Published online Jul 28, 2014. doi: 10.3748/wjg.v20.i28.9506
Revised: April 12, 2014
Accepted: May 23, 2014
Published online: July 28, 2014
Processing time: 164 Days and 9.7 Hours
AIM: To study the effects of preconditioning on inducible nitric oxide synthase (iNOS) and interleukin 1 (IL-1) receptor transcription in rat liver ischemia/reperfusion injury (IRI).
METHODS: Seventy-two male rats were randomized into 3 groups: the one-hour segmental ischemia (IRI, n = 24) group, the ischemic preconditioning (IPC, n = 24) group or the remote ischemic preconditioning (R-IPC, n = 24) group. The IPC and R-IPC were performed as 10 min of ischemia and 10 min of reperfusion. The iNOS and the IL-1 receptor mRNA in the liver tissue was analyzed with real time PCR. The total Nitrite and Nitrate (NOx) in continuously sampled microdialysate (MD) from the liver was analyzed. In addition, the NOx levels in the serum were analyzed.
RESULTS: After 4 h of reperfusion, the iNOS mRNA was significantly higher in the R-IPC (ΔCt: 3.44 ± 0.57) group than in the IPC (ΔCt: 5.86 ± 0.82) group (P = 0.025). The IL-1 receptor transcription activity was reduced in the IPC group (ΔCt: 1.88 ± 0.53 to 4.81 ± 0.21), but not in the R-IPC group, during reperfusion (P = 0.027). In the MD, a significant drop in the NOx levels was noted in the R-IPC group (12.3 ± 2.2 to 4.7 ± 1.2 μmol/L) at the end of ischemia compared with the levels in early ischemia (P = 0.008). A similar trend was observed in the IPC group (11.8 ± 2.1 to 6.4 ± 1.5 μmol/L), although this difference was not statistically significant. The levels of NOx rose quickly during reperfusion in both groups.
CONCLUSION: IPC, but not R-IPC, reduces iNOS and IL-1 receptor transcription during early reperfusion, indicating a lower inflammatory reaction. NOx is consumed in the ischemic liver lobe.
Core tip: Ischemic preconditioning has been shown to reduce ischemia/reperfusion injury (IRI) in the liver. NO has been shown to be important in the preconditioning process as well as in IRI. This study shows that ischemic preconditioning (IPC) but not remote ischemic preconditioning (R-IPC) reduces inducible nitric oxide synthase and interleukin 1 receptor transcription during early reperfusion. As it has been shown that IPC protects the liver more than R-IPC, this reduction in inflammatory activation may be important. In addition, this study shows that nitrite and nitrate are consumed in the liver during ischemia, unlike the levels in peripheral blood.
- Citation: Björnsson B, Winbladh A, Bojmar L, Sundqvist T, Gullstrand P, Sandström P. Conventional, but not remote ischemic preconditioning, reduces iNOS transcription in liver ischemia/reperfusion. World J Gastroenterol 2014; 20(28): 9506-9512
- URL: https://www.wjgnet.com/1007-9327/full/v20/i28/9506.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i28.9506
In a more aggressive liver surgery, Pringle’s manoeuvre is frequently used to occlude the vascular inflow to the liver during resection, thereby reducing intraoperative bleeding. However, the disadvantage to this procedure is that it may simultaneously cause ischemia-reperfusion injury (IRI). The consequences of IRI have been extensively studied, and it has been shown that excessive amounts of reactive oxygen species (ROS) are formed during reperfusion and that they may damage the liver cells[1]. A possible mechanism that triggers this damage is the reaction between ROS and cellular components such as lipids and proteins[1]. Nitric oxide (NO) is thought to be one important factor in the IRI process. It has been shown that during IRI, the availability of NO in the rat liver decreases, and this may be related to subsequent and more severe injury[2]. Furthermore, a model using endothelial nitric oxide synthase (eNOS) knockout mice has shown that an eNOS deficiency increases the IRI[3]. It can therefore be stated that the physiological amounts of NO produced by eNOS most likely serve as a protective agent against IRI. The vasodilatory effect of NO appears to be important, although the mechanisms involved in this protection are still unclear. Studies involving inducible nitric oxide synthase (iNOS) inhibitors have suggested that the inhibition of iNOS decreases IRI[4,5]. Thus, it seems likely that iNOS activation is involved in the IRI process and that it is most likely deleterious to IRI. It has been suggested that a hepatocyte-derived interleukin-1β (IL-1β) in conjunction with tumor necrosis factor-α (TNF-α) is an important mediator of IRI[6]. An up-regulation of the IL-1β receptor appears to promote iNOS transcription, although the downstream mechanisms involved are not clear[7]. In addition, IL-1 has been shown to induce TNF-α synthesis in Kuppfer cells as well as inducing neutrophil recruitment and promoting the production of ROS[8]. Blocking the IL-1 receptor has been shown to reduce IRI, although it is not known if this reduction is related to the reduced iNOS transcription. However, the effect appears to be mediated, at least in part, through improved microcirculation, which may indicate NO involvement[9].
