Published online Aug 28, 2013. doi: 10.3748/wjg.v19.i32.5377
Revised: May 27, 2013
Accepted: June 1, 2013
Published online: August 28, 2013
Processing time: 171 Days and 20.8 Hours
Pulmonary abnormalities are not frequently encountered in patients with inflammatory bowel diseases. However, lung toxicity can be induced by conventional medications used to maintain remission, and similar evidence is also emerging for biologics. We present the case of a young woman affected by colonic Crohn’s disease who was treated with oral mesalamine and became steroid-dependent and refractory to azathioprine and adalimumab. She was referred to our clinic with a severe relapse and was treated with infliximab, an anti-tumor necrosis factor α (TNF-α) antibody, to induce remission. After an initial benefit, with decreases in bowel movements, rectal bleeding and C-reactive protein levels, she experienced shortness of breath after the 5th infusion. Noninfectious interstitial lung disease was diagnosed. Both mesalamine and infliximab were discontinued, and steroids were introduced with slow but progressive improvement of symptoms, radiology and functional tests. This represents a rare case of interstitial lung disease associated with infliximab therapy and the effect of drug withdrawal on these lung alterations. Given the increasing use of anti-TNF-α therapies and the increasing reports of pulmonary abnormalities in patients with inflammatory bowel diseases, this case underlines the importance of a careful evaluation of respiratory symptoms in patients undergoing infliximab therapy.
Core tip: Safety during anti-tumor necrosis factor (TNF)-α therapy is a major concern. Paradoxical inflammatory and autoimmune phenomena can be induced by this treatment and should always be considered. Interstitial lung disease is an emerging complication often observed early after the beginning of treatment, particularly when combination immunosuppressive regimens are employed. This case demonstrates that interstitial lung disease can also occur later during anti-TNF-α treatment and during monotherapy. Thus, great vigilance is recommended when patients start complaining of any respiratory symptom.
- Citation: Caccaro R, Savarino E, D’Incà R, Sturniolo GC. Noninfectious interstitial lung disease during infliximab therapy: Case report and literature review. World J Gastroenterol 2013; 19(32): 5377-5380
- URL: https://www.wjgnet.com/1007-9327/full/v19/i32/5377.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i32.5377
The occurrence of pulmonary involvement in patients with inflammatory bowel disease (IBD) was first described in 1976 and has been explained either as a potential extra-intestinal manifestation of the disease itself or as a secondary effect of medications employed to control inflammation[1-4]. The common embryological origin of both the gastrointestinal tract and the respiratory system could be responsible for the shared antigenicity leading to the pulmonary manifestations. However, noninfectious drug-induced lung disease has been described using sulfasalazine, mesalamine, methotrexate and azathioprine[2,4]. Anti-tumor necrosis factor (TNF)-α agents have also been implicated as a cause of drug-induced interstitial lung disease and account for most of the cases reported in the rheumatology literature[5,6].
We report the case of a noninfectious interstitial pneumonia that occurred during infliximab (IFX) treatment in a young woman with colonic Crohn’s disease (CD).
