Published online Nov 28, 2013. doi: 10.3748/wjg.v19.i44.8065
Revised: August 21, 2013
Accepted: September 16, 2013
Published online: November 28, 2013
Processing time: 257 Days and 20.1 Hours
AIM: To evaluate the effects of disease severity and necrosis on organ dysfunctions in acute pancreatitis (AP).
METHODS: One hundred and nine patients treated as AP between March 2003 and September 2007 with at least 6 mo follow-up were included. Patients were classified according to severity of the disease, necrosis ratio and localization. Subjective clinical evaluation and fecal pancreatic elastase-I (FPE-I) were used for exocrine dysfunction evaluation, and oral glucose tolerance test was completed for endocrine dysfunction. The correlation of disease severity, necrosis ratio and localization with exocrine and endocrine dysfunction were investigated.
RESULTS: There were 58 male and 51 female patients, and mean age was 56.5 ± 15.7. Of the patients, 35.8% had severe AP (SAP) and 27.5% had pancreatic necrosis. Exocrine dysfunction was identified in 13.7% of the patients [17.9% were in SAP, 11.4% were in mild AP (MAP)] and 34.7% of all of the patients had endocrine dysfunction (56.4% in SAP and 23.2% in MAP). In patients with SAP and necrotizing AP (NAP), FPE-Ilevels were lower than the others (P < 0.05 and 0.001 respectively) and in patients having pancreatic head necrosis or near total necrosis, FPE-1 levels were lower than 200 μg/g stool. Forty percent of the patients who had undergone necrosectomy developed exocrine dysfunction. Endocrine dysfunction was more significant in patients with SAP and NAP (P < 0.001). All of the patients in the necrosectomy group had endocrine dysfunction.
CONCLUSION: Patients with SAP, NAP, pancreatic head necrosis and necrosectomy should be followed for pancreatic functions.
Core tip: The aim of this study was to evaluate the effects of disease severity and necrosis on organ dysfunctions in acute pancreatitis (AP). Exocrine and endocrne dysfunctions were investigated according to disease severity and necrosis ratio after acute pancreatitis. Exocrine dysfunction was identified in 13.7% of the patients [17.9% were in severe AP (SAP), 11.4% were in mild AP (MAP) and 34.7% of all of the patients had endocrine dysfunction (56.4% in SAP and 23.2% in MAP)]. Forty percent of the patients who had undergone necrosectomy developed exocrine dysfunction. Endocrine dysfunction was more significant in patients with SAP and NAP. All of the patients in the necrosectomy group had endocrine dysfunction. Patients with SAP, NAP, pancreatic head necrosis and necrosectomy should be followed for pancreatic functions.
- Citation: Garip G, Sarandöl E, Kaya E. Effects of disease severity and necrosis on pancreatic dysfunction after acute pancreatitis. World J Gastroenterol 2013; 19(44): 8065-8070
- URL: https://www.wjgnet.com/1007-9327/full/v19/i44/8065.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i44.8065
Eighty percent of the pancreatic mass is devoted to exocrine function, and the remaining part is responsible for endocrine function, which is crucial to the maintenance of homeostasis of the body[1]. Clinically, the severity of acute pancreatitis (AP) varies significantly. Some patients experience a mild form (mild AP, MAP) of the disease (80%-90% of all cases), which is a self-limiting condition with patients recovering within 3-4 d after onset of the disease. Serious insult occurs in 20%-30% of the cases in the first week after AP attack and mortality can be 30% in the severe form. Pancreatic necrosis develops in 20% of all cases[2,3]. Both the presence and extent of the necrosis affects the clinical course of the disease. Necrosis larger than 50% of the pancreatic mass significantly increases the local and systemic complication rates[4]. There are contradictory results from evaluations of exocrine and endocrine dysfunction in mild and severe cases[5,6]. In 1984 at the Marseille symposium, it was accepted that pancreatic injury is temporary and endocrine and exocrine functions recover during the following month[7]. But there are some contradictory reports claiming that pancreatic injury is persistent[8-10]. Currently, it is accepted that pancreatic function recovers in the absence of pancreatic necrosis and if necrosectomy is not performed[11].
