Published online Feb 7, 2012. doi: 10.3748/wjg.v18.i5.472
Revised: June 30, 2011
Accepted: July 7, 2011
Published online: February 7, 2012
AIM: To develop a pharmacodynamic model of portal hypertension from chronic hepatitis.
METHODS: Pathological changes and collagen depositions were analyzed using morphometry to confirm CCl4-induced chronic hepatitis. At d0, d28, d56 and d84 of the process, the portal perfused velocities (μL/min) in isolated rat livers were exactly controlled with a quantified pump. The pressure (mmHg) was monitored with a Physiological System. The geometric concentrations of phenylephrine or acetylcholine were added to a fixed volume (300 mL) of the circulating perfusate. The equation, the median effective concentration and its 95% confidence intervals of phenylephrine or acetylcholine were regressed with Prism-4 software in non-linear fit and various slopes. In the isolated perfused rat livers with chronic hepatitis, both median effective concentrations were defined as the pharmacodynamic model of portal hypertension.
RESULTS: At d0, d28, d56 and d84, the equations of portal pressure potency from the concentrations of phenylephrine used to constrict the portal vein in isolated perfused rat livers were Y = 0.1732 + 0.3970/[1 + 10(-4.3061-0.4407 X)], Y = -0.004934 + 0.12113/[1 + 10(-3.1247-0.3262 X)], Y = 0.0104 + 0.2643/[1 + 10(-8.8462-0.9579 X)], and Y = 0.01603 + 0.12107/[1 + 10(-5.1134-0.563 X)]; the median effective concentrations were 1.69 × 10-10 mol/L, 2.64 × 10-10 mol/L, 5.82 × 10-10 mol/L, and 8.24 × 10-10 mol/L, respectively. The equations from the concentrations of acetylcholine used to relax the portal vein were Y = -0.4548 + 0.3274/[1 + 10(6.1538 + 0.5554 X)], Y = -0.05391 + 0.06424/[1 + 10(3.8541 + 0.3469 X)], Y = -0.2733 + 0.22978/[1 + 10(3.0472 + 0.3008 X)], and Y = -0.0559 + 0.053178/[1 + 10(5.6336 + 0.5883 X)]; the median effective concentrations were 8.40 × 10-10 mol/L, 7.73 × 10-12 mol/L, 5.98 × 10-11 mol/L, and 2.66 × 10-10 mol/L, respectively.
CONCLUSION: A pharmacodynamic model of portal hypertension in isolated perfused rat livers with chronic hepatitis was defined as the median effective concentrations of phenylephrine and acetylcholine.
- Citation: Zhang T, Xu XY, Zhou H, Zhao X, Song M, Zhang TT, Yin H, Li T, Li PT, Cai DY. A pharmacodynamic model of portal hypertension in isolated perfused rat liver. World J Gastroenterol 2012; 18(5): 472-478
- URL: https://www.wjgnet.com/1007-9327/full/v18/i5/472.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i5.472
Patients with portal hypertension have significant mortality[1]. A lack of drugs[2] to treat this disease is derived from the failure to use the reversible mechanisms in its pathogenesis[3]. Being similar to amiloride, a candidate drug for portal hypertension[4], molecules from medical plants have been demonstrated to affect portal hypertension in rats[5-7] and in patients[8] with chronic hepatitis. The effect of these molecules on relaxation of the extra-hepatic portal rings did not account for the efficacy of these therapies in vivo[9]. A novel mode of portal perfusion has been characterized with both controlled velocity and monitored pressure in the isolated portal perfused rat liver (IPPRL)[10-12]. With the primary velocity and preload at the various advanced stages of CCl4-induced chronic hepatitis in rats, constriction with phenylephrine (PE) and relaxation with acetylcholine (Ach) were more sensitive than those reported previously[13,14]. With standardization of the IPPRL[15], both median effective concentrations of Ach and PE were defined as the pharmacodynamic model of portal hypertension. Both the controlled velocity and monitored pressure made the model sensitive enough for the basis of systems biology in portal regulation[16].
