Published online May 21, 2014. doi: 10.3748/wjg.v20.i19.5867
Revised: July 25, 2013
Accepted: August 16, 2013
Published online: May 21, 2014
Processing time: 359 Days and 17.1 Hours
AIM: To investigate the contribution of ABCB4 mutations to pediatric idiopathic gallstone disease and the potential of hormonal contraceptives to prompt clinical manifestations of multidrug resistance protein 3 deficiency.
METHODS: Mutational analysis of ABCB4, screening for copy number variations by multiplex ligation-dependent probe amplification, genotyping for low expression allele c.1331T>C of ABCB11 and genotyping for variation c.55G>C in ABCG8 previously associated with cholesterol gallstones in adults was performed in 35 pediatric subjects with idiopathic gallstones who fulfilled the clinical criteria for low phospholipid-associated cholelithiasis syndrome (LPAC, OMIM #600803) and in 5 young females with suspected LPAC and their families (5 probands, 15 additional family members). The probands came to medical attention for contraceptive-associated intrahepatic cholestasis.
RESULTS: A possibly pathogenic variant of ABCB4 was found only in one of the 35 pediatric subjects with idiopathic cholesterol gallstones whereas 15 members of the studied 5 LPAC kindreds were confirmed and another one was highly suspected to carry predictably pathogenic mutations in ABCB4. Among these 16, however, none developed gallstones in childhood. In 5 index patients, all young females carrying at least one pathogenic mutation in one allele of ABCB4, manifestation of LPAC as intrahepatic cholestasis with elevated serum activity of gamma-glutamyltransferase was induced by hormonal contraceptives. Variants ABCB11 c.1331T>C and ABCG8 c.55G>C were not significantly overrepresented in the 35 examined patients with suspect LPAC.
CONCLUSION: Clinical criteria for LPAC syndrome caused by mutations in ABCB4 cannot be applied to pediatric patients with idiopathic gallstones. Sexual immaturity even prevents manifestation of LPAC.
Core tip: Mutations in ABCB4 are not overrepresented in children with idiopathic gallstones who fulfill the clinical and laboratory criteria for low phospholipid-associated cholelithiasis syndrome (Gallbladder Disease 1, OMIM #600803). Sexual immaturity prevents manifestation of low phospholipid-associated cholelithiasis. In young females, manifestation of low phospholipid-associated cholelithiasis syndrome such as intrahepatic cholestasis with elevated serum activity of gamma-glutamyltransferase may be induced by hormonal contraceptives.
-
Citation: Jirsa M, Bronský J, Dvořáková L, Šperl J, Šmajstrla V, Horák J, Nevoral J, Hřebíček M.
ABCB4 mutations underlie hormonal cholestasis but not pediatric idiopathic gallstones. World J Gastroenterol 2014; 20(19): 5867-5874 - URL: https://www.wjgnet.com/1007-9327/full/v20/i19/5867.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i19.5867
Low phospholipid-associated cholelithiasis syndrome (LPAC, synonym Gallbladder disease 1, OMIM #600803) has been defined as symptomatic and recurring cholelithiasis associated with mutations in ABCB4 encoding multidrug resistance protein 3 (MDR3), the canalicular phospholipid export pump[1,2]. LPAC should be suspected in patients with symptomatic cholelithiasis in whom at least one minor criterion is present. These minor criteria are proposed as: (a) age below 40 years at the onset of symptoms; (b) recurrence after cholecystectomy; (c) intrahepatic hyperechoic foci with a topography compatible with lipid deposits along the luminal surface of the intrahepatic biliary tree; (d) intrahepatic sludge; (e) microlithiasis; (f) history of gallstones in first-degree relatives; or (g) history of intrahepatic cholestasis of pregnancy[3]. The distribution of associated ABCB4 mutations in conserved regions of the gene, as well as their type, strongly support the role of partial MDR3 deficiency in LPAC, with decreased MDR3 activity and/or expression altering biliary lipid composition.
Apart from LPAC, mutations in ABCB4 that reduce but do not abrogate the activity of MDR3 can cause a variety of milder forms of familial intrahepatic cholestasis type 3 (OMIM #602347), with slowly progressive or non-progressive hepatobiliary disease or anicteric cholestasis with varying liver fibrosis in adulthood[4]. Several reports[5-7] have shown that intrahepatic cholestasis of pregnancy is associated with ABCB4 mutations in some women. Finally, the idea that contraceptive-induced cholestasis (CIC) may be associated with mutations in ABCB4 has also been proposed. Asymptomatic gallstones and clinically silent cirrhosis, diagnosed later as progressive familial intrahepatic cholestasis type 3, became manifest in a 17-year-old girl when cholestasis developed on ingestion of contraceptive pills containing ethinylestradiol 30 μg, and levonorgestrol 150 μg[8], and isolated gallstone disease unmasked by oral contraception and associated with ABCB4 mutation has been reported[1]. In contrast, no mutations in ABCB4 were found in 5 subjects with CIC studied by Lang et al[9].