Previous studies have demonstrated that severity of AP, extent of the pancreatic necrosis and the cause of pancreatitis are closely related to the magnitude of pancreatic dysfunction[12,13]. After severe acute pancreatitis (SAP), Bozkurt et al[14] and Boreham et al[15] reported 85% and 86% pancreatic exocrine dysfunction, respectively. Boreham also reported 13% pancreatic exocrine dysfunction after mild cases. The etiologic factor is also correlated with the level of pancreatic injury; acute pancreatitis due to alcohol consumption may cause pancreatic dysfunction[9]. While it is mostly β cell injury that induces endocrine dysfunction, insulin resistance may also contribute to glucose intolerance[16-18]. Endocrine dysfunction is reported in 15%-35% of the cases[12,14,18].
There is no consensus about the frequency and severity of endocrine and exocrine dysfunction due to acute pancreatitis. Also, experts do not agree on the necessity of enzyme supplementation following the discharge of these patients[15]. Our study aimed to clarify the relationship between pancreatic dysfunction, the severity of the disease and the extent of the pancreatic necrosis.
This study was undertaken in the Uludag University Department of Surgery-Bursa, Turkey. From March 2003 to October 2007, 216 consecutive patients with AP were evaluated in our center. This study was approved by the Institutional Review Board of Uludag University (September 11th 2007, No: 2007-14/64). Patients who died (n = 16) or with less than 6 mo of follow-up after onset of the disease (n = 11) were excluded. All of the patients were invited to the hospital to participate the study by phone or mail. Fifty-five patients could not be contacted due to change of address and 25 patients declined to participate. The remaining 109 patients were included the study, and written informed consent was obtained from each subject.
The data of the patients who were treated for AP were recorded prospectively in previously prepared forms. Diagnosis of AP and determination of its etiology were based on clinical evaluation, serum and urine amylase (higher than three times the upper level of normal was considered diagnostic), liver function tests, serum triglycerides, calcium, alkaline phosphatase and abdominal ultrasound (US) at the admission. Within 72 h following admission, contrast enhanced abdominal computed tomography (CECT) was performed. Patients with gallstones on US were assumed to be cases of biliary pancreatitis; patients consuming large amounts of alcohol (but not having chronic pancreatitis) were considered as having alcoholic pancreatitis. In patients with high levels of serum fat (triglyceride level more than 1000 mg/dL), hyperlipidemia was accepted as the etiological factor. Patients with undetermined etiology were considered to be idiopathic cases. For prognostic evaluation and classification of the severity of the disease, the Acute and Physiology and Chronic Health Evaluation II scoring system (APACHE II) was used. Patients with APACHE II ≥ 8 were accepted as severe AP (SAP). While patients who had < 8 score were accepted as mild AP (MAP) and treated conservatively (fluid resuscitation only), patients who had SAP were treated with aggressive fluid resuscitation, nutritional support (enteral or parenteral) and antibiotic prophylaxis. If the patient’s clinical status deteriorated and CECT findings revealed infected necrosis or if fine needle aspiration cytology demonstrated infection, they were treated surgically. The Beger procedure (open necrosectomy and closed continuous lavage) plus feeding jejunostomy was our treatment of choice. In some of the cases, more conservative procedures (i.e., percutaneous drainage) had to be undertaken due to the patient’s condition.
Following designation of the participating patients and receiving informed consent, specific questions were asked of the patients to evaluate the clinical findings of the pancreatic exocrine insufficiency. The findings were recorded on the Subjective Clinical Evaluation (SCE) form (Table 1). If a patient answered “yes” to even only one of the questions on the SCE form, the test was accepted as positive. Exocrine pancreatic function was also evaluated using the fecal pancreatic elastaz-1 (FPE-1) test in a random stool sample. Patients who were having pancreatic enzyme supplements were instructed to taper their enzyme supplementation 1 mo before the FPE-1 test. Stool samples were collected from the patients and stored at -20 °C. FPE-1 level was measured by a commercially available Enzyme-Linked Immunosorbent Assay kit (Bioserv Diagnostics, BS-86-01, Rostock, Germany 2007) according to manufacturer’s instructions. Stool elastase 1 concentration higher than 200 μg/g stool indicated normal pancreatic function, whereas concentration of 100 to 200 μg/g stool indicated mild to moderate pancreatic insufficiency, and concentrations below 100 μg/g stool were qualified as pointing to severe pancreatic insufficiency[12].