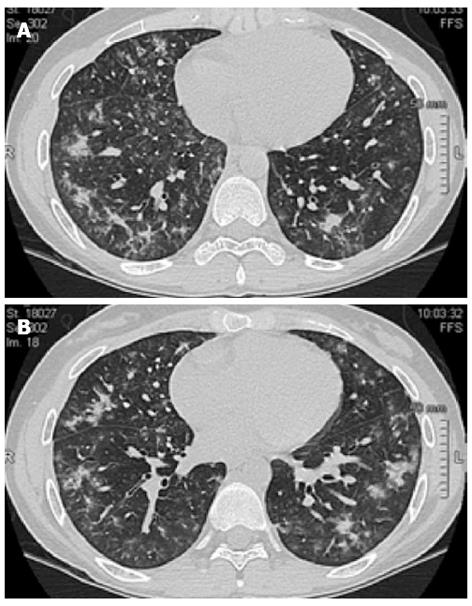
A 25-year-old female was diagnosed with left-sided ulcerative colitis (UC) in 2004 (16-year-old) and treated with oral and rectal mesalamine. She required several courses of oral prednisone during the subsequent 4-year follow up. Azathioprine was introduced in 2008 because of steroid dependency; however, despite the optimization of the dosage up to 2.5 mg/kg, the patient never experienced a full clinical remission. Colonoscopy demonstrated a segmental distribution of the ulcerative lesions, and histology confirmed CD. According to these findings, in December 2010, the patient discontinued azathioprine and was screened for biologics. Adalimumab (ADA) was started with an induction regimen followed by maintenance. After 4 mo, the patient was referred for a new disease flare and did not respond to concomitant therapy with 25 mg of prednisone. Biochemical parameters demonstrated thrombocytosis (810 × 103/μL) and elevated C-reactive protein (25 mg/L) and fecal lactoferrin (538 μg/mL). The new endoscopic assessment showed moderate activity in the left colon and mild lesions in the cecum and terminal ileum (Simple Endoscopic Score for CD 13). The interval between ADA administrations was then reduced to every week for one month, without any significant clinical or biochemical improvement. ADA was stopped, and IFX was started (5 mg/kg) with concomitant steroid tapering. She improved clinically, and her C-reactive protein levels normalized. After the 5th infusion, the patient reported the onset of shortness of breath and fatigue, without concomitant cough or fever. The patient had no history of asthma, atopy or allergy to medications. Chest X-ray did not demonstrate any significant lesion, and thorax auscultation was normal. In accordance with the lung specialist who preliminarily suspected pulmonary sarcoidosis, the 6th dose of IFX was administered, and the patient was admitted to the Pneumology Unit for monitoring. High-resolution computed tomography (HRCT) of the thorax revealed bilateral shadowing nodules and adjacent interstitial thickening with a predominant distribution in the middle and basal regions and relative sparing of the apices (Figure 1). Pulmonary function tests were compatible with a moderately restrictive pattern, without any oximetric deficiency. Bronchoscopy did not demonstrate any endobronchial abnormality, and a bronchoalveolar lavage fluid analysis was negative for Pneumocystis carinii, fungi and alcohol-acid resistant bacilli. Cyto-immunological analysis revealed increased cellularity (213 × 106/L) with a decreased percentage of macrophages (66%) and a well-represented component of eosinophils (20%) (Table 1). Transbronchial biopsy showed a mild chronic, nonspecific, non-granulomatous infiltrate and thickening of the basal membrane. A QuantiFERON-TB Gold test, auto-antibodies, serum angiotensin-converting enzyme, serum precipitins and blood cultures were unremarkable. Mesalamine and infliximab were discontinued, and prednisone was started at a dose of 50 mg/d for 7 d and subsequently tapered to 25 mg/d in association with inhalations of budesonide and a long-acting beta2-agonist. Clinical improvement occurred over the following 6 wk, with mild symptoms still present at 8 wk. HRCT performed after 10 wk showed minimal peripheral irregularities in both apices. All the symptoms had subsided by week 14. The spirometric values during the follow up are reported in Table 2.
| 4-wkfollow up | 8-wkfollow up | 12-wkfollow up | |
| FEV1, L | 2.03 (58%) | 2.24 (64%) | 2.56 (73%) |
| FVC, L | 2.08 (52%) | 2.33 (58%) | 2.62 (66%) |
| TLC, L | 3.04 (56%) | 3.63 (67%) | 3.73 (69%) |
| RV, L | 0.91 (62%) | 1.30 (88%) | 0.97 (66%) |
| FRC, L | 1.62 (57%) | 2.47 (87%) | 2.44 (86%) |
| DLCO, mL/mmHg per minute | NA | 17.3 (58%) | 19.0 (64%) |
Although IBDs are pathologic conditions of the gastro-intestinal tract, they should be considered as systemic diseases because almost all organs can be involved, although the most frequent extra-intestinal manifestations are articular, dermatologic, ophthalmologic and hepatobiliary[7,8].