In our previous study[10] we focused on the role of the common variants c.523A>G (p.Thr175Ala) and c.1954A>G (p.Arg652Gly) in ABCB4, c.1331T>C (p.Val444Ala) in ABCB11 and c.55 G>C (p.Asp19His) in ABCG8 in pediatric gallstone disease. These variants are considered either as potentially pathogenic or as susceptibility alleles for cholesterol cholelithiasis in adults; however, they were not observed to contribute to genetic predisposition to gallstones in childhood[10].
In this study we investigated: (1) the role of ABCB4 mutations in the etiology of pediatric idiopathic gallstones; and (2) the capability of hormonal contraceptives to unmask hitherto clinically silent MDR3 deficiency.
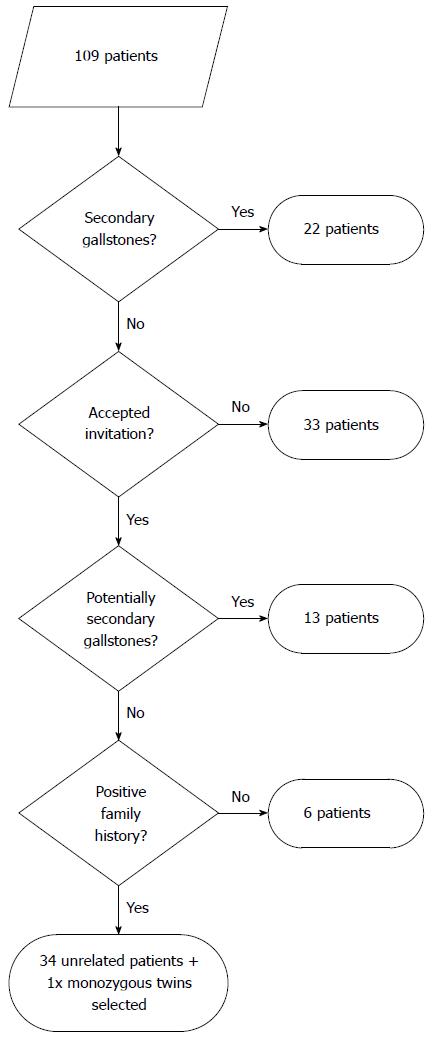
Pediatric patients with gallstones were selected as described[10] (see Figure 1 for the selection algorithm). Briefly, 109 children (53 males and 56 females) with gallbladder gallstones who had been hospitalized at the Department of Pediatrics, Faculty Hospital Motol, Prague, between 1995-2004, were considered. In 22 patients, gallstones were clearly associated with another disease such as Down syndrome, Gaucher disease, cystic fibrosis, hemolytic anemia, inflammatory bowel disease, immune deficiency and Gilbert syndrome. Thirty-three of the 87 invited patients did not respond. In 13 of 54 patients, the etiology of gallstones was uncertain. However, as these 13 patients had at least one of the following: long-term parenteral nutrition, treatment with cephalosporins or furosemide, dyslipidemia, hepatobiliary infectious disease or obesity (BMI > 27), e.g. conditions that could promote gallstone formation, they were not enrolled. In 41 patients, gallstones were most likely idiopathic. For ABCB4 mutation testing, only 35 of these 41 patients (including only one of the monozygous twins) with idiopathic gallstones were selected who had at least one parent or grandparent with gallstones. These subjects (15 males and 20 females with positive family history), all unrelated Caucasians of Czech origin, met the major criterion and minor criteria (a) and (f) of Rosmorduc and Poupon[3]. The mean age at diagnosis of cholelithiasis was 10.7 ± 5.0 years (range 1-17). Nineteen of these 35 patients (13 girls and 6 boys) underwent cholecystectomy with no recurrence after surgery. As of this writing, all cholecystectomized patients are well, without abdominal pain or jaundice.
Five young adult female patients with symptomatic gallstones (age below 40 years at the onset of symptoms), a history of intrahepatic cholestasis, and a family history of gallstones in first-degree relatives were referred for ABCB4 analysis. Their clinical characteristics are summarized in Table 1. None of the patients had hyperechoic foci in the liver parenchyma or proven intrahepatic sludge; duodenal bile was not investigated for microlithiasis. Nonethless, all met the proposed criteria for LPAC[3]. In patient I, intrahepatic cholestasis associated with exposure to an oral contraceptive containing ethinylestradiol 0.030 mg, and levonorgestrel 0.125 mg (Minisiston; Jenapharm, Jena, Germany) was the first clinical symptom of LPAC. Cholestasis resolved rapidly after withdrawal of the contraceptive. However, the patient developed cholecystolithiasis within one year despite ursodeoxycholic acid administration (15 mg/kg) and underwent cholecystectomy. One year later, ursodeoxycholic acid was withdrawn because the patient was completely asymptomatic with normal clinical/laboratory test results. Rechallenge with another oral contraceptive containing ethinylestradiol 0.020 mg and desogestrel 0.150 mg (Mercilon; Organon, Oss, The Netherlands) two years after cholecystectomy was followed within several weeks by a second attack of cholestasis. Clinical and laboratory findings improved again rapidly when the medication was withdrawn. None of the other 4 index patients mentioned any problems associated with the use of contraceptives; the data on contraceptives presented in Table 1 were obtained in part from clinical records and in part by specific questioning.
