Published online Nov 21, 2012. doi: 10.3748/wjg.v18.i43.6269
Revised: September 17, 2012
Accepted: September 22, 2012
Published online: November 21, 2012
AIM: To clarify features of hepatic hemangiomas on gadolinium-ethoxybenzyl-diethylenetriaminpentaacetic acid (Gd-EOB-DTPA)-enhanced magnetic resonance imaging (MRI) compared with enhanced computed tomography (CT).
METHODS: Twenty-six patients with 61 hepatic hemangiomas who underwent both Gd-EOB-DTPA-enhanced MRI and enhanced CT were retrospectively reviewed. Hemangioma appearances (presence of peripheral nodular enhancement, central nodular enhancement, diffuse homogenous enhancement, and arterioportal shunt during the arterial phase, fill-in enhancement during the portal venous phase, and prolonged enhancement during the equilibrium phase) on Gd-EOB-DTPA-enhanced MRI and enhanced CT were evaluated. The degree of contrast enhancement at the enhancing portion within the hemangioma was visually assessed using a five-point scale during each phase. For quantitative analysis, the tumor-muscle signal intensity ratio (SIR), the liver-muscle SIR, and the attenuation value of the tumor and liver parenchyma were calculated. The McNemar test and the Wilcoxon’s signed rank test were used to assess the significance of differences in the appearances of hemangiomas and in the visual grade of tumor contrast enhancement between Gd-EOB-DTPA-enhanced MRI and enhanced CT.
RESULTS: There was no significant difference between Gd-EOB-DTPA-enhanced MRI and enhanced CT in the presence of peripheral nodular enhancement (85% vs 82%), central nodular enhancement (3% vs 3%), diffuse enhancement (11% vs 16%), or arterioportal shunt (23% vs 34%) during arterial phase, or fill-in enhancement (79% vs 80%) during portal venous phase. Prolonged enhancement during equilibrium phase was observed less frequently on Gd-EOB-DTPA-enhanced MRI than on enhanced CT (52% vs 100%, P < 0.001). On visual inspection, there was significantly less contrast enhancement of the enhancing portion on Gd-EOB-DTPA-enhanced MRI than on enhanced CT during the arterial (3.94 ± 0.98 vs 4.57 ± 0.64, respectively, P < 0.001), portal venous (3.72 ± 0.82 vs 4.36 ± 0.53, respectively, P < 0.001), and equilibrium phases (2.01 ± 0.95 vs 4.04 ± 0.51, respectively, P < 0.001). In the quantitative analysis, the tumor-muscle SIR and the liver-muscle SIR observed with Gd-EOB-DTPA-enhanced MRI were 0.80 ± 0.24 and 1.28 ± 0.33 precontrast, 1.92 ± 0.58 and 1.57 ± 0.55 during the arterial phase, 1.87 ± 0.44 and 1.73 ± 0.39 during the portal venous phase, 1.63 ± 0.41 and 1.78 ± 0.39 during the equilibrium phase, and 1.10 ± 0.43 and 1.92 ± 0.50 during the hepatobiliary phase, respectively. The attenuation values in the tumor and liver parenchyma observed with enhanced CT were 40.60 ± 8.78 and 53.78 ± 7.37 precontrast, 172.66 ± 73.89 and 92.76 ± 17.92 during the arterial phase, 152.76 ± 35.73 and 120.12 ± 18.02 during the portal venous phase, and 108.74 ± 18.70 and 89.04 ± 7.25 during the equilibrium phase, respectively. Hemangiomas demonstrated peak enhancement during the arterial phase, and both the SIR with Gd-EOB-DTPA-enhanced MRI and the attenuation value with enhanced CT decreased with time. The SIR of hemangiomas was lower than that of liver parenchyma during the equilibrium and hepatobiliary phases on Gd-EOB-DTPA-enhanced MRI. However, the attenuation of hemangiomas after contrast injection was higher than that of liver parenchyma during all phases of enhanced CT.
CONCLUSION: Prolonged enhancement during the equilibrium phase was observed less frequently on Gd-EOB-DTPA-enhanced MRI than enhanced CT, which may exacerbate differentiating between hemangiomas and malignant tumors.
- Citation: Tateyama A, Fukukura Y, Takumi K, Shindo T, Kumagae Y, Kamimura K, Nakajo M. Gd-EOB-DTPA-enhanced magnetic resonance imaging features of hepatic hemangioma compared with enhanced computed tomography. World J Gastroenterol 2012; 18(43): 6269-6276
- URL: https://www.wjgnet.com/1007-9327/full/v18/i43/6269.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i43.6269
Hepatic hemangioma is the most common benign hepatic neoplasm; it occurs with a reported incidence of 0.4%-7.3% in an autopsy series[1]. Differentiation from malignant liver tumors is very important because hemangiomas usually do not require treatment[2]. Enhanced computed tomography (CT) and gadolinium-diethylenetriaminepentaacetic acid (Gd-DTPA)-enhanced magnetic resonance imaging (MRI) are useful modalities for the diagnosis of hepatic hemangiomas and their differentiation from malignant liver tumors[3-7].
