Published online Nov 28, 2013. doi: 10.3748/wjg.v19.i44.8071
Revised: September 28, 2013
Accepted: October 19, 2013
Published online: November 28, 2013
Processing time: 159 Days and 5.1 Hours
AIM: To evaluate the therapeutic effect of Shugan-decoction (SGD) on visceral hyperalgesia and colon gene expressions using a rat model.
METHODS: Ninety-six adult male Wistar rats were randomized into six equal groups for assessment of SGD effects on psychological stress-induced changes using the classic water avoidance stress (WAS) test. Untreated model rats were exposed to chronic (1 h/d for 10 d consecutive) WAS conditions; experimental treatment model rats were administered with intragastric SGD at 1 h before WAS on consecutive days 4-10 (low-dose: 0.1 g/mL; mid-dose: 0.2 g/mL; high-dose: 0.4 g/mL); control treatment model rats were similarly administered with the irritable bowel syndrome drug, dicetel (0.0042 g/mL); untreated normal control rats received no drug and were not subjected to the WAS test. At the end of the 10-d WAS testing period, a semi-quantitative measurement of visceral sensitivity was made by assessing the abdominal withdrawal reflex (AWR) to colorectal balloon-induced distension (at 5 mmHg increments) to determine the pain pressure threshold (PPT, evidenced by pain behavior). Subsequently, the animals were sacrificed and colonic tissues collected for assessment of changes in expressions of proteins related to visceral hypersensitivity (transient receptor potential vanilloid 1, TRPV1) and sustained visceral hyperalgesia (substance P, SP) by immunohistochemistry and real-time polymerase chain reaction. Inter-group differences were assessed by paired t test or repeated measures analysis of variance.
RESULTS: The WAS test successfully induced visceral hypersensitivity, as evidenced by a significantly reduced AWR pressure in the untreated model group as compared to the untreated normal control group (190.4 ± 3.48 mmHg vs 224.0 ± 4.99 mmHg, P < 0.001). SGD treatments at mid-dose and high-dose and the dicetel treatment significantly increased the WAS-reduced PPT (212.5 ± 2.54, 216.5 ± 3.50 and 217.7 ± 2.83 mmHg respectively, all P < 0.001); however, the low-dose SGD treatment produced no significant effect on the WAS-reduced PPT (198.3 ± 1.78 mmHg, P > 0.05). These trends corresponded to the differential expressions observed for both TRPV1 protein (mid-dose: 1.64 ± 0.08 and high-dose: 1.69 ± 0.12 vs untreated model: 3.65 ± 0.32, P < 0.001) and mRNA (0.44 ± 0.16 and 0.15 ± 0.03 vs 1.39 ± 0.15, P < 0.001) and SP protein (0.99 ± 0.20 and 1.03 ± 0.23 vs 2.03 ± 0.12, P < 0.01) and mRNA (1.64 ± 0.19 and 1.32 ± 0.14 vs 2.60 ± 0.33, P < 0.05). These differential expressions of TRPV1 and SP related to mid- and high-dose SGD treatments were statistically similar to the changes induced by dicetel treatment. No signs of overt damage to the rat system were observed for any of the SGD dosages.
CONCLUSION: Shugan-decoction can reduce chronic stress-induced visceral hypersensitivity in rats, and the regulatory mechanism may involve mediating the expressions of TRPV1 and SP in colon tissues.
Core tip: The classical rat model of chronic stress induction via water avoidance stress (WAS) test was used to investigate the therapeutic effect of the Shugan-decoction (SGD) on visceral hypersensitivity of the gastrointestinal tract and its underlying molecular mechanisms. The study design reflected the therapeutic potential of SGD for treating the stress-related gut aspects of irritable bowel syndrome (IBS) in humans. Mid- and high-dose SGD treatments significantly increased the WAS-reduced pressure thresholds, similarly to those induced by the IBS drug dicetel. The SGD treatments also restored WAS-related changes in transient receptor potential vanilloid 1 and substance P expression in the colon.
- Citation: Shang JJ, Yuan JY, Xu H, Tang RZ, Dong YB, Xie JQ. Shugan-decoction relieves visceral hyperalgesia and reduces TRPV1 and SP colon expression. World J Gastroenterol 2013; 19(44): 8071-8077
- URL: https://www.wjgnet.com/1007-9327/full/v19/i44/8071.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i44.8071
In recent decades, irritable bowel syndrome (IBS) has emerged as a highly prevalent functional gastrointestinal disorder that is strongly associated with high levels of stress in daily life. The spectrum of IBS symptoms, ranging from discomfort associated with altered bowel habits to recurrent abdominal pain, is non-life threatening, but can severely impact an individual’s general wellbeing and severely disrupt daily life. Despite the extensive laboratory- and clinical-based investigations that have been carried out to determine the underlying etiology and pathogenesis of IBS, no precise causative factors have been identified for the onset and progression of this disease. Patients present with an absence of IBS-specific structural and biochemical abnormalities[1,2], but have higher incidences of psychological stress (both acute and chronic), visceral sensory abnormalities, gastrointestinal motility disorders, and gastrointestinal infections.
