Published online May 21, 2014. doi: 10.3748/wjg.v20.i19.5875
Revised: February 11, 2014
Accepted: March 6, 2014
Published online: May 21, 2014
Processing time: 145 Days and 2 Hours
AIM: To investigate the prognostic significance of pretreatment standardized maximum uptake value (SUVmax) and serum carbohydrate antigen (CA)19-9 in pancreatic cancer.
METHODS: From January 2007 to October 2011, 80 consecutive patients with pancreatic cancer who received positron emission/computed tomography before any treatment were enrolled in this study. The pretreatment SUVmax and CA19-9 level of the primary pancreatic tumor were obtained and compared with clinicopathological and prognostic factors. Student’s t test for unpaired data was used to analyze the differences between two groups. Univariate analysis and Cox proportional hazards regression were used to examine the independent effects of each significant variable. Survival was analyzed by the Kaplan-Meier method.
RESULTS: There was a significant correlation between both the SUVmax and serum CA19-9 of pancreatic cancer and R0 surgical resection (P = 0.043 and P = 0.007). Lymph node metastasis was associated with SUVmax (P = 0.017), but not serum CA19-9 (P = 0.172). On the contrary, the tumor stage was significantly related to serum CA19-9 (P = 0.035), but not SUVmax (P = 0.110). The univariate analysis showed that survival time was significantly related to tumor stage (P < 0.001), lymph node metastasis (P = 0.043), R0 surgical resection (P < 0.001), serum CA19-9 (P = 0.001), SUVmax (P < 0.001) and SUVmax plus CA19-9 (P = 0.002). Multivariate analysis clearly showed that only tumor stage (hazard ratio = 0.452; P = 0.020) was an independent prognostic factor for overall survival in pancreatic cancer. Higher SUVmax or CA19-9 showed worse prognosis. We found that high serum CA19-9 plus SUVmax was the most significant variable.
CONCLUSION: Higher pretreatment SUVmax and serum CA19-9 indicates poor prognosis. SUVmax plus serum CA19-9 is the most significant variable in predicting survival.
Core tip: We investigated the clinicopathological characteristics and prognostic significance of standardized maximum uptake value (SUVmax) of pancreatic cancer and serum carbohydrate antigen (CA)19-9. Higher SUVmax or CA19-9 showed worse prognosis, and high serum CA19-9 plus SUVmax was the most significant variable in predicting survival.
- Citation: Zhao JG, Hu Y, Liao Q, Niu ZY, Zhao YP. Prognostic significance of SUVmax and serum carbohydrate antigen 19-9 in pancreatic cancer. World J Gastroenterol 2014; 20(19): 5875-5880
- URL: https://www.wjgnet.com/1007-9327/full/v20/i19/5875.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i19.5875
Despite advances in diagnostic technology and therapeutic methods, pancreatic cancer is still a medical challenge. Even if early diagnosis and surgical resection are achieved, the average 5-year survival rate remains at approximately 5%[1]
Nowadays, after ultrasonography, contrast-enhanced computed tomography (CT) and enhanced magnetic resonance, fluorodeoxyglucose positron emission tomography (FDG-PET)/CT has assumed an important role in preoperative staging of patients with all kinds of cancers, including lung and breast cancer[2,3]. For any kind of pancreatic cancer, the success of surgical treatment does not depend on the histological type of the tumor but rather on its staging. Accurate preoperative staging is difficult and important for evaluating the disease extension and choosing the most appropriate treatment[4,5]. Some studies have concluded that the efficacy of FDG uptake measured as standardized maximum uptake value (SUVmax) in the primary tumor is predictive of an inferior outcome[6,7]; however, other studies have not agreed[8]. Several studies have shown that FDG uptake possibly plays a role in predicting the prognosis of pancreatic cancer[9,10]. However, the prognostic value of FDG uptake in pancreatic cancer remains uncertain because of the small number of studies reported in the literature.
Carbohydrate antigen (CA)19-9 is the most useful tumor marker for pancreatic cancer. Preoperative serum CA19-9 level has been reported as a useful prognostic marker in pancreatic cancer[11]. Some authors have reported that CA19-9 is an important independent prognostic variable in patients with inoperable pancreatic cancer[12,13].