Thirty two healthy male Wistar rats weighing 200-220 g were supplied by the Animal Center of the Chinese Academy of Medical Sciences. Standard rodent pellets for rats were prepared by Beijing Scientific Animal Feedstuff Company. The study was approved by the Animal Study Committee of the Chinese Academy of Medical Sciences. All experimental procedures were performed in accordance with the Guidelines of Animal Experiments from the Committee of Medical Ethics, National Health Department of China. All rats were maintained in a temperature-controlled room (25.0 °C ± 0.2 °C) in the SPF laboratory, with a 12-h/12-h light/dark photoperiod and 45% ± 2% humidity. The rats were fed standard rodent pellets and allowed free access to tap water throughout the experiment.
Carbon tetrachloride (CCl4, MW 153.84, CAS 56-23-5), Olive oil (CAS 8001-25-0) and Heparin sodium (MW 12 000, CAS 9041-08-1) were purchased from Sinopharm Chemical Reagent Company to induce chronic hepatic hepatitis or for anticoagulation.
As the perfusate in portal perfusion, Krebs-Henseleit solution consisted of KCl 4.7, KH2PO4 1.2, CaCl2 2.5, MgSO4 1.2, NaCl 118, and Glucose 11.0 mmol/L in the final concentration at pH 7.35-7.45 equilibrated with 95% O2-5% CO2 and warmed to 37.8 °C before being added to the livers.
Phenylephrine hydrochloride (PE, MW 203.67, CAS 61-76-7) and Acetylcholine chloride (Ach, MW 181.67, CAS 60-31-1) were purchased from Sigma (United States) as the α1-adrenoceptor and M3-cholinoceptor agonist, respectively, used to elevate or reduce portal pressure.
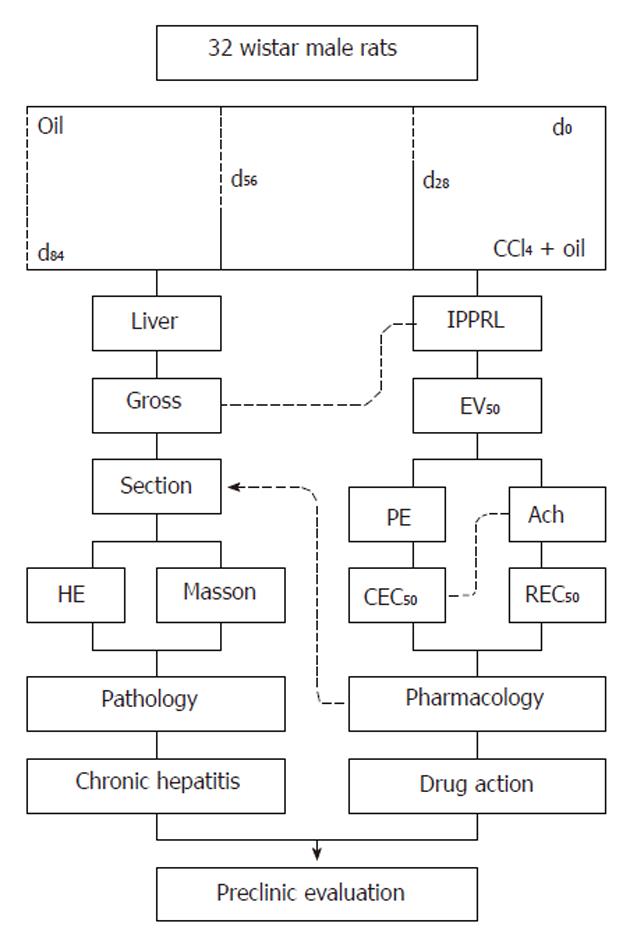
Male Wistar rats were randomly divided into four groups. In four rats, PE was used to constrict the portal vein and in the other rats Ach was used to relax the portal vein in each group. Group 1 was the vehicle control without CCl4 In this group, rats were subcutaneously administered 3 mL/kg olive oil twice weekly for 84 d. Groups 2, 3 and 4 were model groups with CCl4-induced chronic hepatitis, the rats in these groups were subcutaneously administered the same volume of a mixture of 40% (v/v) CCl4 in olive oil twice weekly for 28 d, 56 d and 84 d, beginning at d58, d28, and d0, respectively (Figure 1). Forty-eight hours after the last CCl4 injection, rats were anesthetized with 50 mg/kg pentobarbital sodium subcutaneously; a midline incision was made to expose the liver and its vessels. The hepatic artery, portal vein and hepatic vein were canalized. The remaining blood in the IPPRLs was eliminated using Krebs-Henseleit perfusate through the hepatic artery. When portal perfusion was complete, a small portion of the liver was removed for pathological examination following fixation with 40 g/L formaldehyde solution and subsequent embedding in paraffin.
