscopy for the detection of polyps and cancer. N Engl J Med. 2009;361:264-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 227] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 108. | Ramos L, Alarcón O, Adrian Z, Gimeno-García AZ, Nicolás-Pérez D, Jiménez-Sosa A, Quintero E. One-day versus two-day cleansing for colon capsule endoscopy: a prospective randomized pilot study. Gastroenterol Hepatol. 2014;37:101-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 109. | Kakugawa Y, Saito Y, Saito S, Watanabe K, Ohmiya N, Murano M, Oka S, Arakawa T, Goto H, Higuchi K. New reduced volume preparation regimen in colon capsule endoscopy. World J Gastroenterol. 2012;18:2092-2098. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 40] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 110. | Spada C, Riccioni ME, Hassan C, Petruzziello L, Cesaro P, Costamagna G. PillCam colon capsule endoscopy: a prospective, randomized trial comparing two regimens of preparation. J Clin Gastroenterol. 2011;45:119-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 111. | Spada C, Hassan C, Munoz-Navas M, Neuhaus H, Deviere J, Fockens P, Coron E, Gay G, Toth E, Riccioni ME. Second-generation colon capsule endoscopy compared with colonoscopy. Gastrointest Endosc. 2011;74:581-589.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 171] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 112. | Triantafyllou K, Viazis N, Tsibouris P, Zacharakis G, Kalantzis C, Karamanolis DG, Ladas SD. Colon capsule endoscopy is feasible to perform after incomplete colonoscopy and guides further workup in clinical practice. Gastrointest Endosc. 2014;79:307-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 113. | Negreanu L, Smarandache G, Mateescu RB. Role of capsule endoscopy Pillcam COLON 2 in patients with known or suspected Crohn’s disease who refused colonoscopy or underwent incomplete colonoscopic exam: a case series. Tech Coloproctol. 2014;18:277-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 114. | Alarcón-Fernández O, Ramos L, Adrián-de-Ganzo Z, Gimeno-García AZ, Nicolás-Pérez D, Jiménez A, Quintero E. Effects of colon capsule endoscopy on medical decision making in patients with incomplete colonoscopies. Clin Gastroenterol Hepatol. 2013;11:534-40.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 115. | Spada C, Hassan C, Barbaro B, Iafrate F, Cesaro P, Petruzziello L, Minelli Grazioli L, Senore C, Brizi G, Costamagna I. Colon capsule versus CT colonography in patients with incomplete colonoscopy: a prospective, comparative trial. Gut. 2014;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 94] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 116. | Negreanu L, Babiuc R, Bengus A, Sadagurschi R. PillCam Colon 2 capsule in patients unable or unwilling to undergo colonoscopy. World J Gastrointest Endosc. 2013;5:559-567. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 34] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 117. | Pioche M, de Leusse A, Filoche B, Dalbiès PA, Adenis Lamarre P, Jacob P, Gaudin JL, Coulom P, Letard JC, Borotto E. Prospective multicenter evaluation of colon capsule examination indicated by colonoscopy failure or anesthesia contraindication. Endoscopy. 2012;44:911-916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 118. | Adler SN, Hassan C, Metzger Y, Sompolinsky Y, Spada C. Second-generation colon capsule endoscopy is feasible in the out-of-clinic setting. Surg Endosc. 2014;28:570-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |