Published online Aug 28, 2013. doi: 10.3748/wjg.v19.i32.5227
Revised: July 3, 2013
Accepted: July 17, 2013
Published online: August 28, 2013
Processing time: 141 Days and 4 Hours
Local recurrence (LR) has an adverse impact on rectal cancer treatment. Neoadjuvant chemoradiotherapy (nCRT) is increasingly administered to patients with progressive cancers to improve the prognosis. However, LR still remains a problem and its pattern can alter. Correspondingly, new risk factors have emerged in the context of nCRT in addition to the traditional risk factors in patients receiving non-neoadjuvant therapies. These risk factors are decisive when reviewing treatment options. This review aims to elucidate the distinctive risk factors related to LR of rectal cancers in patients receiving nCRT and to clarify their clinical significance. A search was conducted on PubMed to identify original studies investigating patients with rectal cancer receiving nCRT. Outcomes of interest, especially potential risk factors for LR in patients with nCRT, were then analyzed. The clinical importance of these risk factors is discussed. Remnant cancer cells, lymph-nodes and tumor response were found to be major risk factors. Remnant cancer cells decide the status of resection margins. Local excision following nCRT is promising in ypT0-1N0M0 cases. Dissection of lateral lymph nodes should be considered in advanced low-lying cancers. Although better tumor response resulted in a relatively lower recurrence rate, the evidence available is insufficient to justify a non-operative approach in clinical complete responders to nCRT. LR cannot be totally avoided by current multidisciplinary approaches. The related risk factors resulting from nCRT should be considered when making decisions regarding treatment selection.
Core tip: This review identifies the distinctive risk factors associated with local recurrence (LR) in patients with rectal cancer receiving neoadjuvant therapy. These factors are different from the traditional risk factors seen in patients treated with surgery and/or adjuvant therapy alone. The clinical significance of these risk factors is clarified in detail. To our knowledge, no reviews concerning this topic have been published. The present manuscript might help to understand the origin of LR following neoadjuvant chemoradiotherapy and may receive attention from investigators devoted to improving the prognosis of rectal cancer.
- Citation: Peng JY, Li ZN, Wang Y. Risk factors for local recurrence following neoadjuvant chemoradiotherapy for rectal cancers. World J Gastroenterol 2013; 19(32): 5227-5237
- URL: https://www.wjgnet.com/1007-9327/full/v19/i32/5227.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i32.5227
Local recurrence (LR) is a major problem and threatens the prognosis of rectal cancer patients. For locally progressive tumors, LR can not be prevented just by improving surgical techniques. Therefore, preoperative, also known as neoadjuvant, therapy has been advocated due to its ability to down-stage tumors and thus increase resectability. Multidisciplinary neoadjuvant approaches have been proven to effectively control LR[1,2] and improve overall survival[3,4]. However, LR still occurs[5,6] and its pattern can change[7,8] with regard to time and location. For example, the time from operation to LR is prolonged[9]. Most importantly, neoadjuvant therapy and its downsizing effects on tumors have resulted in the emergence of some LR-associated risk factors unlike those related with only surgery plus adjuvant chemoradiotherapy, such as vascular invasion or tumor differentiation[8,10]. These distinctive risk factors, consisting of isolated remnant cancer cells and tumor response to neoadjuvant chemoradiotherapy (nCRT), have been reported to be associated with the prognosis of patients[11]. Therefore, determination of the characteristics of these factors and their clinical significance would provide very helpful data for clinical practice.
The aim of the present review was to characterize the risk factors in patients receiving neoadjuvant therapy, mainly nCRT. Moreover, the clinical implications of these risk factors in treatment decision-making following nCRT were also explored.
A systematic review was performed in order to explore potential risk factors for LR following nCRT. A literature search was performed in PubMed and EMBASE databases for English-language papers published over the last 10 years, with outcome data limited to humans. The search terms used included “rectal cancer” or “rectal neoplasm”; “neoadjuvant” or “preoperative”; “radiotherapy” or “chemotherapy” or “chemoradiotherapy”; “recurrence” or “local recurrence” or “local control” or “local relapse” or “local failure” or “prognosis”.
The criteria for including potential studies in the systematic review were: (1) randomized clinical trials (RCTs) or cohort studies investigating patients with rectal cancer receiving nCRT; (2) retrospective studies of LR in patients with rectal cancer who were treated with nCRT; and (3) studies evaluating parameters (risk factors) that may influence the outcome in terms of LR in patients with rectal cancer who were treated with nCRT. Articles that did not show LR or investigate the causes of LR were excluded. Furthermore, abstract-only publications and chapters from books were excluded. When the same series of patients were reported by the same authors in different articles, only the series with the longest follow-up was included in the review.
Two reviewers independently reviewed each article, and discrepancies were resolved by discussion and consensus. All data were extracted from the main text, tables, and figures of the articles. Traditional risk factors such as differentiation, vascular invasion, TNM staging and circumferential resection margin status were excluded. Risk factors related to the downsizing effect of nCRT were included.
Analysis of the data from the included studies was carried out. Descriptive statistics (simple counts, means, and medians) were either directly derived from the article or calculated based on the data presented in the article, and used to report studies, patients, and treatment-level data. Outcomes of interest, especially potential risk factors for LR in patients who received nCRT were synthesized by pooling relevant data, and then analyzed. Due to high heterogeneity among the studies and lack of RCTs, a meta-analysis was not deemed appropriate.
To better understand the risk factors, a deep insight into the patterns of LR is required. The patterns of LR can be described by two aspects, namely timing and location. The first aspect is the time interval to development of LR. Habr-Gama et al[9] found that the mean recurrence interval was 52 mo (18-79 mo) in 6 cases with sustained complete clinical response to nCRT. However, Coco et al[6] reported that the time to development of LR was longer than 5 years in approximately one third of cases treated with nCRT (4 of 14 cases). Similar results were observed in studies[12,13], in which only neoadjuvant radiotherapy (nRT) was administered. However, in a study which included patients receiving surgery alone or associated with postoperative chemoradiotherapy (pCRT) with an average follow-up of 10 years, LR occurred in 72% of patients within 18 mo of surgery[14]. These data suggest that neoadjuvant therapy may have an ongoing impact, different from that of pCRT, on the natural history of rectal cancer. This may be the reason why a better response can be induced by nCRT over time[15,16].
