Published online Aug 28, 2013. doi: 10.3748/wjg.v19.i32.5365
Revised: May 23, 2013
Accepted: July 9, 2013
Published online: August 28, 2013
Processing time: 150 Days and 20.6 Hours
AIM: To evaluate the safety and efficacy of laparoscopy-assisted total gastrectomy (LATG) and open total gastrectomy (OTG) for gastric cancer.
METHODS: A comprehensive search of PubMed, Cochrane Library, Web of Science and BIOSIS Previews was performed to identify studies that compared LATG and OTG. The following factors were checked: operating time, blood loss, harvested lymph nodes, flatus time, hospital stay, mortality and morbidity. Data synthesis and statistical analysis were carried out using RevMan 5.1 software.
RESULTS: Nine studies with 1221 participants were included (436 LATG and 785 OTG). Compared to OTG, LATG involved a longer operating time [weighted mean difference (WMD) = 57.68 min, 95%CI: 30.48-84.88; P < 0.001]; less blood loss [standard mean difference (SMD) = -1.71; 95%CI: -2.48 - -0.49; P < 0.001]; earlier time to flatus (WMD= -0.76 d; 95%CI: -1.22 - -0.30; P < 0.001); shorter hospital stay (WMD = -2.67 d; 95%CI: -3.96 - -1.38, P < 0.001); and a decrease in medical complications (RR = 0.41, 95%CI: 0.19-0.90, P = 0.03). The number of harvested lymph nodes, mortality, surgical complications, cancer recurrence rate and long-term survival rate of patients undergoing LATG were similar to those in patients undergoing OTG.
CONCLUSION: Despite a longer operation, LATG can be performed safely in experienced surgical centers with a shorter hospital stay and fewer complications than open surgery.
Core tip: This study evaluated the safety and efficacy of laparoscopy-assisted total gastrectomy (LATG) and open total gastrectomy (OTG) for gastric cancer through systematic review and meta-analysis. The existing research shows that LATG is safe and feasible, which can achieve similar lymph node dissection effects as OTG, characterized by such advantages as less pain, fewer postoperative complications, and rapid recovery, and which is expected to achieve the same effect in oncological treatment as OTG.
- Citation: Chen K, Xu XW, Zhang RC, Pan Y, Wu D, Mou YP. Systematic review and meta-analysis of laparoscopy-assisted and open total gastrectomy for gastric cancer. World J Gastroenterol 2013; 19(32): 5365-5376
- URL: https://www.wjgnet.com/1007-9327/full/v19/i32/5365.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i32.5365
Since it was first reported in 1994[1], laparoscopy-assisted distal gastrectomy (LADG) for gastric cancer has undergone rapid development and gained popularity in the past 20 years. Compared to traditional open gastrectomy, most studies have reported that LADG can achieve better cosmesis, shorter hospital stay, faster postoperative recovery, and better postoperative quality of life[2-6]. However, laparoscopy-assisted total gastrectomy (LATG) is technically demanding and the incidence of upper gastric carcinoma is relatively low in East Asia[7,8]. Therefore, although LADG has been accepted worldwide for tumors located in the lower stomach, LATG for upper and middle gastric cancer has not been generalized. In fact, there are only a few reports on the technical feasibility and safety of LATG and its long-term oncologic outcomes[9-12]. Although several meta-analyses and systematic reviews have been published for LADG[13-19], such studies have not been conducted for the potential benefits and disadvantages of LATG.
In order to assess accurately the current status of LATG, we strictly limited inclusion criteria by focusing exclusively on LATG and carried out a comprehensive meta-analysis. We believe that such research will contribute to a more systematic and objective evaluation of the safety of the LATG in cancer treatment.
We searched PubMed, Cochrane Library, Web of Science and BIOSIS Previews for literature comparing LATG and open total gastrectomy (OTG) published between January 1995 and March 2013, and broadened the search range by browsing the related summary, methods, and references of retrieved articles. The following keywords were used: “laparoscopy”, “laparoscopic”, “gastric cancer”, “gastric carcinoma”, and “gastrectomy”. The language of the publications was confined to English. Two investigators reviewed the titles and abstracts, and assessed the full text to establish eligibility.
All clinical studies should meet the following criteria for the meta-analysis: (1) published in English with data comparing LATG and OTG; (2) clear case selection criteria, containing at least the following information: the number of cases, surgical methods and perioperative data; and (3) if there was overlap between authors or centers, the higher quality or more recent literature were selected. However, articles from the same authors or centers but with different patient cohorts were included. The papers containing any of the following were excluded: (1) totally laparoscopic, laparoscopic hand-assisted, or robot-assisted gastrectomy; (2) non-gastric carcinoma cases; (3) palliative resection cases; and (4) extent of lymphadenectomy was not required for grouping in this study, but the articles with significant differences between the two groups in the extent of lymphadenectomy were excluded.
Two authors independently extracted the data using a unified datasheet, and decided upon the controversial issues through discussion. Extracted data included: author, study period, geographical region, number of patients, operating time, blood loss, number of retrieved lymph nodes, proximal and distal margin distance, time to flatus, time to oral intake, length of hospital stay, morbidity and mortality. Postoperative complications were classified as medical (cardiovascular, respiratory, or metabolic events; nonsurgical infections; deep venous thrombosis; and pulmonary embolism) or surgical (any anastomotic leakage or fistula, any complication that required reoperation, intra-abdominal collections, wound complications, bleeding events, pancreatitis, ileus, delayed gastric emptying, and anastomotic stricture). This classification system is based on the Memorial Sloan-Kettering Cancer Center complication reporting system[20]. If necessary, the first authors were contacted to retrieve further information. Selected documents were rated according to the grading of the Centre of Evidence-Based Medicine (CEBM, Oxford, United Kingdom; http://www.cebm.net), which, in brief, assigns level 1 to randomised controlled trials (RCTs), level 2 to cohort studies, level 3 to case-control studies, level 4 to case series or poor quality observational study and level 5 to expert opinion.
The meta-analysis was performed in line with recommendations from the Cochrane Collaboration and the Quality of Reporting of Meta-Analyses guidelines[21,22]. Continuous variables, when both means and standard deviations were presented, were assessed using weighted mean difference (WMD) or standard mean difference (SMD), the postoperative morbidity and mortality were analyzed using the risk ratio (RR), and the risk difference (RD) was used to evaluate cancer recurrence because there may be no recurrence events in either groups during follow-up. When heterogeneity test showed no significant differences (P > 0.05), we used a fixed-effects model to calculate the summary statistics. When the heterogeneity test showed statistically significant differences (P < 0.05), we used a random effects model based on the DerSimonian and Laird method. Subgroup analysis of intraoperative outcomes, such as operating time, blood loss, and number of retrieved lymph nodes, was conducted for the number of LATG cases performed (40 cases were used as a cut-point), because the learning curve may have an impact on the operative outcomes. Potential publication bias was determined by conducting informal visual inspection of funnel plots based on the complications. Data analyses were performed using Review Manage Version 5.1 (RevMan 5.1) software downloaded from Cochrane Library. P < 0.05 was considered statistically significant.