In this study, we investigated the clinicopathological characteristics and prognostic significance of SUVmax of pancreatic cancer and serum CA19-9.
From January 2007 to October 2011, 80 patients with pancreatic cancer were reviewed at Peking Union Medical College Hospital, China. Clinical, histological, and imaging data of patients were collected and stored in a computerized database. Twelve patients had diabetes. Informed consent was obtained and the study design was approved by the Ethics Committee of the hospital.
The inclusion criteria were as follows: (1) all patients were diagnosed with pancreatic cancer by surgical pathology or endoscopic-ultrasound-guided staging pathology; (2) all patients were examined for tumor markers including CA 19-9; (3) pretreatment FDG-PET/CT was performed in all patients; (4) no previous diagnosis of another malignant disease; (5) no prior antitumor treatment, such as chemotherapy or radiotherapy; and (6) lymph node metastasis was confirmed by surgical pathology. The diagnosis and staging of pancreatic cancer was based on the 7th edition Staging Manual of the American Joint Committee on Cancer. Overall survival was defined as the time from pathological diagnosis to death or loss to follow-up. All patients were followed for 7-35 mo.
Patients were asked to fast for at least 6 h before examination and serum glucose level < 160 mg/dL was ensured. Before and after injection, patients were kept comfortable in the prone position. Scanning was initiated 1 h after administration of the tracer. PET/CT data were prospectively evaluated by consensus by two nuclear medicine physicians who were aware of the clinical and imaging results, but blinded to the pathological results. The SUVmax of the primary tumor was measured and calculated by the software according to standard formulas. The SUVmax of the liver and vascular pool were both measured. The same procedure was performed for two adjacent planes and the average of these data was considered.
Serum CA19-9 concentration was measured by an automated, commercially available enzyme immunoassay on an AxSYM analyzer (Abbott Diagnostics Laboratory). A value of 37 U/mL was used as the upper limit of normal.
Student’s t test for unpaired data was used to analyze the differences between two groups. Univariate analysis was performed using the following factors: patient age, tumor stage, tumor histological type, tumor size, blood vessel invasion, lymph node metastasis, distant metastasis, SUVmax, and serum CA19-9. All tests were two-tailed and P < 0.05 was considered significant. Survival curves were estimated by the Kaplan-Meier method and examined by the log-rank test. Cox proportional hazards regression was used to examine the independent effects of each significant variable. The statistical software SPSS version 13.0 was used for all analyses.
Only 59 patients underwent R0 surgical resection. The patient characteristics including age, sex, histology, tumor location, tumor pathological stage, metastasis, and treatment methods are summarized in Table 1. The mean serum CA19-9 for all patients was 842.1 U/mL (range: 151.3-2937.0 U/mL). The median serum CA19-9 was 604.5 U/mL. The mean SUVmax for all patients was 6.20 (range: 2.20-16.30). The median SUVmax was 5.35.
| n | SUVmax (n = 80) | Serum CA19-9 (n = 80) | |||
| mean ± SE | P value | mean ± SE | P value | ||
| Sex | |||||