Gadolinium-ethoxybenzyl-diethylenetriaminpentaacetic acid (Gd-EOB-DTPA) is a new hepatobiliary contrast agent that improves the detection and characterization of focal liver lesions in clinical studies[8-12]. This contrast agent exhibits high T1 relaxivity in the liver and shows enhancement on both early perfusion phase images and delayed hepatobiliary phase images. Immediately after injection, Gd-EOB-DTPA is distributed into the extracellular fluid space and yields additional diagnostic information for the characterization of liver lesions, similar to performing MRI with extracellular contrast agents, such as Gd-DTPA[13]. Hepatocellular uptake of Gd-EOB-DTPA starts approximately 90 s after injection, and Gd-EOB-DTPA is excreted equally via the biliary route and renal clearance[11,12]. The enhanced liver signal intensity plateaus approximately 20 min after injection, and delayed hepatobiliary phase images have high diagnostic quality for the detection of focal liver lesions[11].
Some studies have shown that Gd-EOB-DTPA-enhanced MRI is more useful than enhanced CT and Gd-DTPA-enhanced MRI for the detection and characterization of focal liver lesions[8,10-12,14]. The dynamic images obtained after injection of Gd-EOB-DTPA are similar in some aspects but not the same as those images obtained using extracellular contrast agents[11,12,14,15]. Recently, the differences in the appearances of Gd-EOB-DTPA-enhanced MRI between hemangiomas and metastases have been reported[16,17]. To our knowledge, however, published reports regarding the findings of hemangiomas observed with Gd-EOB-DTPA-enhanced MRI have been limited[9,16-19]. Moreover, the appearance and the degree of contrast enhancement of hepatic hemangiomas observed with Gd-EOB-DTPA-enhanced MRI compared with enhanced CT have not been elucidated. Therefore, the purpose of the present study was to clarify the features of hepatic hemangiomas observed with Gd-EOB-DTPA-enhanced MRI compared with enhanced CT.
Institutional ethics review board approval was obtained, and informed consent was waived for this retrospective study. From February 2008 to February 2010, 432 consecutive patients underwent Gd-EOB-DTPA-enhanced MRI for the evaluation of suspected liver tumors. Among these patients, 27 were included in this study because they were suspected of having hepatic hemangiomas on three-phase enhanced CT with at least three of the following four well-documented CT characteristic findings of hepatic hemangiomas[4-7]: relative hypoattenuation compared to normal liver on precontrast images, peripheral nodular enhancement during the arterial phase, fill-in enhancement (progressive opacification from the periphery to the center) during the portal venous phase, and prolonged enhancement (showing iso- or hyperattenuation relative to the liver) during the equilibrium phase. One patient was excluded from the study because the hemangioma did not have the typical appearance of bright signal intensity on T2-weighted MRI[20]. Therefore, the final study group comprised 26 patients (8 men, 18 women; age range: 31-77 years; mean age: 53.5 years) with 61 hepatic hemangiomas (size: mean, 26 mm; range 5-120 mm). Four hemangiomas in two patients were diagnosed by histological examination following surgical resections because of the risk of spontaneous rupture.
MRI was performed with a 3-T system (Magnetom Trio; Siemens AG, Erlangen, Germany) with a maximum gradient amplitude of 45 mT/m and a slew rate of 200 T/m.s-1. The coil had four linear elements in a left-to-right direction for both the anterior and posterior components. The standard sequences performed prior to Gd-EOB-DTPA administration included T1-weighted gradient-echo, T2-weighted turbo spin-echo, and respiratory-triggered with navigator-echo technique fat-suppressed T2-weighted turbo spin-echo. Dynamic images using a three-dimensional (3D) fat-suppressed T1-weighted gradient-echo volumetric interpolated breath-hold examination (VIBE) axial series were obtained before and after intravenous contrast injection. The image parameters were as follows: repetition time/echo time, 3.06/1.12; flip angle, 10°; slice thickness, 2 mm; field of view, 350 mm × 280 mm; matrix, 256 × 224; acceleration factor, 2; number of partitions, 80; and acquisition time, 20 s. Before dynamic MRI, a test dose of 1 mL of Gd-EOB-DTPA was injected at a rate of 1 mL/s through a cubital intravenous line, and the bolus was flushed with 40 mL saline using a power injector. During the test injection, the image at the level of the celiac axis in which the aorta was enhanced the most was chosen, and its acquisition time was adopted as the peak aortic enhancement time. For dynamic MRI, 0.025 mmol/kg body weight of Gd-EOB-DTPA was intravenously administered at a flow rate of 1 mL/s, followed by a 40-mL saline solution flush. Breath-hold 3D fat-suppressed T1-weighted VIBE dynamic MRI was repeated at 10 s (arterial phase), 50 s (portal venous phase), 160 s (equilibrium phase), and 20 min (hepatobiliary phase) after the peak aortic enhancement time, which was determined by the test injection. In this study, only precontrast, arterial, portal venous, equilibrium, and hepatobiliary phase T1-weighted VIBE images were evaluated.
