Published online Jul 28, 2013. doi: 10.3748/wjg.v19.i28.4590
Revised: May 20, 2013
Accepted: June 1, 2013
Published online: July 28, 2013
Processing time: 117 Days and 16.3 Hours
AIM: To compare matrix metalloproteinase (MMP)-9 and tissue inhibitor of metalloproteinase (TIMP)-1 in gastric ulcer (GU) and chronic superficial gastritis (CSG).
METHODS: This study enrolled 63 patients with GU and 25 patients with CSG. During upper gastroduodenal endoscopy, we took samples of gastric mucosa from the antrum and ulcer site from patients with GU, and samples of antral mucosa from patients with CSG. Mucosal biopsy tissues were cultured for 24 h, and the culture supernatant was measured for levels of MMP-9 and TIMP-1. After receiving eradication therapy for Helicobacter pylori (H. pylori) and 8 wk proton-pump inhibitor therapy for GU, follow-up endoscopy examination was performed after 6 mo and whenever severe symptoms occurred.
RESULTS: Levels of MMP-9 and TIMP-1 at the ulcer site or in the antrum were significantly higher in GU than CSG patients. MMP-9 levels at the ulcer site were significantly higher than in the antrum in GU patients, and had a significantly positive correlation with TIMP-1. MMP-9 levels were significantly higher in H. pylori-positive than H. pylori-negative GU and CSG patients. Levels of MMP-9 or TIMP-1 at the ulcer site were associated with the histological severity of activity and inflammation. About 57 GU patients were followed up, and seven had GU recurrence. H. pyloriinfection and MMP-9 levels were risk factors for the recurrence of GU adjusted for age and sex by multiple logistic regression analysis.
CONCLUSION: MMP-9 may perform an important function in gastric ulcer formation and recurrence.
Core tip: Gastric ulcer is a multifaceted process including acid secretion, reactive oxygen species generation, prostaglandin inhibition, and extracellular matrix degradation. Gastric mucosal damage is directly associated with extracellular matrix degradation in which matrix metalloproteinases (MMPs) play a crucial role. In this study, the authors compared MMP-9 and tissue inhibitor of metalloproteinase-1 levels in patients with gastric ulcer or chronic superficial gastritis.
- Citation: Li SL, Zhao JR, Ren XY, Xie JP, Ma QZ, Rong QH. Increased expression of matrix metalloproteinase-9 associated with gastric ulcer recurrence. World J Gastroenterol 2013; 19(28): 4590-4595
- URL: https://www.wjgnet.com/1007-9327/full/v19/i28/4590.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i28.4590
Gastric ulcer (GU) is a multifaceted process including acid secretion, reactive oxygen species generation, prostaglandin inhibition, and extracellular matrix (ECM) degradation[1]. Gastric mucosal damage is directly associated with extracellular matrix degradation in which matrix metalloproteinases (MMPs) play a crucial role[2]. MMPs are endopeptidases that perform important functions in ECM remodeling, cell proliferation, and inflammatory processes. Recent studies have indicated that gastric ulceration is associated with cleaving and remodeling of the ECM by MMPs[3,4]. In several animal studies of GU, attention has focused on the role of MMP-1, MMP-2, MMP-3, MMP-9 and MMP-13[4-6]. In particular, MMP-9 is important in the early phase of chronic GU[7]. However, these data are mostly derived from animal studies, and human clinical data remains rare, especially in assessing MMPs expression in GU formation and recurrence. Here, we compared MMP-9 and tissue inhibitor of metalloproteinase (TIMP)-1 in patients with GU or chronic superficial gastritis (CSG), and how they correlated with GU recurrence.
We examined 63 consecutive patients with GU and 25 with CSG who were diagnosed during upper gastroduodenal endoscopic examination at Liaocheng People’s Hospital between January and December 2010. The patients were enrolled in the study if they met the following criteria: (1) age 18-75 years; (2) no nonsteroidal anti-inflammatory drugs (NSAIDs), antibiotics, or bismuth compounds in the 2 wk prior to the study; and (3) acute phase GU. Patients were excluded as follows: (1) a history of gastric or duodenal surgery; (2) allergy to the study drugs; (3) required long-term treatment with NSAIDs, corticosteroids, aspirin, or anticoagulant agents; (4) pregnant women; and (5) active cancer, acute serious medical illness, or terminal illness. The study protocol was approved by the Ethics Committee of our institution. All patients gave written informed consent before participating in the study.
During endoscopic examination, three antral specimens were taken from all patients, including one for rapid urease test (Triwizard, Fujian, China), one for histological examination, and one for in vitro culture for measurement of levels of MMP-9 and TIMP-1. Two additional specimens were taken from the margin of the ulcer in GU patients; one for histological examination and one for in vitro cultures for MMP-9 and TIMP-1.
Helicobacter pylori (H. pylori) infection was confirmed by positive results for at least two of three diagnostic tests, namely rapid urease test, 13C-urea breath test, or identification of the organism on tissue sections by Giemsa stain. Absence of infection was defined by a negative result in all three tests. Cases satisfying at least two test results were defined as positive for infection.
Mucosal biopsy tissues were weighed and then cultured in a 5% CO2 incubator for 24 h in a culture bottle (Xiangya Gene Technology, Changsha, China) containing 5 mL RPMI 1640 medium with 5% heat-inactivated fetal calf serum, 15 mmol/L HEPES buffer, 100 U/mL penicillin-G, 100 mg/mL streptomycin and 10 mg/mL phytohemagglutinin-P. At the end of the culture period, the supernatant was drawn off and stored at -70 °C until measured by enzyme-linked immunosorbent assay for MMP-9 and TIMP-1 (Boster, Wuhan, China). A modified version of the Lowry method was used to assay total protein in biopsy homogenates (Boster). The amount of MMP-9 and TIMP-1 was expressed relative to protein content in the biopsy tissue homogenate (per milligram of biopsy protein).
Tissue sections stained with hematoxylin-eosin were used to assess activity, inflammation, glandular atrophy, and intestinal metaplasia. Grading was done on a four-item scale of 0, 1, 2 and 3, corresponding to none, mild, moderate and severe, respectively, in accordance with the updated Sydney system[8].
H. pylori-positive GU patients received eradication treatment with triple therapy using lansoprazole (30 mg, bid), amoxicillin (1000 mg, bid), and clarithromycin (500 mg, bid) for 1 wk, and subsequently received lansoprazole (30 mg, qd) for 8 wk, whereas H. pylori-negative GU patients received lansoprazole (30 mg, qd) for 8 wk. After that, the presence of the ulcer scar was confirmed by endoscopy, and six patients who still had active ulcer were excluded from follow-up. An additional 13C urea breath test or rapid urease test was conducted to assess the final H. pylori status after 6 mo for all GU patients. Follow-up endoscopy examination was performed at the end of the 6 mo and whenever severe symptoms occurred. Ulcer recurrence was defined as a lesion of white coat with a distinct depressed area and a diameter of ≥ 5 mm.
All data were expressed as mean ± SD. Frequency variables were compared using the χ2 test. Quantitative variables were analyzed using Student’s t test. Correlation was analyzed by Pearson’s correlation or Spearman’s rank correlation. Logistic analysis was used for risk factors for GU recurrence. SPSS version 17.0 (Chicago, IL, United States) was used, and P < 0.05 was regarded as significant.
A total of 88 patients were enrolled. The 63 GU patients included 31 males and 32 females, with an average age of 47.8 years (range, 24-71 years). The 25 CSG patients included 15 males and 10 females, with an average age of 51.3 years (range, 29-68 years). Fifty-four GU patients were positive and nine were negative for H. pylori, and 10 CSG patients were positive and 15 were negative for H. pylori (Table 1). There were no significant difference between the GU and CSG patients in age and sex, except in H. pylori infection (χ2 = 18.86, P < 0.01).