| Male | 48 | 5.73 ± 0.36 | 0.091 | 749.30 ± 72.99 | 0.102 |
| Female | 32 | 6.91 ± 0.64 | 981.31 ± 132.74 | ||
| Age (yr) | |||||
| ≤ 60 | 37 | 5.74 ± 0.42 | 0.206 | 766.43 ± 93.14 | 0.316 |
| > 60 | 43 | 6.60 ± 0.52 | 907.22 ± 101.44 | ||
| Pancreatic location (n) | |||||
| Head | 62 | 6.39 ± 0.41 | 0.293 | 871.46 ± 84.01 | 0.437 |
| Body or tail | 18 | 5.53 ± 0.50 | 740.99 ± 108.47 | ||
| Stage | |||||
| I/II | 55 | 5.83 ± 0.43 | 0.110 | 729.09 ± 71.31 | 0.035 |
| III/IV | 25 | 7.01 ± 0.49 | 1090.74 ± 148.23 | ||
| Pathological differentiation | |||||
| Well | 28 | 5.46 ± 0.52 | 0.110 | 720.83 ± 99.27 | 0.055 |
| Moderate | 18 | 5.81 ± 0.62 | 667.87 ± 126.90 | ||
| Poor | 34 | 0.70 ± 0.57 | 1034.22 ± 119.19 | ||
| Tumor size | |||||
| > 2 cm | 56 | 6.32 ± 0.40 | 0.584 | 901.61 ± 87.16 | 0.193 |
| ≤ 2 cm | 24 | 5.91 ± 0.65 | 703.27 ± 108.27 | ||
| Lymph node metastasis | |||||
| Yes | 34 | 6.54 ± 0.52 | 0.017 | 831.84 ± 90.02 | 0.172 |
| No | 25 | 4.72 ± 0.50 | 634.35 ± 112.96 | ||
| Distant metastasis | |||||
| Yes | 8 | 7.48 ± 0.89 | 0.210 | 1363.26 ± 338.95 | 0.134 |
| No | 72 | 6.06 ± 0.36 | 784.20 ± 65.13 | ||
| R0 surgical resection | |||||
| Yes | 59 | 5.79 ± 0.38 | 0.043 | 731.19 ± 71.35 | 0.007 |
| No | 21 | 7.35 ± 0.67 | 1153.72 ± 156.96 | ||
The mean SUVmax and serum CA19-9 grouped by stage, lymph node status, pathological differentiation, distant metastasis, R0 surgical resection, and other clinicopathological variables including sex, age, tumor size and tumor location are shown in Table 2. There was significant correlation between the SUVmax and serum CA19-9 of pancreatic cancer and R0 surgical resection (P = 0.043 and P = 0.007) (Table 2). Lymph node metastasis was associated with SUVmax (P = 0.017), but not serum CA19-9 (P = 0.172). On the contrary, tumor stage was significantly related to serum CA19-9 (P = 0.035), but not SUVmax (P = 0.110). There was no correlation between SUVmax and serum CA19-9 of pancreatic cancer and sex, age, tumor location, tumor size, pathological differentiation, and distant metastasis.
| Variable | n | P value |
| Sex | ||
| Male | 48 | 0.119 |
| Female | 32 | |
| Age (yr) | ||
| ≤ 60 | 37 | 0.993 |
| > 60 | 43 | |
| Pancreatic location (n) | ||
| Head | 62 | 0.378 |
| Body or tail | 18 | |
| Stage | ||
| I/II | 55 | < 0.001 |
| III/IV | 25 | |
| Pathological differentiation | ||
| Well | 28 | 0.697 |
| Moderate | 18 | |
| Poor | 34 | |
| Tumor size | ||
| > 2cm | 56 | 0.339 |
| ≤ 2cm | 24 | |
| Lymph node metastasis | ||
| Yes | 34 | 0.043 |
| No | 25 | |
| Distant metastasis | ||
| Yes | 8 | < 0.001 |
| No | 72 | |
| Surgical resection | ||
| Yes | 59 | < 0.001 |
| No | 21 | |
| SUVmax | ||
| High | 40 | < 0.001 |
| Low | 40 | |
| CA19-9 | ||
| High | 40 | 0.001 |
| Low | 40 | |
| SUVmax plus CA19-9 | ||
| Both are high | 29 | 0.002 |
| Others | 51 |
Results of Cox regression analysis are shown in Table 3. The univariate analysis showed that survival time was significantly related to tumor stage (P < 0.001), lymph node metastasis (P = 0.043), R0 surgical resection (P < 0.001), serum CA19-9 (P = 0.001), SUVmax (P < 0.001) and SUVmax plus CA19-9 (P = 0.002). However, age, sex, pathological differentiation, tumor location and tumor size were not significantly related to survival time. Multivariate analysis clearly showed that only tumor stage (HR = 0.452; P = 0.020) was an independent prognostic factor for overall survival in pancreatic cancer.