Three-phase enhanced CT was performed with a 16-slice multidetector-row CT scanner (Aquilion, Toshiba Medical Systems, Tokyo, Japan). All scans were conducted from the top to the bottom of the liver with a tube voltage of 120 kVp and gantry rotation speed of 0.5 s. Unenhanced images were acquired using the following parameters: tube current, 350 mA; detector row configuration, 16 mm × 2 mm; and table increment, 30 mm/rotation. The imaging parameters for three-phase enhanced images were as follows: tube current, 440 mA; detector row configuration, 16 mm × 1 mm; and table increment, 15 mm/rotation in the cephalocaudal direction. In all patients, 2 mL/kg body weight of nonionic contrast material with an iodine concentration of 300 mg I/mL was injected over a fixed duration of 30 s, followed by 20 mL of saline was injected at the same rate through a 20-gauge plastic intravenous catheter in an upper extremity vein. Automatic bolus tracking was employed using Sure Start software (Toshiba, Tokyo, Japan) to determine individual scan delays from the injection of the contrast material to commencement of the first pass. For bolus tracking, a series of nonhelical sequential images were obtained 8 s after contrast material administration. These images were acquired with a gantry rotation speed of 0.5 s and a low-dose radiation technique (120 kVp, 50 mA). A circular region of interest with an area of 50 pixels was placed in the aorta at the level of the celiac axis. The arterial phase scan was initiated automatically 20 s after the bolus-tracking program detected the threshold enhancement of 50 Hounsfield units in the aorta. The portal venous and equilibrium phases were obtained 70 s and 300 s, respectively, after the beginning of contrast material injection.
Gd-EOB-DTPA-enhanced MRI and enhanced CT images were qualitatively and quantitatively assessed. Three radiologists (Takumi K, Shindo T and Kumagae Y, with 10, 10 and 9 years of experience, respectively) with knowledge of the diagnosis of hepatic hemangioma assessed the following MRI and CT features in random order on two occasions with an interval of ≥ 2 wk: presence of peripheral nodular enhancement, central nodular enhancement, diffuse homogenous enhancement, and arterioportal shunt during the arterial phase, fill-in enhancement during the portal venous phase, and prolonged enhancement during the equilibrium phase. In the case of discrepancies between the three readers, the discrepancies were discussed during an additional reading session until a consensus was reached.
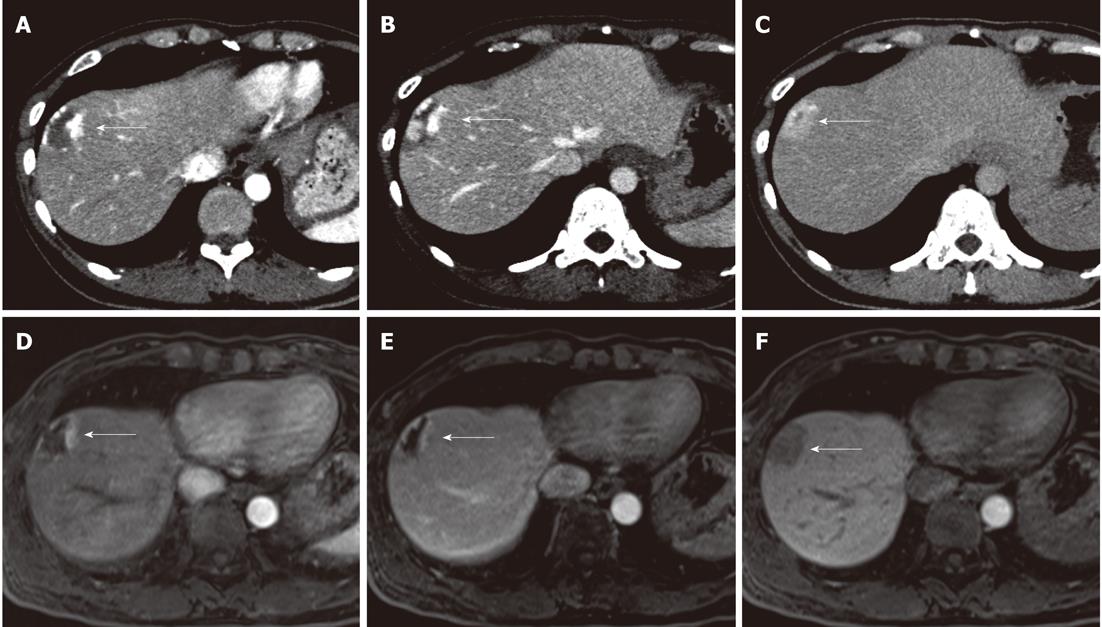
To assess the visual grades of tumor contrast enhancement relative to the surrounding liver parenchyma, the above three radiologists independently determined the degree of contrast enhancement in the enhancing portion of the hemangioma during each phase using the following five-grade scale: grade 5, a prominently greater enhancement than in the liver parenchyma; grade 4, a mildly greater enhancement than in the liver parenchyma; grade 3, an equal enhancement to the liver parenchyma; grade 2, a slightly decreased enhancement compared with the liver parenchyma; and grade 1, a prominently decreased enhancement compared with the liver parenchyma (Figure 1).