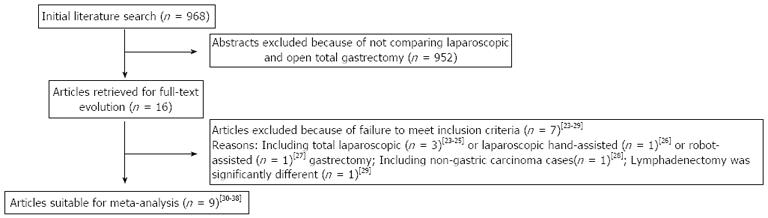
The initial search strategy retrieved 968 publications in English. After the titles and abstracts were reviewed, papers without comparison of LATG and OTG were excluded, which left 16 comparative studies, seven[23-29] of which did not meet the inclusion criteria and were excluded. This left a total of nine comparative observational studies[30-38]. A flow chart of the search strategies is illustrated in Figure 1.
A total of 1221 patients were included in the analysis with 436 undergoing LATG (35.7%) and 785 undergoing OTG (64.3%). Only one study reported a case converted to open surgery because of extensive abdominal adhesions[38]. Regarding the tumor stage, only one study was limited to early stage cancer[33]. In another study, only patients with advanced gastric cancer were described[34]. The other seven studies included both populations. All studies had Asian data from Japan, South Korea and China. In the included studies, four studies was considered as level of evidence 2b, two studies as level of evidence 3b, and the remaining three as level of evidence 4 (according to the grading of the CEBM). The characteristics and methodological quality assessment scores of the included studies are shown in Table 1.
| Ref. | Nation | Study type | Study period | Sample size | Stage | Level of lymphadenectomy | Follow-up (mo) | Level of evidence | ||
| LATG | OTG | LATG | OTG | |||||||
| Kim et al[30] | South Korea | Retro | 2004-2006 | 27 | 33 | EC + AC | D1 + α/β, D2 | NR | NR | 2b |
| Mochiki et al[31] | Japan | Retro | 1999-2007 | 20 | 18 | EC + AC | D1 + β | 31 (3-60) | 46 (13-60) | 2b |
| Sakuramoto et al[32] | Japan | Retro | 2003-2007 | 30 | 44 | EC + AC | D1 + β, D2 | 30 | 4 | |
| Kawamura et al[33] | Japan | Retro | 2003-2008 | 46 | 35 | EC | D2 | NR | NR | 4 |
| Du et al[34] | China | Retro | 2005-2009 | 82 | 94 | AC | D2 | 25 (2-44) | 2b | |
| Kim et al[35] | South Korea | Pros | 2009-2010 | 63 | 127 | EC + AC | D2 | NR | NR | 2b |
| Kunisaki et al[36] | Japan | Pros | 2002-2008 | 27 | 30 | EC + AC | D1 + β | NR | NR | 3b |
| Eom et al[37] | Korea | Retro | 2003-2008 | 100 | 348 | EC + AC | D2 | 52.6 (0.3-95.7) | 4 | |
| Guan et al[38] | China | Pros | 2007-2010 | 41 | 56 | EC + AC | D2 | NR | NR | 3b |
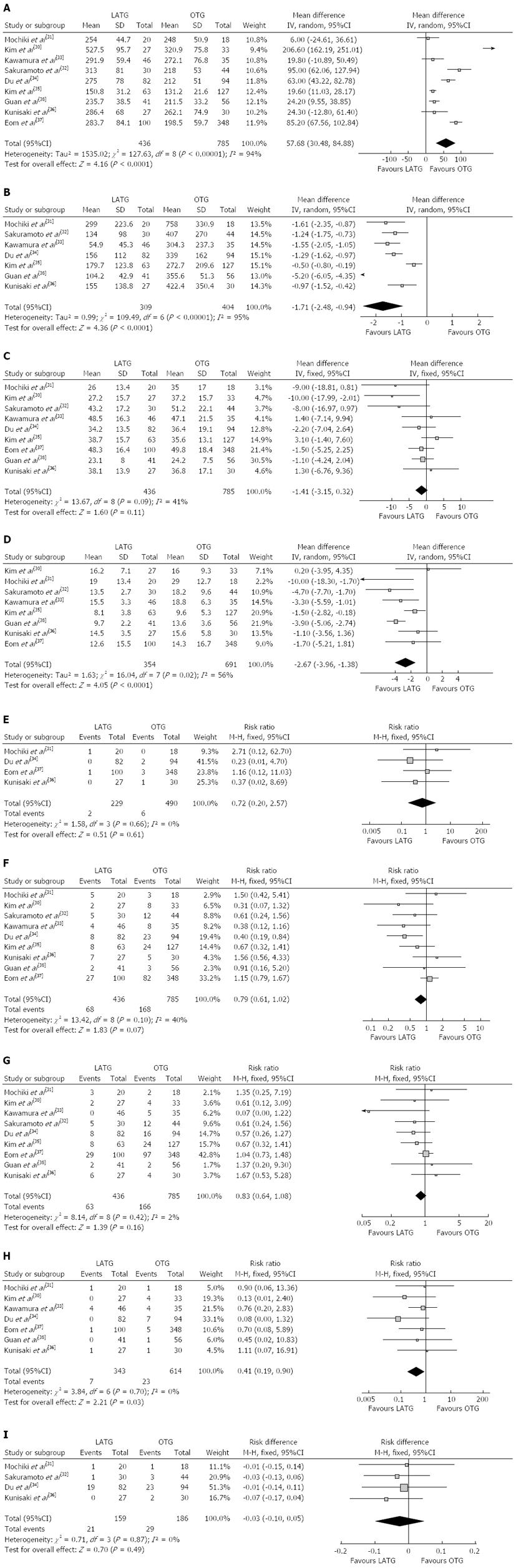
Most of the studies considered suitable for the meta-analysis reported a longer operating time for LATG than for OTG. The mean operating time of LATG was 57.68 min longer than for OTG (WMD = 57.68 min; 95%CI: 30.48-84.88, P < 0.001) (Figure 2A). Two studies[31,32] used grams but the others[33-36,38] used milliliters as the unit of measurement for intraoperative blood loss, therefore, SMD was used to synthesize the data. The intraoperative blood loss was lower in LATG than OTG (SMD = -1.71; 95%CI: -2.48 - -0.94, P < 0.001) (Figure 2B). All studies contained the number of retrieved lymph nodes. The difference in the mean number of retrieved lymph nodes between LATG and OTG was not significant in the pooled data (WMD = -1.41; 95%CI: -3.15 - 0.32, P = 0.11) (Figure 2C). Two studies described the proximal and distal margin distances[35,38]. Meta-analysis of the distal margin distance showed no significant difference between the two groups (WMD = 0.46 cm; 95%CI: -0.40 - 1.32, P = 0.29). However, the proximal margin distance of OTG was longer than that of LATG with a marginal difference (WMD = -0.40 cm; 95%CI: -0.82 - 0.02, P = 0.06). All intraoperative effect outcomes are summarized in Table 2.