When CCl4-induced chronic hepatitis was complete, eight rats from each group were randomized into two subgroups, one for PE constriction and the other for Ach relaxation of the portal vein. Each IPPRL was instrumented for portal pressure measurement.
Each IPPRL was perfused in a recirculation at a fixed temperature of 37.8 °C and equilibrated with 95% O2-5% CO2 mixed gas (Beijing Specialized Mixed Gas Institute), portal velocity was precisely controlled by a quantified BTO1 pump (Beijing Yidaxk Technical Company), and 3935.50, 4720.63, 4753.35, and 5164.16 (μL/min) at d0, d28, d56, and d84, were chosen, respectively, the equation of portal perfusion median velocity (Y) from the day (x) of chronic hepatitis was Y = 13.28x + 4085 (r = 0.935, P < 0.01)[12].
The portal pressure of perfusion (mmHg) was continuously monitored and recorded with a strain-gauge transducer connected to the portal inflow cannula 6 cm proximal to the perfusion cannula with BL-420S Physiological Systems (Taimeng Instruments, Chengdu) according to a previously published method[10-14]. The global viability of livers was assessed by gross appearance and perfusate stable pH.
Perfusions were performed in the recirculating system containing 300 mL Krebs-Henseleit solution. Each preparation was allowed to stabilize for 15 min. The flow rate during each individual perfusion was maintained at a constant rate equalized to the portal perfusion median velocity at d0, d28, d56, and d84, respectively, the average portal pressure during this condition had been designated as the baseline. With a fixed volume of the recirculating perfusate in the portal perfusion system, cumulative geometric concentrations of PE (10-12-10-6 mol/L) were added to elevate portal pressure.
After the median effective concentration of PE to constrict the portal vein was added, cumulative geometric concentrations of Ach (10-13-10-7 mol/L) were added to reduce portal pressure.
Concentration-response curves were obtained following the addition of PE and Ach, and the changes in intra-hepatic resistance expressed as the percentage increase or decrease in perfusion pressure from baseline in the various portal perfused velocities were obtained.
To observe pathological changes after portal perfusion, a portion of the left liver lobe (40 mg) from each liver was fixed in 40 g/L formaldehyde solution for 48 h, embedded in paraffin, sectioned (6 μm), and stained with hematoxylin-eosin and Masson according to standard procedures.
Images were acquired with a Nano Zoomer Digital Pathology system (Hamamatsu, Japan), at a low magnification (× 20); all the compartments of the liver were analyzed. At high magnification (× 40), the collagen density in the liver section was quantified using a computerized image analysis system (Image-Pro Plus v 5.1). The density of collagen in blinded specimens was expressed as a percentage (the ratio of collagen area per total analyzed field area). The average of the score taken from ten random fields was used to generate a single score for each IPPRL.
All primary data are presented as means ± SE for each dosage in each group. Statistical significance was calculated using Student’s t test between groups, P < 0.05 was significant. (1) Dose-effect relationship: The equation, the median effective concentration and its 95% confidence intervals of PE or Ach were calculated by regression analysis using Graph-Pad Prism 4 in non-linear fit and various slopes, to express the dose-effect relationship; and (2) Time-effect relationship: The median effective concentrations of PE or Ach were calculated by linear regression analysis with the duration (0 d, 28 d, 56 d, and 84 d) of chronic hepatitis, to express the time-effect relationship of pathological conditions affecting the portal response to both molecules.