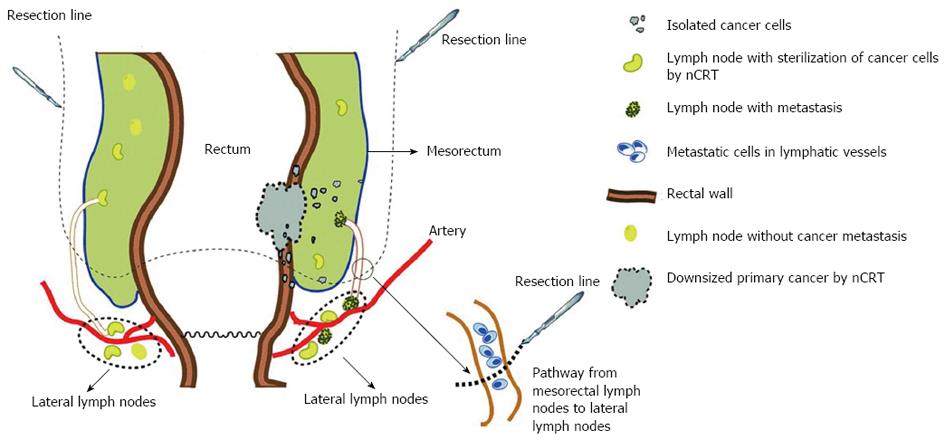
The second pattern is the subtle alteration concerning subsites of LR. It has been shown that the incidence of anastomotic recurrence is declining[12,17]. The two most common sites of LR in nCRT cases are the lower pelvis (56%) and presacral region (22%)[18,19]. Syk et al[20] indicated that the majority of LRs in patients receiving nRT were located anatomically below the S1-S2 interspace. The higher frequency of LR within the presacral area in patients undergoing nRT may be explained by the unique anatomical locations of the mesorectum and lateral lymph nodes (LLNs). The mesorectum is defined as the fatty and fibrous tissues surrounding the rectum. Most mesorectal tissues are located at the dorsal side of the rectum and include lymphatic and vascular vessels to which cancer may disseminate. Furthermore, a recent anatomical study revealed the presence of an alternative lymphatic drainage pathway from mesorectal LNs to LLNs[21] using three-dimensional reconstruction and histological section. This connection may provide a pathway for the cancer cells to spread or escape and LLNs may serve as a harbor for these cells[22,23]. Some isolated cancer cells in the mesorectum or lymphatic tissues (see “Isolated tumor cells”) serve as seeds for LR following nCRT. These cells are inhibited, but not killed, by nRT and rest in the G0 phase[24]. During surgery, cells may be spilled and implanted in the lower pelvis and presacral region resulting in LR.
We hypothesize that the seeds of LR may be the cancer cells at the margin of the mesorectum or within the lymphatic pathway from the mesorectum to LLNs. During a standard total mesorectum resection (TME), these cells may “leak” following complete resection of the mesorectum, implant in the presacral space due to the force of gravity and trigger subsequent LR (Figure 1). This hypothesis may be further confirmed if the tumor cells can be separated from post-operative lymph fluid drainage.
Understanding the altered LR patterns in patients with different neoadjuvant and intraoperative therapies has practical implications. On the one hand, delayed LR occurs in patients receiving nCRT, and thus, the standard 5-year follow-up currently recommended by the European Society for Medical Oncology[25] should be extended to at least 7-8 years and intensified monitoring is required in selected cases[26]. In addition, if delayed LR is expected to occur in a proportion of patients, the observational period in prospective and randomized trials[4,27] should be prolonged in order to draw more definitive conclusions. On the other hand, attention should be paid to common regions involved in LR in patients receiving neoadjuvant therapies which may help us accurately select the area at high risk for radiotherapy and avoid unnecessary irradiation.
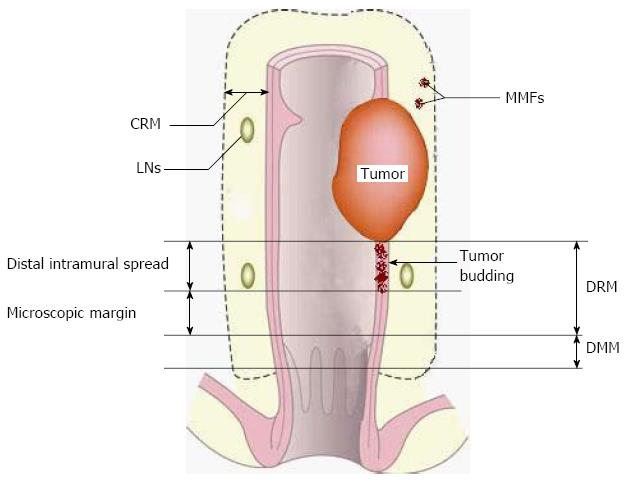
As mentioned above, nCRT may be “suppressive” rather than “destructive” for a certain proportion of cancer cells. Thus, the surviving cells, if not removed by surgery, may restore their viability and evolve into seed cells for LR (Figure 1). These seed cells can be divided into two groups, extranodal and intranodal seed cells, according to their relationship with lymph nodes (LNs). Furthermore, two major types of LR derived from extranodal seed cells, tumor budding (inside the bowel wall) and mesorectal microfoci (MMF), have been reported, according to their locations.
Tumor budding is described as a subset of isolated cancer cells located at the invasive front and extending from the neoplastic gland structures to the adjacent stroma[28]. Tumor budding has been reported to be an independent factor predicting prognosis[29,30]. Research on nCRT cases has shown that tumor budding is always described as isolated or small clusters of remnant cancer cells resulting from tumor regression. A control-case study[24] showed that nRT increased the frequency of budding cells compared with surgery without nRT (mean 54 vs 38, P = 0.03). These cells are always surrounded by fibrosis or an inflammatory reaction induced by nCRT. NCRT-induced tumor budding can be classified into two grades: high grade (clusters of budding cells easily observed by pathological examination) and low grade (minimal or isolated budding barely detected by pathological examination). According to Gavioli et al[31] study of 139 patients with nCRT, LR did not appear in the low grade budding group, while 8.8% of the high grade budding patients developed LR. In a more recent study, patients with low grade budding also had better 5-year disease-free survival than those with high grade budding (87.5% vs 55.6%, P < 0.0001).
It has been shown that the distal intramural spread of tumor budding is discontinuous in 57% of patients receiving nCRT[32]. The nature of this discontinuity is of special clinical importance; the supposed “clean” distal resection margin (DRM) in sphincter-sparing resection may not necessarily be free of cancer cells and longer a DRM may be required in a proportion of patients due to the possible existence of tumor budding. Thus, the focus is now “How far does tumor budding go?” Two studies demonstrated that DRMs less than 10 mm did not compromise LR[33,34]. In contrast, a study with a longer follow-up (5.6 years) demonstrated that a DRM less than 8 mm was associated with increased LR[35]. Why was there discrepancy between these two studies? First, the average period of follow-up may have had an influence. The follow-up time in these two studies may have been too short to draw definite conclusions (both were less than 36 mo). Second, the whole-mount section of the pathological examination was not used in these two studies, making the conclusion less convincing. Studies using whole-mount sections have shown that approximately 90% of patients receiving nCRT have a distal intramural extension of tumor budding within 5 mm, and 8% within 6-10 mm and less than 2% over 10 mm[32,36,37] (Table 1). Correspondingly, it has been suggested that the required length of the DRM should be shortened from 20 to 10 mm due to tumor remission induced by nCRT[36]. A DRM less than 10 mm is not yet justified for cases receiving nCRT based on current evidence. Therefore, following nCRT, the existence of budding cells is discontinuous and a supposed “negative” DRM less than 10 mm may not be a real negative margin for low-lying cancers.
Unlike tumor budding which is intramural, MMF, another risk factor for LR, is mesorectal. MMF is primarily defined as extranodal cancer deposits discontinuous with the primary tumor[38] in the mesorectum. The incidence of MMF is reported to be directly associated with the infiltrating depth of the primary tumor[38].