| Outcome | No. of study | Sample size | Heterogeneity (P, I2) | Overall effect size | 95%CI of overall effect | P value | |
| LATG | OTG | ||||||
| Operating time (min) | 9 | 436 | 785 | < 0.001, 94% | WMD = 57.68 | 30.48-84.88 | < 0.001 |
| Blood loss | 7 | 309 | 404 | < 0.001, 95% | SMD = -1.71 | -2.48 - -0.94 | < 0.001 |
| Retrieved lymph nodes | 9 | 436 | 785 | 0.09, 41% | WMD = -1.41 | -3.15 - 0.32 | 0.11 |
| Proximal margin (cm) | 2 | 163 | 475 | 1.00, 0% | WMD = -0.40 | -0.82 - 0.02 | 0.06 |
| Distal margin (cm) | 2 | 163 | 475 | 0.67, 0% | WMD = 0.46 | -0.40 - 1.32 | 0.29 |
| Analgesics given | 4 | 221 | 300 | < 0.001, 93% | SMD = -0.86 | -1.62 - -0.11 | 0.02 |
| Duration of fever (d) | 2 | 112 | 138 | 0.47, 0% | WMD = -1.58 | -1.80 - -1.37 | < 0.001 |
| Time to first flatus (d) | 7 | 316 | 419 | < 0.001, 91% | WMD = -0.76 | -1.22 - -0.30 | 0.001 |
| Time to oral intake (d) | 4 | 161 | 257 | 0.04, 63% | WMD = -0.81 | -1.26 - -0.35 | < 0.001 |
| Hospital stay (d) | 8 | 354 | 691 | 0.02, 56% | WMD = -2.67 | -3.96 - -1.38 | < 0.001 |
| Overall complications | 9 | 436 | 785 | 0.10, 40% | RR = 0.79 | 0.61-1.02 | 0.07 |
| Surgical complications | 9 | 436 | 785 | 0.42, 2% | RR = 0.83 | 0.64-1.08 | 0.16 |
| Medical complications | 7 | 343 | 614 | 0.70, 0% | RR = 0.41 | 0.19-0.90 | 0.03 |
| Mortality | 4 | 229 | 490 | 0.66, 0% | RR = 0.72 | 0.20-2.57 | 0.61 |
The overall effects of operating time and blood loss remained unchanged in subgroups, although performing > 40 LATG cases demonstrated a moderate reduction in operating time and blood loss. Lymph node retrieval was lower in the subgroup with < 40 LATG cases performed (WMD = -6.12; 95%CI: -10.42 - -1.81, P = 0.005). However, there was no difference when > 40 LATG procedures were performed (WMD = -0.50; 95%CI: -2.4 - 1.39, P = 0.60). The outcomes of subgroup analysis are summarized in Table 3.
| Outcome | No. of study | Sample size | Heterogeneity (P, I2) | Overall effect size | 95%CI of overall effect | P value | |
| LATG | OTG | ||||||
| Operating time (min) | |||||||
| < 40 LATG cases | 4 | 104 | 125 | < 0.001, 95% | WMD = 81.99 | 1.47-162.5 | 0.05 |
| > 40 LATG cases | 5 | 332 | 660 | < 0.001, 93% | WMD = 42.53 | 16.23-68.82 | 0.002 |
| Blood loss | |||||||
| < 40 LATG cases | 3 | 77 | 92 | 0.40, 0% | SMD = -1.22 | -1.55 - -0.88 | < 0.001 |
| > 40 LATG cases | 4 | 232 | 312 | < 0.001, 97% | SMD = -2.07 | -3.35 - -0.79 | 0.002 |
| Retrieved lymph nodes | |||||||
| < 40 LATG cases | 4 | 104 | 125 | 0.20, 36% | WMD = -6.12 | -10.42 - -1.81 | 0.005 |
| > 40 LATG cases | 5 | 332 | 660 | 0.47, 0% | WMD = -0.50 | -2.4 - 1.39 | 0.60 |
Flatus is one of the outcome measures for evaluating postoperative recovery of gastrointestinal functions. The mean time to first flatus was shorter in LATG than in OTG (WMD= -0.76 d; 95%CI: -1.22 - -0.30, P = 0.001), as was the time to restart oral intake after surgery (WMD = -0.81 d; 95%CI: -1.26 - -0.35, P < 0.001). Postoperative analgesic consumption was less in LATG than in OTG (SMD = -0.86; 95%CI: -1.62 - -0.11, P = 0.02). A shorter hospital stay was also observed in the LATG group (WMD = -2.67 d; 95%CI: -3.96 - -1.38, P < 0.001) (Figure 2D). All postoperative outcomes are summarized in Table 2.
Two studies reported inflammatory response index such as white blood cell (WBC) count and C-reactive protein (CRP)[32,33]. A significantly lower WBC count for LATG compared with OTG was found on postoperative days 1, 3, 7[32,33] and 10[33], and lower CRP for LATG was found on postoperative day 1 in both studies[32,33].