To reduce the IRI, Ischemic Preconditioning (IPC) has been proposed, which has a protective effect that has been established in several animal studies[10-14]. Among the mechanisms postulated for the action of IPC are decreased endothelial injury and hepatocyte apoptosis, and it has been shown that adenosine triphosphate (ATP) levels are higher after IPC and ischemia/reperfusion (IR) than after IR alone[15-19]. Several studies have investigated the role of NO in IPC and found that NO is involved in the process, although the exact mechanisms are unclear. It has been shown, however, that IPC reduces sinusoidal endothelial cell death and that this effect is likely to be dependent on NO production[20,21]. Furthermore, eNOS, but not iNOS, expression has been found to be increased during the early (2 h) phase of reperfusion in IPC-treated animals compared with those that have been subjected to ischemia and reperfusion without IPC[22].
The concept of remote-IPC (R-IPC) has recently emerged and has been investigated primarily as a method to protect the myocardium[23]. This method has also been investigated in the context of liver IRI and has been found to provide some protection against IRI in the liver, although it may be less effective than IPC in reducing tissue damage[14,24-26]. Only one study has compared the two methods in the setting of liver IRI, and this study showed that the protective effect on transaminase elevation was less with R-IPC than with IPC[14]. The underlying mechanisms for R-IPC have been less extensively studied than the mechanisms involving “conventional” IPC, although it has been shown that R-IPC increases hepatic blood flow and that the total hepatic vein Nitrite and Nitrate (NOx) is higher in R-IPC treated animals than in the control group (ischemia reperfusion only) at the end of 2 h of reperfusion, which indicates a role for NO[27]. Furthermore, it has been shown that administration of the NO scavenger inhibits the protective effect of R-IPC and that eNOS-/- transgenic mice do not benefit from R-IPC[28,29]. In addition, the hepatic microcirculatory blood flow was found to be better preserved, and thus, the authors conclude that the NO produced by activated eNOS is an essential mediator of the protection that R-IPC provides against IRI[28,29].
The changes in NOx in the liver parenchyma have not been evaluated in the settings of IPC and R-IPC.
In this study, we examine the changes in the NOx (Nitrite and Nitrate) concentrations in serum and microdialysate during IRI after both conventional and remote IPC, as well as the gene expression of iNOS and the IL-1β receptor in rat livers.
The Local Ethics Committee for Animal Experiments at Linköping University, Linköping, Sweden approved the study protocol (Permit number: 2-09). Seventy-two male Sprague-Dawley rats (258-444 g, Scandjur, Sollentuna, Sweden) were randomized into 3 groups; A: ischemia (n = 24), B: IPC + ischemia (n = 24) and C: remote IPC + ischemia (n = 24). The animals within each group were then randomly stratified to no reperfusion, 1 h of reperfusion or 4 h of reperfusion. Before the 1 h segmental ischemia, the animals in group B were preconditioned by 10 min of ischemia to the lobe of the liver that was later subjected to the ischemic insult. This was followed by 10 min of reperfusion. In group C, the preconditioning was applied through a tourniquet to the left hind leg for the same length of time as group B. During this time period, nothing was performed to the animals in group A. The animals were acclimatized for 1 wk at 21 °C on a 12-h light/dark cycle with free access to standard rat food pellets and tap water.
A detailed description of the procedures can be found elsewhere[14]. Briefly, the rats were anesthetized, intubated and ventilated throughout the experiment. Their body temperatures were maintained within 38 °C-39 °C, and the animals were monitored. A laparotomy was performed via a midline incision, and the ligament attachments of the liver were divided. Blood was sampled before the laparotomy and at the end of the experiment. Microdialysis was performed during the entire experiment from two lobes (ischemia and control) of the liver. Immediately after the final blood sampling, the liver was harvested and preserved in liquid nitrogen, tissue for iNOS and IL-1 receptor analysis was sampled from the ischemic liver.