Ratto et al[39] specifically classified MMF into four major subtypes: endovascular (cancer deposits in blood vessels), endolymphatic (cancer deposits in lymphatic vessels but not in lymph nodes), perineural (cancer cell aggregates between the fasciculus and perineurium) and isolated (cancer deposits within the mesorectum, not a continuous extension from the main tumor mass). Clinically, MMF can be identified by careful pathological examination. Studies[39-41] have shown that MMF are detected in 13.8%-44.2% of cases after surgery despite downstaging induced by nCRT. Prabhudesai et al[38] reported that LR occurred in 17.2% (5/29) of patients with MMF and in 3.8% (1/26) of those without MMF, although the difference was not statistically significant.
Similar to tumor budding, MMF may decide the status of the circumferential resection margin (CRM) and distal mesorectal margin (DMM) (Figure 2). However, no data are available regarding the appropriate CRM and DMM after nCRT. Should CRM and DMM be correspondingly shortened? Further pathological studies are required.
Cancer cells harbored within LNs surrounding the rectum may serves as the seeds for LR. Although the nCRT-induced tumor regression does not necessarily parallel the sterilization of LNs metastasis, better tumor response may predict less LNs metastasis. Recent studies have proven that tumors at stage ypT0-1 correlate with a very low incidence of positive LN involvement[31,42-52] (Table 2). With regard to stage ypT2, LN involvement is present in about 20%-30% of cases[44,48].
| Ref. | No. of patients | Neoadjuvant therapy regimen | Time interval1 (wk) | No. of patients with ypT0/T1 | ||
| Radiotherapy (Gy) | Chemotherapy | ypN+/ypT0-1 | ypN+/ypT2-4 | |||
| Zmora et al[42] | 109 | 45-50.4 | 5-Fu | 6 | 4/33 (12.1) | 30/61 (49.2) |
| Read et al[43] | 644 | 20-45 | 5-Fu | NS | 3/87 (3.4) | 217/557 (39.0) |
| Bujko et al[44] | 147 | 5 × 5 | 1 | 0/4 (0.0) | 69/138 (50.0) | |
| Bujko et al[44] | 138 | 50.4 | 5-Fu | 4-6 | 2/33 (6.1) | 41/101 (40.6) |
| Pucciarelli et al[45] | 235 | 45-50.4 | 5-Fu | 6-8 | 3/69 (4.3) | 45/166 (27.1) |
| Tulchinsky et al[46] | 101 | 45 | 5-Fu | 5-7 | 1/22 (4.5) | 29/75 (38.7) |
| Habr-Gama et al[47] | 401 | 50.4 | 5-Fu | 8 | 3/25 (10.7) | 75/224 (33.5) |
| Stipa et al[48] | 187 | 50.4 | 5-Fu | NS | 3/44 (6.8) | 48/143 (33.6) |
| Kundel et al[49] | 320 | 45 | 5-Fu | 4-8 | 3/69 (4.3) | 49/222 (22.1) |
| Gavioli et al[31] | 139 | 50 | 5-Fu | 4 | 2/34 (5.9) | 38/105 (36.2) |
| Kim et al[50] | 282 | 45 | 5-Fu | 4-8 | 2/58 (3.4) | 85/224 (37.9) |
| Lindebjerg et al[51] | 135 | 60 | 5-Fu | 8 | 8/47 (17.0) | 32/88 (36.4) |
| Coco et al[52] | 271 | NS | NS | NS | 3/71 (4.2) | 70/200 (35.0) |
| Total | 3109 | 37/596 (6.2) | 828/2304 (35.9) | |||
With the belief that favorable tumor response may be equal to the disappearance of LNs metastasis, we propose that a proportion of pretreated T3 or T4 tumors might meet the requirements for local excision (LE). Several studies have shown that LR is not observed in ypT0 cases followed by LE, and the LR rate is around 3%-6% in ypT1 cases[53-60]. Moreover, the LR cases can be efficiently salvaged by subsequent radical dissection if early detection is achieved[54,61]. Therefore, LE is recommended by some authors for ypT0 or ypT1 cases due to its efficacy in local control which is equivalent to radical surgery[49,52,53,59,61-64]. Although these results are encouraging, the majority of the above-mentioned studies are retrospective and include small sample sizes. Thus, further prospective, population-based and multi-center investigations are required to confirm these results.
With regard to ypT2 stage, 63% (53/88) of patients with ypT2 are reported to have at least one unfavorable pathological feature in addition to LNs metastases (vascular or perineural invasion, mucinous type and tumor size > 3 cm) for LE[65]. Perez et al[66] reported that the LR rate in patients with ypT2 who underwent LE was 9% (8/88) after nCRT. In cases with ypT3N0 or ypT4N0, the rate was up to 25% (14/25), including 14.7% (n = 8) systemic and 10.3% (n = 6) local relapse despite the absence of LNs micro-metastasis[66]. These findings indicate that ypT2-4 may have more residual cancer cells than detected and these tumor stages are not suitable for LE under the current nCRT regimen.
LLNs are a particular type of lymph nodes and dissection of LLNs is not included during regular TME. The incidence of LLN involvement varies from 7.7% to 20% in low and middle rectal cancer[67-69]. There is evidence to suggest that TME even with nCRT cannot completely remove remnant cancer cells in LLNs (Figure 1), especially in advanced tumors[45,70,71]. Kim et al[72] reported that 9 (7.9%) of 366 patients developed LR after nCRT and TME during a mean follow-up of 5 years, and lateral pelvic recurrence accounted for most (n = 24, 82.7%) of these cases. Patients with positive LLNs had a higher risk of lateral pelvic recurrence, compared with those with negative LLNs (LR rate: 26.6% vs 2.3%). Kusters et al[73] demonstrated that bilateral lateral lymph node dissection (LLND) generally resulted in better local control than unilateral LLND (LR rate: 15.4% vs 8.3%) in patients with advanced cancers after nCRT. When positive LLNs were detected preoperatively, the difference between unilateral and bilateral LLND was still significant (LR rate: 32.8% vs 14.2%). Furthermore, LR was detected on the contralateral side in a proportion of patients who underwent unilateral lymph node dissection. These data indicate that positive LLNs are a vital risk factor causing pelvic recurrence even after nCRT.
There is controversy between Western and Japanese researchers concerning the application of LLND. Western researchers believe that nCRT plus TME may have a comparable outcome to that of LLND[74]. Moreover, resection of LLNs may result in injury to pelvic nerves. Thus, they recommend nCRT plus TME, not LLND. However, Japanese researchers indicate that LLND has a comparable outcome to that of nCRT plus standard TME regarding local control and the incidence of complications[75]. Thus, they recommend LLND. In our opinion, LLNs status is reflective of overall mesenteric LNs status and LLNs positivity may represent the poor response of rectal cancer to nCRT. LLND should be undertaken in selected patients, e.g., those with tumor below the peritoneal reflection and poor tumor response. In addition, laparoscopic technology has unique advantages over laparotomy in terms of decreasing morbidity following LLND due to its high-definition close view in nerve-sparing.
