Published online Aug 14, 2013. doi: 10.3748/wjg.v19.i30.5000
Revised: May 30, 2013
Accepted: July 4, 2013
Published online: August 14, 2013
Processing time: 176 Days and 20.4 Hours
AIM: To evaluate the clinical efficacy of an expanded polytetrafluoro-ethylene-covered Fluency stent compared with that of a polyethylene terephthalate-covered Wallgraft stent for the management of transjugular intrahepatic portosystemic shunt (TIPS) dysfunction.
METHODS: A retrospective review of patients who underwent TIPS revision with stent-grafts between May 2007 and June 2011 was conducted. The patients were divided into two groups according to the stent-grafts implanted: the Fluency stent (Bard Incorporated, Karlsruhe, Germany) and the Wallgraft stent (Boston Scientific, Galway, Ireland). The primary patency rates were calculated and compared using the Kaplan-Meier method.
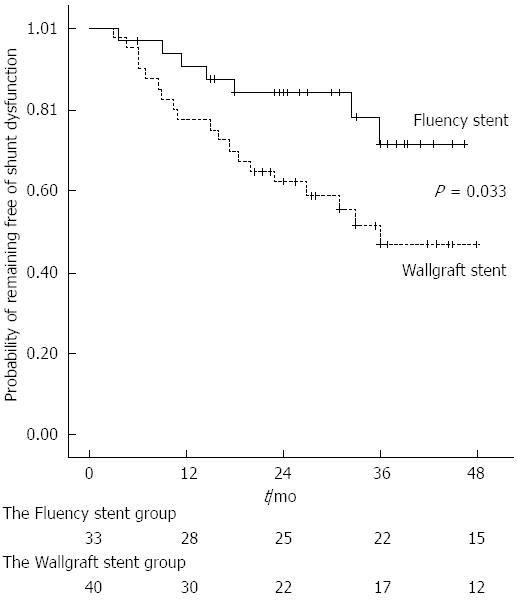
RESULTS: A total of 73 patients were evaluated in this study: 33 with Fluency stents and 40 with Wallgraft stents. The primary patency rates at 12 and 24 mo were 91% and 85%, respectively, in the Fluency stent group and 78% and 63%, respectively, in the Wallgraft stent group. The primary shunt patency rates after TIPS revision were significantly better with the Fluency stent than with the Wallgraft stent (P = 0.033).
CONCLUSION: TIPS revision with the Fluency stent has higher medium-term patency rates than that with the Wallgraft stent.
Core tip: There are few data on the clinical use of expanded polytetrafluoroethylene-covered stent-grafts for the management of transjugular intrahepatic portosystemic shunt (TIPS) dysfunction in the literature. The present study was designed to retrospectively evaluate the clinical efficacy of Fluency stent compared with Wallgraft stent in the treatment of TIPS dysfunction. And the results demonstrated that TIPS revision with the Fluency stent has higher medium-term patency rates than that with the Wallgraft stent.
-
Citation: Luo XF, Nie L, Wang Z, Tsauo J, Liu LJ, Yu Y, Zhou B, Tang CW, Li X. Stent-grafts for the treatment of TIPS dysfunction: Fluency stent
vs Wallgraft stent. World J Gastroenterol 2013; 19(30): 5000-5005 - URL: https://www.wjgnet.com/1007-9327/full/v19/i30/5000.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i30.5000
Transjugular intrahepatic portosystemic shunts (TIPS) have been increasingly used for the management of portal hypertension complications in patients with cirrhosis[1,2]. However, shunt dysfunction is a major drawback of TIPS. The primary patency rate after 24 mo has been reported to be 40%-60% when bare stents were used[1]. Consequently, regular shunt surveillance and reintervention are required to maintain shunt patency.
TIPS dysfunction is the result of acute thrombosis within the stent or of pseudointimal hyperplasia within the TIPS tract in the liver parenchyma or along the outflow hepatic vein[3]. Both phenomena may be associated with a biliary fistula. Several experimental and clinical studies have shown that TIPS creation with an expanded polytetrafluoroethylene (ePTFE)-covered Viatorr stent can remarkably improve the long-term shunt patency[4-6]. Similarly, encouraging results have been obtained when the Viatorr stent was used for the treatment of TIPS dysfunction[7,8].