| Characteristics | GU group (n = 63) | CSG group (n = 25) |
| Sex | ||
| Male | 31 (49.2) | 15 (60.0) |
| Female | 32 (50.8) | 10 (40.0) |
| Age, yr (mean ± SD) | 47.8 ± 12.9 | 51.3 ± 8.5 |
| H. pylori infection | ||
| Positive | 54 (85.7) | 10 (40.0) |
| Negative | 9 (14.3) | 15 (60.0) |
| Position of ulcer | ||
| Corpus | 18 (28.6) | - |
| Antrum | 45 (71.4) | - |
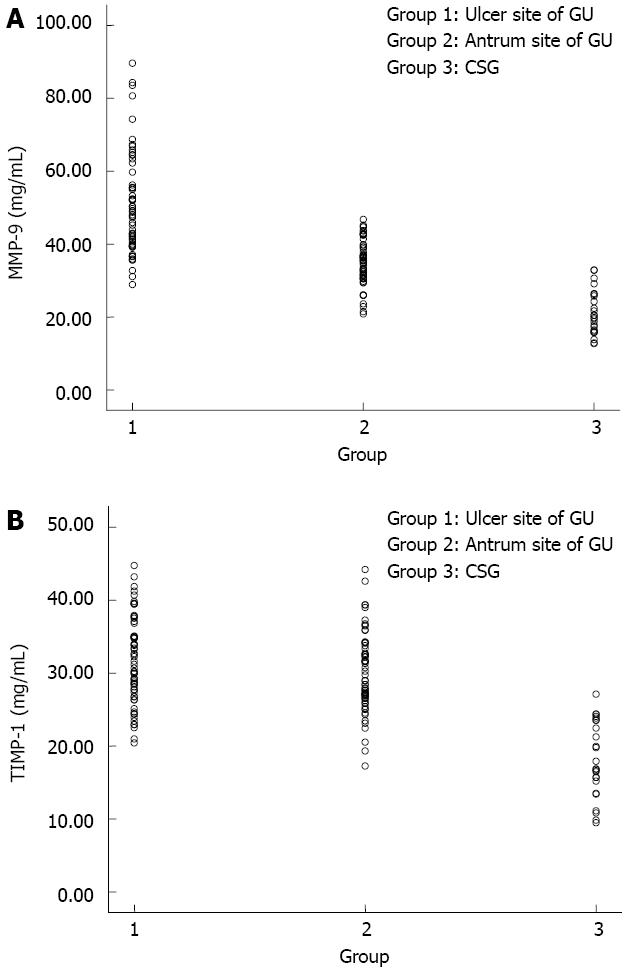
In all patients, MMP-9 levels (Figure 1A) were significantly higher at the margin of the ulcer (50.50 ± 13.72 mg/mL, t = 13.96, P < 0.01) or in the antrum (35.08 ± 6.07 mg/mL, t = 9.78, P < 0.01) of the GU patients than the CSG patients (21.06 ± 6.04 mg/mL). In the GU patients, MMP-9 levels were significantly higher (t = 8.16, P < 0.01) at the margin of the ulcer (50.50 ± 13.72 mg/mL) than in the antrum (35.08 ± 6.07 mg/mL).
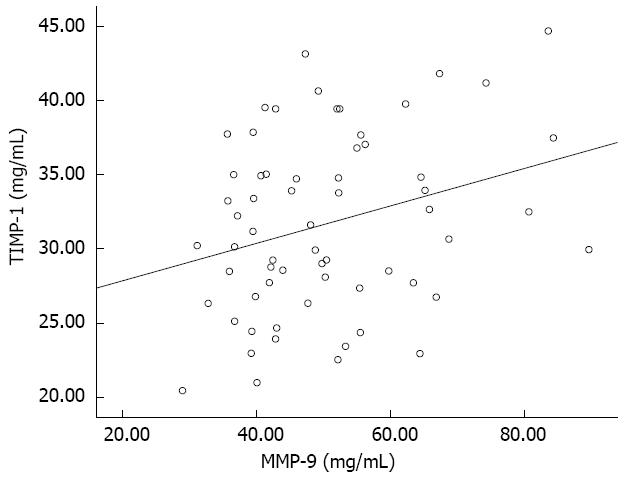
With regard to TIMP-1 levels (Figure 1B), a significant difference was seen between at the margin of the ulcer (18.17 ± 5.14 mg/mL vs 31.71 ± 5.97 mg/mL, t = 9.96, P < 0.01) or in the antrum (18.17 ± 5.14 mg/mL vs 30.07 ± 5.42 mg/mL, t = 9.42, P < 0.01) of the GU and CSG patients. There was no significant difference between the antrum and the margin of the ulcer (30.07 ± 5.42 mg/mL vs 31.71 ± 5.97 mg/mL, t = 1.62, P = 0.108) of the GU patients. A significant positive correlation was observed between levels of MMP-9 and TIMP-1 (50.50 ± 13.72 mg/mL vs 31.71 ± 5.97 mg/mL, r = 0.29, P = 0.021) at the margin of the ulcer in the GU patients (Figure 2).