A better tumor response may predict a more favorable prognosis for patients with advanced rectal cancer[76]. The response to neoadjuvant therapy includes remission in both primary tumor volume and lymphatic or vascular metastasis. Pathologic complete response (pCR) is defined as both ypT0 and ypN0, and the pCR rates range from around 10% to 30% in patients who underwent nCRT[77-80]. The final pathologic stage after nCRT and radical surgery is considered a vital factor in predicting LR. According to Mandard’s Tumor Regression Grade (TRG) criteria[81], patients achieving a significant tumor remission (TRG1-3) displayed a relatively lower LR rate[71,82-87] compared with the non-downstaging group (TRG4-5). This figure decreased to 0%[31,71,82,86,88] (Table 3) in the pCR group. The reason for this may be that a pCR suggests a more favorable biological behavior and increases the chances of R0 resection. Moreover, complete regression of the primary cancer is paralleled with the disappearance of remnant cancer cells either in the mesorectum or lymph nodes[39].
| Ref. | No. of patients | Neoadjuvant therapy regimen | No. of LR | ||
| Radiotherapy (Gy) | Chemotherapy | pCR LR/total | Non-pCR LR/total | ||
| Gavioli et al[31] | 139 | 50 | 5-Fu | 0/25 (0.0) | 8/114 (7.0) |
| Stipa et al[57] | 200 | 50 | 5-Fu | 0/60 (0.0) | 6/140 (4.3) |
| Hughes et al[71] | 130 | 45 | 5-Fu | 0/23 (0.0) | 23/107 (17.7) |
| Kim et al[82] | 114 | 50.4 | 5-Fu | 0/10 (0.0) | 17/104 (16.3) |
| Kuo et al[83] | 248 | 50 | 5-Fu | 2/36 (5.6) | 66/212 (31.1) |
| Chan et al[84] | 128 | 50 | 5-Fu | 0/32 (0.0) | 24/96 (18.4) |
| García-Aguilar et al[86] | 168 | 40-65 | 5-Fu | 0/21 (0.0) | 7/147 (5) |
| Wheeler et al[87] | 63 | 45-50 | 5-Fu | 1/29 (3.4) | 8/34 (23.5) |
| Theodoropoulos et al[88] | 88 | 45 | 5-Fu | 0/16 (0.0) | 3/72 (4.2) |
| Total | 1278 | 3/252 (1.2) | 162/1026 (15.8) | ||
It has been shown that in patients with pCR, no residual cancer is found in resected specimens. This raises the question as to whether immediate radical surgery following nCRT is necessary, or, whether “watch and wait” is an appropriate strategy for these selected patients. Since pathological response can be judged only after tumor resection, a substitute parameter, clinical complete response (cCR), has been used to preoperatively screen potentially suitable patients[9,89]. A single-center study revealed that in patients treated with chemotherapy without surgery, only 5% of cCR cases (5 of 99) developed LR[9], whereas another study found that 8 of 10 patients had LR[90]. How do we explain such a big discrepancy? Actually, the critical premise for the “watch and wait” approach is to correctly identify the “real” suitable responders. A long-term persistent cCR may be a better representative of pCR. Only patients with sustained cCR for at least 12 mo were submitted to non-operative management in the study by Habr-Gama et al[9]. In contrast, the majority (75%) of patients with a short-term cCR (6-12 wk) were reported to have microscopic remnant cancers[70], at high risk of LR if subjected to “watch and wait”. In addition, accuracy of staging in cases pretreated with nCRT is controversial. The absence of palpable tumors is not reliable evidence, nor is an invisible tumor on imaging methods, including transrectal ultrasonography, CT and MRI. Therefore, the overall attitude toward non-operative management remains critical and cautious, although the results from Habr-Gama et al[9,91] are promising. In our opinion, only selected cCR patients may undergo close observation without immediate radical surgery.
With the adoption of TME, LR and survival have improved significantly in patients with rectal cancer, especially in those receiving anterior resection (AR)[92]. In comparison, abdominoperineal resection (APR) is reported to be related to a higher LR rate and poorer prognosis[93,94]. A possible explanation for the inferior outcome after APR is that surgeons often encounter more difficulties when resecting lower-lying tumors within a narrow pelvis[93]. Moreover, for those receiving nCRT, the appropriate surgical plane may be difficult to recognize due to tissue edema and fibrosis. These factors together may lead to inadequate excision of the mesorectum or of the tumor itself. In addition, the incidence of inadvertent intra-operative rectal perforation and post-operative anastomotic leak may increase, resulting in a higher LR rate[95-97].
With regard to AR, there is a legitimate concern about implanting exfoliated tumors cells when using circular staples. Despite the feasibility of low colorectal anastomosis, staples may also lead to implantation of viable tumor cells lying freely in the bowel lumen during staple firing[98,99]. That may also explain the mechanism of anastomotic recurrence in patients receiving nCRT (see Patterns of LR Following nCRT), who were expecting that tumor regression may translate to final sphincter-sparing surgery. Some authors[100,101] recommend intra-operative washout to eliminate exfoliated cancer cells because it is relatively risk-free and adds little to the operative trauma. However, it is difficult for surgeons to accomplish rectal washout in laparoscopic AR, as frequent laparoscopic manipulation probably increases tumor exfoliation, making wash-out even more crucial. Therefore, specific equipment or tools need to be designed to overcome the technical problems of laparoscopic rectal wash-out.
nCRT can downsize rectal cancer and facilitate subsequent radical resection. However, the impact of nCRT on downstaging of rectal cancer may also result in an altered pattern of LR and several distinctive risk factors for LR. These distinctive risk factors and altered patterns of LR are of clinical importance because they are decisive in treatment selection and follow-up. In future studies, we should not only identify but also improve our multidisciplinary approaches to minimize these factors.