| Age/sex: |
| - Did DM develop after AP? |
| (a) yes (b) no |
| - Any abdominal pain, discomfort, steatorrhea, weakness, weight loss, lack of appetite after AP? |
| (a) yes (b) no |
| - Did you use pancreatic enzyme supplementation? If so, how long time did you use it? |
| (a) yes (b) no |
In all of the patients without diagnosed insulin-dependent diabetes mellitus (DM), endocrine pancreatic function was assessed by oral glucose tolerance test (OGTT). Measurement of glucose concentration was made on patients who had fasted overnight and discontinued drugs and food that could have affected the results. After taking blood samples for fasting blood glucose measurement during the OGTT, the patients drank 75 g glucose dissolved in 300 mL, and their blood glucose concentration was measured at 30, 60, 90 and 120 min. Basal blood glucose levels < 120 mg/dL and between 126-200 mg/dL at 120 min were accepted as impaired glucose tolerance. Basal glucose levels > 126 mg/dL and > 200 mg/dL at 120 min were accepted as DM.
The association between pancreatic dysfunction (either SCE or FPE-1 level) and disease severity and necrosis was investigated.
Statistical analysis was performed using SPSS 13.0 for Windows (Chicago, IL). Non-parametric tests were used to analyze the data. When comparing more than 3 groups, the Kruskal-Wallis test was used. Comparison between 2 groups was made with Mann-Whitney U test. The χ2 test was used to compare categorical variables. A P value of < 0.05 was considered significant.
A total of 109 patients with a mean follow-up of 32 mo (range: 6-48 mo) was included in this study. Fifty-eight of the subjects were male (53.2%), and 51 were female (46.8%). The mean age of the patients was 56.5 ± 15.7 (range: 19-89) years. The etiologies were biliary (66%), idiopathic (15.5%), alcohol (8.2%), hyperlipidemia (4.6%), endoscopic retrograde cholangio-pancreatography related (2.7%) and drug-related (2.7%). According to the APACHE II scoring system, 35.8% of the patients were SAP, and the remaining were MAP. Necrosis was found in 27.5% of the patients, and there was only pancreatic edema in 49.6% of the patients on CECT examination. Almost 23% of the CECT findings were noted to be normal. While necrosis was found in 59% of the SAP cases, it was found in 10% of MAP. On the other hand, of the patients who had necrosis according to CECT findings, 76.6% had SAP, and the remaining had MAP according to APACHE II criteria. Twenty-six percent of the patients who had pancreatic edema on CECT scan had SAP.
Five patients were operated on due to infected pancreatic necrosis, and a Beger procedure plus feeding jejunostomy was performed. Cystoenterostomy was performed in 17 cases due to a pancreatic pseudocyst. Percutaneous drainage was performed in 7 cases for pancreatic and peripancreatic abscesses. The remaining patients were treated medically. Before starting the study, 50.4% of the patients were taken pancreatic enzyme supplementation.