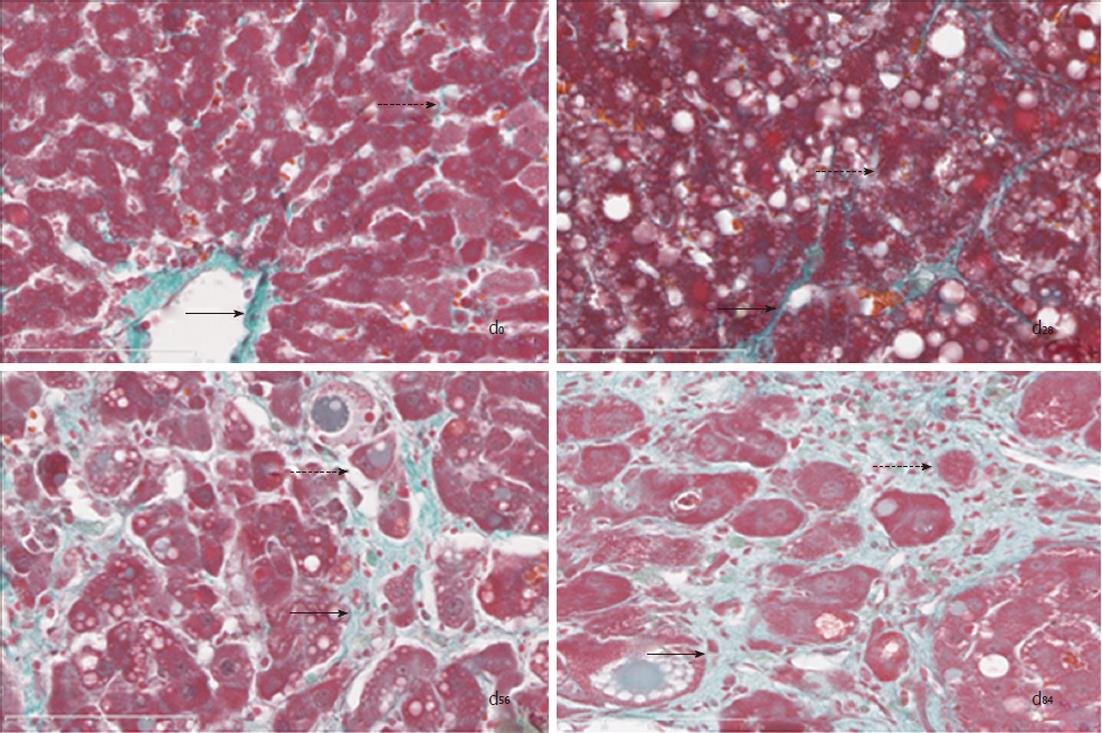
Lobule or pseudo-lobule ratio: Hepatic tissues were clear in hematoxylin and eosin-stained sections. When compared with those in control rats at d0, the lobule ratios (Table 1) in the model rats at d28, d56 and d84 were significantly decreased by 4.04%, 70.22%, and 83.82%, respectively (P < 0.01). When compared with those at d28, the pseudo-lobule ratios in the model rats at d56 and d84 were significantly decreased by 65.35% and 81.00%, respectively (P < 0.01). In addition, these ratios were significantly decreased by 45.67% at d84 compared with those at d56 (P < 0.01).
| Advanced | Lobule ratio | Collagen ratio |
| d0 | 0.38 ± 0.05 | 0.0000700 ± 0.0001180 |
| d28 | 0.33 ± 0.04b | 0.0019658 ± 0.0024864b |
| d56 | 0.11 ± 0.04b,d | 0.0043315 ± 0.0048768b,d |
| d84 | 0.06 ± 0.01b,d,f | 0.0143996 ± 0.0143860b,d,f |
Hepatic collagen distribution: Hepatic histological changes in Masson-stained sections showed collagen depositions along with CCl4-induced chronic hepatitis (Figure 2). (1) Normal structure at d0: The histological structure in control rats showed normal hepatic architecture with some fatty degeneration and less collagen located at the lobules; (2) Degeneration at d28: The pathological changes in the model rats at d28 showed mainly hepatic fatty degeneration and cellular swelling, collagen was deposited around the center veins, thus the enlarged hepatic cords severely narrowed the hepatic sinusoid; (3) Hepatic fibrosis at d56: The pathological changes in the model rats at d56 showed more collagen deposited in the lobules, thus the enlarged hepatic cords led to significant widening of the hepatic sinusoid; and the collagen in interlobular area extended into the lobules, some separating the lobules completely, therefore the direction of the circulating blood did not change in the hepatic sinusoid of the lobules; and (4) Hepatic cirrhosis at d84: The pathological changes in the model rats at d84 showed extensive collagen deposited in the lobules, which were all pseudo-lobules instead of normal lobules, thus the direction of the circulating blood had completely changed in the hepatic sinusoid.