For the GU patients, ulcers were classified according to their anatomical location, that is, 18 patients had corpus or fundus ulcers, and 45 had antral or prepyloric ulcers. Both MMP-9 (47.45 ± 11.92 mg/mL vs 51.72 ± 14.32 mg/mL, t = -1.12, P = 0.267) and TIMP-1 (30.85 ± 5.93 mg/mL vs 32.05 ± 6.02 mg/mL, t = -0.72, P = 0.476) levels were not significantly different at the margin of the ulcer between corpus or fundus ulcers and antral or prepyloric ulcers.
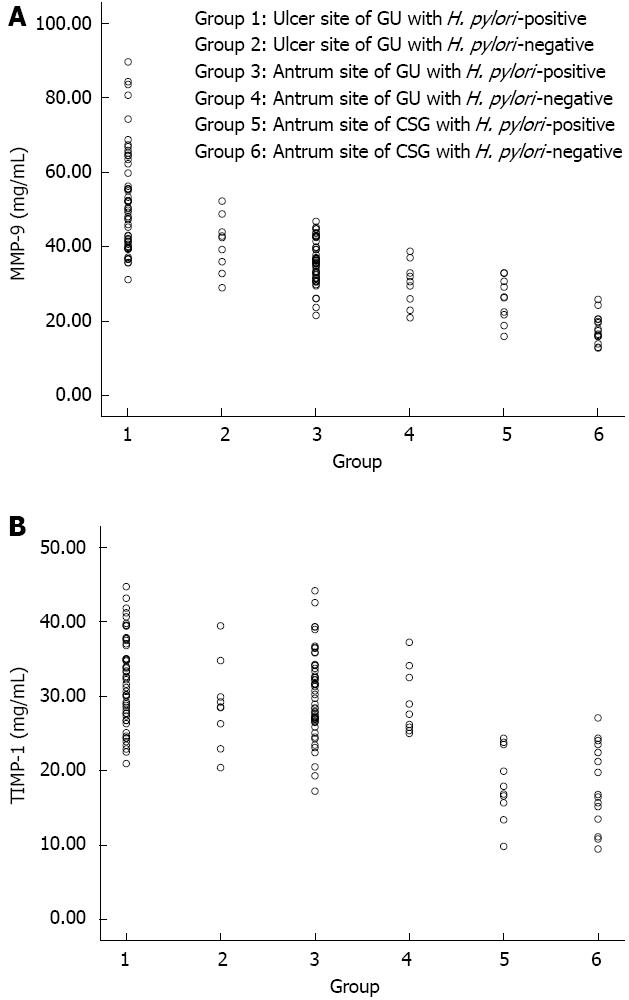
In the GU patients, levels of MMP-9 (Figure 3A) at the margin of the ulcer or in the antrum in the H. pylori-positive patients were significantly higher than in the H. pylori-negative patients (52.12 ± 13.90 mg/mL vs 40.77 ± 7.43 mg/mL, t = 2.38, P = 0.020; 35.92 ± 5.72 mg/mL vs 30.03 ± 6.01 mg/mL, t = 2.84, P = 0.006, respectively). In the CSG patients, levels of MMP-9 in the H. pylori-positive patients were significantly higher than in the H. pylori-negative patients (25.70 ± 5.89 mg/mL vs 17.96 ± 3.82 mg/mL, t = 4.00, P = 0.001).
In the GU patients, levels of TIMP-1(Figure 3B) at the margin of the ulcer or in the antrum in the H. pylori-positive patients did not differ significantly from those in the H. pylori-negative patients (32.18 ± 5.94 mg/mL vs 28.91 ± 5.71 mg/mL, t = 1.53, P = 0.130; 30.21 ± 5.60 mg/mL vs 29.22 ± 4.40 mg/mL, t = 0.50, P = 0.617, respectively). In the CSG patients, levels of TIMP-1 in the H. pylori-positive patients also did not differ significantly from those in the H. pylori-negative patients (18.22 ± 4.76 mg/mL vs 18.13 ± 5.55 mg/mL, t = 0.04, P = 0.967). Levels of MMP-9 (35.92 ± 5.72 mg/mL) and TIMP-1 (30.21 ± 5.60 mg/mL) in the antrum in the H. pylori-positive GU patients were significantly higher (t = 5.17, P < 0.01; t = 6.35, P < 0.01, respectively) than in the H. pylori-positive CSG patients (25.70 ± 5.89 mg/mL and 18.22 ± 4.76 mg/mL, respectively).