The theory of increased visceral sensitivity as a feature of IBS has been addressed by numerous studies. Indeed, IBS patients have been reported to show an enhanced sensitivity to colon and rectal balloon dilatation[3]. The mechanisms underlying such visceral hypersensitivity remain unknown, but are likely multifactorial and complex[4,5]. Under normal physiological conditions, visceral sensitivity is mediated by a variety of neuron-localized ion channels, such as the transient receptor potential (TRP) non-selective cation channels, that also function in the formation and regulation of hyperalgesia.
The transient receptor potential vanilloid 1 (TRPV1) TRP family member plays a key role in modulation of the sensation of pain and thermal hyperalgesia[6] and is widely expressed throughout the gastrointestinal tract[7]. In the colon, substance P (SP)-mediated phosphorylation activates TRPV1, thereby enhancing the probability of channel gating promoting development of visceral hypersensitivity[8]. In this manner, SP itself acts as an important regulator of sustained visceral hyperalgesia, and has been characterized as an etiological factor of the repeated stress rat model system[9].
Clinical observations of IBS patients have indicated that remarkably aggravated disease symptoms occur during times of increased emotional and mental stress[10]. In traditional Chinese medicine (TCM), these disrupted states correspond to liver-depression and spleen-deficiency. Thus, therapies that soothe the liver and strengthen the spleen are applied to IBS patients. One such therapy is the TCM compound Shugan-decoction (SGD), which, when administered orally, has been shown to significantly improve the clinical symptoms of IBS[11]. In this study, a rat model of stress-induced visceral hypersensitivity was employed to investigate the efficacy profile and therapeutic mechanism of SGD in IBS-like conditions.
Ninety-six Wistar rats (150 ± 20 g adult males) were obtained from the Experimental Animal Center of Shanghai University of TCM (China) for analysis. The animals were housed under a 12/12 light cycle, with standard temperature (21-23 °C) and humidity (50% ± 5%) and ad libitum access to standard rat chow and tap water. All consecutive daily experimental procedures were conducted between 8:00-11:00 AM to minimize confounding due to diurnal variations.
The study was designed according to the guidelines of ethical treatment in research published by the Committee of International Association for the Study of Pain and approved by the Committee on the Use of Human and Animal Subjects in Teaching and Research at the Shanghai University of TCM. All protocols were carried out with the aim of minimizing or eliminating discomfort to the animals.
The constituent ingredients of SGD (white atractylodes rhizome, white peony root, dried old orange peel, Ledebouriella root and Radix bupleuri) were purchased as crude herbs from the Yanghetang Pharmacy (Shanghai, China). The aqueous extract of SGD was made by the Herbal Chemistry Lab at the Shanghai University of TCM, using the following steps: decoction of the crude herbs twice, combination of the two filtrate samples, decompression recovery to obtain the final aqueous extract product. The standard IBS pharmaceutical drug dicetel (pinaverium bromide; 50 mg tablets) was obtained from Solvay Pharma (Suresnes, France).
Repeated water avoidance stress (WAS) was conducted as previously described to induce chronic psychological stress with gastric disruption[12]. Briefly, rats were placed on a clear glass platform (10 × 8 × 8 cm) in the middle of a plexiglass tank (45 × 25 × 25 cm) filled with water at 25 °C (to fill the tank up to 1 cm below the top of the platform), and remained on the platform for 1 h. The WAS procedure was repeated once daily for 10 consecutive days.
Untreated model rats (n = 16) were exposed to chronic (1 h/d for 10 d consecutive) WAS conditions. Experimental treatment model rats (n = 16 each dosage group) were administered with intragastric SGD at 1 h before WAS on consecutive days 4-10 (low-dose: 0.1 g/mL; mid-dose: 0.2 g/mL; high-dose: 0.4 g/mL). Control treatment model rats (n = 16) were similarly administered the IBS drug, dicetel (0.0042 g/mL). Untreated normal control rats (n = 16) received no drug and were not subjected to the WAS test.