Deposited collagen ratio: Compared with the control rats (Table 1), the collagen ratio in the model rats at 28 d, 56 d and 84 d was significantly increased by 2707.65%, 60 860.51%, and 20 466.49%, respectively (P < 0.01). Compared with the model rats at 28 d, the collagen ratio in the model rats at 56 d and 84 d was significantly increased by 120.34% and 632.52%, respectively (P < 0.01). The collagen ratio in the model rats at 84 d increased by 232.44% compared to that at 56 d (P < 0.01).
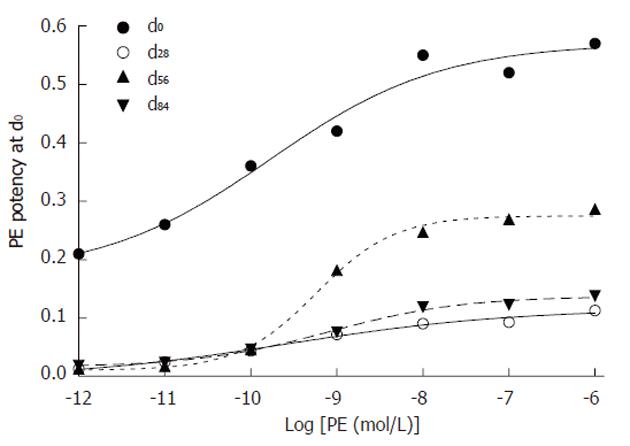
Phenylephrine elevated portal pressure: Geometric concentrations of PE to activate the α1 receptor were added to the recirculating perfusate to elevate perfused portal pressure (Table 2 and Figure 3). The equation, the median effective concentration of PE and its 95% confidence intervals were regressed: (1) dose-effect at d0: The data showed that the equation of PE was Y = 0.1732 + 0.3970/[1 + 10(-4.3061-0.4407 x)] (r = 0.9701, P < 0.01); the median effective concentration with its 95% confidence intervals was 1.69 × 10-10 (4.9769 × 10-12 - 5.7599 × 10-9) mol/L; (2) dose-effect at d28: The data showed that the equation of PE was Y = -0.004934 + 0.121134/[1 + 10(-3.1247-0.3262 x)] (r = 0.9937, P < 0.01); the median effective concentration with its 95% confidence intervals was 2.64 × 10-10 (7.1864 × 10-12 - 9.6834 × 10-9) mol/L; (3) dose-effect at d56: The data showed that the equation of PE was Y = 0.0104 + 0.2643/[1 + 10(-8.8462-0.9579 x)] (r = 0.9980, P < 0.01); the median effective concentration with its 95% confidence intervals was 5.82 × 10-10 (3.0691 × 10-10 - 1.1031 × 10-9) mol/L; (4) dose-effect at d84: The data showed that the equation of PE was Y = 0.01603 + 0.12107/[1 + 10(-5.1134-0.563 x)] (r = 0.9963, P < 0.01); the median effective concentration with its 95% confidence intervals was 8.24 × 10-10 (2.2476 × 10-10 - 3.0207 × 10-9) mol/L; and (5) time-effect: The linear regression equation was Y = 0.081 x + 1.173 (r = 0.981, P < 0.01) between the median effective concentrations of PE (1.69, 2.64, 5.82 and 8.24) × 10-10 mol/L and the durations (0 d, 28 d, 56 d and 84 d) of chronic hepatic hepatitis.