For the GU patients, we compared levels of MMP-9 and TIMP-1 in vitro with the severity of histological gastritis (activity, inflammation, atrophy, and metaplasia) at the margin of the ulcer. A significant association was identified between levels of MMP-9 or TIMP-1 and the histological degree of activity and inflammation, but not with the degree of glandular atrophy or intestinal metaplasia (Table 2).
Of the 63 GU patients, six were excluded because they still had active ulcer after 8 wk PPI treatment. Among 57 follow-up patients, seven (12.3%) had recurrence at the time of or before endoscopy examination at the end of 6 mo. There were nine patients with H. pylori infection. A multivariate logistic regression analysis adjusted for age and sex demonstrated that H. pylori infection (OR = 17.705, 95%CI: 2.091-149.929, P = 0.008) and MMP-9 levels (OR = 1.078, 95%CI: 1.007-1.154, P = 0.031) were GU recurrence risk factors.
We found that MMP-9 production was increased in the gastric mucosa at the margin of the ulcer in GU patients. This increase had a significant positive correlation with production of TIMP-1, an MMP-9 inhibitor. Several studies have investigated the association between MMPs and GU. Indomethacin-induced ulcerated gastric tissues exhibited about 12-fold higher pro-MMP-9 activity as compared to control tissues. Similarly, ethanol induced about 22-fold higher pro-MMP-9 activities in rat gastric tissues[5]. One study showed that significant up-regulation of MMP-9 expression in indomethacin-induced GU in mice was correlated with increased activity of activator protein-1, and oxidative stress was preceded by chronic inflammation that enhanced expression of MMP-9[9].
MMPs have recently been shown to be up-regulated in gastric epithelial cells infected with H. pylori, and might contribute to the pathogenesis of peptic ulcer. Our study showed that MMP-9 levels were associated with H. pylori infection. Significantly elevated serum levels of MMP-9 and reduced serum levels of TIMP-1 have been demonstrated in patients with H. pylori gastritis as compared to H. pylori-negative controls[10]. H. pylori-infected GUs had even higher MMP-9 and TIMP-1 expression in epithelial cells than in NSAID-related GU[11]. One study showed that there were no significant differences in serum levels of MMP-9 between H. pylori-positive and H. pylori-negative children[12].
We showed that levels of MMP-9 correlated with the histological degree of activity and inflammation at the margin of the ulcer. In BALB/c mice, NSAIDs caused dose-dependent induction in MMP-9 activity and expression in ulcerated gastric tissues, along with significant infiltration of inflammatory cells and disruption of the gastric mucosal layer[13]. GU is associated with infiltration of the gastric mucosa by neutrophils, lymphocytes, monocytes, and plasma cells. Inflammatory cells secrete an array of pro-inflammatory cytokines and growth factors (epidermal growth factor, platelet-derived growth factor, transforming growth factor-β, vascular endothelial growth factor, angiopoietins). MMPs can be induced by the activity of pro-inflammatory cytokines such as tumor necrosis factor-α, interleukin (IL)-1, IL-6 and IL-8[1,9,14]. Oxidative stress is preceded by chronic inflammation that enhances the expression of MMP-9. By decreased synthesis and secretion of MMP-9, as well as infiltration of inflammatory cells and oxidative damage in gastric tissues, we may block or heal acute GU[3,13].
The C/C genotype of MMP-9-1562 C/T gene polymorphism might be associated with H. pylori infection[15]. H. pylori infection increases the secretion of MMPs in the gastric mucosa, leading to severe mucosal damage. Genetic variations in the MMP-9 gene may be part of a complex genetic risk profile to develop GU in chronic H. pylori infection[16]. MMP-9 levels decrease consistently and significantly after successful H. pylori eradication, whereas the elevated levels remain unchanged when treatment fails[17].
We found that patients with high levels of MMP-9 and H. pylori infection were the risk factors for GU recurrence. H. pylori infection associated with GU recurrence has been verified[18]. Some studies found that severity of GU was strongly correlated with increased secretion of proMMP-9 in ethanol-induced acute gastric ulceration in rats[19,20]. Higher levels of MMP-9 in chronic wound fluid correlate with a clinically worse wound[21]. Measurements of MMP-9 and TIMP-1 may help to identify diabetic foot ulcers at risk of poor healing[22]. These findings suggest that MMP-9 may be indicative of inflammation and poor wound healing, and that we can reduce GU recurrence by inhibition of MMP-9 activity.