For quantitative analysis, one radiologist (Tateyama A, with 8 years of experience) who did not attend each reading session to minimize the bias of the measurements measured signal intensities of the enhancing portion of the hemangioma, liver parenchyma, and paravertebral muscle on Gd-EOB-DTPA-enhanced MRI and attenuation of the enhancing portions of the hemangioma and liver parenchyma on enhanced CT using operator-defined circular region of interests (ROIs). In the enhancing portion of the hemangioma during each phase, the largest possible ROIs were selected to measure signal intensity and attenuation. The ROIs of the liver parenchyma at the level of the hilum of the liver were 100-200 mm2 in size and were drawn in the lateral, anterior, and posterior segments, avoiding blood vessels and artifacts; the intensity and attenuation measurements were averaged. The ROIs of paravertebral muscle were 100 mm2 in size. The tumor-muscle and liver-muscle signal intensity ratios (SIRs) were calculated respectively by dividing the signal intensity of the enhancing portion of the hemangioma and the liver by the signal intensity of the paravertebral muscle. The tumor-liver contrast (TLC) on Gd-EOB-DTPA-enhanced MRI was calculated with the following equation: TLC = (signal intensity of the enhancing portion - signal intensity of the liver parenchyma)/signal intensity of the paravertebral muscle. The TLC on enhanced CT was calculated as the difference in attenuation between the enhancing portion of the hemangioma and the liver parenchyma.
Statistical analyses were performed using SPSS 14.0 software for Windows (SPSS Version 14.0, Chicago, IL). The McNemar test was used to assess the significance of differences in the appearances of hemangiomas between Gd-EOB-DTPA-enhanced MRI and enhanced CT. The Wilcoxon’s signed rank test was used to assess the significance of differences in the visual grade of tumor contrast enhancement between Gd-EOB-DTPA-enhanced MRI and enhanced CT. To assess interobserver variability in the visual analysis of tumor contrast enhancement, the weighted κ test of concordance was applied to measure the degree of agreement between the three radiologists. Agreement was graded as poor (κ value < 0.20), moderate (≥ 0.20 and <0.40), fair (≥ 0.40 and < 0.60), good (≥ 0.60 and < 0.80), or very good (≥ 0.8-1). The relationship between the visual grades of tumor contrast enhancement and TLC was analyzed using the Spearman rank correlation coefficient (Rs). For all statistical analyses, P < 0.05 was considered significant.
There were no significant differences between GdEOB-DTPA-enhanced MRI and enhanced CT in the presence of peripheral nodular enhancement (85% vs 82%), central nodular enhancement (3% vs 3%), diffuse enhancement (11% vs 16%), or arterioportal shunt (23% vs 34%) during the arterial phase. There was also no difference in fill-in enhancement (79% vs 80%, respectively) during the portal venous phase. Prolonged enhancement during the equilibrium phase was observed significantly less frequently on Gd-EOB-DTPA-enhanced MRI than on enhanced CT (52% vs 100%, respectively, P < 0.001) (Table 1).
| Appearance | Tumors | P value | |
| Gd-EOB-DTPA-enhanced MRI | Enhanced CT | ||
| Peripheral nodular enhancement | 52 (85) | 50 (82) | 0.7541 |
| Central nodular enhancement | 2 (3) | 2 (3) | 1.0001 |
| Diffuse homogenous enhancement | 7 (11) | 10 (16) | 0.5081 |
| Arterioportal shunt | 14 (23) | 21 (34) | 0.0651 |
| Fill-in enhancement | 48 (79) | 49 (80) | 1.0001 |
| Prolonged enhancement | 32 (52) | 61 (100) | < 0.0011 |
| Visual grade | |||
| Arterial phase | 3.94 ± 0.98 | 4.57 ± 0.64 | < 0.0012 |
| Portal venous phase | 3.72 ± 0.82 | 4.36 ± 0.53 | < 0.0012 |
| Equilibrium phase | 2.01 ± 0.95 | 4.04 ± 0.51 | < 0.0012 |
The visual grade of tumor contrast enhancement was significantly less on Gd-EOB-DTPA-enhanced MRI than enhanced CT during the arterial phase (3.94 ± 0.98 vs 4.57 ± 0.64, respectively, P < 0.001), the portal venous phase (3.72 ± 0.82 vs 4.36 ± 0.53, respectively, P < 0.001), and the equilibrium phase (2.01 ± 0.95 vs 4.04 ± 0.51, respectively, P < 0.001) (Table 1). There was good interobserver agreement for the visual grade of tumor contrast enhancement, with weighted κ values ranging from 0.59 to 0.92.