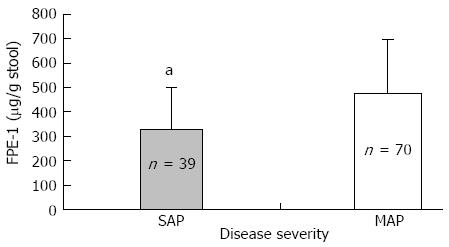
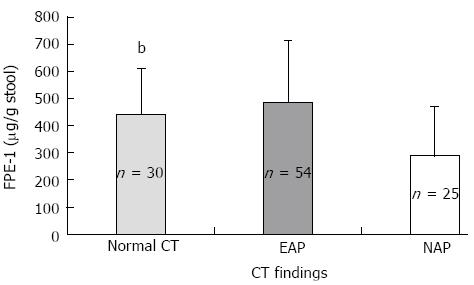
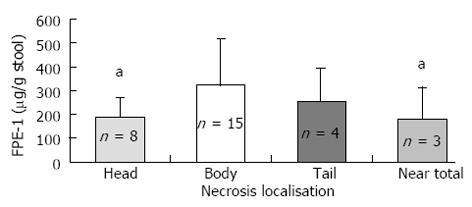
Exocrine dysfunction was detected in 13.7% of the patients according to both subjective clinical evaluation and the FPE-1 test. It was found in 17.9% with SAP and in 11.4% with MAP. Four patients in the SAP group had severe exocrine dysfunction according to FPE-1 measurement, and 11 patients had moderate exocrine dysfunction (7 of them in the MAP and 4 in the SAP group). Disease severity and necrosis were not associated with subjective clinical evaluation (Table 2). FPE-1 was lower in the SAP than MAP group and was lower in patients with necrotizing AP (NAP) than those without necrosis (Figures 1 and 2). FPE-1 was lower in cases with pancreatic head or near total necrosis than patients with necrosis at other localizations (Figure 3). This was under the critical level of FPE-1 (200 mg/g stool). There was no significant correlation between FPE-I level and subjective clinical evaluation.
| Disease severity and CT findings | Exocrine dysfunction | Pvalue |
| SAP | 7/39 (17.9) | NS |
| MAP | 8/70 (11.4) | NS |
| NAP | 8/30 (26.6) | |
| EAP | 5/54 (9.2) | NS |
| Normal CT | 2/25 (8.0) |
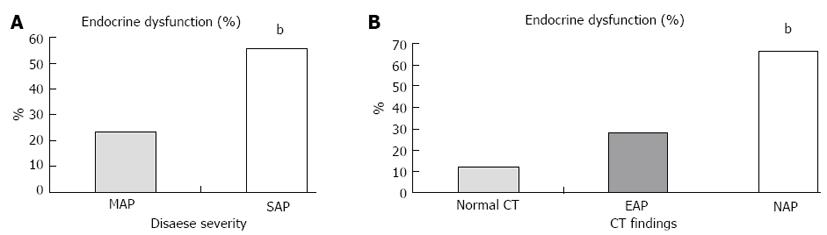
DM was detected in 11.9% of the cases before the AP attack. DM and impaired glucose tolerance were detected in 30.2% and 4.5% of the remaining cases, respectively, according to the OGTT test. Therefore, endocrine dysfunction was noted to be present in 34.7% of the cases (56.4% with SAP and 23.2% with MAP). According to CECT findings, patients with necrosis had more severe endocrine dysfunction than patients without necrosis (endocrine dysfunction rate was 66.6% in NAP, 27.8% in Edematous AP (EAP) and 12% in normal CECT) (Figure 4).
AP is a mediator disease caused by proinflammatory cytokine release and has a clinical picture ranging from mild disease to multiorgan failure and sepsis. The clinical picture is serious in 15%-20% of patients; complications can develop and mortality can be seen in these patients. Pancreatic necrosis develops in 20%-30% of the patients[3]. In this series, SAP was diagnosed in 35.8% and NAP was determined in 27.5% of the AP cases. The fact that SAP and NAP rates in our series are higher than those in the literature might be due to our hospital being the tertiary care referral center.
Currently, there is no consensus on whether or not pancreatic functions recover and to what extent after an AP attack. Full pancreatic functional recovery has been reported in some studies[6,19,20], whereas others have reported that both endocrine and exocrine insufficiency might develop[8-10]. The research is not conclusive or sufficient to answer the question. The results of previous studies remain conflicting because of very small patient numbers and non-homogenous etiologies that affect pancreatic functions. Using ineffective and non-standardized tests can also lead to some mistakes and controversies[11]. In the current study, we used subjective clinical evaluation and the FPE-1 test, which has a relatively high sensitivity for exocrine function. Pancreatic elastase is a specific protease of humans and it undergoes minimal breakdown during intestinal transit. Strong parallelism between stool FPE-1 and amylase, lipase and trypsin in pancreatic juice has been reported[21,22]. The sensitivity of the FPE-I test is 90% in SAP and 60%-70% in mild disease[23]. Although the test is easy and used widely in many reference laboratories, it is not an ideal test. But, FPE-1 test is more reliable than the direct tests which are more expensive, invasive and time-consuming.