However, the Viatorr stent is not available in many countries. The Fluency stent, which is a non-dedicated ePTFE-covered stent-graft, has been utilized to establish a transjugular intrahepatic portosystemic shunt[9]. Currently, there is no relevant report on the clinical use of the Fluency stent for the management of TIPS dysfunction in the literature. In this study, we retrospectively evaluated the clinical efficacy of the Fluency stent and of the Wallgraft stent in TIPS revision.
This retrospective study was approved by the ethics committees of West China Hospital, Sichuan University. Between May 2007 and June 2011, patients who underwent TIPS revision by implantation of stent-grafts were analyzed. Patients were excluded if they already had a previous TIPS revision. Patients who underwent the insertion of a parallel shunt due to failed original shunt revision were also excluded. Thus, a total of 73 patients were evaluated in this study. This study group was further divided into two subgroups according to the stent-grafts received: 33 patients who underwent TIPS revision by implantation of the Fluency stent and 40 patients who underwent TIPS revision by implantation of the Wallgraft stent.
Written consent was obtained from each patient before the procedure. All procedures were performed by two experienced interventional radiologists. The patients were prepared and draped in the angiographic suite using local anesthesia. After puncturing the right internal jugular vein, a standard 10-F TIPS set (Cook Incorporated, Bloomington, United States) was introduced into the inferior vena cava. Once the previous shunt was accessed through the sheath using an angled hydrophilic guidewire (Terumo Company, Fijinomiya, Japan), a 5-F Cobra catheter (Terumo Company) was advanced into the superior mesenteric or splenic vein. Portography was performed with the 5-F catheter placed in the portal region, and the portosystemic pressure gradient (PPG) was measured. After dilating the stenotic or occluded shunt with a 10 mm angioplasty balloon catheter (Cordis, LJ Roden, the Netherlands), we advanced the 10-F sheath into the main portal vein. The stent-graft delivery set, which was either a Fluency stent or a Wallgraft stent, was then introduced into the sheath. The sheath was withdrawn into the inferior vena cava, and the stent-graft was released to cover the entire length of the shunt up to the junction of the hepatic vein and the inferior vena cava. Shunt venography was performed, and the PPG was measured again. Additional shunt dilation or an additional stent-graft implantation was performed if necessary. Patients with a PPG higher than 12 mmHg despite sufficient dilation of the shunt received prophylactic embolization of the varices with metal coils.
All patients received a single prophylactic dose of a second-generation cephalosporin 1 h before the procedure. Intravenous heparin (3000 U) was administered immediately after successful TIPS revision, except for those patients with a coagulation disorder. Subsequently, antiplatelet therapy with aspirin was maintained for life.
All patients were evaluated by the same medical team in the gastroenterology clinic according to the follow-up schedule. Doppler duplex ultrasonography (US) was performed 24 h and 1, 3 and 6 mo after the procedure, followed by every 6 mo thereafter or whenever recurrent TIPS dysfunction was suspected clinically. TIPS dysfunction was suspected on the US if the intrastent flow velocity was less than 60 cm/s or higher than 120 cm/s or if there was a change in the direction of the flow in the intrahepatic portal branches compared with previous US findings. TIPS dysfunction was defined as a shunt narrowing of more than 50%, a PPG higher than 12 mmHg or both. Primary patency was defined as the interval of time without an intervention.
The results were expressed as the mean ± SD. The 12 and 24 mo primary patency rates were analyzed and compared using the Kaplan-Meier method. The results were compared with the log-rank test. A P value of less than 0.05 was considered statistically significant. All calculations were performed using SPSS version 20.0 software for Windows.
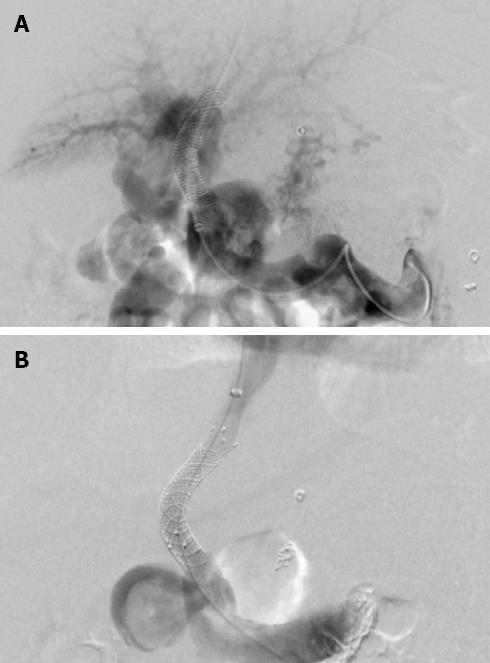
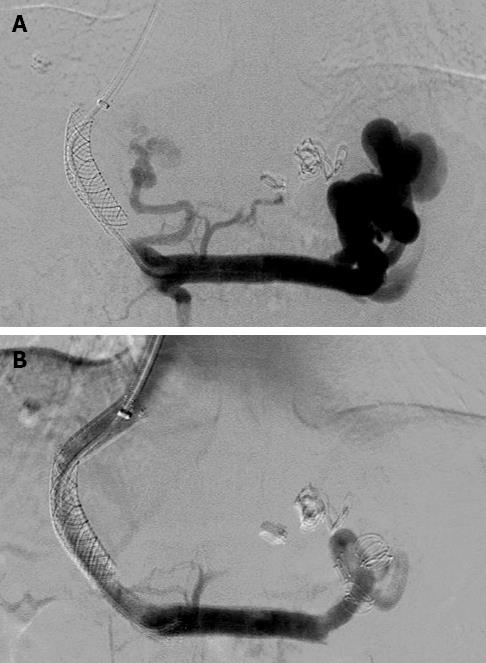
The patient characteristics at the time of the TIPS revision and indications for TIPS revision are documented and summarized in Table 1. Technical success was achieved in all 73 patients, and no severe complications occurred (Figures 1 and 2). Four patients had transient discomfort in the upper abdominal area. A total of 15 patients developed new hepatic encephalopathy after the procedure, including nine patients with the Fluency stent and six with the Wallgraft stent. Eleven of these patients were successfully managed by protein restriction and lactulose administration. One patient was treated with shunt reduction by implantation of an additional Fluency stent, which raised the PPG from 7 to 10 mmHg. Hepatic encephalopathy failed to improve in the remaining three patients.
| Characteristics | Fluency endoprosthesis | Wallgraft endoprosthesis | P value |
| Patients (n) | 33 | 40 | |
| Age (yr) | 51.4 ± 5.7 | 50.2 ± 8.5 | 0.491 |
| Sex (male/female) | 25/8 | 29/11 | 0.752 |
| Etiology | 0.242 | ||
| Hepatitis B | 21 | 31 | |
| Alcohol | 7 | 4 | |
| Other | 5 | 5 | |
| Child-Pugh classification | 0.564 | ||
| A | 6 | 8 | |
| B | 19 | 25 | |
| C | 8 | 7 | |
| TIPS indication | 0.946 | ||
| Variceal bleeding | 25 | 31 | |
| Ascites | 6 | 5 | |
| Other | 2 | 4 | |
| Indications for TIPS dysfunction | 0.762 | ||
| Abnormal US findings | 12 | 15 | |
| Variceal bleeding | 18 | 23 | |
| Ascites | 3 | 2 |
In the 33 TIPS revisions with the Fluency stent, the mean PPG was reduced from 18.5 ± 4.9 mmHg (range: 13-47 mmHg) to 7.4 ± 5.7 mmHg (range: 5-15 mmHg) after the procedure. A single stent-graft was used in 32 cases, and two stent-grafts were used in the other case because the stent was not terminated at the hepatocaval junction. All Fluency stents measured 10 mm in diameter. A PPG value below 12 mmHg after TIPS revision was not achieved in five patients, even though no obvious shunt stenosis was observed. Prophylactic embolization of the varices with metal coils was then performed in these patients.
In the 40 TIPS revisions with the Wallgraft stent, the mean PPG was reduced from 20.8 ± 3.7 mmHg (range: 12-51 mmHg) to 8.6 ± 3.1 mmHg (range: 6-17 mmHg) after the procedure. A single Wallgraft stent was used in 38 patients. Two patients were implanted with an additional stent because the shunt was not extended to the inferior vena cava. All Wallgraft stents measured 10 mm in diameter. The stent-grafts were placed after shunt dilation with 4 mm × 10 mm angioplasty balloons. In three patients, the PPG was not reduced to below 12 mmHg. These three patients received prophylactic embolization of the varices with metal coils. Another patient was treated with embolization of a massive splenorenal shunt to maintain sufficient portal flow in the stent (Figure 2).
The mean follow-up time in the Fluency stent group was 27.7 ± 11.6 mo (range: 3.5-46.5 mo). Of the 33 patients in this group, seven patients developed recurrent TIPS dysfunction during this period. Three patients presented with gastrointestinal hemorrhage, and the other four were diagnosed by US findings (Table 2). Angioplasty and subsequent implantation of a second stent-graft were performed in four of these patients, resulting in the restoration of blood flow within the shunt. One patient underwent orthotopic liver transplantation without revision. The other two patients refused TIPS revision. The primary shunt patency rates after TIPS revision with the Fluency stent were 91% at 12 mo and 85% at 24 mo.
| Fluency endoprosthesis | Wallgraft endoprosthesis | P value | |
| Patients (n) | 33 | 40 | |
| Hepatic encephalopathy | 9 | 6 | 0.196 |
| Variceal rebleeding | 7 | 19 | 0.020 |
| Shunt dysfunction | |||
| 12 mo | 3 | 9 | 0.722 |
| 24 mo | 4 | 15 | 0.039 |
The mean follow-up time in the Wallgraft stent group was 25.6 ± 13.2 mo (range: 3-48 mo). Of the 40 patients in this group, 19 patients developed recurrent TIPS dysfunction during this period. Eight patients presented with gastrointestinal hemorrhage, three presented with recurrent ascites, and the remaining eight were diagnosed by US findings (Table 2). Angioplasty and subsequent implantation of a second stent-graft were performed in the nine patients who had shunt restenosis or occlusion. Five patients were solely treated with angioplasty because the portography did not show any stenosis or portal vein thrombosis. Three patients were not revised because of overt hepatic encephalopathy. One patient underwent orthotopic liver transplantation. The primary shunt patency rates after TIPS revision with the Wallgraft stent were 78% at 12 mo and 63% at 24 mo. The primary shunt patency rates after TIPS revision were significantly better with the Fluency stent than with the Wallgraft stent (Log-rank test, P = 0.033, Figure 3).
The causes of TIPS dysfunction include acute thrombosis and pseudointimal hyperplasia in the parenchymal tract of the shunt or in the outflow hepatic vein[3,10]. During TIPS creation with bare stents, the dilatation of the liver parenchymal tract may cause laceration of the bile ducts, resulting in biliary-TIPS fistulas, which have been observed frequently in patients with acute thrombosis or recurrent shunt occlusions. The fibrotic or inflammatory healing response to the trauma of shunt creation induces the fibroblasts from adjacent liver stroma to differentiate into myofibroblasts and to then migrate through the stent mesh into the shunt lumen[3,10]. The overgrowth of pseudointimal hyperplasia could be responsible for stenosis or occlusion within the parenchymal tract.
The preliminary stent position within the outflow hepatic vein plays an important role in TIPS patency. The turbulence and shear stress from increased shunt flow could provoke the acceleration of pseudointimal hyperplasia and predispose the patient to shunt dysfunction. Additionally, late shortening of the self-expanding stent-grafts may occur, leaving the outflow hepatic vein at risk of subsequent intimal hyperplasia or the recoiling of an unsupported outflow section of the parenchymal tract[11]. Clark et al[12] demonstrated that the initial stent position within the hepatic venous outflow was predictive of shunt patency, with TIPS extending to the junction of the hepatic vein and the inferior vena cava having longer lifespans than shunts terminating in the hepatic vein. In this study, the primary patency rates at 12 mo were 36% ± 10% among patients with the outflow portion of the stent-grafts terminating in the hepatic vein and 58% ± 8% among patients with the outflow portion of the stent-grafts terminating at the hepatocaval junction[12]. Based on previous studies and our experience, we were careful about bridging the complete tract to the inferior vena cava in the present series.
A few experimental and clinical studies have verified the application of ePTFE-covered stent-grafts in de novo TIPS creation and TIPS revision[4,7,8]. ePTFE was utilized as a cover material for stent-grafts, separating the blood flow within the shunt from the liver parenchyma and from the injured outflow hepatic vein. After TIPS creation with the ePTFE-covered stent-graft, the shunt flow was maintained by inhibiting the overgrowth of pseudointimal hyperplasia in the parenchymal tract or along the outflow hepatic vein. Echenagusia et al described the application of ePTFE-covered stent-grafts in the treatment of TIPS stenosis or occlusion in 12 patients. After TIPS revision, the primary patency rates were 100% at 12 mo and 88.8% at 24 mo[7]. More recently, Jirkovsky et al reported a clinical study in which 121 episodes of dysfunctional TIPS were evaluated retrospectively. The primary patency rates after 12 and 24 mo were 49.7% and 25.3%, respectively, in conventional angioplasty, 74.9% and 64.9%, respectively, with bare stents, 75.2% and 64.5%, respectively, with ePTFE-covered stent-grafts and 88.1% and 80.8%, respectively, with Viatorr stent-grafts. These results showed a tendency favoring ePTFE-covered stent-grafts, especially the Viatorr stent[13]. Unfortunately, the Viatorr stent is not commercially available in mainland China.
Compared with previously published reports, our study has a relatively large number of patients who underwent TIPS revision (n = 73). In our series, the primary shunt patency rates at 12 and 24 mo were 91% and 85%, respectively, with the Fluency stent and 78% and 63%, respectively, with the Wallgraft stent. After TIPS revision by implantation of a second stent-graft, the primary shunt patency rates were significantly better with the Fluency stent than with the Wallgraft stent. The long-term patency of the Fluency stent compared well with the previously published results of ePTFE-covered stent-grafts[13]. To the best of our knowledge, TIPS revision with angioplasty alone may have barely satisfactory short-term patency but results in a high incidence of recurrent shunt dysfunction. Lining the dysfunctional TIPS with a second stent-graft not only enables the restoration of shunt function but may also improve the configuration of the TIPS to prevent future restenosis.
The Fluency stent is a PTFE-encapsulated grid-like cylinder composed of a biocompatible nickel-titanium alloy. The deployment of a Fluency stent is easy because the position of this type of stent-graft is very precise. Based on our data, one patient (3%) with the Fluency stent and two patients (5%) with the Wallgraft stent required additional stent-graft implantation, partly due to previous stent malposition. The Fluency stent is one of the two commercially available stent-grafts in mainland China, the other being the Wallgraft stent[9,14]. Wu et al[9] performed a retrospective study on shunt patency in patients treated with TIPS creation using the Fluency stent. The rates of recurrent bleeding, shunt occlusion, hepatic encephalopathy and mortality were 0.03%, 0.0%, 16.7% and 0% after 6.16 ± 3.89 mo of follow-up. Although the follow-up time was not long enough, these results suggested that the Fluency stent was effective in TIPS creation and had a favorable patency rate. The Wallgraft stent is a polyethylene terephthalate-covered stent-graft and has been considered unsuitable for initial TIPS creation according to experimental studies[12,15]. However, the role of the Wallgraft stent in TIPS revision remains unknown.
There are several limitations of the present study that warrant consideration. This study is a single-institution, retrospective study, which increases the likelihood of systematic bias. Ideally, a randomized two-arm clinical trial should be designed to compare the Fluency stent with the Wallgraft stent. Additionally, the decision on the stent-graft selected for revision was based on the operator`s preference.
This study represents one of the largest published case series of patients with dysfunctional TIPS who underwent shunt revision with stent-grafts. Our results suggest that completing TIPS revision with the Fluency stent is safe and effective. Although large prospective studies with longer follow-up periods are needed, our analysis indicates that TIPS revision using the Fluency stent provided better shunt patency than that using the Wallgraft stent in the medium-term. Based on our institutional experience and on results from the literature, the ePTFE-covered Fluency stent could be a valuable solution for TIPS revision.
Transjugular intrahepatic portosystemic shunts (TIPS) has been widely used in the treatment of complications of portal hypertension. However, shunt dysfunction is a main defect of TIPS. Previously clinical studies have demonstrated that de novo TIPS creation and shunt revision with an expanded polytetrafluoroethylene (ePTFE)-covered stent could improve the long-term shunt patency.
In this study, the authors demonstrated that TIPS revision with the ePTFE-covered Fluency stent has higher medium-term patency rates than that with the polyethylene terephthalate-covered Wallgraft stent which are commercially available in mainland China.
There are few data on the clinical use of Fluency stent or Wallgraft strent for the management of TIPS dysfunction in the literature. This is believed to be the first study to report that TIPS revision with Fluency stent could provide better second shunt patency.
Considering TIPS dysfunction would still be an imprortant clinical issue in the near future, this study may illustrate a useful management strategy in the treatment of TIPS dysfunction.
The Fluency stent is a polytetrafluoroethylene-encapsulated grid-like cylinder composed of a biocompatible nickel-titanium alloy.