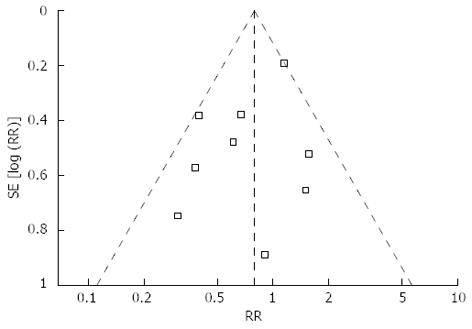
Mortality was described in four studies, and there was no significant difference in postoperative mortality (RR = 0.72, 95%CI: 0.20-2.57, P = 0.61) (Figure 2E). Morbidity was addressed and specified in all studies with exception of Kunisaki’s study[36]. We contacted the authors of this study to get information about the specific complications. The rate of overall postoperative complications was lower for LATG with a marginal difference (RR = 0.79, 95%CI: 0.61-1.02, P = 0.07) (Figure 2F). Visual inspection of the funnel plot revealed symmetry, indicating no serious publication bias (Figure 3). After further analysis, surgical complications were similar between the two groups (RR = 0.83, 95%CI: 0.64-1.08, P = 0.16) (Figure 2G), without the exception of any specific complications such as anastomotic leakage, intra-abdominal collections, bleeding, or anastomotic stricture. LATG was associated, however, with a significant reduction in medical complications (RR = 0.41, 95%CI: 0.19-0.90, P = 0.03) (Figure 2H) with a possible contribution from respiratory complications (RR = 0.34, 95%CI: 0.11-1.03, P = 0.06). The outcomes of mortality and morbidity are summarized in Table 2.
During the follow-up period, cancer recurrence was observed in four studies[31,32,34,36]. The recurrence risk in LATG was 13.2% (21/159) and 15.6% (29/186) in OTG, but the difference between LATG and OTG was not significant (RD = -0.03, 95%CI: -0.10-0.05, P = 0.49) (Figure 2I).
Three trials reported the long-term survival rate[31,36,37]. Mochiki et al[31] have reported that there was no significant difference in the cumulative or disease-specific 5-year survival rates between LATG and OTG (cumulative: 95% in LATG, 90.9% in OTG; disease-specific: 100% in LATG, 91.7% in OTG, P = 0.81). Eom et al[37] have reported that the survival rates were similar between groups; the hazard ratio of LATG vs OTG was 0.43 (95%CI: 0.15-1.20; P = 0.107) for overall survival and 0.47 (95%CI: 0.19-1.18; P = 0.106) for disease-free survival. Kunisaki et al[36] also have reported that there was no significant differences in overall and disease-specific survival between groups.
RCTs are the most ideal tools for meta-analysis. However, no RCTs on LATG have yet been conducted because the history and popularity of LATG are insufficient compared with LADG, due to the fact that it is difficult to dissect splenic hilar lymph nodes and mobilize the esophagus under a laparoscope, while it is demanding to perform Roux-en-Y esophagojejunostomy through mini-laparotomy. Thus, our meta-analysis synthesized the existing observational studies with strictly limiting inclusion and exclusion criteria. The included studies were primarily derived from the countries with the most widespread use of laparoscopic gastrectomy (four from Japan, three from Korea, and two from China), and all published in the past 5 years (2008-2012), and the total number of cases incorporated in the study was 1221. The meta-analysis conducted based on this point will contribute a more comprehensive and objective evaluation for the current LATG surgical status.
Similar to most reports comparing laparoscopic and open surgery in many different clinical situations, the intraoperative blooding in the LATG group was less than that in the OTG group, as is the need for transfusions. The reduced length of incision wound and the application of energy-dividing devices, such as the Harmonic Scalpel and Ligasure, contribute to the reduction in blood loss. Lack of blood is a common problem faced by many hospitals, especially in developing countries such as China. Therefore, less-invasive laparoscopic surgery can reduce the clinical requirement for blood and lower the rate of complications associated with blood transfusions such as virus infection and allergic reaction. In addition, some researchers have suggested that transfusions are associated with increased perioperative mortality and morbidity[39].
Regarding the operating time, LATG is more time-consuming than OTG. LATG combined with lymphadenectomy is a complex operation and needs a lot of technical expertise. Almost all of the studies included in this meta-analysis demonstrated prolonged operating time in LATG, despite significant heterogeneity. Learning curve which related to the surgeon’s experience, familiarity with instruments, and assistant compliance could influence some outcomes studied, such as operating time or lymph node retrieval[40]. Because several of the researches included in this study reported on their initial experience, so we performed a subgroup analysis using 40 LATG cases as a cut-point and demonstrated a moderate reduction in LATG operating time. Another reason for the prolonged operating time for LATG may be related to the reconstructive step, which is more difficult to complete through minilaparotomy than open surgery because of the narrow operating window for manual suture or anvil insertion and application of other instruments, especially in obese patients. To overcome these potential problems, various modified techniques have been reported, such as laparoscopic purse-string suture technique using Endo Stitch (Covidien, Mansfield, MA, United States)[41], Endo-PSI (Hope Electronics Co., Ltd, Shenzhen, China)[42], or a hemi-double stapling technique[43]. Another two intracorporeal reconstruction methods may be most representative; one using a transorally inserted anvil (OrVil; Covidien) to make an end-to-side esophagojejunostomy[44], the other using linear staplers to make a side-to-side anastomosis[45]. These methods not only avoid auxiliary incision, but also help to simplify the procedure of reconstruction and shorten the operating time[46,47].
The inflammatory stress reaction is an inevitable outcome of operative trauma and is an important index for measuring its extent. Some studies have compared inflammatory cytokines such as interleukin (IL)-6, IL-10 and CRP in plasma of patients who have undergone laparoscopic or laparotomic resection for gastroenteric cancer. The postoperative level of IL-6, IL-10 and CRP increased but the levels in the laparoscopic group are significantly lower than in the laparotomic group[48-50]. A meta-analysis of laparoscopic colectomy has also demonstrated that the postoperative IL-6 level of laparotomic group patients was significantly lower than that of laparotomic group[51]. The studies included in this research show that the WBC count and CRP of patients in the LATG group were lower than those in the OTG group, and serum protein was higher[32,33], indicating that LATG imposes few inflammatory stimuli on patients and consumes less protein. Kawamura et al[33] have also found that postoperative blood glucose in OTG patients is significantly higher than that in LATG patients when the same amount of calories was ingested, indicating that LATG has a lower effect on sugar metabolism.
The most striking finding was a reduced number of complications in the LATG vs OTG group, which may have resulted from a reduction in medical complications. It was conceivable that surgical complications were similar between groups because LATG results in the same organ and lymphatic resection as OTG. However, it is worth noting that some studies have found that there is a high risk of anastomotic stricture after LATG[10,52], whereas our study found morbidity associated with anastomotic stricture was similar between the two groups. Prevention of anastomotic stricture has long been one of the main tasks in total gastrectomy and also should not be ignored in LATG. Some researchers hold that side-to-side esophagojejunostomy could be used to reduce the risk of anastomotic stricture because a larger anastomotic stoma can be made from it[45,53]. Besides, the significantly decreased medical complications could be explained by the reduced invasiveness of the laparoscopic technique and less postoperative pain. We also found that respiratory complications occurred in LATG less often than in OTG, although the difference was not significant (P = 0.06). The pain caused by large incision as well as the use of tension sutures and abdominal bandages after laparotomy can make it difficult for patients to cough, expectorate and perform exercise breathing effectively, thus leading to such complications as pulmonary infection[54]. Pain after surgery was less serious in LATG than in OTG due to the shorter duration or the lower dosage of analgesic application[32-35]. The time to first flatus was also earlier in LATG than in OTG, which indicated a rapid recovery of gastrointestinal function after LATG. Reduced use of analgesic drugs, shortened time of abdominal cavity exposure, alleviated inflammatory reactions, and earlier postoperative activities are considered to be the main reasons for earlier gastrointestinal recovery from LATG; all of which may also contribute to shortening the duration of postoperative hospital stay.