Long term results after AP, in terms of pancreatic functions are heterogeneous. The pancreatic dysfunction rate after AP ranges between 11%-85% in SAP and 13%-55% in MAP[12,15,16,24]. Bozkurt et al[14] reported the results of their relatively small series. They observed mild-moderate exocrine dysfunction rates of 74% and 81% at 1 and 18 mo follow-up, respectively. Severe exocrine dysfunction following AP was noted in 26% at 1 mo and 6% at 18 mo. On the other hand, Ibars et al[11] observed normal pancreatic exocrine function after AP in their study of the same size. In our study, the exocrine dysfunction rate was found to be 13.7% among the patients. This rate was higher in the NAP group and lower in edematous cases and among patients with normal CECT findings. The duration between the AP attack and FPE-1 test of patients having exocrine dysfunction was relatively long (most of them longer than 24 mo). The relation between pancreatic exocrine dysfunction and pancreatic necrosis and its localization is not clear. Although no relation was found between necrosis and subjective clinical evaluation, the FPE-1 level was low in SAP, NAP and patients with pancreatic head necrosis. FPE-1 was noted to be under the critical threshold (< 200 μg/g stool) in patients having head necrosis. Kemppainen et al[25] reported that in patients with pancreatic head and body necrosis, the complication rate was much higher than the others and they attributed this to proximal obstruction.
As seen in some of our cases, the occurrence of exocrine dysfunction or enzyme insufficiency after AP may be independent of organ necrosis and severity of disease. We can only speculate about the cause of this finding. It is not ethical to biopsy the pancreas after AP. On the other hand, measuring tissue microcirculation and organ perfusion is not practical. We can speculate that fibrosis and the loss of functional units might occur during the healing period under cytokine cascade after AP attack. It has been reported that pancreatic exocrine dysfunction is relatively more common in the long term, especially after NAP, and in patients with necrosectomy[15,26]. Exocrine dysfunction was observed in 26.6% of NAP cases and 40% of patients who had necrosectomy in our study. We did not analyze the FPE-1 level in different time periods. Therefore, from our data, we cannot draw conclusions about the course of enzyme insufficiency over the long term.
We have very limited data about pancreatic enzyme supplementation and its dosage and duration after AP. Approximately 50% of our cases were on enzyme supplementation before starting the study. Interestingly, only 12.8% of these patients had exocrine dysfunction after one month tapering of enzyme supplementation. On the other hand, only 3 of 15 patients with exocrine dysfunction had been taking enzyme supplementation. Therefore these results showed that enzyme supplementation regimes should be questioned.
The reported rate of endocrine dysfunction after AP is 15%-35%[18,27]. The rate in the present study was relatively high (34.7%). In previous studies, endocrine dysfunction has been reported to occur in up to 50%-70% of cases of necrosis or necrosectomy[5,17,28]. In our study, endocrine dysfunction was related to disease severity and presence of necrosis but was not related to the necrosis ratio and localization. This finding can be also explained with pancreatic fibrosis occurring as a result of inflammation, leading to exocrine dysfunction. In addition to beta cell loss, increased insulin resistance is thought to be another causal factor for endocrine dysfunction after AP[17]. This factor was demonstrated in an experimental study[29]. In contrast to exocrine dysfunction, endocrine dysfunction was not related to pancreatic head necrosis. This also can be explained by the fact that islet cells are disseminated homogenously through the organ. Therefore islet cells localized in uninjured regions of the organ can compensate. Endocrine dysfunction can occur without necrosis if the whole pancreas is affected by fibrosis.