| Variable | Hazard ratio (95%CI) | P value |
| Stage | 0.452 (0.192-1.064) | 0.020 |
| Lymph node metastasis | 0.786 (0.418-1.478) | 0.455 |
| Surgical resection | 11.521 (0.799-166.191) | 0.073 |
| SUVmax | 0.553 (0.216-1.413) | 0.216 |
| CA19-9 | 0.452 (0.192-1.064) | 0.069 |
| SUVmax plus CA19-9 | 1.558 (0.439-5.525) | 0.493 |
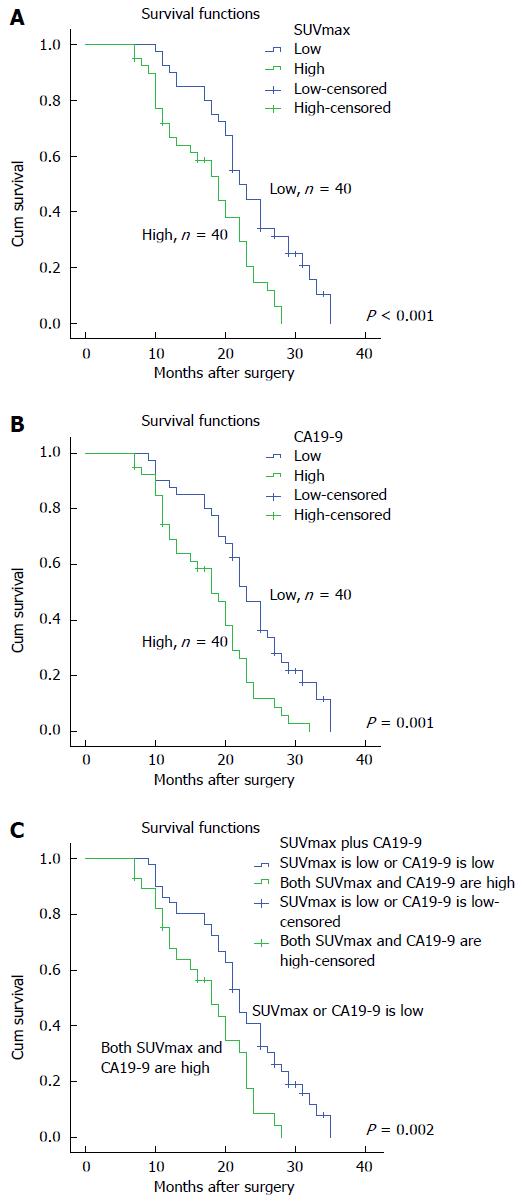
Figure 1 shows the survival curves for all patients who underwent pretreatment CA19-9 and SUVmax analysis of the primary tumor. The SUVmax and CA19-9 cutoff values for the primary tumor as determined by the median value were 5.35 and 604.5 U/mL, respectively. In patients with SUVmax and serum CA19-9 more than cutoff value, survival time was 17.69 ± 1.08 and 17.80 ± 1.08 mo, respectively. In patients with SUVmax and serum CA19-9 less than cutoff value, survival time was significantly longer (23.47 ± 1.19 and 23.33 ± 1.22 mo, respectively). Figure 1C shows the survival time of the patients in the following four groups: high SUVmax plus serum CA19-9; high SUVmax plus low serum CA19-9; low SUVmax plus high serum CA19-9; and low SUVmax plus serum CA19-9. Survival time in the high SUVmax plus serum CA19-9 group was shorter than in the other groups (17.37 ± 1.23 vs 22.32 ± 1.08 mo).
For prognostic factors for pancreatic cancer, the tumor stage and grade, R0 surgical resection, serum tumor marker levels, size of the primary lesion, and lymph node metastasis have been reported[14,15]. The increased glycolytic activity of the tumor detected by SUVmax may represent tumor growth and biological behavior[16]. FDG-PET possibly plays a role in predicting the prognosis of pancreatic cancer[9]. However, the significance of FDG-PET imaging in the diagnosis, staging, or predicting prognosis of pancreatic cancer has not yet been established.