The adequacy of the radical resection should be evaluated by the extent of lymph node dissection performed and the number of harvested lymph nodes, as well as the length of the resection margins. We found that fewer lymph nodes were obtained after LATG than in OTG, even though the difference was not significant. However, the subgroup analysis with 40 cases in LATG showed that the difference was shrinking. The number of laparoscopic lymph nodes dissected was closely related to the level of surgical technique. In recent years, with increasingly mature techniques, some researchers have reported not only a similar number of overall retrieved lymph nodes between LADG and open distal gastrectomy, but also a similar number of specific lymph nodes, such as group 7, 8a, 9, 11p, 12a and 14v, which used to be considered difficult for laparoscopic dissection[55,56]. Splenic hilar lymph node dissection is one of the difficulties in radical total gastrectomy, which is because the splenic vessels run circuitously, and the branches vary substantially and they are in a narrow space at a very deep location. It is easy to cause hemorrhage because of splenic vascular injury or cause spleen ischemia and further necrosis by accidental cutting of the splenic artery branches of when dissecting the lymph nodes in this area. Compared to laparotomy, laparoscopy allows the operator to complete the spleen hilum lymph node dissection under a clear field of view and helps to improve surgical safety[57].
With regard to the length of the resection margin, we found that the proximal margin in LATG was shorter than that of OTG. Such result may relate to the nature for LATG which should resect specimen and make reconstruction all through mini-laparotomy; and it is difficult to pull the proximal stomach using a narrow incision, which may influence the distance of proximal margin. Therefore, patients with smaller neoplasms are more likely to receive LATG instead of OTG, thus allowing the surgeon to choose a smaller excision extension.
Cancer recurrence and long-term survival rate are two critical outcomes for evaluating surgical interventions in oncological therapy. LATG is not superior to LADG in both history and popularity, and only three studies have compared the long-term survival rate between the two groups[31,36,37], and another two have performed a descriptive analysis of cancer recurrence[32,34]. Based on these data, postoperative cancer recurrence and long-term survival rate in LATG were similar to those in OTG. However, as the cases in the studies included in our analysis were mostly concerned with early gastric cancer, the effect of LATG for early gastric cancer should be affirmed. Some RCTs and meta-analyses have demonstrated that long-term follow-up outcome of laparoscopic gastrectomy for advanced gastric cancer is similar to that of laparotomy[58,59]. Recently, Park et al[60] have analyzed the follow-up results of 239 cases of advanced gastric cancer treated with laparoscopic radical gastrectomy. Among these cases, 130 were T2 stage, 63 were T3, and 46 were T4, and the 5-year survival rates were 86.6%, 77.4% and 58.7%, respectively. The result is similar to that for concurrent laparotomy and is encouraging. However, there should be an attitude of caution for laparoscopic resection of advanced gastric cancer because relevant studies and clinical evidence are still deficient.
During our research, a similar article by Haverkamp et al[61] was published, which had several limitations. The clinical heterogeneity could have been caused by the different underlying conditions and interventions. It is well known that gastric submucosal tumors (SMTs) such as lymphoma, leiomyosarcoma, and gastrointestinal stromal tumors are significantly different from adenocarcinoma in terms of biological characteristics, clinical diagnosis, and treatment. In our study, only patients who underwent gastrectomy for gastric adenocarcinoma were included, but Haverkamp et al included 8 patients undergoing total gastrectomy for SMTs; this may influence the reliability the results[28]. The difference in surgical methods is a major cause of clinical heterogeneity. In laparoscopy-assisted gastrectomy (LAG), an incision is almost always required for extracting a relatively large specimen and involves some complicated steps. However, totally laparoscopic gastrectomy (TLG) is considered to be incisionless, except for the trocar wounds, and it is a laparoscopic approach for intracorporeal anastomosis without auxiliary incision and touching the tumor. Hence, these are two different operative methods. Furthermore, some studies have shown that TLG may be less invasive than LAG, with the disadvantage of prolonged operating time[47,62-66]. Therefore, it is inappropriate to pool trials that differ in terms of these two methods in a meta-analysis. However, the existing meta-analysis included a study in which the TLG was performed using a totally laparoscopic method[23]. In addition, for the trials without the mean and standard deviation, Haverkamp et al used the median and range to estimate them based on the Hozo method[67]. However, this method may lead to deviation, especially when the sample size is small or the samples exhibit serious skewness. In the study of Topal for example[23], the median intraoperative blood in the laparoscopic group (n = 38) was 10 (5-400) mL, so the estimated mean blood loss was 10 mL. In fact, however, even the minimum mean blood loss could be 15.4 mL, which differed from the estimated value. Besides, since the study by Haverkamp et al[61] was published, several clinical observational studies have become available. The larger the number of patients in a meta-analysis, the greater its power to detect a possible treatment effect. Therefore, our comprehensive meta-analysis will contribute to a more systematic and objective evaluation for the safety and cancer treatment of LATG.
In conclusion, the existing research shows that LATG is safe and feasible, which can achieve similar lymph node dissection effects as OTG, characterized by such advantages as less pain, fewer postoperative complications, and rapid recovery, and which is expected to achieve the same effect in oncological treatment as OTG. However, most of the published studies were retrospective, the sample sizes were relatively small, most of the cases were early gastric cancer, the follow-up periods were not long enough, and the results exhibited substantial heterogeneity. Therefore, the results mentioned above should be subject to verification by strictly designed, large-sample, multicenter, RCTs.
Since it was first reported in 1994, laparoscopy-assisted distal gastrectomy (LADG) for gastric cancer has undergone rapid development and gained popularity in the past 20 years. Compared with traditional open gastrectomy, LADG can achieve better cosmesis, shorter hospital stay, faster postoperative recovery, and better postoperative quality of life. Although LADG has been accepted worldwide for tumors located in the lower stomach, laparoscopy-assisted total gastrectomy (LATG) for upper and middle gastric cancer has not been generalized. Although several meta-analyses and systematic reviews have been published for LADG, such studies have not been conducted for the potential benefits and disadvantages of LATG.