Necrosis is a risk factor for pancreatic dysfunction; however, pancreatic dysfunction can occur without necrosis over the long term. It is not easy to detect pancreatic dysfunction, especially exocrine dysfunction. Reasons for this include: (1) There is no ideal test to detect exocrine dysfunction; (2) Pancreatic dysfunction can be explained by apoptosis in patients who did not have necrosis; (3) Ultrastructural changes and fibrosis due to the healing process in the ductal system can cause dysfunction; and (4) Recurrent attacks might be a source of morphologic changes and dysfunction.
In conclusion, long-term quality of life after AP should be evaluated in SAP, NAP, patients having necrosectomy and patients with pancreatic head necrosis.
Eighty percent of the pancreatic mass is devoted to exocrine function, and the remaining part is responsible for endocrine function, which is crucial to the maintenance of homeostasis of the body. This study aimed to clarify the relationship between pancreatic dysfunction, the severity of the disease and the extent of the pancreatic necrosis.
To evaluate the effects of disease severity and necrosis on organ dysfunctions in acute pancreatitis.
Necrosis is a risk factor for pancreatic dysfunction; however, pancreatic dysfunction can occur without necrosis over the long term. It is not easy to detect pancreatic dysfunction, especially exocrine dysfunction.
In this study the authors evaluate the effects of disease severity and necrosis on organ dysfunction in acute pancreatitis. Fecal pancreatic Elastaz-1 and oral glucose tolerance test are used to measure exocrine and endocrine insufficiency. This is an interesting manuscript dealing with an area of investigation poorly explored in the past.
| 1. | Gardner TB. Acute Pancreatitis. Available from: http://emedicine.medscape.com/article/181364-overview. |
| 2. | Beger HG, Rau B, Mayer J, Pralle U. Natural course of acute pancreatitis. World J Surg. 1997;21:130-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 325] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 3. | Heinrich S, Schäfer M, Rousson V, Clavien PA. Evidence-based treatment of acute pancreatitis: a look at established paradigms. Ann Surg. 2006;243:154-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 208] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 4. | Rau B, Pralle U, Uhl W, Schoenberg MH, Beger HG. Management of sterile necrosis in instances of severe acute pancreatitis. J Am Coll Surg. 1995;181:279-288. [PubMed] |
| 5. | Bavare C, Prabhu R, Supe A. Early morphological and functional changes in pancreas following necrosectomy for acute severe necrotizing pancreatitis. Indian J Gastroenterol. 2004;23:203-205. [PubMed] |
| 6. | Angelini G, Cavallini G, Pederzoli P, Bovo P, Bassi C, Di Francesco V, Frulloni L, Sgarbi D, Talamini G, Castagnini A. Long-term outcome of acute pancreatitis: a prospective study with 118 patients. Digestion. 1993;54:143-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 47] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Singer MV, Gyr K, Sarles H. Revised classification of pancreatitis. Report of the Second International Symposium on the Classification of Pancreatitis in Marseille, France, March 28-30, 1984. Gastroenterology. 1985;89:683-685. [PubMed] |
| 8. | Büchler M, Malfertheiner P, Block S, Maier W, Beger HG. [Morphologic and functional changes in the pancreas following acute necrotizing pancreatitis]. Z Gastroenterol. 1985;23:79-83. [PubMed] |
| 9. | Büchler M, Hauke A, Malfertheiner P. Follow up after acute pancreatitis: morphology and function. Acute pancreatitis. Berlin/Heidelberg: Springer-Verlag 1987; 367-374. |
| 10. | Seidensticker F, Otto J, Lankisch PG. Recovery of the pancreas after acute pancreatitis is not necessarily complete. Int J Pancreatol. 1995;17:225-229. [PubMed] |
| 11. | Ibars EP, Sánchez de Rojas EA, Quereda LA, Ramis RF, Sanjuan VM, Peris RT. Pancreatic function after acute biliary pancreatitis: does it change? World J Surg. 2002;26:479-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Symersky T, van Hoorn B, Masclee AA. The outcome of a long-term follow-up of pancreatic function after recovery from acute pancreatitis. JOP. 2006;7:447-453. [PubMed] |