| Patient ID | Year of birth | Age at cholecystectomy (yr) | Age at liver biopsy (yr) | Age at pregnancies (yr) | Age (yr), contraceptive, complication |
| I | 1980 | 20 | 19, periportal fibrosis | Nulliparous | 18 Minisiston, withdrawn for CIC |
| 22 Mercilon, withdrawn for CIC | |||||
| II | 1978 | 17 | 21, periportal fibrosis | Nulliparous | 18 Tri-regol, withdrawn for CIC |
| III | 1973 | 22 | 22, periportal fibrosis | 30, with ICP | 20-22 Cilest, withdrawn for CIC |
| IV | 1967 | 28 | 31, normal histology | 19, without ICP | 28 Tri-regol, withdrawn for CIC |
| 39-now Lunafem, tolerated | |||||
| V | 1973 | 20 | 20, periportal fibrosis | 23, without ICP | 19-?, withdrawn for CIC |
| 32, without ICP | 24-31 Marvelon, tolerated | ||||
| 32-now Mirena, permanent pruritus, GGT twice normal |
The patient studies were approved by the Institutional Review Board of the Faculty Hospital Motol. Either both parents or the examined subjects, when aged over 15 years, gave written informed consent before blood sampling.
Twenty-seven fragments covering all exonic (protein-coding) regions of ABCB4 and including portions of adjacent intronic sequences were amplified from genomic DNA by PCR (primer sequences are available from the corresponding author). The DNA sequence of purified PCR products was analyzed on an ABI-PRISM 3100-Avant automated DNA sequencer (Applied Biosystems, Foster City, CA). Ensembl Acc. No. ENSG00000005471 and GenBank Acc. No. NM_018849.2 served as genomic and cDNA reference sequences. Mutations found by DNA sequencing were independently confirmed by restriction fragment length polymorphism analysis after digestion of the corresponding PCR product with restriction enzymes. In addition, ABCB4 was scanned for deletions/duplications by multiplex ligation-dependent probe amplification, using SALSA MLPA KIT P109 ABCB4 (MRC-Holland, Amsterdam, The Netherlands) according to manufacturer’s instructions.
The low expression allele c.1331T>C of ABCB11[11] was detected as the presence of a PCR-BsuRI restriction fragment length polymorphism. The variation c.55G>C in ABCG8 associated with cholesterol gallstones in adults[12] was detected as described by Hubáček et al[13]. Pathogenicity of missense variations was predicted in silico by SIFT[14], PMut[15], PolyPhen-2[16] and MutationTaster[17].
The data are presented as mean and standard deviation, or as frequencies when appropriate. We used chi-square testing to check whether genotype frequencies were consistent with Hardy-Weinberg equilibrium. Differences between genotype frequencies were analyzed by two-sided Fisher exact testing, using the approximation of Katz, with the InStat3 program (GraphPad Software, La Jolla, CA).
In the group of pediatric patients with idiopathic gallstones selected for genetic examination, analysis of protein-coding exons and intron/exon junctions of ABCB4 identified no obvious pathogenic mutations. In patient 31, a novel heterozygous variation was found (c.2222C>T, leading to predicted conservative amino acid substitution p.Pro741Leu in the extracellular loop between transmembrane domains 7 and 8). The substitution was rated as neutral by all four pathogenicity prediction programs. Another predicted amino acid substitution (p.Gly773Val, localized in transmembrane domain 8 and caused by the novel mutation c.2318G>T) was found in a heterozygous state in patient 32. This conservative substitution was rated as disease-causing by MutationTaster, possibly pathogenic by PolyPhen-2, and neutral by SIFT and PMut. In addition, 6 known coding (5 synonymous) and 6 known non-coding variations were found (Table 2). None of these changes is reportedly associated with hepatobiliary disease, with the possible exception of c.1954A>G (p.Arg652Gly), found previously in a heterozygous state in subjects 4, 8 and 26[10]. However, the c.1954A>G variant was not overrepresented (3/70, allelic frequency 0.043) in our patients as compared with a healthy adult Czech Caucasian population (allelic frequency 0.090, 27 heterozygotes in 150 controls, OR = 0.48, 95%CI: 0.16-1.48, P = 0.17).
| Patient. ID | Variations in the coding sequence of ABCB4 | ABCB11 low expression allele | ABCG8 variation | |||||
| c.147C>T | c.175C>T | c.459T>C | c.504C>T | c.711A>T | c.1954A>G | c.1331T>C | c.55G>C | |
| Ser49Ser | Leu59Leu | Phe153Phe | Asn168Asn | Ile237Ile | Arg652Gly | Val444Ala | Asp19His | |
| rs8187789 | rs2302387 | rs2230027 | rs1202283 | rs2109505 | rs2230028 | rs2287622 | rs11887534 | |
| 1 | CC | CC | TT | TT | AA | AA | CC | GG |
| 2 | CC | CC | TT | CT | AA | AA | TC | GG |
| 3 | CC | CC | TT | CT | AA | AA | TC | GG |
| 4 | CC | CC | TT | CT | AT | AG | TC | GG |
| 5 | CC | CC | TT | TT | AA | AA | CC | GG |
| 6 | CC | CC | TT | CC | AA | AA | TC | GG |
| 7 | CC | CC | TT | CT | AA | AA | TC | GG |
| 8 | CC | CC | TT | CC | AA | AG | TC | GG |
| 9 | CC | CC | TT | TT | AA | AA | TT | GG |
| 10 | CC | CC | TT | TT | AA | AA | CC | GG |
| 11 | CC | CC | TT | TT | AA | AA | TC | GG |
| 12 | CC | CC | TT | TT | AA | AA | CC | GG |
| 13 | CC | CC | TT | CT | AA | AA | TC | GG |
| 14 | CC | CC | TT | CC | AA | AA | CC | GG |
| 15 | CC | CC | TT | TT | AA | AA | TC | GC |
| 16 | CC | CC | TT | CC | AA | AA | TC | GC |
| 17 | CC | CC | TT | CC | AA | AA | TC | GG |
| 18 | CC | CC | TT | TT | AA | AA | TC | GC |
| 19 | CC | CC | TT | CT | AA | AA | TC | GC |
| 20 | CC | CC | TT | CT | AA | AA | TT | GG |
| 21 | CC | CC | TT | TT | AA | AA | TC | GG |
| 22 | CC | CC | TT | CT | AA | AA | TT | GG |
| 23 | CC | CC | TT | CT | AA | AA | TT | GG |
| 24 | CC | CT | TT | CC | AT | AA | CC | GG |
| 25 | CC | CC | TT | CC | AA | AA | TC | GG |
| 26 | CT | CT | TC | CC | AA | AG | TC | GG |
| 27 | CC | CC | TT | TT | AA | AA | TC | GG |
| 28 | CC | CC | TT | TT | AA | AA | TT | GG |
| 29 | CC | CC | TT | CT | AA | AA | TT | GG |
| 30 | CC | CC | TT | TT | AA | AA | CC | GC |
| 31 | CC | CC | TT | CT | AA | AA | TT | GC |
| 32 | CC | CC | TT | CT | AA | AA | TT | GG |
| 33 | CC | CC | TT | TT | AA | AA | TC | GG |
| 34 | CC | CC | TT | CC | AA | AA | TT | GG |
| 35 | CC | CC | TT | TT | AA | AA | CC | GG |
| Allelic frequency of variant alleles in patients with gallstones, HapMap populations and Czech population controls | ||||||||
| Allele | T | T | C | T | T | G | C | G |
| Gallstone patients | 0.014 | 0.029 | 0.014 | 0.571 | 0.029 | 0.043 | 0.471 | 0.086 |
| HapMap CEU | 0 | 0.112 | 01 | 0.664 | 0.175 | 0.075 | 0.408 | 0.085 |
| HapMap HCB | 0 | 0.167 | 01 | 0.344 | 0.222 | 0.023 | 0.333 | 0.022 |
| HapMap JPT | 0 | 0.273 | 01 | 0.442 | 0.300 | 0.023 | 0.261 | 0.011 |
| HapMap YRI | 0.042 | 0.525 | 0.11 | 0 | 0.362 | 0.392 | 0.425 | 0.042 |
| Czech controls (n = 150) | n.d. | n.d. | n.d. | n.d. | n.d. | 0.090 | 0.400 | 0.0672 |
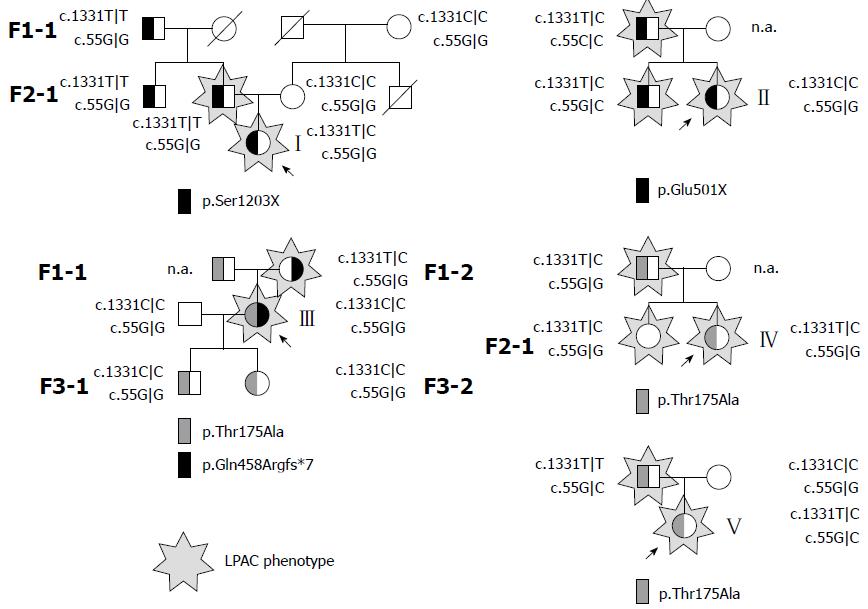
Two of the five probands carried a single heterozygous nonsense mutation, two were heterozygotes for the missense mutation c.523A>G (p.Thr175Ala, rs58238559), and one was a compound heterozygote for the same missense mutation (c.523A>G) and for the frameshift mutation c.1371delG (p.Gln458Argfs*7) (Figure 2). The variation c.523A>G was found on 30% (3/10) of alleles in patients with LPAC, whereas only 2.7% of control alleles from the Czech population carried guanine at the position 523 (8/300, 8 heterozygotes in 150 control individuals, OR = 8.00, CI: 2.20-29.24, P = 0.012). While the number of patients was too low to make the result fully convincing, this observation suggests that p.Thr175Ala at least confers susceptibility to hepatobiliary disease. All three null mutations were novel to our best knowledge.