NOx (the sum of NO2- and NO3-) was analyzed in the serum and microdialysate following the instructions in the commercial “Nitrite/Nitrate Fluorometric Assay Kit” (Cayman Chemical Company, Ann Arbor, MI, United States). Prior to the analysis, the serum was ultrafiltrated through a 10 kDalton cut-off filter (Millipore, Solna, Sweden). The microdialysate, on the other hand, was analyzed without any processing. The standard curves were plotted. For nitrate, 10 μL of the sample was diluted in 70 μL of the assay buffer. Aliquots of 10 μL enzyme cofactor and 10 μL of nitrate reductase mixture were added to the buffered sample. After 30 min of incubation at room temperature, 10 μL of DAN (2,3-diaminonaphthalene) were added and incubated for another 10 min. For nitrite, 10 μL of the sample was diluted with 90 μL of assay buffer, and 10 μL of DAN was added. Both the nitrate and nitrite samples were then incubated for 10 min, and 20 μL of NaOH was added. All of the samples were read in threes using fluorometry with an excitation wavelength of 355 nm and an emission wavelength of 430 nm.
Approximately 10 mg of dry weight liver tissue was disrupted and homogenized in the Micro-Dismembrator (Braun Biotech, Allentown PA, United States) at 2900 rpm for 30 s. The RNA extraction was performed in accordance with the manufacturer’s protocol (Qiagene, Valencia CA, United States). However, no β-mercaptoethanol was used. The RNA was eluted in 300 μL RNAse-free water; the RNA concentration was measured at 260 nm and the purity (about 2.0) was 260/280 nm (NanoDrop, Thermo Scientific, Erembodegem, Belgium). It was then stored at -70 °C until assayed. 0.5 μg RNA were used in the reverse transcriptase cDNA conversion with a High Capacity cDNA Reverse Transcription Kit in a total volume of 20 μL in accordance with the manufacturer’s protocol (Applied Biosystems, CA, United States). cDNA samples were stored at -20 °C until assayed. For the real-time PCR reaction, a 2 μL sample containing 50 ng cDNA (0.025 μg/μL) was used in a total reaction volume of 20 μL. The samples were analyzed in triplicate with Fast Master Mix and TaqMan Gene Expression Assay (Applied Biosystems) in a 7500 Fast Instrument (Applied Biosystems). In the results, the mean of the samples’ Ct has been normalized against the mean Ct for GAPDH, presented as ΔCt.
The quantitative PCR for IL1R was performed with Fast SYBR Green Master Mix (Applied Biosystems). The final concentration of the primers was 100 nmol/L. In the negative controls, the cDNA was replaced with distilled water. The final amount of cDNA in each reaction was comparable to 1 ng of RNA. The PCR program was set at 40 cycles at 95 °C for 20 s, 95 °C for 1 s and 60 °C for 20 s. At the end of the reaction, a dissociation curve analysis was performed. Specific primers for rat IL-1R (Invitrogen) were used. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and beta-actin (b-actin) (CyberGene AB, Stockholm, Sweden) were used as housekeeping genes. The primers were designed using Primer Express (Applied Biosystems). All reactions were performed in duplicate by the EpMotion-pipetting robot (Eppendorf, Hamburg, Germany), including none-template controls and endogenous control probes. The results were analyzed using the ΔCt method and presented as a relative gene expression. The 7900 Fast Real-Time PCR system with 7900 System SDS 2.3 Software (Applied Biosystems) was used following the manufacturer’s protocol.
The details of the probes, pumps, fluids and rates, as well as the sampling intervals, have been published previously[14]. After sampling, the fluid was frozen at -70 °C for later analysis, as described above.
Data are presented as the mean ± SE unless otherwise stated. A P value < 0.05 was considered statistically significant. Groups were compared with ANOVA followed by Fisher’s post hoc test when appropriate, while temporal changes within the groups were analyzed using the t test or the Mann-Whitney U test. Statistica 8.0 software (StatSoft Inc, Tulsa, OK, United States) was used for the statistical calculations.
The comparison of se-AST and se-ALT has been published earlier[14]. The greatest rise in both the AST and ALT was observed in the IRI group, followed by the R-IPC group, and finally by the IPC group. As there was a significant difference between the R-IPC and the IRI group regarding AST, it was concluded that R-IPC offers some protection against liver IR in the rat liver.