To estimate distal colonic motility, fecal pellet output was measured as previously described[9]. Briefly, fecal pellets found in the WAS tank were counted at the end of each 1 h WAS test. For the untreated normal control rats, the amount of fecal pellets left in the home cage were counted over a 60 min period of time. Data are presented as mean ± SE (n = 16).
At the end of the 10-d WAS testing period, a semi-quantitative measurement of visceral sensitivity was made in each group (n = 8 each group) by assessing the abdominal withdrawal reflex to colorectal balloon-induced distension to determine the pressure threshold (evidenced by pain behavior)[10]. Briefly, rats were lightly sedated with halothane and a deflated latex balloon (4-5 cm diameter at full inflation) was inserted intra-anally with its end 1 cm proximal to the anus into the descending colon and rectum. Animals were then placed into a small lucite cubicle (20 × 8 × 8 cm) and allowed to wake up and adapt for 30 min prior to initiation of colorectal distension (CRD). The CRD was performed by progressive inflation of the colorectally-inserted balloon at 5 mmHg increments, and stopped when the animal exhibited pain behavior. The pressure pain threshold (PPT) value was recorded as the mmHg pressure that evoked contraction of the animal’s abdominal muscles following balloon-mediated CRD delivered for 30 s duration at 4 min intervals. All the measurements were observed by two investigators (Shi HL and Qian W) working independently and blinded to the animals’ grouping.
All rats were sacrificed by cervical dislocation immediately after visceral sensitivity measurements were completed so that the descending colon (2 cm above the anus, which had not undergone CRD) could be removed by dissection. The tissue sample was then divided into two parts: one was fixed with 10% formalin [for subsequent immunohistochemical (IHC) analysis] and the other was snap-frozen and stored at -80 °C [for subsequent real-time polymerase chain reaction (PCR) analysis].
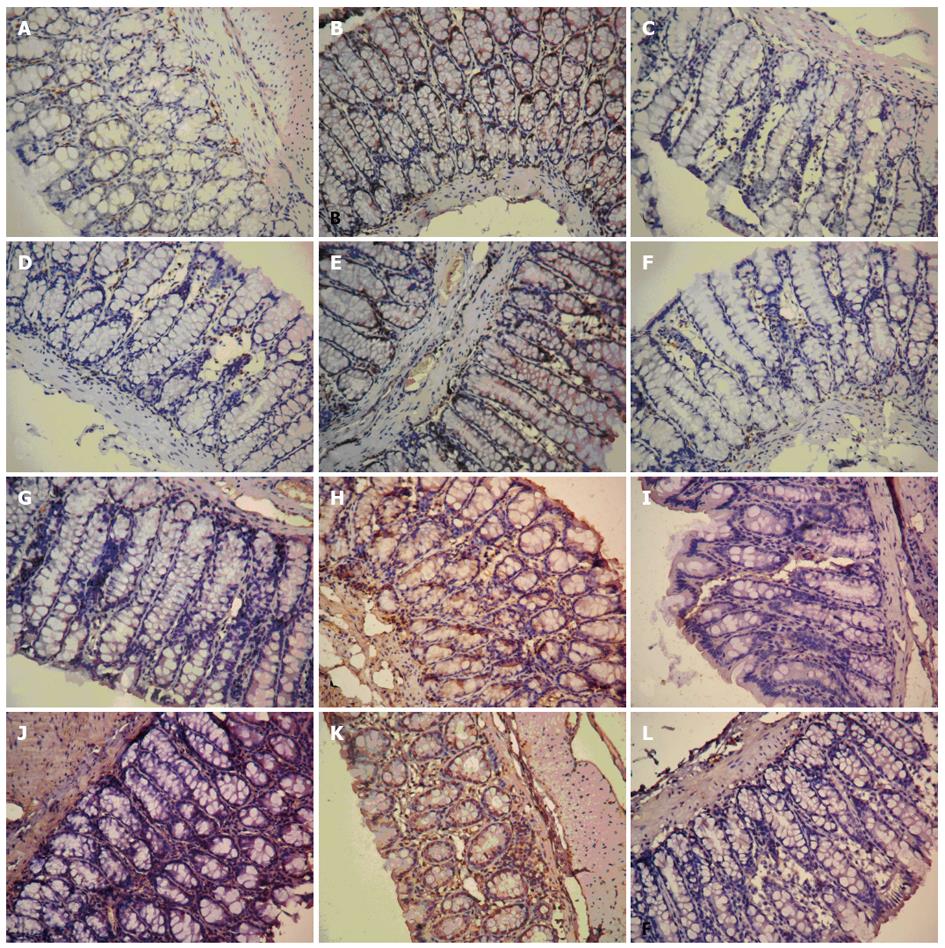
The IHC analysis of TRPV1 and SP protein expression in colon tissues was performed using the EnVision + System two-step horseradish peroxidase staining technique (DakoCytomation, Glostrup, Denmark) with targeted polyclonal rabbit anti-human primary antibodies (1:100 dilutions; Santa Cruz Biotechnology, Inc., Santa Cruz, CA,United States). Negative controls were run with the primary antibodies omitted from the procedure. Positive detection was indicated by visualization of a brown stain in the cytoplasm. Three randomly selected × 200 magnification fields were evaluated using a BH2 microscope (Olympus, Tokyo, Japan) equipped with a Nikon 4500 digital camera (Tokyo, Japan). The computer-aided image analysis system by Qiu Wei Inc. (Shanghai, China) assessed the area and optical density (OD) of TRPV1 and SP-positive cells in each field. The IHC index was calculated as the average integral optical density: [(positive area × OD)/total area]. Data are presented as mean ± SE (n = 6).