No deletions/duplications in ABCB4 were detected in index patients by multiplex ligation-dependent probe amplification.
Two probands were homozygous and the other three probands were heterozygous for the low-expression ABCB11 variant c.1331T>C (p.Val444Ala) (Figure 2). One proband had a c.55G|C genotype while four other probands were homozygous for the wildtype allele c.55G in ABCG8. To assess the segregation of the genotype and phenotype in the families of all index patients, first degree relatives were examined. As can be seen from the family trees depicted in Figure 2, the parents in families I, II, IV, and V who carried the same mutation as the probands in a heterozygous state were symptomatic. This indicates that the null mutations in families I - III and even the missense mutation leading to p.Thr175Ala in families IV and V all are likely sufficient in a heterozygous state to promote the LPAC phenotype. In contrast, variations ABCB11 c.1331T>C and ABCG8 c.55G>C, found in probands and 11 family members carrying mutations in ABCB4, do not seem to affect the penetrance of LPAC (Figure 2).
The only possible pathogenic mutation in ABCB4 found in pediatric patients with idiopathic gallstones who met clinical criteria for the diagnosis of LPAC was the variation c.2318G>T (p.Gly773Val ) found in a heterozygous state in only one affected subject. The nucleotide change c.1954A>G found in 3 other pediatric gallstone subjects is common in the European, Caucasian, and African general population, but it has also been found in a patient with LPAC and low biliary phospholipid in whom its predicted consequence p.Arg652Gly was hypothesized to be conditionally penetrant, leading to clinical symptoms only under certain circumstances, such as pregnancy, or when combined with another mutation[18]. In contrast, no correlation of the ABCB4 genotype c.1954A|G with the MDR3 expression level in the liver, as measured by Western blot, was observed in a study by Meier et al[11] and the substitution was rated as neutral by all software tools used. Our finding that the genotype c.1954A|G was neither overrepresented nor significantly underrepresented in patients with gallstones may indicate the negligible role of this variation in etiology of pediatric idiopathic gallstones. Similar conclusions could be drawn for both carriers and homozygotes for the low expression variant of the bile salt export pump and for the carriers of the ABCG8 variation c.55G>C.
Interestingly, the ABCB4 variation c.523A>G (p.Thr175Ala), found in three index patients with LPAC, was not present in any of our patients with pediatric gallstones. The allele c.523G is linked to cholestatic disease[1] although it is also found in healthy Caucasian populations at an allelic frequency of 0.025-0.032[9,19,20]. The threonine residue at position 175 is highly conserved, lying in a Thr-Arg-Leu-Thr cluster required for MDR3 adenosine triphosphatase (ATPase) activity. While the functional consequences of replacement of threonine at position 175 by a neutral amino-acid residue having a hydrophobic side chain were not evaluated in MDR3, they were studied in yeast in the close homologue P-glycoprotein[21], in which the substitution p.Thr169Ile resulted in a complete loss of substrate-induced P-glycoprotein ATPase activity. The substitution p.Thr175Ala, predicted uniformly to impair protein function by SIFT, PMut, PolyPhen-2 and MutationTaster is thus considered a disease-associated mutation[3] with incomplete penetrance.