| Log[PE (mol/L)] | d0 | d2 | d56 | d84 |
| -12 | 0.210 ± 0.19 | 0.013 ± 0.02 | 0.013 ± 0.02 | 0.019 ± 0.03 |
| -11 | 0.260 ± 0.16 | 0.025 ± 0.03 | 0.017 ± 0.02 | 0.024 ± 0.04 |
| -10 | 0.360 ± 0.18 | 0.044 ± 0.06 | 0.046 ± 0.06 | 0.047 ± 0.07 |
| -9 | 0.420 ± 0.24 | 0.072 ± 0.09 | 0.182 ± 0.25 | 0.076 ± 0.09 |
| -8 | 0.550 ± 0.37 | 0.090 ± 0.12 | 0.247 ± 0.27 | 0.119 ± 0.10 |
| -7 | 0.520 ± 0.37 | 0.093 ± 0.12 | 0.269 ± 0.27 | 0.123 ± 0.11 |
| -6 | 0.570 ± 0.24 | 0.113 ± 0.13 | 0.286 ± 0.28 | 0.138 ± 0.12 |
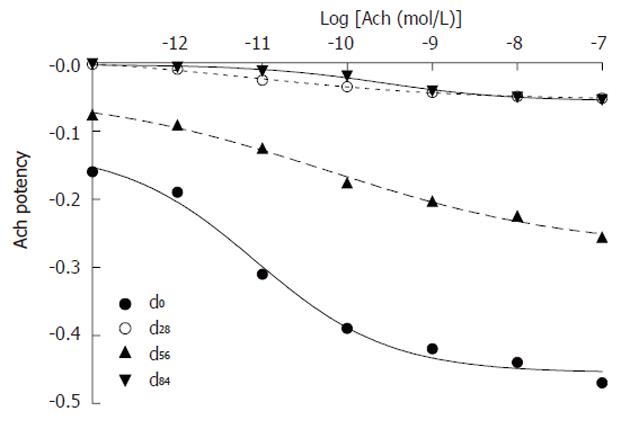
Acetylcholine reduced portal pressure: Geometric con-centrations of Ach to activate the M3 receptor were added to the circulating perfusate to reduce perfused portal pressure. The equation, the median effective concentration of Ach and its 95% confidence intervals of Ach were regressed (Table 3 and Figure 4): (1) dose-effect at d0: The data showed that the equation of Ach was Y = -0.4548 + 0.3274/[1 + 10(6.1538 + 0.5554 x)] (r = 0.9950, P < 0.01); the median effective concentration with its 95% confidence intervals was 8.40 × 10-10 (1.3263 × 10-12 - 5.3240 × 10-11) mol/L; (2) dose-effect at d28: The data showed that the equation of Ach was Y = -0.05391 + 0.06424/[1 + 10(3.8541 + 0.3469 x)] (r = 0.9982, P < 0.01); the median effective concentration with its 95% confidence intervals was 7.73 × 10-12 (7.3614 × 10-13 - 8.1095 × 10-11) mol/L; (3) dose-effect at d56: The data showed that the equation of Ach was Y = -0.2733 + 0.22978/[1 + 10(3.0472 + 0.3008 x)] (r = 0.9964, P < 0.01); the median effective concentration with its 95% confidence intervals was 5.98 × 10-11 (4.2797 × 10-12 - 8.3556 × 10-11) mol/L; (4) dose-effect at d84: The data showed that the equation of Ach was Y = -0.0559 + 0.053178/[1 + 10(5.6336 + 0.5883 x)] (r = 0.9956, P < 0.01); the median effective concentration with its 95% confidence intervals was 2.66 × 10-10 (6.5887 × 10-11 - 1.0701 × 10-9) mol/L; and (5) time-effect: The linear regression equation was Y = 0.046 X -1.470 (r = 0.945, P < 0.05) between the median effective concentrations of Ach (0.0773, 0.598 and 2.66) × 10-10 mol/L and the durations (28 d, 56 d and 84 d) of chronic hepatitis.
| Log[Ach (mol/L)] | d0 | d28 | d56 | d84 |
| -13 | -0.16 ± 0.12 | -0.002 ± 0.01 | -0.076 ± 0.08 | -0.001 ± 0.01 |
| -12 | -0.19 ± 0.10 | -0.009 ± 0.01 | -0.091 ± 0.10 | -0.006 ± 0.01 |
| -11 | -0.31 ± 0.07 | -0.025 ± 0.01 | -0.125 ± 0.15 | -0.012 ± 0.01 |
| -10 | -0.39 ± 0.08 | -0.035 ± 0.18 | -0.176 ± 0.23 | -0.019 ± 0.01 |
| -9 | -0.42 ± 0.08 | -0.043 ± 0.04 | -0.203 ± 0.26 | -0.041 ± 0.02 |
| -8 | -0.44 ± 0.12 | -0.049 ± 0.05 | -0.225 ± 0.26 | -0.050 ± 0.03 |
| -7 | -0.47 ± 0.14 | -0.052 ± 0.07 | -0.256 ± 0.28 | -0.054 ± 0.03 |
Patients with portal hypertension have significant morbidity and mortality[1] without special drugs[2] based on the reversible pathogenesis of this disease[3]. Some candidate drugs from chemicals and medical plants have demonstrated effects on portal hypertension in animal experiments and in clinical trials[4-8]. Data from the extra-hepatic portal rings failed to account for these effects[9]. Consequently, sensitive portal perfusion for intra-hepatic portal resistance has been developed with both controlled velocity and monitored pressure in IPPRLs[10-14]. The pharmacodynamic model of portal hypertension has further been defined as the median effective concentrations of Ach and PE in the IPPRLs at various stages of CCl4-induced chronic hepatitis.