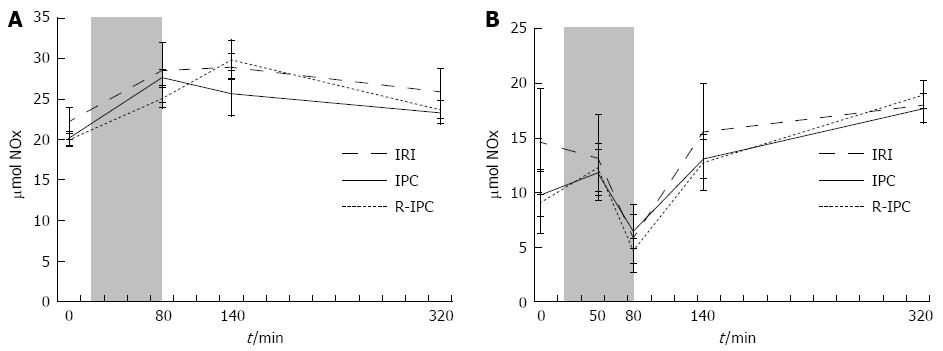
The combined amounts of nitrite (NO2-) and nitrate (NO3-) were measured in serum and referred to as S-NOx. In the IPC group, an initial rise (20.2-27.7 μmol/L) was found during ischemia followed by a decline (27.7-23.4 μmol/L) that started immediately in the reperfusion phase. In the R-IPC group, an initial rise (20.0-25.2 μmol/L) during the ischemia and 1 h of reperfusion was followed by a decline (25.2-23.7 μmol/L) during later parts of the reperfusion. In both of the groups, the rise and decline in S-NOx was statistically significant (both groups, P < 0.001) but no significant difference was noted between the groups (Figure 1A). The IRI group followed a similar pattern, although neither the initial rise nor the later decline was statistically significant.
In the R-IPC group, there was a significant fall (12.3-4.7 μmol/L) at the end of ischemia (P = 0.008) compared with the levels in early ischemia. Similar trends were observed in the IRI group (13.2-5.8 μmol/L) and the IPC group (11.8-6.4 μmol/L), although the change was not statistically significant. During reperfusion, the levels rose quickly again, to significantly (P < 0.01) higher levels in the IPC and R-IPC groups, while the rise in the IRI group was not statistically significant (P = 0.065) (Figure 1B). A comparison among the groups did not reveal any statistically significant differences.
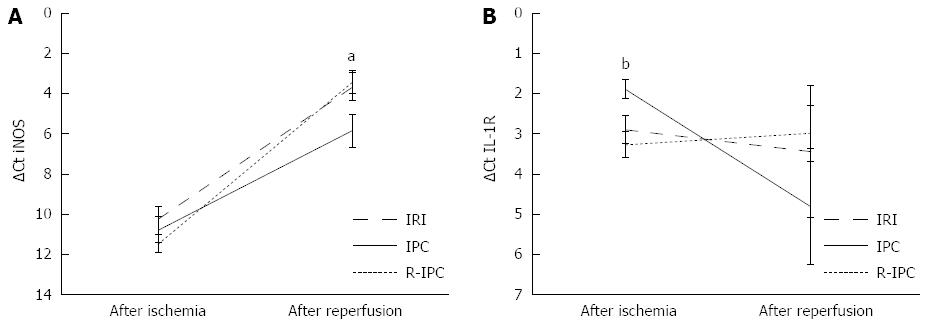
The iNOS mRNA in the liver tissue from the ischemic lobe was measured with real time PCR and increased from the end of the ischemia to the end of the reperfusion, as shown in Figure 2A. The transcription of iNOS at the end of the reperfusion was significantly higher in all of the groups, IRI (ΔCt = 3.66), IPC (ΔCt = 5.86) and R-IPC (ΔCt = 3.44) compared with at the end of the ischemia (ΔCt = 10.21, 10.78 and 11.49, respectively) (P < 0.001). Moreover, the mRNA was also significantly lower in the IPC group than in the IRI group or the R-IPC group at the end of the reperfusion (P = 0.039 respective P = 0.025) (Figure 2A).
The expression of the IL-1 receptor in the liver tissue from the ischemic lobe was measured with semi-quantitative real-time PCR. In the IPC group, significantly (P = 0.027) reduced IL-1 mRNA transcription at the end of reperfusion compared with the end of ischemia was noted (ΔCt: 1.88-4.81). In the IRI and R-IPC groups, this decrease was not observed, as the transcription activity changed minimally (ΔCt: 2.89-3.43 in the IRI group and ΔCt: 3.26-2.99 in the R-IPC group, ns). When the groups are compared, the IPC group has significantly more IL-1 receptor mRNA at the end of ischemia than do the IRI and R-IPC groups (P = 0.029 and P = 0.0044, respectively). At the end of reperfusion, no statistically significant differences were found between the groups (Figure 2B).