Total RNA was extracted from the thawed colon tissue samples using the TRIzol Reagent (Invitrogen Life Technologies, Carlsbad, CA, United States) and reverse transcribed to cDNA by using the Prime-Script™Reagent Kit (Takara, Tokyo, Japan), according to the manufacturers’ instructions. The following primer sets (forward and reverse, respectively) were used for gene-specific amplifications: TRPV1 (GenBank accession No. NM_031982): 5’-CCACACAAGTGCCGGGGGTC-3’ and 5’-CCAGGTCGCCCATGCCGATG-3’; SP (GenBank accession No. NM_053844): 5’-CTTCCTGGACGCGATGGGCTG-3’ and 5’-TGGAAATCCTGGCAGGCCCCTT-3’; GAPDH (normalizing control; primers were synthesized by Dawei Biotechnology Co., Shijiazhuang, China): 5’-GCCACAGCACTCCATCGAC-3’ and 5’-GTCTCCGATCTGGAAAACGC-3’. The real-time PCR was carried out with Synergy Brands Green I dye (Qiagen GmbH, Hilden, Germany) using a Prism 7500 System (Applied Biosystems Inc., Foster City, CA, United States) under the following conditions: 40 cycles of 94 °C for 30 s, 57 °C for 30 s and 72 °C for 30 s, followed by a single final extension cycle of 72 °C for 7 min. Samples were run in triplicate and the normalized values were averaged. Data are presented as mean ± SE (n = 6).
All statistical analyses were carried out with the GraphPad Prism v5.0 software (GraphPad Software Inc, La Jolla, CA, United States). Inter-group differences were assessed by a paired t test or repeated measures analysis of variance. A P value of < 0.05 was set as the threshold for statistical significance.
The WAS test successfully induced visceral hypersensitivity, as evidenced by a sustained significant increase in fecal pellet output (distal colonic motility) from the untreated model group as compared with the untreated normal control group not subject to the WAS test (day 3: 8.69 ± 0.60 vs 2.31 ± 0.66 and day 10: 8.56 ± 0.63 vs 0.56 ± 0.29, both P < 0.001). After 7 d of SGD treatment, significant relief of the WAS-stimulated increase in fecal output was achieved by the mid-dose (day 3: 8.38 ± 0.77 vs day 10: 4.31 ± 0.42, P < 0.001) and high-dose (day 3: 8.19 ± 0.62 vs day 10: 3.63 ± 0.39, P < 0.001). Although the extent of relief in these groups was similar to that achieved with the dicetel control treatment (day 3: 8.75 ± 0.53 vs day 10: 4.00 ± 0.35, P < 0.001 for all vs corresponding mid- and high-dose SGD values), none of the treatments reduced fecal output to untreated normal control group levels by day 10. The low-dose SGD treatment produced no significant effect on WAS-stimulated fecal output increase (day 3: 8.94 ± 0.84 and day 10: 6.88 ± 0.51; both P < 0.001 vs untreated normal control group; P > 0.05 for day 3 vs day 10).
The same WAS-induced and SGD-relieved trends were seen for visceral hyperalgesia. The untreated model group showed significantly lower PPT than the untreated normal control group (190.40 ± 3.48 mmHg vs 224.00 ± 4.99 mmHg, P < 0.001), which was relieved by the mid- and high-dose SGD treatments (212.50 ± 2.54 mmHg and 216.50 ± 3.50 mmHg) to a similar extent achieved with dicetel control treatment (217.70 ± 2.83 mmHg) (all P < 0.001 vs untreated model group). Again, the low-dose SGD treatment produced no significant effect on the WAS-reduced pressure threshold (198.30 ± 1.78 mmHg, P > 0.05 vs untreated normal control group and P < 0.001 vs untreated model group).