Pulmonary involvement can manifest with different patterns[9]. A significant proportion of IBD patients show abnormal functional tests compared to healthy matched controls, suggesting the potential presence of subclinical pulmonary dysfunctions[2,10-13]. In addition and more importantly, HRCT scans performed in 2 series of consecutive IBD patients were pathological in 53.00% and 64.10% of patients, respectively[14,15]. Interestingly, these findings were unrelated to the presence of respiratory symptoms.
Our patient had never previously experienced respiratory symptoms; she did not smoke and did not suffer from asthma or atopy. The previous existence of subclinical respiratory defects cannot be excluded because the patient did not perform a functional respiratory test before the onset of pulmonary symptoms. Nonetheless, it is reasonable to predict the absence of abnormalities because the baseline chest-X ray performed before starting anti-TNF-α therapy was unremarkable.
In addition to IBD-associated pulmonary manifestations, the occurrence of drug-induced effects has to be considered, particularly according to the cyto-immunological analysis of bronchoalveolar lavage fluid, which in our case, was consistent with subacute respiratory illness compatible with either nonspecific interstitial pneumonia or cryptogenic pneumonia[16-18]. Several cases of pulmonary toxicity induced by sulfasalazine and mesalamine have been reported, particularly eosinophilic pneumonia, which is characterized by eosinophilic infiltration of the lungs with or without peripheral eosinophilia[2]. In our patient, we detected normal levels of peripheral eosinophils, and transbronchial biopsy did not reveal an abnormal number of these cells; however, the examination of specimens obtained by fiberoptic bronchoscopy is sub-optimal for the diagnosis of interstitial lung diseases. Most of the reported reactions occurred between 2 and 6 mo after the introduction of the drug, with rare cases occurring later on (44 mo)[2,19,20]. Peripheral eosinophilia was often present, and the resolution of symptoms (dyspnea, fever, chest pain, cough) with the discontinuation of the drug was prompt[2]. When our patient first developed shortness of breath, she had been under mesalamine treatment for 8 years. The possibility of mesalamine-induced pneumonia seems unlikely. However, reports of lung toxicity associated with TNF-α antagonists have recently appeared, particularly in the rheumatology literature[5,6]. Our patient was exposed to two different biologics: ADA for 1 year and subsequently IFX for 6 mo. The pulmonary toxicity of ADA is controversial: there are reported cases of induced interstitial pneumonia[21,22] as well as reports of efficacy in the treatment of rheumatoid arthritis- and dermatomyositis-associated lung disease[21,23]. Our patient did not experience any respiratory symptoms during treatment with ADA, and she started complaining of dyspnea on exertion after the 5th dose of IFX. This timing is delayed compared with the previously reported experiences that occurred in the majority of rheumatology cases after the 2nd-3rd infusion[24-28]. Similarly, in the CD patient described by Weatherhead et al, symptoms appeared after the first infusion and were exacerbated after the 2nd[27]. In the most recently reported case of nonspecific interstitial pneumonia in a young female with UC, symptoms appeared after the 2nd infusion of IFX[30]. The timing of respiratory symptoms after the 5th infusion of IFX observed in our patient is similar to that reported by Wiener and colleagues in a 63-year-old woman affected by UC[31]. Most of the reported cases received anti-TNF α associated with other immunomodulators[6]; additionally, in the reports by Weatherhead and Wiener, the patients were taking other medications for IBD (azathioprine and balsalazide, respectively)[29,31]. Thus, it was hypothesized that TNF-α blocking agents might provide a favorable environment for the induction and/or the progression of iatrogenic lung disease through modulation of the immune system[32].