The metabolic activity measured by FDG-PET, usually through SUVmax, seems to be useful in evaluating the prognosis of pancreatic cancer. Most authors consider SUVmax to be an independent prognostic factor that is expressed in tumor growth and biological behavior: higher SUV predicts worse prognosis[17]. In particular, Maemura et al[18] have shown that pancreatic cancer with distant metastases has a higher SUVmax than that without metastases. Sperti et al[10] have demonstrated that SUV > 4 is associated with shorter survival. Nakata et al[19] have shown in patients with unresectable disease that higher SUV is correlated with shorter survival. Tomita et al[20] have demonstrated that preoperative SUVmax and serum carcinoembryonic antigen (CEA) level are independent prognostic factors for survival in non-small cell lung cancer, and combined use of the two indicators might be better prognostic factors. Schellenberg et al[21] have shown that median survival for patients with a low and high SUVmax value was 15.3 and 9.8 mo, respectively, and patients with SUVmax < 5 had longer progression-free and overall survival than those with SUVmax > 5.
Sperti et al[22] have found that patients with preoperative CA19-9 level < 200 U/mL have significantly better prognosis than those with CA19-9 > 200 U/mL. After surgical resection, survival of patients with normal serum CA19-9 is significantly longer than that of patients with persistently elevated CA19-9. It has also been found that serum CA19-9 is an important independent prognostic variable in patients with inoperable pancreatic cancer, and baseline CA19-9 more or less than the median value is an independent prognostic factor for overall survival[13]. Ni et al[23] have shown that higher serum CA19-9, CA242 and CEA is related to distant metastasis, advanced stage, and worse prognosis of pancreatic cancer.
In the current study, we found that pretreatment SUVmax and serum CA19-9 in patients with pancreatic cancer were significantly associated with R0 surgical resection. Furthermore, we showed that lymph node metastasis was associated with SUVmax but not serum CA19-9. On the contrary, tumor stage was significantly related to serum CA19-9 but not SUVmax. Moreover, we found that the SUVmax in poorly differentiated cancer was near to 0. Thus, we suggest that SUVmax is associated with pancreatic cancer differentiation. Our univariate analysis demonstrated that survival time was significantly associated with tumor stage, and lymph node metastasis, R0 surgical resection, serum CA19-9, SUVmax. SUVmax plus serum CA19-9 were the main risk factors for prognosis of pancreatic cancer. However, although there was a clear correlation of CA19-9 and SUVmax with survival, multivariate analysis clearly showed that only tumor stage, and not CA19-9 and SUVmax, was an independent prognostic factor for overall survival in pancreatic cancer. We think that these results may have been related to the small number of patients.
Kaplan-Meier analysis revealed that SUVmax and serum CA19-9 were associated with prognosis in pancreatic cancer. The results showed that high SUVmax (≥ 5.35) and serum CA19-9 (≥ 604.5 U/mL) were correlated with worse prognosis. More importantly, we found that SUVmax plus serum CA19-9 was the most significant variable in predicting survival. Thus, we think that the combination of SUVmax plus serum CA19-9 is more meaningful in predicting survival of pancreatic cancer.
Although this study was retrospective, it did shed some light on the usefulness of SUVmax and serum CA19-9 in prognosis of pancreatic cancer. We can draw the following conclusions. Tumor stage is an independent predictor of survival in pancreatic cancer. Higher pretreatment SUVmax and serum CA19-9 level in pancreatic cancer indicate worse prognosis. SUVmax plus serum CA19-9 are significantly associated with poor prognosis. These findings may help to guide the treatment of pancreatic cancer and evaluate its prognosis.
Despite advances in diagnostic technology and therapeutic methods, pancreatic cancer is still a medical challenge. Several researchers have found that fluorodeoxyglucose (FDG) uptake possibly plays a role in predicting the prognosis of pancreatic cancer. However, the prognostic value of FDG uptake in pancreatic cancer remains uncertain because of the small number of reports in the literature. In this study, the authors investigated the clinicopathological characteristics and prognostic significance of standardized maximum uptake value (SUVmax) of pancreatic cancer and serum carbohydrate antigen (CA)19-9. CA19-9 is the most useful tumor marker for pancreatic cancer.
The prognostic value of FDG uptake in pancreatic cancer remains uncertain. CA19-9 is the most useful tumor marker for pancreatic cancer. The authors evaluated the significance of FDG-positron emission tomography (PET) imaging and CA19-9 in predicting the prognosis of pancreatic cancer.