IHC detection of TRPV1 and SP in colon tissues of untreated normal control rats showed that their expressions were mainly localized to the mucosa and submucosa (Figure 1). The untreated model group showed significantly higher AOID levels than the untreated normal controls for both TRPV1 (3.65 ± 0.32 vs 0.86 ± 0.11, P < 0.001) and SP (2.03 ± 0.12 vs 0.64 ± 0.11, P < 0.001). These WAS-stimulated increases in protein levels were significantly reduced by the SGD treatments at mid-dose (TRPV1: 1.64 ± 0.08 and SP: 0.99 ± 0.20) and high-dose (TRPV1: 1.69 ± 0.12 and SP: 1.03 ± 0.23) compared with the untreated model group (TRPV1: P < 0.001 and SP: P < 0.01). Furthermore, the extent of reduction was similar to that achieved with dicetel control treatment (TRPV1: 1.46 ± 1.60 and SP: 0.76 ± 0.11; both P < 0.001 vs untreated model group). The low-dose SGD treatment produced no significant effect on the WAS-stimulated increases in TRPV1 (3.48 ± 0.33, P < 0.001 vs untreated model group) or SP (1.69 ± 0.22, P < 0.01 vs untreated model group).
The same WAS-induced and SGD-relieved trends were seen for the gene expressions of TRPV1 and SP. The untreated model group showed significantly higher relative expressions of both genes compared with the untreated normal control group (TRPV1: 1.39 ± 0.15 vs 0.14 ± 0.03 and SP: 2.60 ± 0.33 vs 0.70 ± 0.12, both P < 0.001). The mid- and high-dose SGD treatments significantly reduced the WAS-increased mRNA expression of TRPV1 (0.44 ± 0.16 and 0.15 ± 0.03, both P < 0.001 vs untreated model group) and SP (1.64 ± 0.19 and 1.32 ± 0.14, both P < 0.05 vs untreated model group), with the SP levels being uniquely reduced by mid-dose SGD to levels similar to those of the untreated normal controls (P < 0.05). Furthermore, the trends in SGD-mediated relief were similar to those observed with the dicetel control treatment (TRPV1: 0.22 ± 0.02, P < 0.001 vs untreated model group and SP: 1.35 ± 0.13, P < 0.01 vs untreated model group). Again, the low-dose SGD treatment produced no significant effect on the WAS-stimulated increase in mRNA expression of TRPV1 (0.99 ± 0.16) and SP (2.34 ± 0.19) (both P < 0.001 vs untreated normal control group).
In the present study, the well-established animal model of chronic water avoidance stress was used to stimulate the gastrointestinal tract hypersensitivity that is characteristic of human IBS. The WAS-induced physical manifestations (i.e., increased fecal output and lower PPT) were accompanied by differential expression patterns of genes/proteins related to visceral hypersensitivity (TRPV1) and hyperalgesia (SP) in colon tissues. In addition, the model was used to evaluate the therapeutic efficacy of SGD, as a TCM alternative to dicetel, the pharmacologic agent most commonly used to treat IBS in humans. The findings indicated SGD was able to relieve the WAS-induced visceral hypersensitivity and hyperalgesia, as well as restore the perturbed TRPV1 and SP expressions.
Visceral pain, related to CRD and visceral hypersensitivity, is a hallmark feature of IBS and is often the factor precipitating a patient’s presentation to the clinic[13-16]. However, the underlying molecular mechanisms of the IBS pain response are poorly understood, which has inhibited development of effective pain management strategies[17,18]. The demonstration of TRPV1 as a contributor to WAS-induced colonic hypersensitivity, suggests its potential as a target of molecular therapies that may not only reduce the overactive distal colonic motility, but also relieve the associated lower PPT. Indeed, when TRPV1 was knocked-out in mice, the visceral sensitivity to CRD was significantly reduced[19], and enhanced TRPV1 expression has been observed in a variety of gastrointestinal diseases[20,21], including human cases of IBS[22].
An increased amount of TRPV1-expressing nerve fibers have been reported in IBS-affected tissues from human patients[22], and may represent a physiological link between increased TRPV1 transcription and the pain response in IBS[23,24]. In addition, inflammatory factors are known agonists of TRPV1 channels[25] and might explain the common feature of low-grade inflammation in IBS. Considering a previous finding that development of fecal urgency and rectal hypersensitivity correlated with increased immunoreactivity to TRPV1 within the gastrointestinal tract[20], it is possible that therapeutic antagonism of TRPV1 channels may result in antihyperalgesic effects without hypoalgesic activity, and might be beneficial in the treatment of IBS visceral pain[26].