At d0, d28, d56, and d84 in CCl4-induced chronic hepatitis, there were similar portal pressure potency equations with various coefficients due to the concentrations of PE and Ach in the IPPRLs. The median effective concentrations of PE increased geometrically during the process, suggesting that the function of portal smooth muscle cells gradually decreased. A similar effect was noted with the median effective concentrations of Ach in advanced stages, which suggested that portal endothelia were gradually damaged. During portal perfusion with both controlled pressure and monitored velocity, as reported previously, the effective range of PE and Ach concentrations was from 10-3 mol/L to 10-8 mol/L[15,16]. In this novel model of portal perfusion with both controlled velocity and monitored pressure, the effective range was from 10-6 mol/L to 10-12 mol/L, which indicated that this novel mode was more sensitive than the previous mode by 103-106 times in IPPRLs.
Hepatocyte injuries originate from the free radicals of CCl4 metabolites[3]. Amiloride reduced intra-hepatic portal resistance through inhibition of the Rho kinase pathway in hepatic stellate cells[4]. Glycyrrhizinate and Salvianolic acid B are representative molecules from medical plants used for portal hypertension in rats[5-7] and patients[8] with chronic hepatitis, however, their biomolecular mechanisms are not yet clear. PE, as a α1-adrenoceptor agonist, constricts vascular smooth muscle[13] and Ach, as a M3-cholinoceptor agonist in endothelia, relaxes vascular smooth muscle[14]. Due to these mechanisms in IPPRLs, the median perfused velocity in portal pressure has been defined as the primary flow rate of portal perfusion in this novel mode, the median effective concentration of PE for elevating portal pressure as the preload, the median effective concentration of Ach for reducing the portal pressure as the positive action at the classic stages of the pathological process in chronic hepatitis.
The pharmacodynamic model of portal hypertension has been defined as both the median effective concentrations of PE and Ach in the IPPRLs with advanced chronic hepatitis in this study. This model may be used to evaluate the preclinical effects of candidate drugs for the treatment of portal hypertension. Both controlled velocity and monitored pressure[10-12] made this model more sensitive than previous models[13-14]. Based on the standardization[15] of the IPPRL, this sensitive model is considered the basis of systems biology for portal regulation in an isolated setting[16].
Portal hypertension results in significant mortality without the administration of special drugs. Candidate drugs for this condition require serious pre-clinical evaluation using suitable methods.
The recently identified reversible pathogenesis of portal hypertension may allow the development of new drugs for this disease. Candidate drugs derived from medical plants used in Chinese medical practices have confirmed its reversible pathogenesis. A sensitive pressure transducer was used here for exploiting the reversible pathogenesis as the pharmacological models. The optimal conditions for each step of the procedure can be defined as an available model.
Reversible portal hypertension was replicated in the advanced stages of chronic hepatitis in rats using CCl4. A pharmacological model was developed using the median primary velocity of perfused flow as the anatomical preload, median effective concentrations of phenylephrine to constrict portal veins as the physiological preload, and the median effective concentrations of acetylcholine to relax portal veins in IPPRLs with chronic hepatitis.
This novel pharmacological model can be used to evaluate candidate drugs for the treatment of portal hypertension.
This novel mode of portal perfusion is characterized by both controlled velocity and monitored pressure in the isolated portal perfused rat livers. The controlled velocity creates the optimal conditions for research purposes, and the monitored pressure gives exact data from vascular smooth muscle or endothelia.
The authors investigated to develop a pharmacodynamic model for portal hypertension from chronic hepatitis. They have developed a pharmacodynamic model for portal hypertension in rats with chronic hepatitis and demonstrated that the model had been defined as the median effective concentrations of phenylephrine and acetylcholine. The results are clear and informative for the study on portal hypertension.