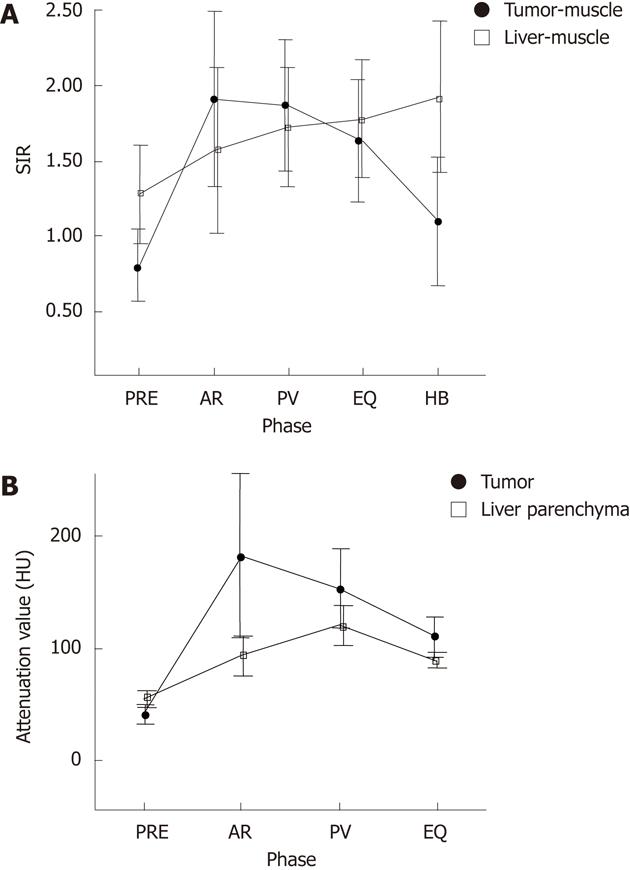
In the quantitative analysis, the tumor-muscle SIR and the liver muscle SIR observed with Gd-EOB-DTPA-enhanced MRI were, respectively, 0.80 ± 0.24 and 1.28 ± 0.33 precontrast, 1.92 ± 0.58 and 1.57 ± 0.55 during the arterial phase, 1.87 ± 0.44 and 1.73 ± 0.39 during the portal venous phase, 1.63 ± 0.41 and 1.78 ± 0.39 during the equilibrium phase, and 1.10 ± 0.43 and 1.92 ± 0.50 during the hepatobiliary phase (Figure 2A). The attenuation values in the tumor and liver parenchyma observed with enhanced CT were, respectively, 40.60 ± 8.78 and 53.78 ± 7.37 precontrast, 172.66 ± 73.89 and 92.76 ± 17.92 during the arterial phase, 152.76 ± 35.73 and 120.12 ± 18.02 during the portal venous phase, and 108.74 ± 18.70 and 89.04 ± 7.25 during the equilibrium phase (Figure 2B). Hemangiomas demonstrated peak enhancement during the arterial phase, and both the SIR observed with Gd-EOB-DTPA-enhanced MRI and the attenuation value observed with enhanced CT decreased with time. The SIR of hemangiomas was lower than the SIR of liver parenchyma during the equilibrium and hepatobiliary phases observed with Gd-EOB-DTPA-enhanced MRI (Figure 2). The TLC observed with Gd-EOB- DTPA-enhanced MRI were -0.48 ± 0.20 precontrast, 0.35 ± 0.53 during the arterial phase, 0.15 ± 0.43 during the portal venous phase, and -0.15 ± 0.28 during the equilibrium phase. In comparison, the TLC observed with enhanced CT were -13.18 ± 7.79 precontrast, 79.9 ± 35.9 during the arterial phase, 32.6 ± 17.4 during the portal venous phase, and 19.7 ± 13.5 during the equilibrium phase. The visual assessment of tumor contrast enhancement correlated well with the TLC in the quantitative analysis of both Gd-EOB-DTPA-enhanced MRI and enhanced CT, with Rs values ranging from 0.45 to 0.71 (all P < 0.001).
Enhanced CT and Gd-DTPA-enhanced MRI findings of hepatic hemangiomas have been well documented in many reports, and the dynamic characteristics observed using both modalities are similar[3-7]. The typical findings of hepatic hemangioma on enhanced CT are hypoattenuation similar to the attenuation of vessels on precontrast images, peripheral nodular enhancement during the arterial phase, fill-in enhancement during the portal venous phase, and prolonged enhancement during the equilibrium phase[4-7].