In the present case, it is reasonable to suspect drug-induced interstitial lung disease attributable to IFX for several reasons: (1) the onset of respiratory symptoms shortly after IFX introduction; (2) the 8-year treatment with mesalamine without any symptoms; and (3) the slow improvement after mesalamine discontinuation (despite its rapid wash-out period) and after IFX discontinuation (consistent with its long wash-out period). Given the seriousness of the adverse event, definite proof is unrealistic because a re-challenge with the drug would be unethical and dangerous; however, the lack of an anatomical diagnosis using open-lung biopsy limited the differential diagnosis.
In conclusion, there is emerging evidence that anti-TNF-α agents might induce lung toxicity even in the long term. High vigilance is recommended for the occurrence of respiratory symptoms in patients undergoing biological treatment.
| 1. | Kraft SC, Earle RH, Roesler M, Esterly JR. Unexplained bronchopulmonary disease with inflammatory bowel disease. Arch Intern Med. 1976;136:454-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 137] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 2. | Storch I, Sachar D, Katz S. Pulmonary manifestations of inflammatory bowel disease. Inflamm Bowel Dis. 2003;9:104-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 134] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 3. | Vennera MC, Picado C. [Pulmonary manifestations of inflammatory bowel disease]. Arch Bronconeumol. 2005;41:93-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Schleiermacher D, Hoffmann JC. Pulmonary abnormalities in inflammatory bowel disease. J Crohns Colitis. 2007;1:61-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 5. | Ramos-Casals M, Brito-Zerón P, Muñoz S, Soria N, Galiana D, Bertolaccini L, Cuadrado MJ, Khamashta MA. Autoimmune diseases induced by TNF-targeted therapies: analysis of 233 cases. Medicine (Baltimore). 2007;86:242-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 512] [Cited by in RCA: 522] [Article Influence: 27.5] [Reference Citation Analysis (1)] |
| 6. | Perez-Alvarez R, Perez-de-Lis M, Diaz-Lagares C, Pego-Reigosa JM, Retamozo S, Bove A, Brito-Zeron P, Bosch X, Ramos-Casals M. Interstitial lung disease induced or exacerbated by TNF-targeted therapies: analysis of 122 cases. Semin Arthritis Rheum. 2011;41:256-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 202] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 7. | Rothfuss KS, Stange EF, Herrlinger KR. Extraintestinal manifestations and complications in inflammatory bowel diseases. World J Gastroenterol. 2006;12:4819-4831. [PubMed] |
| 8. | Larsen S, Bendtzen K, Nielsen OH. Extraintestinal manifestations of inflammatory bowel disease: epidemiology, diagnosis, and management. Ann Med. 2010;42:97-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 204] [Article Influence: 12.8] [Reference Citation Analysis (1)] |
| 9. | Tzanakis NE, Tsiligianni IG, Siafakas NM. Pulmonary involvement and allergic disorders in inflammatory bowel disease. World J Gastroenterol. 2010;16:299-305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | Godet PG, Cowie R, Woodman RC, Sutherland LR. Pulmonary function abnormalities in patients with ulcerative colitis. Am J Gastroenterol. 1997;92:1154-1156. [PubMed] |
| 11. | Heatley RV, Thomas P, Prokipchuk EJ, Gauldie J, Sieniewicz DJ, Bienenstock J. Pulmonary function abnormalities in patients with inflammatory bowel disease. Q J Med. 1982;51:241-250. [PubMed] |
| 12. | Herrlinger KR, Noftz MK, Dalhoff K, Ludwig D, Stange EF, Fellermann K. Alterations in pulmonary function in inflammatory bowel disease are frequent and persist during remission. Am J Gastroenterol. 2002;97:377-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 91] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 13. | Dierkes-Globisch A, Mohr H. Pulmonary function abnormalities in respiratory asymptomatic patients with inflammatory bowel disease. Eur J Intern Med. 2002;13:385. [PubMed] |