Neither the 14 confirmed and 1 suspected heterozygous carriers of ABCB4 mutations investigated in the second part of our study nor the heterozygotes reported previously by others[1,2,4,22-24] developed symptomatic gallstones without progressive familial intrahepatic cholestasis in childhood. This suggests that other pathogeneses of idiopathic gallstones in childhood should be sought. Since we did not assay phospholipid and cholesterol concentrations in bile from our 35 pediatric subjects, we cannot definitively claim that they did not have LPAC; only that, if they had LPAC, it was associated neither with demonstrable ABCB4 mutation (this study) nor with the studied variations in ABCB11 and ABCG8[10]. We suggest that to carry out ABCB4 sequencing in pediatric patients with idiopathic cholesterol gallstones who meet only some of the present criteria for assigning the diagnosis of LPAC may be unproductive. We believe that the validity of these criteria for LPAC associated with ABCB4 mutation should be re-assessed, in pediatric patients at least, and propose that the present criteria at this juncture be considered to apply only to adults aged less than 40 years.
The observations that LPAC syndrome becomes manifest after middle adolescence and that young females heterozygous for pathogenic mutations in ABCB4 developed CIC and/or manifested previously asymptomatic gallstones during administration of combined oral contraceptives are most likely explained by known changes in biliary lipid composition during the second decade of life. Gallstones hardly ever occur in children, but are frequent in adults; this difference seems to be due to the low concentrations of cholesterol in the bile of children[25]. Children have reduced biliary cholesterol:bile salt excretion ratios[26]. Therefore, even at low rates of phospholipid secretion caused by incomplete MDR3 deficiency, bile is not saturated with cholesterol. The known increase in the biliary cholesterol saturation index in young adults[26], together with the decreased biliary secretion rate of phosphatidylcholine in carriers of mutations in ABCB4, shifts the cholesterol-solubility equilibrium to the borderline. Even the low load of exogenous hormones contained in contraceptives or other hormonally active drugs, which inhibit bile salt secretion[27] and further decrease secretion of phospholipids into bile proportionally to bile salt flow[28], can precipitate cholestasis and promote cholesterol crystallization from supersaturated bile, with formation of intrahepatic sludge and of gallstones.
A practical question can be raised on the safety of contraceptives in women with MDR3 deficiency. Our patients heterozygous for null mutations developed CIC rapidly and consequently contraceptives had to be withdrawn. In contrast, two patients heterozygous for the missense MDR3 variant p.Thr175Ala tolerated long-term administration of oral contraceptives after cholecystectomy without apparent worsening in hepatobiliary disease. Interestingly, patient V reported pruritus and her serum levels of GGT were repeatedly increased twofold when she used estrogen-free intrauterine contraception. We therefore believe that the heterozygous state for missense mutations in ABCB4 is not an a priori contraindication to oral contraception. However, monitoring of clinical status and clinical-laboratory indices of hepatobiliary injury is essential in such cases.
In conclusion, our findings indicate that clinical criteria for LPAC caused by mutations in ABCB4 cannot be applied to pediatric patients with idiopathic gallstones. Sexual immaturity prevents manifestation of LPAC even in carriers of pathogenic mutations in ABCB4. In young females, manifestation of LPAC as intrahepatic cholestasis with elevated serum activity of gamma-glutamyltransferase may be triggered by hormonal contraceptives.
We thank A S Knisely, Institute of Liver Studies, King’s College Hospital, London, United Kingdom, for comments on the manuscript, and Lucie Budišová, IKEM, and Michaela Boučková, Institute of Inherited Metabolic Diseases, both Prague, Czech Republic, for technical assistance.
Mutations in ABCB4, the variation c.55G>C in ABCG8 and the low expression allele c.1331T>C of ABCB11 may affect biliary lipid composition and increase saturation of bile with cholesterol. Mutations in ABCB4 are known to cause low phospholipid-associated cholelithiasis (LPAC) in young adults. The variation c.55G>C in ABCG8 has been linked with gallstones in adults.
Since saturation of bile with cholesterol in children is lower than in adults, the authors anticipated a strong contribution of the above listed genetic variations to pediatric idiopathic gallstones and conducted a genetic study in pediatric LPAC-like gallstone patients and in young adults with suspect LPAC who came to medical attention due to contraceptive-induced cholestasis. Whereas young adult females with clinically defined LPAC carried mutations in ABCB4, no association with the studied variants was found in pediatric LPAC-like subjects.
Sexual immaturity prevents manifestation of LPAC even in carriers of pathogenic mutations in ABCB4.