Serum CA19-9 is the most useful tumor marker for pancreatic cancer. FDG-PET also possibly plays a role in predicting the prognosis of pancreatic cancer. Most authors think that SUVmax is an independent prognostic factor. Higher SUVmax predicts worse prognosis. However, the significance of FDG-PET imaging in the diagnosis, staging, or prognosis of pancreatic cancer has not yet been established. The significance of combination of SUVmax and CA19-9 has seldom been evaluated for predicting prognosis of pancreatic cancer. In this study, the authors investigated the clinicopathological characteristics and prognostic significance of SUVmax and serum CA 19-9 of pancreatic cancer.
Their results suggest that higher pretreatment SUVmax and serum CA19-9 level of pancreatic cancer indicate worse prognosis. Moreover, the combination of SUVmax plus serum CA19-9 is significantly associated with poor prognosis. These may help to guide the treatment of pancreatic cancer and evaluate its prognosis.
FDG-PET/CT is the fastest growing diagnostic modality in oncology. It is based on the concept that proliferating tumors have increased uptake and metabolism of glucose, and therefore, preferentially take up tracers, such as 18F-FDG, compared with normal tissue. Not only can this form of functional imaging detect malignant disease, irrespective of lesion morphology, it might also convey prognostic information related to the metabolic activity of the cancer.
The topic of exploring the relevance of SUVmax and CA19-9 in the prediction of prognosis and overall survival in pancreatic cancer is interesting. It is noted that most papers regarding this issue were from western countries. In the present study, the authors analyzed the data of SUVmax and CA19-9 from Chinese pancreatic cancer patients with follow-up time. Their results provide some useful information for the diagnosis and prognosis of pancreatic cancer. The prognostic value of SUVmax and serum CA19-9 in pancreatic cancer has already been reported before, thus, the emphasis of this article may be put on the combination of these two parameters.
| 1. | Yeo TP, Lowenfels AB. Demographics and epidemiology of pancreatic cancer. Cancer J. 2012;18:477-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 2. | De Leyn P, Lardinois D, Van Schil PE, Rami-Porta R, Passlick B, Zielinski M, Waller DA, Lerut T, Weder W. ESTS guidelines for preoperative lymph node staging for non-small cell lung cancer. Eur J Cardiothorac Surg. 2007;32:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 305] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 3. | Morris PG, Ulaner GA, Eaton A, Fazio M, Jhaveri K, Patil S, Evangelista L, Park JY, Serna-Tamayo C, Howard J. Standardized uptake value by positron emission tomography/computed tomography as a prognostic variable in metastatic breast cancer. Cancer. 2012;118:5454-5462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 4. | Miura F, Takada T, Amano H, Yoshida M, Furui S, Takeshita K. Diagnosis of pancreatic cancer. HPB (Oxford). 2006;8:337-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 94] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 5. | Parsons CM, Sutcliffe JL, Bold RJ. Preoperative evaluation of pancreatic adenocarcinoma. J Hepatobiliary Pancreat Surg. 2008;15:429-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Allal AS, Dulguerov P, Allaoua M, Haenggeli CA, El-Ghazi el A, Lehmann W, Slosman DO. Standardized uptake value of 2-[(18)F] fluoro-2-deoxy-D-glucose in predicting outcome in head and neck carcinomas treated by radiotherapy with or without chemotherapy. J Clin Oncol. 2002;20:1398-1404. [PubMed] |
| 7. | Downey RJ, Akhurst T, Gonen M, Vincent A, Bains MS, Larson S, Rusch V. Preoperative F-18 fluorodeoxyglucose-positron emission tomography maximal standardized uptake value predicts survival after lung cancer resection. J Clin Oncol. 2004;22:3255-3260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 267] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 8. | Hoang JK, Hoagland LF, Coleman RE, Coan AD, Herndon JE, Patz EF. Prognostic value of fluorine-18 fluorodeoxyglucose positron emission tomography imaging in patients with advanced-stage non-small-cell lung carcinoma. J Clin Oncol. 2008;26:1459-1464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 102] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 9. | Pakzad F, Groves AM, Ell PJ. The role of positron emission tomography in the management of pancreatic cancer. Semin Nucl Med. 2006;36:248-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 88] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 10. | Sperti C, Pasquali C, Chierichetti F, Ferronato A, Decet G, Pedrazzoli S. 18-Fluorodeoxyglucose positron emission tomography in predicting survival of patients with pancreatic carcinoma. J Gastrointest Surg. 2003;7:953-959; discussion 959-960. [PubMed] |