| 1. | Roberts SE, Goldacre MJ, Yeates D. Trends in mortality after hospital admission for liver cirrhosis in an English population from 1968 to 1999. Gut. 2005;54:1615-1621. [PubMed] |
| 2. | Bosch J, Abraldes JG, Fernández M, García-Pagán JC. Hepatic endothelial dysfunction and abnormal angiogenesis: new targets in the treatment of portal hypertension. J Hepatol. 2010;53:558-567. [PubMed] |
| 3. | Xu YL, Cai DY, Tang CS. Mechanism of CCl4-induced hepatocirrhosis and portal hypertension. Shijie Huaren Xiaohua Zazhi. 2005;13:235-238. |
| 4. | Steib CJ, Hennenberg M, Beitinger F, Hartmann AC, Bystron M, De Toni EN, Gerbes AL. Amiloride reduces portal hypertension in rat liver cirrhosis. Gut. 2010;59:827-836. [PubMed] |
| 5. | Liu SF, Cai DY, Li PT, Xiang PR. Study on compatibility of Ganshen decoction in moderateing Hepatic Fibrosis. Zhonghua Zhongyiyao Zazhi. 2005;20:373-375. |
| 6. | Deng XL, Wang QQ, Zhang XJ, Liu YN, Zeng YM, Jia L, Li PT, Cai DY. Effective mechanisms of Glycyrrhetinic acid with Salvianolic acid B on immunological hepatic fibrosis in rat. Zhongguo Yaoshi. 2007;10:741-744. |
| 7. | Du QH, Li PT. Pathophysiology and clinical practice analysis on endothelin system and portal hypertension. Shijie Huaren Xiaohua Zazhi. 2008;16:1092-1097. |
| 8. | Qin G, Shi GF, Song YY, Chen MQ. Meta-analysis of document on diammonium Glycyrrhizinate in treatment of patients with chronic hepatitis B. Zhonghua Chuanranbing Zazhi. 2005;23:333-337. |
| 9. | Ren LW, Zang XJ, Deng XL, Liu SF, Liu YN, Cai DY. Wave characteristics of portal pressure and their affected factors. Beijing Zhongyiyao Daxue Xuebao. 2006;29:840-843. |
| 10. | Wu K, Xu XY, Wang SX, Zhou H, Zhang WL, Zhang WT, Lu AN, Song M, Zhang BC, Li PT. A rat model for monitoring isolated perfused hepatic artery or portal vein pressure in vitro. Zhongguo Yaoshi. 2010;13:1390-1393. |
| 11. | Zhou H, Zhang T, Xu XY, Wang SX, Wu K, Xu J, Chen M, Li PT, Cai DY. Salvianolic acid B or Glycyrrhizinate acting on rat infused portal pressure. Zhangguo Zhongxiyi Jiehe Zazhi. 2010;30:1084-1086. |
| 12. | Xu XY, Zhang T, Zhou H, Zhao X, Zhang TT, Yin H, Li T, Li PT, Cai DY. Portal pressure determined by perfusion velocity in isolated rat with chronic injury liver in vitro. Shijie Huaren Xiaohua Zazhi. 2010;18:2745-2749. |
| 13. | Laviña B, Gracia-Sancho J, Rodríguez-Vilarrupla A, Chu Y, Heistad DD, Bosch J, García-Pagán JC. Superoxide dismutase gene transfer reduces portal pressure in CCl4 cirrhotic rats with portal hypertension. Gut. 2009;58:118-125. [PubMed] |
| 14. | Hennenberg M, Trebicka J, Sauerbruch T, Heller J. Mechanisms of extrahepatic vasodilation in portal hypertension. Gut. 2008;57:1300-1314. [PubMed] |
| 15. | Bessems M, 't Hart NA, Tolba R, Doorschodt BM, Leuvenink HG, Ploeg RJ, Minor T, van Gulik TM. The isolated perfused rat liver: standardization of a time-honoured model. Lab Anim. 2006;40:236-246. [PubMed] |
| 16. | Hood L, Rowen L, Galas DJ, Aitchison JD. Systems biology at the Institute for Systems Biology. Brief Funct Genomic Proteomic. 2008;7:239-248. [PubMed] |
Peer reviewers: Dr. Orhan Sezgin, Professor, Gastroenteroloji Bilim Dalı, Mersin Üniversitesi Tıp Fakültesi, Mersin 33190, Turkey; Yoshiaki Iwasaki, MD, PhD, Associate Professor, Health Service Center, Okayama University, 2-1-1, Tsushima-Naka, Kita-ku, Okayama 700-8530, Japan
S- Editor Zhang SJ L- Editor Webster JR E- Editor Zhang DN