Clinical criteria for LPAC caused by mutations in ABCB4 cannot be applied to pediatric patients with idiopathic gallstones and to carry out ABCB4 sequencing in pediatric patients with idiopathic cholesterol gallstones may be unproductive. Heterozygous state for some missense mutations in ABCB4 is not an a priori contraindication to oral contraception; however, monitoring of clinical status and clinical/laboratory indices of hepatobiliary injury is essential in such cases.
Biliary lipid secretion is mediated by three ABC transporters: ABCB11 encodes the bile salt export pump, ABCB4 encodes the canalicular lecithin pump MDR3 (multidrug resistance protein 3) and the genes ABCG5 and ABCBG8 encode two proteins named sterolins which form a heterodimeric ABC transporter responsible for biliary secretion of cholesterol and plant sterols.
It is an excellent manuscript submitted to reevaluate the criteria for LAPC associated with ABCB4 mutation and provide the data of both pediatric idiopathic gallstone and young women with LAPC hormonal cholestasis by oral contraceptives. The mechanism of cholelithiasis formation with ABCB11 and ABCG8 mutation was also considered and 5 probands with detailed pedigrees were presented. Ethics of the research was given by written informed consent.
| 1. | Rosmorduc O, Hermelin B, Poupon R. MDR3 gene defect in adults with symptomatic intrahepatic and gallbladder cholesterol cholelithiasis. Gastroenterology. 2001;120:1459-1467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 212] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 2. | Rosmorduc O, Hermelin B, Boelle PY, Parc R, Taboury J, Poupon R. ABCB4 gene mutation-associated cholelithiasis in adults. Gastroenterology. 2003;125:452-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 187] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 3. | Rosmorduc O, Poupon R. Low phospholipid associated cholelithiasis: association with mutation in the MDR3/ABCB4 gene. Orphanet J Rare Dis. 2007;2:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 102] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 4. | Ziol M, Barbu V, Rosmorduc O, Frassati-Biaggi A, Barget N, Hermelin B, Scheffer GL, Bennouna S, Trinchet JC, Beaugrand M. ABCB4 heterozygous gene mutations associated with fibrosing cholestatic liver disease in adults. Gastroenterology. 2008;135:131-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 123] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 5. | Floreani A, Carderi I, Paternoster D, Soardo G, Azzaroli F, Esposito W, Variola A, Tommasi AM, Marchesoni D, Braghin C. Intrahepatic cholestasis of pregnancy: three novel MDR3 gene mutations. Aliment Pharmacol Ther. 2006;23:1649-1653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 43] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 6. | Schneider G, Paus TC, Kullak-Ublick GA, Meier PJ, Wienker TF, Lang T, van de Vondel P, Sauerbruch T, Reichel C. Linkage between a new splicing site mutation in the MDR3 alias ABCB4 gene and intrahepatic cholestasis of pregnancy. Hepatology. 2007;45:150-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 56] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 7. | Floreani A, Carderi I, Paternoster D, Soardo G, Azzaroli F, Esposito W, Montagnani M, Marchesoni D, Variola A, Rosa Rizzotto E. Hepatobiliary phospholipid transporter ABCB4, MDR3 gene variants in a large cohort of Italian women with intrahepatic cholestasis of pregnancy. Dig Liver Dis. 2008;40:366-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 52] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 8. | Ganne-Carrié N, Baussan C, Grando V, Gaudelus J, Cresteil D, Jacquemin E. Progressive familial intrahepatic cholestasis type 3 revealed by oral contraceptive pills. J Hepatol. 2003;38:693-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Lang C, Meier Y, Stieger B, Beuers U, Lang T, Kerb R, Kullak-Ublick GA, Meier PJ, Pauli-Magnus C. Mutations and polymorphisms in the bile salt export pump and the multidrug resistance protein 3 associated with drug-induced liver injury. Pharmacogenet Genomics. 2007;17:47-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 225] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 10. | Bronský J, Jirsa M, Nevoral J, Hrebícek M. Role of common canalicular transporter gene variations in aetiology of idiopathic gallstones in childhood. Folia Biol (Praha). 2010;56:9-13. [PubMed] |
| 11. | Meier Y, Pauli-Magnus C, Zanger UM, Klein K, Schaeffeler E, Nussler AK, Nussler N, Eichelbaum M, Meier PJ, Stieger B. Interindividual variability of canalicular ATP-binding-cassette (ABC)-transporter expression in human liver. Hepatology. 2006;44:62-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 165] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 12. | Buch S, Schafmayer C, Völzke H, Becker C, Franke A, von Eller-Eberstein H, Kluck C, Bässmann I, Brosch M, Lammert F. A genome-wide association scan identifies the hepatic cholesterol transporter ABCG8 as a susceptibility factor for human gallstone disease. Nat Genet. 2007;39:995-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 241] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 13. | Hubácek JA, Berge KE, Stefková J, Pitha J, Skodová Z, Lánská V, Poledne R. Polymorphisms in ABCG5 and ABCG8 transporters and plasma cholesterol levels. Physiol Res. 2004;53:395-401. [PubMed] |