The current study’s finding of chronic WAS-induced changes in SP colon expression agree with other recent studies using the same model system that have implicated this neuropeptide in the maintenance of visceral hyperalgesia[9,27]. As a critical neurotransmitter of injurious signals, SP effectively links the gut nervous system to the immune system, stimulating a wide range of effector cells in the stomach and intestine to facilitate proper gastrointestinal motility, sensibility, secretion and absorption. The mechanism by which SP mediates visceral hypersensitivity may involve a myriad of cellular processes and signaling cascades, including promotion of the mast cell degranulation response, the release of histamines, leukotrienes, prostaglandins and bradykinin, all of which can cause inflammatory reactions leading to neuropathic pain[28].
The clinical observations of increased SP expression in the intestinal mucosa of IBS patients[29,30], coupled with the previous demonstration of SP’s ability to activate TRPV1 via phosphorylation, thereby enhancing the probability of channel gating[9], suggested that SP might be a vital mediator of chronic stress-induced visceral hyperalgesia through the modulation of TRPV1 channels. When TRPV1 channels are activated, a large Ca2+ influx can lead to cellular depolarization[31], with neurons releasing an array of neurotransmitters to trigger the downstream response of visceral hypersensitivity.
Dicetel is the most commonly applied pharmacotherapy of IBS, yet it is associated with a wide range of side effects, such as itching, rash, nausea and dry mouth. In addition, its widespread adoption in clinical practices worldwide has been hampered by its high monetary cost. In the current study, SGD treatment led to decreased expression of the WAS-stimulated TRPV1 and SP proteins and mRNAs in the hypersensitive colon, and increased the pain threshold of the rats. Thus, SGD appears to be an effective alternative to the pharmacologic agent dicetel for treating IBS by affecting the transcription and translation (and presumably secretion) of TRPV1 and SP in the colon.
In conclusion, the TCM SGD is an effective agent for reducing WAS-induced expressions of TRPV1 and SP in rat colons, thereby reducing visceral hypersensitivity and hyperalgesia. However, the chronic WAS testing (10 consecutive days) used in this study caused no overt damage to the colon’s histological structure (data not shown), which may be a limitation in the study’s findings, because human IBS is accompanied by significant structural changes (likely associated with the inflammatory component of IBS). Nonetheless, the present findings indicate an underlying mechanism of stress-induced disruption of distal colon motility and pain, which may represent useful targets for molecular based therapies to treat the pain and sensitivity symptoms of abdominal diseases, such as IBS.
Visceral hypersensitivity has been proposed as a significant contributor to the pathophysiology of irritable bowel syndrome (IBS). Activation of the transient receptor potential vanilloid 1 (TRPV1) channel on neurons, by such effector molecules as the neurotransmitter substance P (SP), increases the probability of channel gating and promotes the formation of visceral hypersensitivity. Therefore, SP-mediated activation of TRPV1 might play a role in the visceral hypersensitivity and hyperalgesia induced by chronic stress conditions, as in IBS. The traditional Chinese medicine (TCM) compound Shugan-decoction (SGD) has been shown to significantly improve the clinical symptoms of IBS patients; however, the therapeutic mechanism of SGD remains unknown.
The molecular mechanisms underlying IBS remain to be fully elucidated, and may represent useful targets of therapies to relieve not only the symptoms associated with visceral hypersensitivity (increased distal colonic motility), but also those related to visceral hyperalgesia (abdominal pain, possibly related to an overactive inflammatory response). In this study, the classical rat model of chronic stress inducement via the water avoidance stress (WAS) test was used to investigate the underlying molecular mechanisms of visceral hypersensitivity and hyperalgesia in the gastrointestinal tract and to evaluate the related therapeutic effect of SGD for treating the stress-related gut aspects of IBS in humans. Mid- and high-dose SGD treatments significantly increased the WAS-reduced pressure thresholds and restored WAS-related changes in TRPV-1 and SP expression in the colon, suggesting this TCM compound as a feasible alternative to the pharmacological agent dicetel.
This study provided novel insights into the molecular mechanisms underlying the observations of SGD-mediated improvements in the clinical symptoms of IBS. Specifically, SGD was demonstrated to reduce WAS-induced perturbations in TRPV1 and SP expressions in the colon that accompany visceral hypersensitivity and hyperalgesia.