In order to assess accurately the current status of LATG, the authors strictly limited inclusion criteria by focusing exclusively on LATG and carried out a comprehensive meta-analysis. This will contribute to a more systematic and objective evaluation of the safety of the LATG in cancer treatment.
LATG is safe and feasible, which can achieve similar lymph node dissection effects as open total gastrectomy (OTG), characterized by such advantages as less pain, fewer postoperative complications, and rapid recovery, and which is expected to achieve the same effect in oncological treatment as OTG.
Despite a longer operation, LATG can be performed safely in experienced surgical centers with a shorter hospital stay and fewer complications than open surgery.
This is a well written paper which will add a great deal to the literature on the subject. One of the most significant conclusions from this work is the lack of randomised controlled trials surrounding the field. Future research should compare LADG and LATG to further verify the safety and feasibility of LATG.
| 1. | Kitano S, Iso Y, Moriyama M, Sugimachi K. Laparoscopy-assisted Billroth I gastrectomy. Surg Laparosc Endosc. 1994;4:146-148. [PubMed] |
| 2. | Lee JH, Han HS, Lee JH. A prospective randomized study comparing open vs laparoscopy-assisted distal gastrectomy in early gastric cancer: early results. Surg Endosc. 2005;19:168-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 329] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 3. | Cui M, Xing JD, Yang W, Ma YY, Yao ZD, Zhang N, Su XQ. D2 dissection in laparoscopic and open gastrectomy for gastric cancer. World J Gastroenterol. 2012;18:833-839. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 4. | Zhao Y, Yu P, Hao Y, Qian F, Tang B, Shi Y, Luo H, Zhang Y. Comparison of outcomes for laparoscopically assisted and open radical distal gastrectomy with lymphadenectomy for advanced gastric cancer. Surg Endosc. 2011;25:2960-2966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 76] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 5. | Kim HH, Hyung WJ, Cho GS, Kim MC, Han SU, Kim W, Ryu SW, Lee HJ, Song KY. Morbidity and mortality of laparoscopic gastrectomy versus open gastrectomy for gastric cancer: an interim report--a phase III multicenter, prospective, randomized Trial (KLASS Trial). Ann Surg. 2010;251:417-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 562] [Cited by in RCA: 635] [Article Influence: 39.7] [Reference Citation Analysis (0)] |
| 6. | Kim YW, Baik YH, Yun YH, Nam BH, Kim DH, Choi IJ, Bae JM. Improved quality of life outcomes after laparoscopy-assisted distal gastrectomy for early gastric cancer: results of a prospective randomized clinical trial. Ann Surg. 2008;248:721-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Jeong O, Park YK. Clinicopathological features and surgical treatment of gastric cancer in South Korea: the results of 2009 nationwide survey on surgically treated gastric cancer patients. J Gastric Cancer. 2011;11:69-77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 185] [Cited by in RCA: 193] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 8. | Kitano S, Shiraishi N. Current status of laparoscopic gastrectomy for cancer in Japan. Surg Endosc. 2004;18:182-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 90] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 9. | Jeong GA, Cho GS, Kim HH, Lee HJ, Ryu SW, Song KY. Laparoscopy-assisted total gastrectomy for gastric cancer: a multicenter retrospective analysis. Surgery. 2009;146:469-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 82] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 10. | Lee SE, Ryu KW, Nam BH, Lee JH, Kim YW, Yu JS, Cho SJ, Lee JY, Kim CG, Choi IJ. Technical feasibility and safety of laparoscopy-assisted total gastrectomy in gastric cancer: a comparative study with laparoscopy-assisted distal gastrectomy. J Surg Oncol. 2009;100:392-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 91] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 11. | Shinohara T, Kanaya S, Taniguchi K, Fujita T, Yanaga K, Uyama I. Laparoscopic total gastrectomy with D2 lymph node dissection for gastric cancer. Arch Surg. 2009;144:1138-1142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 88] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 12. | Wada N, Kurokawa Y, Takiguchi S, Takahashi T, Yamasaki M, Miyata H, Nakajima K, Mori M, Doki Y. Feasibility of laparoscopy-assisted total gastrectomy in patients with clinical stage I gastric cancer. Gastric Cancer. 2013;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 13. | Hosono S, Arimoto Y, Ohtani H, Kanamiya Y. Meta-analysis of short-term outcomes after laparoscopy-assisted distal gastrectomy. World J Gastroenterol. 2006;12:7676-7683. [PubMed] |
| 14. | Yakoub D, Athanasiou T, Tekkis P, Hanna GB. Laparoscopic assisted distal gastrectomy for early gastric cancer: is it an alternative to the open approach? Surg Oncol. 2009;18:322-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 82] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 15. | Chen XZ, Hu JK, Yang K, Wang L, Lu QC. Short-term evaluation of laparoscopy-assisted distal gastrectomy for predictive early gastric cancer: a meta-analysis of randomized controlled trials. Surg Laparosc Endosc Percutan Tech. 2009;19:277-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 16. | Ohtani H, Tamamori Y, Noguchi K, Azuma T, Fujimoto S, Oba H, Aoki T, Minami M, Hirakawa K. A meta-analysis of randomized controlled trials that compared laparoscopy-assisted and open distal gastrectomy for early gastric cancer. J Gastrointest Surg. 2010;14:958-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 99] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 17. | Ohtani H, Tamamori Y, Noguchi K, Azuma T, Fujimoto S, Oba H, Aoki T, Minami M, Hirakawa K. Meta-analysis of laparoscopy-assisted and open distal gastrectomy for gastric cancer. J Surg Res. 2011;171:479-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | Ding J, Liao GQ, Liu HL, Liu S, Tang J. Meta-analysis of laparoscopy-assisted distal gastrectomy with D2 lymph node dissection for gastric cancer. J Surg Oncol. 2012;105:297-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 19. | Zeng YK, Yang ZL, Peng JS, Lin HS, Cai L. Laparoscopy-assisted versus open distal gastrectomy for early gastric cancer: evidence from randomized and nonrandomized clinical trials. Ann Surg. 2012;256:39-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 164] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 20. | Grobmyer SR, Pieracci FM, Allen PJ, Brennan MF, Jaques DP. Defining morbidity after pancreaticoduodenectomy: use of a prospective complication grading system. J Am Coll Surg. 2007;204:356-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 205] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 21. | Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008-2012. [PubMed] |