An interesting publication in which authors show that TIPS revision with the Fluency stent has higher medium-term patency rates than that with the Wallgraft stent. This study will be of interest and the paper is clearly written.
| 1. | Feldstein VA, Patel MD, LaBerge JM. Transjugular intrahepatic portosystemic shunts: accuracy of Doppler US in determination of patency and detection of stenoses. Radiology. 1996;201:141-147. [PubMed] |
| 2. | Garcia-Tsao G, Bosch J. Management of Varices and Variceal Hemorrhage in Cirrhosis. N Engl J Med. 2010;362:823-832. [RCA] [DOI] [Full Text] [Cited by in Crossref: 639] [Cited by in RCA: 649] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 3. | Cura M, Cura A, Suri R, El-Merhi F, Lopera J, Kroma G. Causes of TIPS dysfunction. AJR Am J Roentgenol. 2008;191:1751-1757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 80] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 4. | Haskal ZJ. Improved patency of transjugular intrahepatic portosystemic shunts in humans: creation and revision with PTFE stent-grafts. Radiology. 1999;213:759-766. [PubMed] |
| 5. | Hausegger KA, Karnel F, Georgieva B, Tauss J, Portugaller H, Deutschmann H, Berghold A. Transjugular intrahepatic portosystemic shunt creation with the Viatorr expanded polytetrafluoroethylene-covered stent-graft. J Vasc Interv Radiol. 2004;15:239-248. [PubMed] |
| 6. | Vignali C, Bargellini I, Grosso M, Passalacqua G, Maglione F, Pedrazzini F, Filauri P, Niola R, Cioni R, Petruzzi P. TIPS with expanded polytetrafluoroethylene-covered stent: results of an Italian multicenter study. AJR Am J Roentgenol. 2005;185:472-480. [PubMed] |
| 7. | Echenagusia M, Rodriguez-Rosales G, Simo G, Camuñez F, Bañares R, Echenagusia A. Expanded PTFE-covered stent-grafts in the treatment of transjugular intrahepatic portosystemic shunt (TIPS) stenoses and occlusions. Abdom Imaging. 2005;30:750-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Cejna M, Peck-Radosavljevic M, Thurnher S, Schoder M, Rand T, Angermayr B, Lammer J. ePTFE-covered stent-grafts for revision of obstructed transjugular intrahepatic portosystemic shunt. Cardiovasc Intervent Radiol. 2002;25:365-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 37] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Wu X, Ding W, Cao J, Han J, Huang Q, Li N, Li J. Favorable clinical outcome using a covered stent following transjugular intrahepatic portosystemic shunt in patients with portal hypertension. J Hepatobiliary Pancreat Sci. 2010;17:701-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Siegerstetter V, Huber M, Ochs A, Blum HE, Rössle M. Platelet aggregation and platelet-derived growth factor inhibition for prevention of insufficiency of the transjugular intrahepatic portosystemic shunt: a randomized study comparing trapidil plus ticlopidine with heparin treatment. Hepatology. 1999;29:33-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Tesdal IK, Jaschke W, Bühler M, Adamus R, Filser T, Holm E, Georgi M. Transjugular intrahepatic portosystemic shunting (TIPS) with balloon-expandable and self-expanding stents: technical and clinical aspects after 3 1/2 years’ experience. Cardiovasc Intervent Radiol. 1997;20:29-37. [PubMed] |
| 12. | Clark TW, Agarwal R, Haskal ZJ, Stavropoulos SW. The effect of initial shunt outflow position on patency of transjugular intrahepatic portosystemic shunts. J Vasc Interv Radiol. 2004;15:147-152. [PubMed] |
| 13. | Jirkovsky V, Fejfar T, Safka V, Hulek P, Krajina A, Chovanec V, Raupach J, Lojik M, Vanasek T, Renc O. Influence of the secondary deployment of expanded polytetrafluoroethylene-covered stent grafts on maintenance of transjugular intrahepatic portosystemic shunt patency. J Vasc Interv Radiol. 2011;22:55-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Wu X, Ding W, Cao J, Fan X, Li J. Clinical outcome using the fluency stent graft for transjugular intrahepatic portosystemic shunt in patients with portal hypertension. Am Surg. 2013;79:305-312. [PubMed] |
| 15. | Krajina A, Lojik M, Chovanec V, Raupach J, Hulek P. Stent-grafts in TIPS. Abdom Imaging. 2004;29:53-59. [PubMed] |
P- Reviewers Elena V, Hasegawa K, Kadusevicius E, Zapata R S- Editor Gou SX L- Editor A E- Editor Li JY