| 13. | Endlicher E, Völk M, Feuerbach S, Schölmerich J, Schäffler A, Messmann H. Long-term follow-up of patients with necrotizing pancreatitis treated by percutaneous necrosectomy. Hepatogastroenterology. 2003;50:2225-2228. [PubMed] |
| 14. | Bozkurt T, Maroske D, Adler G. Exocrine pancreatic function after recovery from necrotizing pancreatitis. Hepatogastroenterology. 1995;42:55-58. [PubMed] |
| 15. | Boreham B, Ammori BJ. A prospective evaluation of pancreatic exocrine function in patients with acute pancreatitis: correlation with extent of necrosis and pancreatic endocrine insufficiency. Pancreatology. 2003;3:303-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 77] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 16. | Appelros S, Lindgren S, Borgström A. Short and long term outcome of severe acute pancreatitis. Eur J Surg. 2001;167:281-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 75] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 17. | Buscher HC, Jacobs ML, Ong GL, van Goor H, Weber RF, Bruining HA. Beta-cell function of the pancreas after necrotizing pancreatitis. Dig Surg. 1999;16:496-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Malecka-Panas E, Gasiorowska A, Kropiwnicka A, Zlobinska A, Drzewoski J. Endocrine pancreatic function in patients after acute pancreatitis. Hepatogastroenterology. 2002;49:1707-1712. [PubMed] |
| 19. | Mitchell CJ, Playforth MJ, Kelleher J, McMahon MJ. Functional recovery of the exocrine pancreas after acute pancreatitis. Scand J Gastroenterol. 1983;18:5-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 43] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Glasbrenner B, Büchler M, Uhl W, Malfertheiner P. Exocrine pancreatic function in the early recover phase of acute edematous pancreatitis. Eur J Gastroenterol Hepatol. 1992;4:563. |
| 21. | Stein J, Jung M, Sziegoleit A, Zeuzem S, Caspary WF, Lembcke B. Immunoreactive elastase I: clinical evaluation of a new noninvasive test of pancreatic function. Clin Chem. 1996;42:222-226. [PubMed] |
| 22. | Lüth S, Teyssen S, Forssmann K, Kölbel C, Krummenauer F, Singer MV. Fecal elastase-1 determination: ‘gold standard’ of indirect pancreatic function tests? Scand J Gastroenterol. 2001;36:1092-1099. [PubMed] |
| 23. | Chowdhury RS, Forsmark CE. Review article: Pancreatic function testing. Aliment Pharmacol Ther. 2003;17:733-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 118] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 24. | Tsiotos GG, Luque-de León E, Sarr MG. Long-term outcome of necrotizing pancreatitis treated by necrosectomy. Br J Surg. 1998;85:1650-1653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 104] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 25. | Kemppainen E, Sainio V, Haapiainen R, Kivisaari L, Kivilaakso E, Puolakkainen P. Early localization of necrosis by contrast-enhanced computed tomography can predict outcome in severe acute pancreatitis. Br J Surg. 1996;83:924-929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 68] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Reddy MS, Singh S, Singh R, Singh K, Singh G. Morphological and functional outcome after pancreatic necrosectomy and lesser sac lavage for necrotizing pancreatitis. Indian J Gastroenterol. 2007;26:217-220. [PubMed] |
| 27. | Kaya E, Dervisoglu A, Polat C. Evaluation of diagnostic findings and scoring systems in outcome prediction in acute pancreatitis. World J Gastroenterol. 2007;13:3090-3094. [PubMed] |
| 28. | Sabater L, Pareja E, Aparisi L, Calvete J, Camps B, Sastre J, Artigues E, Oviedo M, Trullenque R, Lledó S. Pancreatic function after severe acute biliary pancreatitis: the role of necrosectomy. Pancreas. 2004;28:65-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | Yeo CJ, Bastidas JA, Schmieg RE, Walfisch S, Couse NF, Olson JL, Andersen DK, Zinner MJ. Pancreatic structure and glucose tolerance in a longitudinal study of experimental pancreatitis-induced diabetes. Ann Surg. 1989;210:150-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
P- Reviewer: Crippa S S- Editor: Wen LL L- Editor: A E- Editor: Wu HL