In conclusion, we observed increased expression of MMP-9 and TIMP-1 in GU patients and found a significantly positive correlation between MMP-9 and TIMP-1 production at the margin of the ulcer. Increased production of MMP-9 was significantly correlated with increased GU recurrence. These results suggest that MMP-9 may play an important role in the occurrence of GU. A clearer understanding of the significance and implications of these findings may provide insights into ulcer healing. Further study is needed to clarify the roles of MMP-9 and elucidate any potential clinical implications in the healing of GU.
Gastric ulcer (GU) is a multifaceted process including acid secretion, reactive oxygen species generation, prostaglandin inhibition, and extracellular matrix (ECM) degradation. Gastric mucosal damage is directly associated with ECM degradation in which matrix metalloproteinases (MMPs) play a crucial role. In several animal studies of GU, attention has focused on the role of MMP-1, MMP-2, MMP-3, MMP-9 and MMP-13. However, these data are mostly derived from animal studies, and human clinical data remains rare, especially in assessing MMPs expression in GU formation and recurrence.
In this study, the authors compared levels of MMP-9 and tissue inhibitor of metalloproteinase (TIMP)-1 in GU patients, and how they correlated with GU recurrence.
This study enrolled 63 patients with GU and 25 patients with superficial gastritis (CSG). Samples of gastric mucosa from the antrum and the ulcer site were harvested from GU patients and samples of antral mucosa were taken from CSG patients during upper gastroduodenal endoscopy. Levels of MMP-9 and TIMP-1 at the ulcer site or in the antrum were significantly higher in GU than CSG patients. MMP-9 levels at the ulcer site were significantly higher than in the antrum in GU patients, and had a significantly positive correlation with TIMP-1. MMP-9 levels were significantly higher in Helicobacter-pylori-positive than -negative GU and CSG patients. Levels of MMP-9 or TIMP-1 at the ulcer site were associated with the histological severity of activity and inflammation.
The authors found that the MMP-9 may perform an important function in gastric ulcer formation and recurrence.
This study compared MMP-9 and TIMP-1 levels in GU and CSG patients. The authors measured the levels of MMP-9 and TIMP-1 from the tissues. The levels of MMP-9 and TIMP-1 at the ulcer site or in the antrum were significantly higher in GU than CSG patients. MMP-9 levels at the ulcer site were significantly higher than in the antrum in GU patients, and had a significantly positive correlation with TIMP-1. They concluded that MMP-9 may perform an important function in gastric ulcer formation and recurrence.
| 1. | Tarnawski AS, Ahluwalia A. Molecular mechanisms of epithelial regeneration and neovascularization during healing of gastric and esophageal ulcers. Curr Med Chem. 2012;19:16-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 98] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 2. | Ganguly K, Kundu P, Banerjee A, Reiter RJ, Swarnakar S. Hydrogen peroxide-mediated downregulation of matrix metalloprotease-2 in indomethacin-induced acute gastric ulceration is blocked by melatonin and other antioxidants. Free Radic Biol Med. 2006;41:911-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 68] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 3. | Chakraborty S, Stalin S, Das N, Choudhury ST, Ghosh S, Swarnakar S. The use of nano-quercetin to arrest mitochondrial damage and MMP-9 upregulation during prevention of gastric inflammation induced by ethanol in rat. Biomaterials. 2012;33:2991-3001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 103] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 4. | Kim SJ, Park YS, Paik HD, Chang HI. Effect of anthocyanins on expression of matrix metalloproteinase-2 in naproxen-induced gastric ulcers. Br J Nutr. 2011;106:1792-1801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | Singh LP, Mishra A, Saha D, Swarnakar S. Doxycycline blocks gastric ulcer by regulating matrix metalloproteinase-2 activity and oxidative stress. World J Gastroenterol. 2011;17:3310-3321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Pradeepkumar Singh L, Vivek Sharma A, Swarnakar S. Upregulation of collagenase-1 and -3 in indomethacin-induced gastric ulcer in diabetic rats: role of melatonin. J Pineal Res. 2011;51:61-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Kim SJ, Lee HJ, Kim BS, Lee D, Lee SJ, Yoo SH, Chang HI. Antiulcer activity of anthocyanins from Rubus coreanus via association with regulation of the activity of matrix metalloproteinase-2. J Agric Food Chem. 2011;59:11786-11793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 8. | Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3221] [Cited by in RCA: 3622] [Article Influence: 120.7] [Reference Citation Analysis (6)] |