| 1. | Garcia-Aguilar J, Smith DD, Avila K, Bergsland EK, Chu P, Krieg RM. Optimal timing of surgery after chemoradiation for advanced rectal cancer: preliminary results of a multicenter, nonrandomized phase II prospective trial. Ann Surg. 2011;254:97-102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 265] [Cited by in RCA: 262] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 2. | Lim YK, Law WL, Liu R, Poon JT, Fan JF, Lo OS. Impact of neoadjuvant treatment on total mesorectal excision for ultra-low rectal cancers. World J Surg Oncol. 2010;8:23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Topova L, Hellmich G, Puffer E, Schubert C, Christen N, Boldt T, Wiedemann B, Witzigmann H, Stelzner S. Prognostic value of tumor response to neoadjuvant therapy in rectal carcinoma. Dis Colon Rectum. 2011;54:401-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 4. | Roh MS, Colangelo LH, O’Connell MJ, Yothers G, Deutsch M, Allegra CJ, Kahlenberg MS, Baez-Diaz L, Ursiny CS, Petrelli NJ. Preoperative multimodality therapy improves disease-free survival in patients with carcinoma of the rectum: NSABP R-03. J Clin Oncol. 2009;27:5124-5130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 627] [Cited by in RCA: 714] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 5. | Kusters M, Holman FA, Martijn H, Nieuwenhuijzen GA, Creemers GJ, Daniels-Gooszen AW, van den Berg HA, van den Brule AJ, van de Velde CJ, Rutten HJ. Patterns of local recurrence in locally advanced rectal cancer after intra-operative radiotherapy containing multimodality treatment. Radiother Oncol. 2009;92:221-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 6. | Coco C, Valentini V, Manno A, Mattana C, Verbo A, Cellini N, Gambacorta MA, Covino M, Mantini G, Miccichè F. Long-term results after neoadjuvant radiochemotherapy for locally advanced resectable extraperitoneal rectal cancer. Dis Colon Rectum. 2006;49:311-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 7. | Fucini C, Pucciani F, Elbetti C, Gattai R, Russo A. Preoperative radiochemotherapy in t3 operable low rectal cancers: a gold standard? World J Surg. 2010;34:1609-1614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Law WL, Chu KW. Local recurrence following total mesorectal excision with double-stapling anastomosis for rectal cancers: analysis of risk factors. World J Surg. 2002;26:1272-1276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Habr-Gama A, Perez RO, Proscurshim I, Campos FG, Nadalin W, Kiss D, Gama-Rodrigues J. Patterns of failure and survival for nonoperative treatment of stage c0 distal rectal cancer following neoadjuvant chemoradiation therapy. J Gastrointest Surg. 2006;10:1319-1328; discussion 1319-1328;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 279] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 10. | Dresen RC, Peters EE, Rutten HJ, Nieuwenhuijzen GA, Demeyere TB, van den Brule AJ, Kessels AG, Beets-Tan RG, van Krieken JH, Nagtegaal ID. Local recurrence in rectal cancer can be predicted by histopathological factors. Eur J Surg Oncol. 2009;35:1071-1077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 66] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 11. | Maas M, Nelemans PJ, Valentini V, Das P, Rödel C, Kuo LJ, Calvo FA, García-Aguilar J, Glynne-Jones R, Haustermans K. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol. 2010;11:835-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1189] [Cited by in RCA: 1507] [Article Influence: 94.2] [Reference Citation Analysis (0)] |
| 12. | Kusters M, Marijnen CA, van de Velde CJ, Rutten HJ, Lahaye MJ, Kim JH, Beets-Tan RG, Beets GL. Patterns of local recurrence in rectal cancer; a study of the Dutch TME trial. Eur J Surg Oncol. 2010;36:470-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 254] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 13. | Ahmad NR, Nagle D. Long-term results of preoperative radiation therapy alone for stage T3 and T4 rectal cancer. Br J Surg. 1997;84:1445-1448. [PubMed] |
| 14. | Chmielarz A, Kryj M, Wloch J, Półtorak S, Sacher A, Lasek-Kryj M. Prognostic factors for the time of occurrence and dynamics of distant metastases and local recurrences after radical treatment in patients with rectal cancer. Med Sci Monit. 2001;7:1263-1269. [PubMed] |
| 15. | Francois Y, Nemoz CJ, Baulieux J, Vignal J, Grandjean JP, Partensky C, Souquet JC, Adeleine P, Gerard JP. Influence of the interval between preoperative radiation therapy and surgery on downstaging and on the rate of sphincter-sparing surgery for rectal cancer: the Lyon R90-01 randomized trial. J Clin Oncol. 1999;17:2396. [PubMed] |
| 16. | Tulchinsky H, Shmueli E, Figer A, Klausner JM, Rabau M. An interval > 7 weeks between neoadjuvant therapy and surgery improves pathologic complete response and disease-free survival in patients with locally advanced rectal cancer. Ann Surg Oncol. 2008;15:2661-2667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 252] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 17. | Nijkamp J, Kusters M, Beets-Tan RG, Martijn H, Beets GL, van de Velde CJ, Marijnen CA. Three-dimensional analysis of recurrence patterns in rectal cancer: the cranial border in hypofractionated preoperative radiotherapy can be lowered. Int J Radiat Oncol Biol Phys. 2011;80:103-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | Yu TK, Bhosale PR, Crane CH, Iyer RB, Skibber JM, Rodriguez-Bigas MA, Feig BW, Chang GJ, Eng C, Wolff RA. Patterns of locoregional recurrence after surgery and radiotherapy or chemoradiation for rectal cancer. Int J Radiat Oncol Biol Phys. 2008;71:1175-1180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 51] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 19. | den Dulk M, Collette L, van de Velde CJ, Marijnen CA, Calais G, Mineur L, Maingon P, Radosevic-Jelic L, Daban A, Bosset JF. Quality of surgery in T3-4 rectal cancer: involvement of circumferential resection margin not influenced by preoperative treatment. Results from EORTC trial 22921. Eur J Cancer. 2007;43:1821-1828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 20. | Syk E, Torkzad MR, Blomqvist L, Nilsson PJ, Glimelius B. Local recurrence in rectal cancer: anatomic localization and effect on radiation target. Int J Radiat Oncol Biol Phys. 2008;72:658-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 70] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 21. | Kusters M, Wallner C, Lange MM, DeRuiter MC, van de Velde CJ, Moriya Y, Rutten HJ. Origin of presacral local recurrence after rectal cancer treatment. Br J Surg. 2010;97:1582-1587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Wang C, Zhou ZG, Yu YY, Li Y, Lei WZ, Cheng Z, Chen ZX. Patterns of lateral pelvic lymph node metastases and micrometastases for patients with lower rectal cancer. Eur J Surg Oncol. 2007;33:463-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Homma Y, Hamano T, Otsuki Y, Shimizu S, Kobayashi H, Kobayashi Y. Severe tumor budding is a risk factor for lateral lymph node metastasis in early rectal cancers. J Surg Oncol. 2010;102:230-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Syk E, Lenander C, Nilsson PJ, Rubio CA, Glimelius B. Tumour budding correlates with local recurrence of rectal cancer. Colorectal Dis. 2011;13:255-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | Glimelius B, Oliveira J. Rectal cancer: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol. 2009;20 Suppl 4:54-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 26. | Merkel S, Mansmann U, Hohenberger W, Hermanek P. Time to locoregional recurrence after curative resection of rectal carcinoma is prolonged after neoadjuvant treatment: a systematic review and meta-analysis. Colorectal Dis. 2011;13:123-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 27. | Sebag-Montefiore D, Stephens RJ, Steele R, Monson J, Grieve R, Khanna S, Quirke P, Couture J, de Metz C, Myint AS. Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC-CTG C016): a multicentre, randomised trial. Lancet. 2009;373:811-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1219] [Cited by in RCA: 1130] [Article Influence: 66.5] [Reference Citation Analysis (6)] |