| 14. | Songür N, Songür Y, Tüzün M, Doğan I, Tüzün D, Ensari A, Hekimoglu B. Pulmonary function tests and high-resolution CT in the detection of pulmonary involvement in inflammatory bowel disease. J Clin Gastroenterol. 2003;37:292-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 82] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 15. | Yilmaz A, Yilmaz Demirci N, Hoşgün D, Uner E, Erdoğan Y, Gökçek A, Cağlar A. Pulmonary involvement in inflammatory bowel disease. World J Gastroenterol. 2010;16:4952-4957. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 41] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 16. | Meyer KC. The role of bronchoalveolar lavage in interstitial lung disease. Clin Chest Med. 2004;25:637-649, v. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 34] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Müller NL, White DA, Jiang H, Gemma A. Diagnosis and management of drug-associated interstitial lung disease. Br J Cancer. 2004;91 Suppl 2:S24-S30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 83] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 18. | Allen JN. Drug-induced eosinophilic lung disease. Clin Chest Med. 2004;25:77-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 67] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | Foster RA, Zander DS, Mergo PJ, Valentine JF. Mesalamine-related lung disease: clinical, radiographic, and pathologic manifestations. Inflamm Bowel Dis. 2003;9:308-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 61] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 20. | Bitton A, Peppercorn MA, Hanrahan JP, Upton MP. Mesalamine-induced lung toxicity. Am J Gastroenterol. 1996;91:1039-1040. [PubMed] |
| 21. | Komiya K, Ishii H, Fujita N, Oka H, Iwata A, Sonoda H, Kadota J. Adalimumab-induced interstitial pneumonia with an improvement of pre-existing rheumatoid arthritis-associated lung involvement. Intern Med. 2011;50:749-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 22. | Yamazaki H, Isogai S, Sakurai T, Nagasaka K. A case of adalimumab-associated interstitial pneumonia with rheumatoid arthritis. Mod Rheumatol. 2010;20:518-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 23. | Park JK, Yoo HG, Ahn DS, Jeon HS, Yoo WH. Successful treatment for conventional treatment-resistant dermatomyositis-associated interstitial lung disease with adalimumab. Rheumatol Int. 2012;32:3587-3590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Mori S, Imamura F, Kiyofuji C, Sugimoto M. Development of interstitial pneumonia in a rheumatoid arthritis patient treated with infliximab, an anti-tumor necrosis factor α-neutralizing antibody. Mod Rheumatol. 2006;16:251-255. [PubMed] |
| 25. | Ostör AJ, Chilvers ER, Somerville MF, Lim AY, Lane SE, Crisp AJ, Scott DG. Pulmonary complications of infliximab therapy in patients with rheumatoid arthritis. J Rheumatol. 2006;33:622-628. [PubMed] |
| 26. | Villeneuve E, St-Pierre A, Haraoui B. Interstitial pneumonitis associated with infliximab therapy. J Rheumatol. 2006;33:1189-1193. [PubMed] |
| 27. | Taki H, Kawagishi Y, Shinoda K, Hounoki H, Ogawa R, Sugiyama E, Tobe K. Interstitial pneumonitis associated with infliximab therapy without methotrexate treatment. Rheumatol Int. 2009;30:275-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 28. | Pataka A, Tzouvelekis A, Bouros D. Infliximab-induced non-specific interstitial pneumonia and lupus-like eruption. Eur J Intern Med. 2006;17:520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 29. | Weatherhead M, Masson S, Bourke SJ, Gunn MC, Burns GP. Interstitial pneumonitis after infliximab therapy for Crohn’s disease. Inflamm Bowel Dis. 2006;12:427-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 30. | Sen S, Peltz C, Jordan K, Boes TJ. Infliximab-induced nonspecific interstitial pneumonia. Am J Med Sci. 2012;344:75-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 31. | Wiener CM, Muse VV, Mark EJ. Case records of the Massachusetts General Hospital. Case 33-2008. A 63-year-old woman with dyspnea on exertion. N Engl J Med. 2008;359:1823-1832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
P- Reviewer D'Ovidio V S- Editor Gou SX L- Editor A E- Editor Zhang DN