This is the first study to compare NO involvement in conventional and remote IPC in the setting of rat liver IRI. We have previously shown that the elevation of transaminase during IRI is less pronounced with IPC than with R-IPC, although R-IPC appears to offer some protection against I/R in the rat liver[14]. This most likely represents differences in the protection against IRI offered by these different methods of preconditioning. Therefore, it is important to investigate further the mechanisms involved.
The iNOS transcription after 4 h of reperfusion was shown to be more active in the R-IPC group than in the IPC group, although the levels of NOx did not differ between the groups. Earlier work has failed to show an expression of the iNOS protein after 2 h of reperfusion in a rat model similar to the present[22]. This discrepancy in findings is most likely explained by the time factor and the fact that the present study measures gene expression, which is expected to be observed earlier than the protein expression reported previously.
The activation of iNOS transcription in the liver during early reperfusion is significantly more apparent with remote IPC than with conventional IPC. This activation, on the other hand, does not lead to detectable differences in the NOx levels whether they are measured in the local environment of the liver or in serum. This may indicate that after only 4 h of reperfusion, the increased mRNA transcription has not resulted in increased NO production, and thus this increase may be a result of different degrees of tissue damage rather than an explanation for it. In this study, the step between the transcription and its product, the enzyme, was not analyzed, thus making it difficult to draw conclusions about the actual effects of iNOS in the early phase of reperfusion. If and how this increase in iNOS transcription affects the later stage of reperfusion injury may only be speculated upon.
Significantly greater transcription of the IL-1 receptor was observed in the IPC group than in the other groups at the end of ischemia (Figure 2B). As the ROS and H2O2 that are generated in response to ischemia and reperfusion are known to contribute to the formation of the active IL-1 receptor, this difference is likely to be caused by the IPC[30]. IL-1 has been shown to activate protein kinase B/akt, which in turn is known to phosphorylate eNOS and thus increase the bioavailability of NO in the rat liver during early ischemia[31,32]. The significant decrease in the IL-1 receptor transcription observed during reperfusion in the IPC group may be explained by the inherent negative feedback in the IL-1 pathway[14,33]. Therefore, at 4 h of reperfusion, we have a lower level of iNOS and IL-1 receptor transcription in the IPC group that may correspond to the reduction in IRI in this group.
This study has shown that the levels of NOx dramatically decrease in the liver tissue, measured directly with microdialysis, during segmental ischemia of the liver. This suggests that the NOx is consumed in the ischemic liver. Despite this, a rise in serum NOx was observed in both groups during ischemia. In an environment where the circulation is closed, the NOx could be expected to remain near the level it was before the occlusion. One of the possible explanations for the decrease in the NOx in the liver found in this study is that nitrate is reduced to nitrite, which is reduced to NO. This has previously been shown to take place in physiological conditions with enhanced acidic environments[34]. This source of NO could be important within the context of IRI and has previously been shown in a mouse model[35].
The classic view that NOS is the only source of NO has been challenged by recent results from experiments in the cardiovascular system, which show that nitrite is reduced to NO by several enzymatic and non-enzymatic pathways, a process that is enhanced in acidic environments[34]. The existence of this pathway appears to make NOx a less suitable proxy for NO than previously thought[36]. Furthermore, the current findings, obtained with microdialysis directly from the liver tissue, show that serum NOx does not reflect the liver parenchymal concentration of NOx. This supports earlier results that have shown significant differences between study groups in the NOx obtained from the hepatic vein, whereas no difference was noted in the samples obtained from the hepatic artery[27]. This supports the conclusion that serum NOx may not accurately reflect the level of NO production in the liver. Earlier studies on the involvement of the NO system in liver IRI have relied partially on the serum measurement of NO2- and NO3-[36,37]. The current results, along with the earlier results from others, therefore, challenge the conclusions drawn from this approximation[27].
As increased IL-1 receptor formation has been shown to precede an increase in iNOS transcription, the reduction observed in the IPC group may explain the lower transcription of iNOS found in this group compared with the R-IPC group[7]. Another possible explanation as to why iNOS transcription is lower in the IPC group than in the R-IPC group is that the NO produced during IPC rapidly degrades to NOx, which in turn may constitute a biological pool for later nitrite reduction to NO, thus prolonging the increased bioavailability of NO in the liver. As NO has been shown to inhibit iNOS transcription, the increased bioavailability of NO may explain the results found in the IPC group[38]. Administering nitrite before ischemia may increase the availability of NO during liver ischemia and thereby decrease later reperfusion injury, as has been shown in the setting of myocardial ischemia[39]. This needs to be tested in the liver in an experimental model.
In conclusion, IPC reduces the transcription of iNOS during early reperfusion, possibly through the early transcription of the IL-1 receptor, but this effect is not observed with R-IPC. NOx is consumed in the ischemic liver and is possibly reduced to NO. Serum NOx does not reflect the tissues’ NOx during liver IRI.