In previous reports, peripheral nodular enhancement during the arterial phase was identified in 55%-87% of hemangiomas[4,5,7,21],and fill-in enhancement during the portal venous phase was observed in 59-96% of hemangiomas observed with enhanced CT[4,5,7,22,23]. In the present study, peripheral nodular enhancement during the arterial phase and fill-in enhancement during the portal venous phase on enhanced CT were observed in 50 (82%) and 49 (80%) of 61 hemangiomas, respectively. Goshima et al[16] reported that peripheral nodular enhancement with fill-in enhancement was observed in 28% who underwent Gd-EOB-DTPA-enhanced MRI. To our knowledge, no reports have compared the Gd-EOB-DTPA-enhanced MRI findings of hemangiomas with enhanced CT. In the present study, peripheral nodular enhancement was identified in 52 (85%) and fill-in enhancement was shown in 48 (79%) of 61 hemangiomas visualized with Gd- EOB-DTPA-enhanced MRI. No significant differences were observed in the presence of peripheral nodular and fill-in enhancement between Gd-EOB-DTPA-enhanced MRI and enhanced CT. In the quantitative analysis, enhanced portions of the hemangiomas demonstrated peak contrast enhancement during the arterial phase, and the contrast enhancement decreased with time on both Gd-EOB-DTPA-enhanced MRI and enhanced CT. However, tumor contrast enhancement during the arterial and portal venous phases was significantly reduced on Gd-EOB-DTPA-enhanced MRI compared with enhanced CT by both visual assessment and quantitative analysis. This result may be influenced by the lower gadolinium dose used with Gd-EOB-DTPA[12,14].
Hemangiomas showing diffuse homogenous enhancement during the arterial phase can mimic hypervascular malignant tumors such as hepatocellular carcinomas or hypervascular metastases[21,22,24,25]. In previous reports, diffuse homogenous enhancement during the arterial phase was observed in 8%-35% of hemangiomas on enhanced CT[22,24] and 34% on Gd-EOB-DTPA-enhanced MRI[16]. In the present study, diffuse homogenous enhancement during the arterial phase was observed in 10 (16%) and 7 (11%) of 61 hemangiomas on enhanced CT and Gd-EOB-DTPA-enhanced MRI, respectively. There were no significant differences between Gd-EOB-DTPA-enhanced MRI and enhanced CT in this respect.
Prolonged enhancement during the equilibrium phase is present in approximately 59%-96% of hemangiomas observed with enhanced CT[22-24] and 37%-72% of hemangiomas observed with Gd-EOB-DTPA-enhanced MRI[16,17]. This difference in the presence of prolonged enhancement on Gd-EOB-DTPA-enhanced MRI may be explained by differences in case selection. In addition, on enhanced CT, once the regions in the hemangioma enhance during the arterial phase, they also show prolonged enhancement[25]. In the present study, prolonged enhancement was observed on enhanced CT in all 61 hemangiomas, whereas Gd-EOB-DTPA-enhanced MRI showed prolonged enhancement only in 52% of the hemangiomas. The contrast enhancement was significantly reduced when using Gd-EOB-DTPA-enhanced MRI instead of enhanced CT. Ringe et al[18] reported that hemangiomas appear iso- or hypointense compared to the liver parenchyma during the equilibrium and hepatobiliary phases on Gd-EOB-DTPA-enhanced MRI. They suggest that this reduction is because of Gd-EOB-DTPA uptake in the surrounding normal liver parenchyma, the lower gadolinium dose, and the shorter plasma half-life of Gd-EOB-DTPA. Hemangiomas showing diffuse homogenous enhancement during the arterial phase can be differentiated from hypervascular malignant tumors on enhanced CT and Gd-DTPA-enhanced MRI because hemangiomas show isoattenuation or hyperattenuation relative to the liver parenchyma during the equilibrium phase[3,22,25]. In the present study, however, none of the hemangiomas with diffuse homogenous enhancement during the arterial phase showed prolonged enhancement during the equilibrium phase of Gd-EOB-DTPA-enhanced MRI. Moreover, 22 (40%) of 54 hemangiomas without diffuse enhancement during the arterial phase did not show prolonged enhancement during the equilibrium phase of Gd-EOB-DTPA-enhanced MRI. Hypovascular metastatic tumors show relatively low signal intensity during the equilibrium phase of Gd-EOB-DTPA-enhanced MRI[9,11,16]. Therefore, the present results suggest that it may be difficult to differentiate hemangiomas from malignant tumors such as hepatocellular carcinomas and metastatic tumors on Gd-EOB-DTPA-enhanced MRI because approximately half the hemangiomas exhibited relatively low signal intensity during the equilibrium phase. Enhanced MRI with extracellular contrast agents, such as Gd-DTPA, may be more useful for the diagnosis of hepatic hemangiomas. However, Gd-EOB-DTPA-enhanced MRI is widely used for evaluating the presence of hepatocellular carcinoma or metastasis in patients with liver cirrhosis or an extrahepatic malignancy because of its higher detectability compared with Gd-DTPA-enhanced MRI[11,12]. The differentiation of hemangioma from HCC or metastases on Gd-EOB-DTPA-enhanced MRI is frequently required in a clinical practice.