The finding that SGD may reduce WAS-induced visceral hypersensitivity and hyperalgesia through regulation of the colonic expressions of TRPV1 and SP confirm this TCM compound as a useful prescription for the treatment of abdominal pain in IBS.
Shugan-decoction is made according to the classic Tongxieyaofang recipe and is reported to soothe the liver soothing and strengthen the spleen. Irritable bowel syndrome is a functional gastrointestinal disorder that is associated with high levels of stress in daily life, and manifests as altered bowel habits and recurrent abdominal pain. The water avoidance stress test is a well-established technique for inducing chronic psychological stress with gastric disruption in a rat model system. TRPV1 is widely expressed on neurons throughout the gastrointestinal tract and modulates visceral sensitivity and hyperalgesia. SP is a neurotransmitter that activates TRPV1 and regulates visceral hyperalgesia.
The authors investigated the therapeutic potential and underlying molecular mechanisms of the TCM compound SGD, in comparison to the common IBS pharmacologic agent dicetel, to relieve stress-induced visceral hypersensitivity and hyperalgesia. This is an interesting manuscript, and the general design is acceptable.
| 1. | Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130:1480-1491. [PubMed] |
| 2. | Su AM, Shih W, Presson AP, Chang L. Characterization of symptoms in irritable bowel syndrome with mixed bowel habit pattern. Neurogastroenterol Motil. 2013;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 3. | Mulak A, Paradowski L. Anorectal function and dyssynergic defecation in different subgroups of patients with irritable bowel syndrome. Int J Colorectal Dis. 2010;25:1011-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Azpiroz F, Bouin M, Camilleri M, Mayer EA, Poitras P, Serra J, Spiller RC. Mechanisms of hypersensitivity in IBS and functional disorders. Neurogastroenterol Motil. 2007;19:62-88. [PubMed] |
| 5. | Blackshaw LA, Brookes SJ, Grundy D, Schemann M. Sensory transmission in the gastrointestinal tract. Neurogastroenterol Motil. 2007;19:1-19. [PubMed] |
| 6. | Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2577] [Cited by in RCA: 2726] [Article Influence: 104.8] [Reference Citation Analysis (1)] |
| 7. | Neri M. Irritable bowel syndrome, inflammatory bowel disease and TRPV1: how to disentangle the bundle. Eur J Pain. 2013;17:1263-1264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Gazzieri D, Trevisani M, Springer J, Harrison S, Cottrell GS, Andre E, Nicoletti P, Massi D, Zecchi S, Nosi D. Substance P released by TRPV1-expressing neurons produces reactive oxygen species that mediate ethanol-induced gastric injury. Free Radic Biol Med. 2007;43:581-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 65] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 9. | Bradesi S, Kokkotou E, Simeonidis S, Patierno S, Ennes HS, Mittal Y, McRoberts JA, Ohning G, McLean P, Marvizon JC. The role of neurokinin 1 receptors in the maintenance of visceral hyperalgesia induced by repeated stress in rats. Gastroenterology. 2006;130:1729-1742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 10. | Spence MJ, Moss-Morris R. The cognitive behavioural model of irritable bowel syndrome: a prospective investigation of patients with gastroenteritis. Gut. 2007;56:1066-1071. [PubMed] |
| 11. | Pan XX, Xie JQ. Clinical observe of Shuganyin in treatment of irritable bowel syndrome. Shanghai Zhongyiyao Daxue Xuebao. 2006;20:48-50. |
| 12. | Bradesi S, Schwetz I, Ennes HS, Lamy CM, Ohning G, Fanselow M, Pothoulakis C, McRoberts JA, Mayer EA. Repeated exposure to water avoidance stress in rats: a new model for sustained visceral hyperalgesia. Am J Physiol Gastrointest Liver Physiol. 2005;289:G42-G53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 235] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 13. | Chaloner A, Greenwood-Van Meerveld B. Sexually dimorphic effects of unpredictable early life adversity on visceral pain behavior in a rodent model. J Pain. 2013;14:270-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 14. | Al-Chaer ED, Kawasaki M, Pasricha PJ. A new model of chronic visceral hypersensitivity in adult rats induced by colon irritation during postnatal development. Gastroenterology. 2000;119:1276-1285. [PubMed] |
| 15. | Mayer EA, Naliboff BD, Chang L, Coutinho SV. V. Stress and irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2001;280:G519-G524. [PubMed] |