Ischemia-Reperfusion Injury (IRI) is known to result from the closure of hepatic circulation that is sometimes used during liver surgery. IRI appears to be multifactorial, and research has shown that Nitric Oxide (NO) is involved in the process, although its role is still somewhat unclear. Among the methods to reduce IRI is ischemic preconditioning (IPC), which has been shown to reduce IRI. Remote Ischemic Preconditioning (R-IPC) is a variant of IPC that is less studied and may offer a lesser degree of protection. Changes in NO formation have been found with both types of preconditioning, but it remains unclear if iNOS activation differs in these settings.
IPC has been shown to offer some protection against IRI in clinical settings, but the lack of an understanding of the involved mechanisms makes the further development of protective strategies difficult; this remains an important area in research on liver IRI. R-IPC has not yet been tested in the clinical setting, and there is very limited knowledge about the mechanisms involved in the protection observed in animal models.
Earlier research has shown diverging results in the matter of the involvement of NO and Nitric Oxide Synthase (NOS) in the IRI process as well as in preconditioning. This can be explained partly by different models used in the studies. Although most results indicate that endothelial NOS (eNOS) has a protective role in IRI, the role of inducible (iNOS) is less certain.
The findings of this basic research article add to the understanding of the mechanisms involved in IRI and preconditioning. This new information may serve as grounds for further research in the area, although a clinical application is not within reach. The findings do give rise in particular to the hypothesis that administering nitrite before IRI may reduce the damage.
IRI is an ill-defined term that refers to the damage that occurs in the liver when the circulation is closed and then reopened. The extent of the damage is typically measured by the amount of liver transaminases in the serum. IPC and R-IPC are two methods of preparing an organ for ischemic insult. By closing the circulation to the organ for a short period of time followed by reperfusion, IPC protects the organ against IRI. R-IPC is based on the same principle, although in this case the circulation to another organ or body part is closed to provoke the protective response.
This is an elected experimental study on the physiological basis of ischemia-reperfusion (IR) injury of the liver. Authors focused on the effects of remote organ ischemic preconditioning on the liver IR injury. The topic of the investigation was IL-1 transcription. The inflammatory cascade was evaluated with the additional help of NOx measurement (serum and liver tissue) and iNOS transcription (liver tissue). As reported previously by other investigators, serum NO or NOx measurement was not reflected tissue damage in this study. Authors concluded that ischemic preconditioning reduces the transcription of iNOS during early reperfusion, possibly through the early transcription of IL-1 receptor, but this effect is not seen with remote organ- ischemic preconditioning. The experimental design and methodology are well designed. Statistical analyses are found suitable for healthy evaluation of the results.
| 1. | Jaeschke H. Molecular mechanisms of hepatic ischemia-reperfusion injury and preconditioning. Am J Physiol Gastrointest Liver Physiol. 2003;284:G15-G26. [PubMed] |
| 2. | Köken T, Inal M. The effect of nitric oxide on ischemia-reperfusion injury in rat liver. Clin Chim Acta. 1999;288:55-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | Hines IN, Harada H, Flores S, Gao B, McCord JM, Grisham MB. Endothelial nitric oxide synthase protects the post-ischemic liver: potential interactions with superoxide. Biomed Pharmacother. 2005;59:183-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 4. | Inoue I, Nagase H, Kishi K, Higuti T. ATP-sensitive K+ channel in the mitochondrial inner membrane. Nature. 1991;352:244-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 553] [Cited by in RCA: 538] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 5. | Takamatsu Y, Shimada K, Yamaguchi K, Kuroki S, Chijiiwa K, Tanaka M. Inhibition of inducible nitric oxide synthase prevents hepatic, but not pulmonary, injury following ischemia-reperfusion of rat liver. Dig Dis Sci. 2006;51:571-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Ben-Ari Z, Avlas O, Fallach R, Schmilovitz-Weiss H, Chepurko Y, Pappo O, Hochhauser E. Ischemia and reperfusion liver injury is reduced in the absence of Toll-like receptor 4. Cell Physiol Biochem. 2012;30:489-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Teshima S, Nakanishi H, Nishizawa M, Kitagawa K, Kaibori M, Yamada M, Habara K, Kwon AH, Kamiyama Y, Ito S. Up-regulation of IL-1 receptor through PI3K/Akt is essential for the induction of iNOS gene expression in hepatocytes. J Hepatol. 