| 14. | Ng PC, Henikoff S. Predicting deleterious amino acid substitutions. Genome Res. 2001;11:863-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1814] [Cited by in RCA: 2127] [Article Influence: 85.1] [Reference Citation Analysis (0)] |
| 15. | Ferrer-Costa C, Gelpí JL, Zamakola L, Parraga I, de la Cruz X, Orozco M. PMUT: a web-based tool for the annotation of pathological mutations on proteins. Bioinformatics. 2005;21:3176-3178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 389] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 16. | Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, Kondrashov AS, Sunyaev SR. A method and server for predicting damaging missense mutations. Nat Methods. 2010;7:248-249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10971] [Cited by in RCA: 10749] [Article Influence: 671.8] [Reference Citation Analysis (0)] |
| 17. | Schwarz JM, Rödelsperger C, Schuelke M, Seelow D. MutationTaster evaluates disease-causing potential of sequence alterations. Nat Methods. 2010;7:575-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2146] [Cited by in RCA: 2424] [Article Influence: 151.5] [Reference Citation Analysis (0)] |
| 18. | Jacquemin E, De Vree JM, Cresteil D, Sokal EM, Sturm E, Dumont M, Scheffer GL, Paul M, Burdelski M, Bosma PJ. The wide spectrum of multidrug resistance 3 deficiency: from neonatal cholestasis to cirrhosis of adulthood. Gastroenterology. 2001;120:1448-1458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 331] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 19. | Pauli-Magnus C, Kerb R, Fattinger K, Lang T, Anwald B, Kullak-Ublick GA, Beuers U, Meier PJ. BSEP and MDR3 haplotype structure in healthy Caucasians, primary biliary cirrhosis and primary sclerosing cholangitis. Hepatology. 2004;39:779-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 118] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 20. | Pauli-Magnus C, Lang T, Meier Y, Zodan-Marin T, Jung D, Breymann C, Zimmermann R, Kenngott S, Beuers U, Reichel C. Sequence analysis of bile salt export pump (ABCB11) and multidrug resistance p-glycoprotein 3 (ABCB4, MDR3) in patients with intrahepatic cholestasis of pregnancy. Pharmacogenetics. 2004;14:91-102. [PubMed] |
| 21. | Kwan T, Gros P. Mutational analysis of the P-glycoprotein first intracellular loop and flanking transmembrane domains. Biochemistry. 1998;37:3337-3350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Gendrot C, Bacq Y, Brechot MC, Lansac J, Andres C. A second heterozygous MDR3 nonsense mutation associated with intrahepatic cholestasis of pregnancy. J Med Genet. 2003;40:e32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 46] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Lucena JF, Herrero JI, Quiroga J, Sangro B, Garcia-Foncillas J, Zabalegui N, Sola J, Herraiz M, Medina JF, Prieto J. A multidrug resistance 3 gene mutation causing cholelithiasis, cholestasis of pregnancy, and adulthood biliary cirrhosis. Gastroenterology. 2003;124:1037-1042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 127] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 24. | Gotthardt D, Runz H, Keitel V, Fischer C, Flechtenmacher C, Wirtenberger M, Weiss KH, Imparato S, Braun A, Hemminki K. A mutation in the canalicular phospholipid transporter gene, ABCB4, is associated with cholestasis, ductopenia, and cirrhosis in adults. Hepatology. 2008;48:1157-1166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 84] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 25. | Niessen KH, Theisen M. [Why do children rarely have gallstones? Examinations of the lithoindices and the bile acid pattern in infants and children in health and disease (author’s transl)]. Monatsschr Kinderheilkd. 1980;128:551-557. [PubMed] |
| 26. | Heubi JE, Soloway RD, Balistreri WF. Biliary lipid composition in healthy and diseased infants, children, and young adults. Gastroenterology. 1982;82:1295-1299. [PubMed] |
| 27. | Stieger B, Fattinger K, Madon J, Kullak-Ublick GA, Meier PJ. Drug- and estrogen-induced cholestasis through inhibition of the hepatocellular bile salt export pump (Bsep) of rat liver. Gastroenterology. 2000;118:422-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 435] [Cited by in RCA: 380] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 28. | Oude Elferink RP, Ottenhoff R, van Wijland M, Smit JJ, Schinkel AH, Groen AK. Regulation of biliary lipid secretion by mdr2 P-glycoprotein in the mouse. J Clin Invest. 1995;95:31-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 178] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
P- Reviewer: Han TQ S- Editor: Wen LL L- Editor: O’Neill M E- Editor: Liu XM