| 11. | Berger AC, Meszoely IM, Ross EA, Watson JC, Hoffman JP. Undetectable preoperative levels of serum CA 19-9 correlate with improved survival for patients with resectable pancreatic adenocarcinoma. Ann Surg Oncol. 2004;11:644-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 107] [Article Influence: 4.9] [Reference Citation Analysis (1)] |
| 12. | Halm U, Schumann T, Schiefke I, Witzigmann H, Mössner J, Keim V. Decrease of CA 19-9 during chemotherapy with gemcitabine predicts survival time in patients with advanced pancreatic cancer. Br J Cancer. 2000;82:1013-1016. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 131] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 13. | Maisey NR, Norman AR, Hill A, Massey A, Oates J, Cunningham D. CA19-9 as a prognostic factor in inoperable pancreatic cancer: the implication for clinical trials. Br J Cancer. 2005;93:740-743. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 121] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 14. | Lim JE, Chien MW, Earle CC. Prognostic factors following curative resection for pancreatic adenocarcinoma: a population-based, linked database analysis of 396 patients. Ann Surg. 2003;237:74-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 15. | Shimada K, Sakamoto Y, Sano T, Kosuge T. The role of paraaortic lymph node involvement on early recurrence and survival after macroscopic curative resection with extended lymphadenectomy for pancreatic carcinoma. J Am Coll Surg. 2006;203:345-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 82] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 16. | Maher JC, Savaraj N, Priebe W, Liu H, Lampidis TJ. Differential sensitivity to 2-deoxy-D-glucose between two pancreatic cell lines correlates with GLUT-1 expression. Pancreas. 2005;30:e34-e39. [PubMed] |
| 17. | Grassetto G, Rubello D. Role of FDG-PET/CT in diagnosis, staging, response to treatment, and prognosis of pancreatic cancer. Am J Clin Oncol. 2011;34:111-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Maemura K, Takao S, Shinchi H, Noma H, Mataki Y, Kurahara H, Jinnouchi S, Aikou T. Role of positron emission tomography in decisions on treatment strategies for pancreatic cancer. J Hepatobiliary Pancreat Surg. 2006;13:435-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 19. | Nakata B, Nishimura S, Ishikawa T, Ohira M, Nishino H, Kawabe J, Ochi H, Hirakawa K. Prognostic predictive value of 18F-fluorodeoxyglucose positron emission tomography for patients with pancreatic cancer. Int J Oncol. 2001;19:53-58. [PubMed] |
| 20. | Tomita M, Shimizu T, Ayabe T, Onitsuka T. Maximum SUV on positron emission tomography and serum CEA level as prognostic factors after curative resection for non-small cell lung cancer. Asia Pac J Clin Oncol. 2012;8:244-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 21. | Schellenberg D, Quon A, Minn AY, Graves EE, Kunz P, Ford JM, Fisher GA, Goodman KA, Koong AC, Chang DT. 18Fluorodeoxyglucose PET is prognostic of progression-free and overall survival in locally advanced pancreas cancer treated with stereotactic radiotherapy. Int J Radiat Oncol Biol Phys. 2010;77:1420-1425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 93] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 22. | Sperti C, Pasquali C, Catalini S, Cappellazzo F, Bonadimani B, Behboo R, Pedrazzoli S. CA 19-9 as a prognostic index after resection for pancreatic cancer. J Surg Oncol. 1993;52:137-141. [PubMed] |
| 23. | Ni XG, Bai XF, Mao YL, Shao YF, Wu JX, Shan Y, Wang CF, Wang J, Tian YT, Liu Q. The clinical value of serum CEA, CA19-9, and CA242 in the diagnosis and prognosis of pancreatic cancer. Eur J Surg Oncol. 2005;31:164-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 220] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
P- Reviewers: Ramia JM, Shah OJ, Tu H, Teo M, Zhang ZM S- Editor: Gou SX L- Editor: O’Neill M E- Editor: Zhang DN