| 28. | Prall F. Tumour budding in colorectal carcinoma. Histopathology. 2007;50:151-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 287] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 29. | Sy J, Fung CL, Dent OF, Chapuis PH, Bokey L, Chan C. Tumor budding and survival after potentially curative resection of node-positive colon cancer. Dis Colon Rectum. 2010;53:301-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 30. | Kanazawa H, Mitomi H, Nishiyama Y, Kishimoto I, Fukui N, Nakamura T, Watanabe M. Tumour budding at invasive margins and outcome in colorectal cancer. Colorectal Dis. 2008;10:41-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 31. | Gavioli M, Luppi G, Losi L, Bertolini F, Santantonio M, Falchi AM, D’Amico R, Conte PF, Natalini G. Incidence and clinical impact of sterilized disease and minimal residual disease after preoperative radiochemotherapy for rectal cancer. Dis Colon Rectum. 2005;48:1851-1857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 57] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 32. | Chmielik E, Bujko K, Nasierowska-Guttmejer A, Nowacki MP, Kepka L, Sopylo R, Wojnar A, Majewski P, Sygut J, Karmolinski A. Distal intramural spread of rectal cancer after preoperative radiotherapy: the results of a multicenter randomized clinical study. Int J Radiat Oncol Biol Phys. 2006;65:182-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 33. | Kuvshinoff B, Maghfoor I, Miedema B, Bryer M, Westgate S, Wilkes J, Ota D. Distal margin requirements after preoperative chemoradiotherapy for distal rectal carcinomas: are & lt; or = 1 cm distal margins sufficient? Ann Surg Oncol. 2001;8:163-169. [PubMed] |
| 34. | Moore HG, Riedel E, Minsky BD, Saltz L, Paty P, Wong D, Cohen AM, Guillem JG. Adequacy of 1-cm distal margin after restorative rectal cancer resection with sharp mesorectal excision and preoperative combined-modality therapy. Ann Surg Oncol. 2003;10:80-85. [PubMed] |
| 35. | Nash GM, Weiss A, Dasgupta R, Gonen M, Guillem JG, Wong WD. Close distal margin and rectal cancer recurrence after sphincter-preserving rectal resection. Dis Colon Rectum. 2010;53:1365-1373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 58] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 36. | Guillem JG, Chessin DB, Shia J, Suriawinata A, Riedel E, Moore HG, Minsky BD, Wong WD. A prospective pathologic analysis using whole-mount sections of rectal cancer following preoperative combined modality therapy: implications for sphincter preservation. Ann Surg. 2007;245:88-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 101] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 37. | Mezhir JJ, Smith KD, Fichera A, Hart J, Posner MC, Hurst RD. Presence of distal intramural spread after preoperative combined-modality therapy for adenocarcinoma of the rectum: what is now the appropriate distal resection margin? Surgery. 2005;138:658-663; discussion 663-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 38. | Prabhudesai A, Arif S, Finlayson CJ, Kumar D. Impact of microscopic extranodal tumor deposits on the outcome of patients with rectal cancer. Dis Colon Rectum. 2003;46:1531-1537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 39. | Ratto C, Ricci R, Valentini V, Castri F, Parello A, Gambacorta MA, Cellini N, Vecchio FM, Doglietto GB. Neoplastic mesorectal microfoci (MMF) following neoadjuvant chemoradiotherapy: clinical and prognostic implications. Ann Surg Oncol. 2007;14:853-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 40. | Ratto C, Ricci R, Rossi C, Morelli U, Vecchio FM, Doglietto GB. Mesorectal microfoci adversely affect the prognosis of patients with rectal cancer. Dis Colon Rectum. 2002;45:733-42; discussion 742-3. [PubMed] |
| 41. | Umlaufová D, Skapa P, Hoch J, Prausová J. [Incidence and prognostic significance of mesorectal extranodal tumor deposits in patients with rectal carcinoma following neoadjuvant therapy]. Rozhl Chir. 2009;88:326-329. [PubMed] |
| 42. | Zmora O, Dasilva GM, Gurland B, Pfeffer R, Koller M, Nogueras JJ, Wexner SD. Does rectal wall tumor eradication with preoperative chemoradiation permit a change in the operative strategy? Dis Colon Rectum. 2004;47:1607-1612. [PubMed] |
| 43. | Read TE, Andujar JE, Caushaj PF, Johnston DR, Dietz DW, Myerson RJ, Fleshman JW, Birnbaum EH, Mutch MG, Kodner IJ. Neoadjuvant therapy for rectal cancer: histologic response of the primary tumor predicts nodal status. Dis Colon Rectum. 2004;47:825-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 102] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 44. | Bujko K, Nowacki MP, Nasierowska-Guttmejer A, Kepka L, Winkler-Spytkowska B, Suwiński R, Oledzki J, Stryczyńska G, Wieczorek A, Serkies K. Prediction of mesorectal nodal metastases after chemoradiation for rectal cancer: results of a randomised trial: implication for subsequent local excision. Radiother Oncol. 2005;76:234-240. [PubMed] |
| 45. | Pucciarelli S, Capirci C, Emanuele U, Toppan P, Friso ML, Pennelli GM, Crepaldi G, Pasetto L, Nitti D, Lise M. Relationship between pathologic T-stage and nodal metastasis after preoperative chemoradiotherapy for locally advanced rectal cancer. Ann Surg Oncol. 2005;12:111-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 71] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 46. | Tulchinsky H, Rabau M, Shacham-Shemueli E, Goldman G, Geva R, Inbar M, Klausner JM, Figer A. Can rectal cancers with pathologic T0 after neoadjuvant chemoradiation (ypT0) be treated by transanal excision alone? Ann Surg Oncol. 2006;13:347-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 32] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 47. | Habr-Gama A, Perez RO, Proscurshim I, Rawet V, Pereira DD, Sousa AH, Kiss D, Cecconello I. Absence of lymph nodes in the resected specimen after radical surgery for distal rectal cancer and neoadjuvant chemoradiation therapy: what does it mean? Dis Colon Rectum. 2008;51:277-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 76] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 48. | Stipa F, Zernecke A, Moore HG, Minsky BD, Wong WD, Weiser M, Paty PB, Shia J, Guillem JG. Residual mesorectal lymph node involvement following neoadjuvant combined-modality therapy: rationale for radical resection? Ann Surg Oncol. 2004;11:187-191. [PubMed] |
| 49. | Kundel Y, Brenner R, Purim O, Peled N, Idelevich E, Fenig E, Sulkes A, Brenner B. Is local excision after complete pathological response to neoadjuvant chemoradiation for rectal cancer an acceptable treatment option? Dis Colon Rectum. 2010;53:1624-1631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 50. | Kim DW, Kim DY, Kim TH, Jung KH, Chang HJ, Sohn DK, Lim SB, Choi HS, Jeong SY, Park JG. Is T classification still correlated with lymph node status after preoperative chemoradiotherapy for rectal cancer? Cancer. 2006;106:1694-1700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 62] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 51. | Lindebjerg J, Spindler KL, Ploen J, Jakobsen A. The prognostic value of lymph node metastases and tumour regression grade in rectal cancer patients treated with long-course preoperative chemoradiotherapy. Colorectal Dis. 2009;11:264-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 52. | Coco C, Manno A, Mattana C, Verbo A, Rizzo G, Valentini V, Gambacorta MA, Vecchio FM, D’Ugo D. The role of local excision in rectal cancer after complete response to neoadjuvant treatment. Surg Oncol. 2007;16 Suppl 1:S101-S104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 53. | Bonnen M, Crane C, Vauthey JN, Skibber J, Delclos ME, Rodriguez-Bigas M, Hoff PM, Lin E, Eng C, Wong A. Long-term results using local excision after preoperative chemoradiation among selected T3 rectal cancer patients. Int J Radiat Oncol Biol Phys. 2004;60:1098-1105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 135] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 54. | Hershman MJ, Myint AS, Makin CA. Multi-modality approach in curative local treatment of early rectal carcinomas. Colorectal Dis. 2003;5:445-450. [PubMed] |