| 22. | Clarke M, Horton R. Bringing it all together: Lancet-Cochrane collaborate on systematic reviews. Lancet. 2001;357:1728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 206] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 23. | Topal B, Leys E, Ectors N, Aerts R, Penninckx F. Determinants of complications and adequacy of surgical resection in laparoscopic versus open total gastrectomy for adenocarcinoma. Surg Endosc. 2008;22:980-984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 83] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 24. | Siani LM, Ferranti F, De Carlo A, Quintiliani A. Completely laparoscopic versus open total gastrectomy in stage I-III/C gastric cancer: safety, efficacy and five-year oncologic outcome. Minerva Chir. 2012;67:319-326. [PubMed] |
| 25. | Kim HS, Kim BS, Lee IS, Lee S, Yook JH, Kim BS. Comparison of totally laparoscopic total gastrectomy and open total gastrectomy for gastric cancer. J Laparoendosc Adv Surg Tech A. 2013;23:323-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 26. | Usui S, Yoshida T, Ito K, Hiranuma S, Kudo SE, Iwai T. Laparoscopy-assisted total gastrectomy for early gastric cancer: comparison with conventional open total gastrectomy. Surg Laparosc Endosc Percutan Tech. 2005;15:309-314. [PubMed] |
| 27. | Arrington AK, Nelson R, Chen SL, Ellenhorn JD, Garcia-Aguilar J, Kim J. The evolution of surgical technique for total gastrectomy over a 12-year period: a single institution’s experience. Am Surg. 2012;78:1054-1058. [PubMed] |
| 28. | Dulucq JL, Wintringer P, Stabilini C, Solinas L, Perissat J, Mahajna A. Laparoscopic and open gastric resections for malignant lesions: a prospective comparative study. Surg Endosc. 2005;19:933-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 87] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 29. | Jeong O, Jung MR, Kim GY, Kim HS, Ryu SY, Park YK. Comparison of short-term surgical outcomes between laparoscopic and open total gastrectomy for gastric carcinoma: case-control study using propensity score matching method. J Am Coll Surg. 2013;216:184-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 68] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 30. | Kim SG, Lee YJ, Ha WS, Jung EJ, Ju YT, Jeong CY, Hong SC, Choi SK, Park ST, Bae K. LATG with extracorporeal esophagojejunostomy: is this minimal invasive surgery for gastric cancer? J Laparoendosc Adv Surg Tech A. 2008;18:572-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 31. | Mochiki E, Toyomasu Y, Ogata K, Andoh H, Ohno T, Aihara R, Asao T, Kuwano H. Laparoscopically assisted total gastrectomy with lymph node dissection for upper and middle gastric cancer. Surg Endosc. 2008;22:1997-2002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 100] [Article Influence: 5.6] [Reference Citation Analysis (1)] |
| 32. | Sakuramoto S, Kikuchi S, Futawatari N, Katada N, Moriya H, Hirai K, Yamashita K, Watanabe M. Laparoscopy-assisted pancreas- and spleen-preserving total gastrectomy for gastric cancer as compared with open total gastrectomy. Surg Endosc. 2009;23:2416-2423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 95] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 33. | Kawamura H, Yokota R, Homma S, Kondo Y. Comparison of invasiveness between laparoscopy-assisted total gastrectomy and open total gastrectomy. World J Surg. 2009;33:2389-2395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 34. | Du J, Zheng J, Li Y, Li J, Ji G, Dong G, Yang Z, Wang W, Gao Z. Laparoscopy-assisted total gastrectomy with extended lymph node resection for advanced gastric cancer--reports of 82 cases. Hepatogastroenterology. 2010;57:1589-1594. [PubMed] |
| 35. | Kim MG, Kim BS, Kim TH, Kim KC, Yook JH, Kim BS. The effects of laparoscopic assisted total gastrectomy on surgical outcomes in the treatment of gastric cancer. J Korean Surg Soc. 2011;80:245-250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 36. | Kunisaki C, Makino H, Kosaka T, Oshima T, Fujii S, Takagawa R, Kimura J, Ono HA, Akiyama H, Taguri M. Surgical outcomes of laparoscopy-assisted gastrectomy versus open gastrectomy for gastric cancer: a case-control study. Surg Endosc. 2012;26:804-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 37. | Eom BW, Kim YW, Lee SE, Ryu KW, Lee JH, Yoon HM, Cho SJ, Kook MC, Kim SJ. Survival and surgical outcomes after laparoscopy-assisted total gastrectomy for gastric cancer: case-control study. Surg Endosc. 2012;26:3273-3281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 38. | Guan G, Jiang W, Chen Z, Liu X, Lu H, Zhang X. Early results of a modified splenic hilar lymphadenectomy in laparoscopy-assisted total gastrectomy for gastric cancer with stage cT1-2: a case-control study. Surg Endosc. 2013;27:1923-1931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 39. | Wu WC, Smith TS, Henderson WG, Eaton CB, Poses RM, Uttley G, Mor V, Sharma SC, Vezeridis M, Khuri SF. Operative blood loss, blood transfusion, and 30-day mortality in older patients after major noncardiac surgery. Ann Surg. 2010;252:11-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 166] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 40. | Kim MC, Jung GJ, Kim HH. Learning curve of laparoscopy-assisted distal gastrectomy with systemic lymphadenectomy for early gastric cancer. World J Gastroenterol. 2005;11:7508-7511. [PubMed] |
| 41. | Takiguchi S, Sekimoto M, Fujiwara Y, Miyata H, Yasuda T, Doki Y, Yano M, Monden M. A simple technique for performing laparoscopic purse-string suturing during circular stapling anastomosis. Surg Today. 2005;35:896-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 42. | Usui S, Nagai K, Hiranuma S, Takiguchi N, Matsumoto A, Sanada K. Laparoscopy-assisted esophagoenteral anastomosis using endoscopic purse-string suture instrument “Endo-PSI (II)” and circular stapler. Gastric Cancer. 2008;11:233-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 73] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 43. | Omori T, Oyama T, Mizutani S, Tori M, Nakajima K, Akamatsu H, Nakahara M, Nishida T. A simple and safe technique for esophagojejunostomy using the hemidouble stapling technique in laparoscopy-assisted total gastrectomy. Am J Surg. 2009;197:e13-e17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 68] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 44. | Jeong O, Park YK. Intracorporeal circular stapling esophagojejunostomy using the transorally inserted anvil (OrVil) after laparoscopic total gastrectomy. Surg Endosc. 2009;23:2624-2630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 150] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 45. | Ziqiang W, ZhiMin C, Jun C, Xiao L, Huaxing L, PeiWu Y. A modified method of laparoscopic side-to-side esophagojejunal anastomosis: report of 14 cases. Surg Endosc. 