The present study has several potential limitations. First, pathological proof was obtained only for four hemangiomas and was not obtained for the majority of the lesions. Tissue biopsies were not obtained because hemangiomas are benign lesions and usually do not require invasive procedures. Therefore, case selection depended solely on imaging findings, but the typical findings on enhanced CT and bright signal intensity on T2-weighted MRI are accepted as diagnostic for hepatic hemangioma. Second, the present study may have a potential selection bias because atypical hemangiomas that did not meet the present selection criteria were not included. Therefore, further investigation will be necessary to elucidate the appearance of atypical hemangiomas on Gd-EOB- DTPA-enhanced MRI. Third, all the lesions were hemangiomas; other liver tumors were not included in the present study. Therefore, this selection may bias the interpretation of the Gd-EOB-DTPA-enhanced MRI and enhanced CT findings. However, the purpose of the present study was to compare the features of hepatic hemangiomas on Gd-EOB-DTPA-enhanced with enhanced CT. Fourth, the imaging parameters, injection rate, duration of the contrast material, and scanning timing were not identical for Gd-EOB-DTPA-enhanced MRI and enhanced CT. However, it is impractical to make them uniform because they have been optimized for each imaging modality.
In conclusion, the typical findings of hemangiomas, such as peripheral nodular enhancement, central nodular enhancement, diffuse enhancement, or arterioportal shunt during the arterial phase, or fill-in enhancement during the portal venous phase, are useful for the diagnosis of hemangiomas using Gd-EOB-DTPA-enhanced MRI and enhanced CT. However, prolonged enhancement during the equilibrium phase was observed significantly less frequently on Gd-EOB-DTPA-enhanced MRI. Some hemangiomas show relatively low signal intensity during the equilibrium phase, which may mimic malignant tumors. Knowledge of Gd-EOB-DTPA-enhanced MRI findings is important for arriving at the correct diagnosis of hepatic hemangioma.
Hepatic hemangioma is the most common benign hepatic neoplasm, and its differentiation from malignant liver tumors is very important. The most common enhanced computed tomography (CT) and gadolinium-diethylenetriaminepentaacetic acid (Gd-DTPA)-enhanced magnetic resonance imaging (MRI) features of hepatic hemangiomas are peripheral nodular enhancement during the arterial phase, fill-in enhancement during the portal venous phase, and prolonged enhancement during the equilibrium phase. Recently, gadolinium-ethoxybenzyl-diethylenetriaminpentaacetic acid (Gd-EOB-DTPA)-enhanced MRI has been used to evaluate liver lesions.
It is important to clarify the features of hepatic hemangiomas observed with Gd-EOB-DTPA-enhanced MRI. However, limited reports describe the findings of hemangiomas observed with Gd-EOB-DTPA-enhanced MRI. Moreover, the appearance and the degree of contrast enhancement of hepatic hemangiomas observed with Gd-EOB-DTPA-enhanced MRI compared with enhanced CT have not been elucidated.
The tumor-to-liver contrast enhancement during each phase was significantly lower on Gd-EOB-DTPA-enhanced MRI than enhanced CT. Prolonged enhancement during the equilibrium phase, which is an important finding for diagnosing hepatic hemangiomas, was observed less frequently on Gd-EOB-DTPA-enhanced MRI than enhanced CT.
Some hemangiomas show relatively low signal intensity during the equilibrium phase and may mimic malignant tumors. Knowledge of Gd-EOB-DTPA-enhanced MRI findings is important for arriving at the correct diagnosis of hepatic hemangioma.
Gd-EOB-DTPA is a new hepatobiliary contrast agent that exhibits high T1 relaxivity in the liver and shows enhancement on both early perfusion phase images and delayed hepatobiliary phase images. Gd-EOB-DTPA-enhanced MRI is more useful for the detection and characterization of focal liver lesions than enhanced CT and Gd-DTPA-enhanced MRI.
The authors compare the liver-tumor contrasts obtained for hepatic hemangioma with hepatobiliary contrast in MRI with extracellular contrast in CT, which is of clinical interest.