| 55. | Lezoche E, Guerrieri M, Paganini AM, Baldarelli M, De Sanctis A, Lezoche G. Long-term results in patients with T2-3 N0 distal rectal cancer undergoing radiotherapy before transanal endoscopic microsurgery. Br J Surg. 2005;92:1546-1552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 116] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 56. | Schell SR, Zlotecki RA, Mendenhall WM, Marsh RW, Vauthey JN, Copeland EM. Transanal excision of locally advanced rectal cancers downstaged using neoadjuvant chemoradiotherapy. J Am Coll Surg. 2002;194:584-590; discussion 590-591. [PubMed] |
| 57. | Stipa F, Chessin DB, Shia J, Paty PB, Weiser M, Temple LK, Minsky BD, Wong WD, Guillem JG. A pathologic complete response of rectal cancer to preoperative combined-modality therapy results in improved oncological outcome compared with those who achieve no downstaging on the basis of preoperative endorectal ultrasonography. Ann Surg Oncol. 2006;13:1047-1053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 134] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 58. | Ruo L, Guillem JG, Minsky BD, Quan SH, Paty PB, Cohen AM. Preoperative radiation with or without chemotherapy and full-thickness transanal excision for selected T2 and T3 distal rectal cancers. Int J Colorectal Dis. 2002;17:54-58. [PubMed] |
| 59. | Kim CJ, Yeatman TJ, Coppola D, Trotti A, Williams B, Barthel JS, Dinwoodie W, Karl RC, Marcet J. Local excision of T2 and T3 rectal cancers after downstaging chemoradiation. Ann Surg. 2001;234:352-358; discussion 352-358. [PubMed] |
| 60. | Borschitz T, Wachtlin D, Möhler M, Schmidberger H, Junginger T. Neoadjuvant chemoradiation and local excision for T2-3 rectal cancer. Ann Surg Oncol. 2008;15:712-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 184] [Article Influence: 9.7] [Reference Citation Analysis (1)] |
| 61. | Nair RM, Siegel EM, Chen DT, Fulp WJ, Yeatman TJ, Malafa MP, Marcet J, Shibata D. Long-term results of transanal excision after neoadjuvant chemoradiation for T2 and T3 adenocarcinomas of the rectum. J Gastrointest Surg. 2008;12:1797-1805; discussion 1805-1806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 73] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 62. | Callender GG, Das P, Rodriguez-Bigas MA, Skibber JM, Crane CH, Krishnan S, Delclos ME, Feig BW. Local excision after preoperative chemoradiation results in an equivalent outcome to total mesorectal excision in selected patients with T3 rectal cancer. Ann Surg Oncol. 2010;17:441-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 77] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 63. | Park C, Lee W, Han S, Yun S, Chun HK. Transanal local excision for preoperative concurrent chemoradiation therapy for distal rectal cancer in selected patients. Surg Today. 2007;37:1068-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 64. | Yeo SG, Kim DY, Kim TH, Chang HJ, Oh JH, Park W, Choi DH, Nam H, Kim JS, Cho MJ. Pathologic complete response of primary tumor following preoperative chemoradiotherapy for locally advanced rectal cancer: long-term outcomes and prognostic significance of pathologic nodal status (KROG 09-01). Ann Surg. 2010;252:998-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 145] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 65. | Perez RO, Habr-Gama A, Proscurshim I, Campos FG, Kiss D, Gama-Rodrigues J, Cecconello I. Local excision for ypT2 rectal cancer--much ado about something. J Gastrointest Surg. 2007;11:1431-1438; discussion 1431-1438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 66. | Perez RO, Habr-Gama A, Nishida Arazawa ST, Rawet V, Coelho Siqueira SA, Kiss DR, Gama-Rodrigues JJ. Lymph node micrometastasis in stage II distal rectal cancer following neoadjuvant chemoradiation therapy. Int J Colorectal Dis. 2005;20:434-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 67. | Koda K, Saito N, Oda K, Takiguchi N, Sarashina H, Miyazaki M. Evaluation of lateral lymph node dissection with preoperative chemo-radiotherapy for the treatment of advanced middle to lower rectal cancers. Int J Colorectal Dis. 2004;19:188-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 68. | Ueno M, Oya M, Azekura K, Yamaguchi T, Muto T. Incidence and prognostic significance of lateral lymph node metastasis in patients with advanced low rectal cancer. Br J Surg. 2005;92:756-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 264] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 69. | Moriya Y, Sugihara K, Akasu T, Fujita S. Importance of extended lymphadenectomy with lateral node dissection for advanced lower rectal cancer. World J Surg. 1997;21:728-732. [PubMed] |
| 70. | Hiotis SP, Weber SM, Cohen AM, Minsky BD, Paty PB, Guillem JG, Wagman R, Saltz LB, Wong WD. Assessing the predictive value of clinical complete response to neoadjuvant therapy for rectal cancer: an analysis of 488 patients. J Am Coll Surg. 2002;194:131-135; discussion 131-135. [PubMed] |
| 71. | Hughes R, Glynne-Jones R, Grainger J, Richman P, Makris A, Harrison M, Ashford R, Harrison RA, Livingstone JI, McDonald PJ. Can pathological complete response in the primary tumour following pre-operative pelvic chemoradiotherapy for T3-T4 rectal cancer predict for sterilisation of pelvic lymph nodes, a low risk of local recurrence and the appropriateness of local excision? Int J Colorectal Dis. 2006;21:11-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 98] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 72. | Kim TH, Jeong SY, Choi DH, Kim DY, Jung KH, Moon SH, Chang HJ, Lim SB, Choi HS, Park JG. Lateral lymph node metastasis is a major cause of locoregional recurrence in rectal cancer treated with preoperative chemoradiotherapy and curative resection. Ann Surg Oncol. 2008;15:729-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 262] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 73. | Kusters M, van de Velde CJ, Beets-Tan RG, Akasu T, Fujita S, Yamamoto S, Moriya Y. Patterns of local recurrence in rectal cancer: a single-center experience. Ann Surg Oncol. 2009;16:289-296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 74. | Georgiou P, Tan E, Gouvas N, Antoniou A, Brown G, Nicholls RJ, Tekkis P. Extended lymphadenectomy versus conventional surgery for rectal cancer: a meta-analysis. Lancet Oncol. 2009;10:1053-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 232] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 75. | Sugihara K, Kobayashi H, Kato T, Mori T, Mochizuki H, Kameoka S, Shirouzu K, Muto T. Indication and benefit of pelvic sidewall dissection for rectal cancer. Dis Colon Rectum. 2006;49:1663-1672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 344] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 76. | Farnault B, Moureau-Zabotto L, de Chaisemartin C, Esterni B, Lelong B, Viret F, Giovannini M, Monges G, Delpero JR, Bories E. [Predictive factors of tumour response after neoadjuvant chemoradiation for locally advanced rectal cancer and correlation of these factors with survival]. Cancer Radiother. 2011;15:279-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 77. | Luna-Pérez P, Rodríguez-Ramírez S, Hernández-Pacheco F, Gutiérrez De La Barrera M, Fernández R, Labastida S. Anal sphincter preservation in locally advanced low rectal adenocarcinoma after preoperative chemoradiation therapy and coloanal anastomosis. J Surg Oncol. 2003;82:3-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 78. | Janjan NA, Khoo VS, Abbruzzese J, Pazdur R, Dubrow R, Cleary KR, Allen PK, Lynch PM, Glober G, Wolff R. Tumor downstaging and sphincter preservation with preoperative chemoradiation in locally advanced rectal cancer: the M. D. Anderson Cancer Center experience. Int J Radiat Oncol Biol Phys. 1999;44:1027-1038. [PubMed] |