| 9. | Ganguly K, Swarnakar S. Chronic gastric ulceration causes matrix metalloproteinases-9 and -3 augmentation: alleviation by melatonin. Biochimie. 2012;94:2687-2698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 10. | Rautelin HI, Oksanen AM, Veijola LI, Sipponen PI, Tervahartiala TI, Sorsa TA, Lauhio A. Enhanced systemic matrix metalloproteinase response in Helicobacter pylori gastritis. Ann Med. 2009;41:208-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 67] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 11. | Cheng HC, Yang HB, Chang WL, Chen WY, Yeh YC, Sheu BS. Expressions of MMPs and TIMP-1 in gastric ulcers may differentiate H. pylori-infected from NSAID-related ulcers. Scientific World Journal. 2012;2012:539316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Rautelin H, Tervahartiala T, Lauhio A, Sorsa T, Kolho KL. Assessment of systemic matrix metalloproteinase and their regulator response in children with Helicobacter pylori gastritis. Scand J Clin Lab Invest. 2010;70:492-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Ganguly K, Swarnakar S. Induction of matrix metalloproteinase-9 and -3 in nonsteroidal anti-inflammatory drug-induced acute gastric ulcers in mice: regulation by melatonin. J Pineal Res. 2009;47:43-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 14. | Tarnawski AS. Cellular and molecular mechanisms of gastrointestinal ulcer healing. Dig Dis Sci. 2005;50 Suppl 1:S24-S33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 294] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 15. | Shan QW, Jing CX, Wang LL, Lu ZL, Tang Q, Yun X, Lian SJ. Relationship between gene polymorphisms in MMP-9 and Helicobacter pylori-related upper gastrointestinal disease in children. Zhongguo Dangdai Erke Zazhi. 2010;12:262-266. [PubMed] |
| 16. | Hellmig S, Ott S, Rosenstiel P, Robert Fölsch U, Hampe J, Schreiber S. Genetic variants in matrix metalloproteinase genes are associated with development of gastric ulcer in H. Pylori infection. Am J Gastroenterol. 2006;101:29-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Kubben FJ, Sier CF, Schram MT, Witte AM, Veenendaal RA, van Duijn W, Verheijen JH, Hanemaaijer R, Lamers CB, Verspaget HW. Eradication of Helicobacter pylori infection favourably affects altered gastric mucosal MMP-9 levels. Helicobacter. 2007;12:498-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Malfertheiner P, Megraud F, O’Morain C, Bazzoli F, El-Omar E, Graham D, Hunt R, Rokkas T, Vakil N, Kuipers EJ. Current concepts in the management of Helicobacter pylori infection: the Maastricht III Consensus Report. Gut. 2007;56:772-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1396] [Cited by in RCA: 1357] [Article Influence: 71.4] [Reference Citation Analysis (1)] |
| 19. | Pradeepkumar Singh L, Kundu P, Ganguly K, Mishra A, Swarnakar S. Novel role of famotidine in downregulation of matrix metalloproteinase-9 during protection of ethanol-induced acute gastric ulcer. Free Radic Biol Med. 2007;43:289-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 20. | Swarnakar S, Mishra A, Ganguly K, Sharma AV. Matrix metalloproteinase-9 activity and expression is reduced by melatonin during prevention of ethanol-induced gastric ulcer in mice. J Pineal Res. 2007;43:56-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 62] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 21. | Rayment EA, Upton Z, Shooter GK. Increased matrix metalloproteinase-9 (MMP-9) activity observed in chronic wound fluid is related to the clinical severity of the ulcer. Br J Dermatol. 2008;158:951-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 181] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 22. | Liu Y, Min D, Bolton T, Nubé V, Twigg SM, Yue DK, McLennan SV. Increased matrix metalloproteinase-9 predicts poor wound healing in diabetic foot ulcers. Diabetes Care. 2009;32:117-119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 245] [Cited by in RCA: 284] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
P- Reviewers Hellmig S, Morante A, Sawicki G S- Editor Gou SX L- Editor Kerr C E- Editor Li JY