2004;40:616-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 8. | Kato A, Gabay C, Okaya T, Lentsch AB. Specific role of interleukin-1 in hepatic neutrophil recruitment after ischemia/reperfusion. Am J Pathol. 2002;161:1797-1803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 82] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 9. | Shirasugi N, Wakabayashi G, Shimazu M, Shito M, Kawachi S, Kitajima M. Interleukin-1 receptor blockade attenuates oxygen-derived free radical production and microcirculatory disturbances in ischemia/reperfusion injury in the liver. Transplant Proc. 1997;29:371-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Ajamieh HH, Candelario-Jalil E, Fernández OS, Gerbes AL. Ischaemic and pharmacological preconditionings protect liver via adenosine and redox status following hepatic ischaemia/reperfusion in rats. Clin Sci (Lond). 2008;115:69-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Caban A, Oczkowicz G, Abdel-Samad O, Cierpka L. Influence of ischemic preconditioning and nitric oxide on microcirculation and the degree of rat liver injury in the model of ischemia and reperfusion. Transplant Proc. 2006;38:196-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Chattopadhyay P, Verma N, Verma A, Kamboj T, Khan NA, Wahi AK. L-arginine protects from pringle manoeuvere of ischemia-reperfusion induced liver injury. Biol Pharm Bull. 2008;31:890-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Koti RS, Seifalian AM, McBride AG, Yang W, Davidson BR. The relationship of hepatic tissue oxygenation with nitric oxide metabolism in ischemic preconditioning of the liver. FASEB J. 2002;16:1654-1656. [PubMed] |
| 14. | Björnsson B, Winbladh A, Bojmar L, Trulsson LM, Olsson H, Sundqvist T, Gullstrand P, Sandström P. Remote or conventional ischemic preconditioning--local liver metabolism in rats studied with microdialysis. J Surg Res. 2012;176:55-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Ishii S, Abe T, Saito T, Tsuchiya T, Kanno H, Miyazawa M, Suzuki M, Motoki R, Gotoh M. Effects of preconditioning on ischemia/reperfusion injury of hepatocytes determined by immediate early gene transcription. J Hepatobiliary Pancreat Surg. 2001;8:461-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Peralta C, Bartrons R, Riera L, Manzano A, Xaus C, Gelpí E, Roselló-Catafau J. Hepatic preconditioning preserves energy metabolism during sustained ischemia. Am J Physiol Gastrointest Liver Physiol. 2000;279:G163-G171. [PubMed] |
| 17. | Peralta C, Bartrons R, Serafin A, Blázquez C, Guzmán M, Prats N, Xaus C, Cutillas B, Gelpí E, Roselló-Catafau J. Adenosine monophosphate-activated protein kinase mediates the protective effects of ischemic preconditioning on hepatic ischemia-reperfusion injury in the rat. Hepatology. 2001;34:1164-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 145] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 18. | Yadav SS, Sindram D, Perry DK, Clavien PA. Ischemic preconditioning protects the mouse liver by inhibition of apoptosis through a caspase-dependent pathway. Hepatology. 1999;30:1223-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 199] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 19. | Yoshizumi T, Yanaga K, Soejima Y, Maeda T, Uchiyama H, Sugimachi K. Amelioration of liver injury by ischaemic preconditioning. Br J Surg. 1998;85:1636-1640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 87] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 20. | Peralta C, Hotter G, Closa D, Gelpí E, Bulbena O, Roselló-Catafau J. Protective effect of preconditioning on the injury associated to hepatic ischemia-reperfusion in the rat: role of nitric oxide and adenosine. Hepatology. 1997;25:934-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 264] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 21. | Arai M, Thurman RG, Lemasters JJ. Contribution of adenosine A(2) receptors and cyclic adenosine monophosphate to protective ischemic preconditioning of sinusoidal endothelial cells against Storage/Reperfusion injury in rat livers. Hepatology. 2000;32:297-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 92] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 22. | Koti RS, Tsui J, Lobos E, Yang W, Seifalian AM, Davidson BR. Nitric oxide synthase distribution and expression with ischemic preconditioning of the rat liver. FASEB J. 2005;19:1155-1157. [PubMed] |