| 79. | Medich D, McGinty J, Parda D, Karlovits S, Davis C, Caushaj P, Lembersky B. Preoperative chemoradiotherapy and radical surgery for locally advanced distal rectal adenocarcinoma: pathologic findings and clinical implications. Dis Colon Rectum. 2001;44:1123-1128. [PubMed] |
| 80. | Habr-Gama A, Perez RO, Nadalin W, Nahas SC, Ribeiro U, Silva E Sousa AH, Campos FG, Kiss DR, Gama-Rodrigues J. Long-term results of preoperative chemoradiation for distal rectal cancer correlation between final stage and survival. J Gastrointest Surg. 2005;9:90-99; discussion 99-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 163] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 81. | Mandard AM, Dalibard F, Mandard JC, Marnay J, Henry-Amar M, Petiot JF, Roussel A, Jacob JH, Segol P, Samama G. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer. 1994;73:2680-2686. [PubMed] |
| 82. | Kim NK, Baik SH, Seong JS, Kim H, Roh JK, Lee KY, Sohn SK, Cho CH. Oncologic outcomes after neoadjuvant chemoradiation followed by curative resection with tumor-specific mesorectal excision for fixed locally advanced rectal cancer: Impact of postirradiated pathologic downstaging on local recurrence and survival. Ann Surg. 2006;244:1024-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 134] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 83. | Kuo LJ, Liu MC, Jian JJ, Horng CF, Cheng TI, Chen CM, Fang WT, Chung YL. Is final TNM staging a predictor for survival in locally advanced rectal cancer after preoperative chemoradiation therapy? Ann Surg Oncol. 2007;14:2766-2772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 137] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 84. | Chan AK, Wong A, Jenken D, Heine J, Buie D, Johnson D. Posttreatment TNM staging is a prognostic indicator of survival and recurrence in tethered or fixed rectal carcinoma after preoperative chemotherapy and radiotherapy. Int J Radiat Oncol Biol Phys. 2005;61:665-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 138] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 85. | Vecchio FM, Valentini V, Minsky BD, Padula GD, Venkatraman ES, Balducci M, Miccichè F, Ricci R, Morganti AG, Gambacorta MA. The relationship of pathologic tumor regression grade (TRG) and outcomes after preoperative therapy in rectal cancer. Int J Radiat Oncol Biol Phys. 2005;62:752-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 333] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 86. | García-Aguilar J, Hernandez de Anda E, Sirivongs P, Lee SH, Madoff RD, Rothenberger DA. A pathologic complete response to preoperative chemoradiation is associated with lower local recurrence and improved survival in rectal cancer patients treated by mesorectal excision. Dis Colon Rectum. 2003;46:298-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 87. | Wheeler JM, Dodds E, Warren BF, Cunningham C, George BD, Jones AC, Mortensen NJ. Preoperative chemoradiotherapy and total mesorectal excision surgery for locally advanced rectal cancer: correlation with rectal cancer regression grade. Dis Colon Rectum. 2004;47:2025-2031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 102] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 88. | Theodoropoulos G, Wise WE, Padmanabhan A, Kerner BA, Taylor CW, Aguilar PS, Khanduja KS. T-level downstaging and complete pathologic response after preoperative chemoradiation for advanced rectal cancer result in decreased recurrence and improved disease-free survival. Dis Colon Rectum. 2002;45:895-903. [PubMed] |
| 89. | Nyasavajjala SM, Shaw AG, Khan AQ, Brown SR, Lund JN. Neoadjuvant chemo-radiotherapy and rectal cancer: can the UK watch and wait with Brazil? Colorectal Dis. 2010;12:33-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 90. | Nakagawa WT, Rossi BM, de O Ferreira F, Ferrigno R, David Filho WJ, Nishimoto IN, Vieira RA, Lopes A. Chemoradiation instead of surgery to treat mid and low rectal tumors: is it safe? Ann Surg Oncol. 2002;9:568-573. [PubMed] |
| 91. | Habr-Gama A, Perez RO, Nadalin W, Sabbaga J, Ribeiro U, Silva e Sousa AH, Campos FG, Kiss DR, Gama-Rodrigues J. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: long-term results. Ann Surg. 2004;240:711-717; discussion 711-717. [PubMed] |
| 92. | van Gijn W, Marijnen CA, Nagtegaal ID, Kranenbarg EM, Putter H, Wiggers T, Rutten HJ, Påhlman L, Glimelius B, van de Velde CJ. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol. 2011;12:575-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1138] [Cited by in RCA: 1385] [Article Influence: 92.3] [Reference Citation Analysis (0)] |
| 93. | den Dulk M, Putter H, Collette L, Marijnen CA, Folkesson J, Bosset JF, Rödel C, Bujko K, Påhlman L, van de Velde CJ. The abdominoperineal resection itself is associated with an adverse outcome: the European experience based on a pooled analysis of five European randomised clinical trials on rectal cancer. Eur J Cancer. 2009;45:1175-1183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 136] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 94. | den Dulk M, Marijnen CA, Putter H, Rutten HJ, Beets GL, Wiggers T, Nagtegaal ID, van de Velde CJ. Risk factors for adverse outcome in patients with rectal cancer treated with an abdominoperineal resection in the total mesorectal excision trial. Ann Surg. 2007;246:83-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 106] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 95. | Bülow S, Christensen IJ, Iversen LH, Harling H. Intra-operative perforation is an important predictor of local recurrence and impaired survival after abdominoperineal resection for rectal cancer. Colorectal Dis. 2011;13:1256-1264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 96. | Jörgren F, Johansson R, Damber L, Lindmark G. Anastomotic leakage after surgery for rectal cancer: a risk factor for local recurrence, distant metastasis and reduced cancer-specific survival? Colorectal Dis. 2011;13:272-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 97. | Harris LJ, Phillips BR, Maxwell PJ, Isenberg GA, Goldstein SD. Outcomes of low anterior resection anastomotic leak after preoperative chemoradiation therapy for rectal cancer. Am Surg. 2010;76:747-751. [PubMed] |
| 98. | Hurst PA, Prout WG, Kelly JM, Bannister JJ, Walker RT. Local recurrence after low anterior resection using the staple gun. Br J Surg. 1982;69:275-276. [PubMed] |
| 99. | Anderberg B, Enblad P, Sjödahl R, Wetterfors J. Recurrent rectal carcinoma after anterior resection and rectal stapling. Br J Surg. 1984;71:98-100. [PubMed] |
| 100. | Kodeda K, Holmberg E, Jörgren F, Nordgren S, Lindmark G. Rectal washout and local recurrence of cancer after anterior resection. Br J Surg. 2010;97:1589-1597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 101. | Maeda K, Maruta M, Hanai T, Sato H, Horibe Y. Irrigation volume determines the efficacy of “rectal washout”. Dis Colon Rectum. 2004;47:1706-1710. [PubMed] |
P- Reviewers Choi Y, Pottgen C, Sugimura H S- Editor Wen LL L- Editor A E- Editor Zhang DN