| 16. | Zhu Y, Zheng X, Cong Y, Chu H, Fried M, Dai N, Fox M. Bloating and distention in irritable bowel syndrome: the role of gas production and visceral sensation after lactose ingestion in a population with lactase deficiency. Am J Gastroenterol. 2013;108:1516-1525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 100] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 17. | Qu R, Tao J, Wang Y, Zhou Y, Wu G, Xiao Y, Hu CY, Jiang X, Xu GY. Neonatal colonic inflammation sensitizes voltage-gated Na(+) channels via upregulation of cystathionine β-synthetase expression in rat primary sensory neurons. Am J Physiol Gastrointest Liver Physiol. 2013;304:G763-G772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 18. | Crouzet L, Gaultier E, Del’Homme C, Cartier C, Delmas E, Dapoigny M, Fioramonti J, Bernalier-Donadille A. The hypersensitivity to colonic distension of IBS patients can be transferred to rats through their fecal microbiota. Neurogastroenterol Motil. 2013;25:e272-e282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 212] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 19. | Jones RC, Xu L, Gebhart GF. The mechanosensitivity of mouse colon afferent fibers and their sensitization by inflammatory mediators require transient receptor potential vanilloid 1 and acid-sensing ion channel 3. J Neurosci. 2005;25:10981-10989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 336] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 20. | Chan CL, Facer P, Davis JB, Smith GD, Egerton J, Bountra C, Williams NS, Anand P. Sensory fibres expressing capsaicin receptor TRPV1 in patients with rectal hypersensitivity and faecal urgency. Lancet. 2003;361:385-391. [PubMed] |
| 21. | Facer P, Knowles CH, Tam PK, Ford AP, Dyer N, Baecker PA, Anand P. Novel capsaicin (VR1) and purinergic (P2X3) receptors in Hirschsprung’s intestine. J Pediatr Surg. 2001;36:1679-1684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Akbar A, Yiangou Y, Facer P, Walters JR, Anand P, Ghosh S. Increased capsaicin receptor TRPV1-expressing sensory fibres in irritable bowel syndrome and their correlation with abdominal pain. Gut. 2008;57:923-929. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 359] [Cited by in RCA: 401] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 23. | Keszthelyi D, Troost FJ, Jonkers DM, Helyes Z, Hamer HM, Ludidi S, Vanhoutvin S, Venema K, Dekker J, Szolcsányi J. Alterations in mucosal neuropeptides in patients with irritable bowel syndrome and ulcerative colitis in remission: a role in pain symptom generation? Eur J Pain. 2013;17:1299-1306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 24. | Suckow SK, Anderson EM, Caudle RM. Lesioning of TRPV1 expressing primary afferent neurons prevents PAR-2 induced motility, but not mechanical hypersensitivity in the rat colon. Neurogastroenterol Motil. 2012;24:e125-e135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | Gunthorpe MJ, Chizh BA. Clinical development of TRPV1 antagonists: targeting a pivotal point in the pain pathway. Drug Discov Today. 2009;14:56-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 185] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 26. | Ravnefjord A, Brusberg M, Kang D, Bauer U, Larsson H, Lindström E, Martinez V. Involvement of the transient receptor potential vanilloid 1 (TRPV1) in the development of acute visceral hyperalgesia during colorectal distension in rats. Eur J Pharmacol. 2009;611:85-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 27. | Liang C, Luo H, Liu Y, Cao J, Xia H. Plasma hormones facilitated the hypermotility of the colon in a chronic stress rat model. PLoS One. 2012;7:e31774. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 28. | Lan C, Tang CW. Effects of substance P on the activity of intestinal mucosal mast cells in rats with multiple organ failure. Zhonghua Xiaohua Zazhi. 2003;23:271-274. |
| 29. | Palsson OS, Morteau O, Bozymski EM, Woosley JT, Sartor RB, Davies MJ, Johnson DA, Turner MJ, Whitehead WE. Elevated vasoactive intestinal peptide concentrations in patients with irritable bowel syndrome. Dig Dis Sci. 2004;49:1236-1243. [PubMed] |
| 30. | Li J, Micevych P, McDonald J, Rapkin A, Chaban V. Inflammation in the uterus induces phosphorylated extracellular signal-regulated kinase and substance P immunoreactivity in dorsal root ganglia neurons innervating both uterus and colon in rats. J Neurosci Res. 2008;86:2746-2752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 31. | Andrew D, Greenspan JD. Mechanical and heat sensitization of cutaneous nociceptors after peripheral inflammation in the rat. J Neurophysiol. 1999;82:2649-2656. [PubMed] |
P- Reviewer: Bian ZX S- Editor: Zhai HH L- Editor: Stewart GJ E- Editor: Wu HL