| 23. | Tapuria N, Kumar Y, Habib MM, Abu Amara M, Seifalian AM, Davidson BR. Remote ischemic preconditioning: a novel protective method from ischemia reperfusion injury--a review. J Surg Res. 2008;150:304-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 278] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 24. | Lai IR, Chang KJ, Chen CF, Tsai HW. Transient limb ischemia induces remote preconditioning in liver among rats: the protective role of heme oxygenase-1. Transplantation. 2006;81:1311-1317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 83] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 25. | Tapuria N, Junnarkar SP, Dutt N, Abu-Amara M, Fuller B, Seifalian AM, Davidson BR. Effect of remote ischemic preconditioning on hepatic microcirculation and function in a rat model of hepatic ischemia reperfusion injury. HPB (Oxford). 2009;11:108-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 26. | Wang F, Birch SE, He R, Tawadros P, Szaszi K, Kapus A, Rotstein OD. Remote ischemic preconditioning by hindlimb occlusion prevents liver ischemic/reperfusion injury: the role of High Mobility Group-Box 1. Ann Surg. 2010;251:292-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 27. | Kanoria S, Jalan R, Davies NA, Seifalian AM, Williams R, Davidson BR. Remote ischaemic preconditioning of the hind limb reduces experimental liver warm ischaemia-reperfusion injury. Br J Surg. 2006;93:762-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 83] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 28. | Abu-Amara M, Yang SY, Quaglia A, Rowley P, de Mel A, Tapuria N, Seifalian A, Davidson B, Fuller B. Nitric oxide is an essential mediator of the protective effects of remote ischaemic preconditioning in a mouse model of liver ischaemia/reperfusion injury. Clin Sci (Lond). 2011;121:257-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 29. | Abu-Amara M, Yang SY, Quaglia A, Rowley P, Fuller B, Seifalian A, Davidson B. Role of endothelial nitric oxide synthase in remote ischemic preconditioning of the mouse liver. Liver Transpl. 2011;17:610-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 30. | Li Q, Harraz MM, Zhou W, Zhang LN, Ding W, Zhang Y, Eggleston T, Yeaman C, Banfi B, Engelhardt JF. Nox2 and Rac1 regulate H2O2-dependent recruitment of TRAF6 to endosomal interleukin-1 receptor complexes. Mol Cell Biol. 2006;26:140-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 190] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 31. | Sizemore N, Leung S, Stark GR. Activation of phosphatidylinositol 3-kinase in response to interleukin-1 leads to phosphorylation and activation of the NF-kappaB p65/RelA subunit. Mol Cell Biol. 1999;19:4798-4805. [PubMed] |
| 32. | Miyake T, Yokoyama Y, Kokuryo T, Mizutani T, Imamura A, Nagino M. Endothelial nitric oxide synthase plays a main role in producing nitric oxide in the superacute phase of hepatic ischemia prior to the upregulation of inducible nitric oxide synthase. J Surg Res. 2013;183:742-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 33. | Virtue A, Wang H, Yang XF. MicroRNAs and toll-like receptor/interleukin-1 receptor signaling. J Hematol Oncol. 2012;5:66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 34. | Zweier JL, Li H, Samouilov A, Liu X. Mechanisms of nitrite reduction to nitric oxide in the heart and vessel wall. Nitric Oxide. 2010;22:83-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 98] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 35. | Duranski MR, Greer JJ, Dejam A, Jaganmohan S, Hogg N, Langston W, Patel RP, Yet SF, Wang X, Kevil CG. Cytoprotective effects of nitrite during in vivo ischemia-reperfusion of the heart and liver. J Clin Invest. 2005;115:1232-1240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 27] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 36. | Saijo F, Milsom AB, Bryan NS, Bauer SM, Vowinkel T, Ivanovic M, Andry C, Granger DN, Rodriguez J, Feelisch M. On the dynamics of nitrite, nitrate and other biomarkers of nitric oxide production in inflammatory bowel disease. Nitric Oxide. 2010;22:155-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 37. | Liu P, Yin K, Nagele R, Wong PY. Inhibition of nitric oxide synthase attenuates peroxynitrite generation, but augments neutrophil accumulation in hepatic ischemia-reperfusion in rats. J Pharmacol Exp Ther. 1998;284:1139-1146. [PubMed] |
| 38. | Taylor BS, Kim YM, Wang Q, Shapiro RA, Billiar TR, Geller DA. Nitric oxide down-regulates hepatocyte-inducible nitric oxide synthase gene expression. Arch Surg. 1997;132:1177-1183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 127] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 39. | Gonzalez FM, Shiva S, Vincent PS, Ringwood LA, Hsu LY, Hon YY, Aletras AH, Cannon RO, Gladwin MT, Arai AE. Nitrite anion provides potent cytoprotective and antiapoptotic effects as adjunctive therapy to reperfusion for acute myocardial infarction. Circulation. 2008;117:2986-2994. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 141] [Cited by in RCA: 131] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
P- Reviewer: Jin S, Topaloglu S S- Editor: Ma YJ L- Editor: A E- Editor: Ma S