2008;22:2091-2094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 46. | Liao GQ, Ou XW, Liu SQ, Zhang SR, Huang W. Laparoscopy-assisted total gastrectomy with trans-orally inserted anvil (OrVil™): a single institution experience. World J Gastroenterol. 2013;19:755-760. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 47. | Kim HS, Kim MG, Kim BS, Lee IS, Lee S, Yook JH, Kim BS. Comparison of totally laparoscopic total gastrectomy and laparoscopic-assisted total gastrectomy methods for the surgical treatment of early gastric cancer near the gastroesophageal junction. J Laparoendosc Adv Surg Tech A. 2013;23:204-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 48. | Veenhof AA, Sietses C, von Blomberg BM, van Hoogstraten IM, vd Pas MH, Meijerink WJ, vd Peet DL, vd Tol MP, Bonjer HJ, Cuesta MA. The surgical stress response and postoperative immune function after laparoscopic or conventional total mesorectal excision in rectal cancer: a randomized trial. Int J Colorectal Dis. 2011;26:53-59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 101] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 49. | Hildebrandt U, Kessler K, Plusczyk T, Pistorius G, Vollmar B, Menger MD. Comparison of surgical stress between laparoscopic and open colonic resections. Surg Endosc. 2003;17:242-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 117] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 50. | Natsume T, Kawahira H, Hayashi H, Nabeya Y, Akai T, Horibe D, Shuto K, Akutsu Y, Matsushita K, Nomura F. Low peritoneal and systemic inflammatory response after laparoscopy-assisted gastrectomy compared to open gastrectomy. Hepatogastroenterology. 2011;58:659-662. [PubMed] |
| 51. | Sammour T, Kahokehr A, Chan S, Booth RJ, Hill AG. The humoral response after laparoscopic versus open colorectal surgery: a meta-analysis. J Surg Res. 2010;164:28-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 66] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 52. | Kachikwu EL, Trisal V, Kim J, Pigazzi A, Ellenhorn JD. Minimally invasive total gastrectomy for gastric cancer: a pilot series. J Gastrointest Surg. 2011;15:81-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 53. | Okabe H, Obama K, Tsunoda S, Tanaka E, Sakai Y. Advantage of Completely Laparoscopic Gastrectomy With Linear Stapled Reconstruction: A Long-term Follow-up Study. Ann Surg. 2013;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 89] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 54. | Ephgrave KS, Kleiman-Wexler R, Pfaller M, Booth B, Werkmeister L, Young S. Postoperative pneumonia: a prospective study of risk factors and morbidity. Surgery. 1993;114:815-89; discussion 815-89;. [PubMed] |
| 55. | Song KY, Kim SN, Park CH. Laparoscopy-assisted distal gastrectomy with D2 lymph node dissection for gastric cancer: technical and oncologic aspects. Surg Endosc. 2008;22:655-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 94] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 56. | Chen QY, Huang CM, Lin JX, Zheng CH, Li P, Xie JW, Wang JB, Lu J. Laparoscopy-assisted versus open D2 radical gastrectomy for advanced gastric cancer without serosal invasion: a case control study. World J Surg Oncol. 2012;10:248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 57. | Jia-Bin W, Chang-Ming H, Chao-Hui Z, Ping L, Jian-Wei X, Jian-Xian L. Laparoscopic spleen-preserving No. 10 lymph node dissection for advanced proximal gastric cancer in left approach: a new operation procedure. World J Surg Oncol. 2012;10:241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 58. | Huscher CG, Mingoli A, Sgarzini G, Sansonetti A, Di Paola M, Recher A, Ponzano C. Laparoscopic versus open subtotal gastrectomy for distal gastric cancer: five-year results of a randomized prospective trial. Ann Surg. 2005;241:232-237. [PubMed] |
| 59. | Martínez-Ramos D, Miralles-Tena JM, Cuesta MA, Escrig-Sos J, Van der Peet D, Hoashi JS, Salvador-Sanchís JL. Laparoscopy versus open surgery for advanced and resectable gastric cancer: a meta-analysis. Rev Esp Enferm Dig. 2011;103:133-141. [PubMed] |
| 60. | Park do J, Han SU, Hyung WJ, Kim MC, Kim W, Ryu SY, Ryu SW, Song KY, Lee HJ, Cho GS. Long-term outcomes after laparoscopy-assisted gastrectomy for advanced gastric cancer: a large-scale multicenter retrospective study. Surg Endosc. 2012;26:1548-1553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 140] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 61. | Haverkamp L, Weijs TJ, van der Sluis PC, van der Tweel I, Ruurda JP, van Hillegersberg R. Laparoscopic total gastrectomy versus open total gastrectomy for cancer: a systematic review and meta-analysis. Surg Endosc. 2013;27:1509-1520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 141] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 62. | Song KY, Park CH, Kang HC, Kim JJ, Park SM, Jun KH, Chin HM, Hur H. Is totally laparoscopic gastrectomy less invasive than laparoscopy-assisted gastrectomy?: prospective, multicenter study. J Gastrointest Surg. 2008;12:1015-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 137] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 63. | Ikeda O, Sakaguchi Y, Aoki Y, Harimoto N, Taomoto J, Masuda T, Ohga T, Adachi E, Toh Y, Okamura T. Advantages of totally laparoscopic distal gastrectomy over laparoscopically assisted distal gastrectomy for gastric cancer. Surg Endosc. 2009;23:2374-2379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 119] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 64. | Kinoshita T, Shibasaki H, Oshiro T, Ooshiro M, Okazumi S, Katoh R. Comparison of laparoscopy-assisted and total laparoscopic Billroth-I gastrectomy for gastric cancer: a report of short-term outcomes. Surg Endosc. 2011;25:1395-1401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 83] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 65. | Kim MG, Kawada H, Kim BS, Kim TH, Kim KC, Yook JH, Kim BS. A totally laparoscopic distal gastrectomy with gastroduodenostomy (TLDG) for improvement of the early surgical outcomes in high BMI patients. Surg Endosc. 2011;25:1076-1082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 82] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 66. | Kim BS, Yook JH, Choi YB, Kim KC, Kim MG, Kim TH, Kawada H, Kim BS. Comparison of early outcomes of intracorporeal and extracorporeal gastroduodenostomy after laparoscopic distal gastrectomy for gastric cancer. J Laparoendosc Adv Surg Tech A. 2011;21:387-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 67. | Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4895] [Cited by in RCA: 7266] [Article Influence: 346.0] [Reference Citation Analysis (1)] |
P- Reviewers Braga S, Clark J, Zhang WH S- Editor Wen LL L- Editor A E- Editor Zhang DN