| 1. | Ishak KG, Rabin L. Benign tumors of the liver. Med Clin North Am. 1975;59:995-1013. [PubMed] |
| 2. | Freeny PC, Vimont TR, Barnett DC. Cavernous hemangioma of the liver: ultrasonography, arteriography, and computed tomography. Radiology. 1979;132:143-148. [PubMed] |
| 3. | Hamm B, Fischer E, Taupitz M. Differentiation of hepatic hemangiomas from metastases by dynamic contrast-enhanced MR imaging. J Comput Assist Tomogr. 1990;14:205-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 63] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 4. | Päivänsalo M, Lähde S, Jalovaara P. Computed tomography of hepatic haemangiomas: a chance for a definite diagnosis. Bildgebung. 1991;58:29-32. [PubMed] |
| 5. | Hanafusa K, Ohashi I, Himeno Y, Suzuki S, Shibuya H. Hepatic hemangioma: findings with two-phase CT. Radiology. 1995;196:465-469. [PubMed] |
| 6. | Freeny PC, Marks WM. Patterns of contrast enhancement of benign and malignant hepatic neoplasms during bolus dynamic and delayed CT. Radiology. 1986;160:613-618. [PubMed] |
| 7. | Ashida C, Fishman EK, Zerhouni EA, Herlong FH, Siegelman SS. Computed tomography of hepatic cavernous hemangioma. J Comput Assist Tomogr. 1987;11:455-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Hammerstingl R, Huppertz A, Breuer J, Balzer T, Blakeborough A, Carter R, Fusté LC, Heinz-Peer G, Judmaier W, Laniado M. Diagnostic efficacy of gadoxetic acid (Primovist)-enhanced MRI and spiral CT for a therapeutic strategy: comparison with intraoperative and histopathologic findings in focal liver lesions. Eur Radiol. 2008;18:457-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 317] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 9. | Reimer P, Rummeny EJ, Daldrup HE, Hesse T, Balzer T, Tombach B, Peters PE. Enhancement characteristics of liver metastases, hepatocellular carcinomas, and hemangiomas with Gd-EOB-DTPA: preliminary results with dynamic MR imaging. Eur Radiol. 1997;7:275-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 133] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 10. | Huppertz A, Balzer T, Blakeborough A, Breuer J, Giovagnoni A, Heinz-Peer G, Laniado M, Manfredi RM, Mathieu DG, Mueller D. Improved detection of focal liver lesions at MR imaging: multicenter comparison of gadoxetic acid-enhanced MR images with intraoperative findings. Radiology. 2004;230:266-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 323] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 11. | Vogl TJ, Kümmel S, Hammerstingl R, Schellenbeck M, Schumacher G, Balzer T, Schwarz W, Müller PK, Bechstein WO, Mack MG. Liver tumors: comparison of MR imaging with Gd-EOB-DTPA and Gd-DTPA. Radiology. 1996;200:59-67. [PubMed] |
| 12. | Kühn JP, Hegenscheid K, Siegmund W, Froehlich CP, Hosten N, Puls R. Normal dynamic MRI enhancement patterns of the upper abdominal organs: gadoxetic acid compared with gadobutrol. AJR Am J Roentgenol. 2009;193:1318-1323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Hamm B, Staks T, Mühler A, Bollow M, Taupitz M, Frenzel T, Wolf KJ, Weinmann HJ, Lange L. Phase I clinical evaluation of Gd-EOB-DTPA as a hepatobiliary MR contrast agent: safety, pharmacokinetics, and MR imaging. Radiology. 1995;195:785-792. [PubMed] |
| 14. | Halavaara J, Breuer J, Ayuso C, Balzer T, Bellin MF, Blomqvist L, Carter R, Grazioli L, Hammerstingl R, Huppertz A. Liver tumor characterization: comparison between liver-specific gadoxetic acid disodium-enhanced MRI and biphasic CT--a multicenter trial. J Comput Assist Tomogr. 2006;30:345-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 140] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 15. | Zizka J, Klzo L, Ferda J, Mrklovský M, Bukac J. Dynamic and delayed contrast enhancement in upper abdominal MRI studies: comparison of gadoxetic acid and gadobutrol. Eur J Radiol. 2007;62:186-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 16. | Goshima S, Kanematsu M, Watanabe H, Kondo H, Shiratori Y, Onozuka M, Moriyama N. Hepatic hemangioma and metastasis: differentiation with gadoxetate disodium-enhanced 3-T MRI. AJR Am J Roentgenol. 2010;195:941-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 57] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 17. | Tamada T, Ito K, Yamamoto A, Sone T, Kanki A, Tanaka F, Higashi H. Hepatic hemangiomas: evaluation of enhancement patterns at dynamic MRI with gadoxetate disodium. AJR Am J Roentgenol. 2011;196:824-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Ringe KI, Husarik DB, Sirlin CB, Merkle EM. Gadoxetate disodium-enhanced MRI of the liver: part 1, protocol optimization and lesion appearance in the noncirrhotic liver. AJR Am J Roentgenol. 2010;195:13-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 264] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 19. | Doo KW, Lee CH, Choi JW, Lee J, Kim KA, Park CM. "Pseudo washout" sign in high-flow hepatic hemangioma on gadoxetic acid contrast-enhanced MRI mimicking hypervascular tumor. AJR Am J Roentgenol. 2009;193:W490-W496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 118] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 20. | Bennett GL, Petersein A, Mayo-Smith WW, Hahn PF, Schima W, Saini S. Addition of gadolinium chelates to heavily T2-weighted MR imaging: limited role in differentiating hepatic hemangiomas from metastases. AJR Am J Roentgenol. 2000;174:477-485. [PubMed] |
| 21. | Leslie DF, Johnson CD, Johnson CM, Ilstrup DM, Harmsen WS. Distinction between cavernous hemangiomas of the liver and hepatic metastases on CT: value of contrast enhancement patterns. AJR Am J Roentgenol. 1995;164:625-629. [PubMed] |
| 22. | Choi BI, Han JK, Cho JM, Choi DS, Han MC, Lee HS, Kim CY. Characterization of focal hepatic tumors. Value of two-phase scanning with spiral computed tomography. Cancer. 1995;76:2434-2442. [PubMed] |
| 23. | Duan CX, Lu TZ, Tao WZ, Wang JJ, Han XY. Hepatic cavernous hemangioma. CT findings and pathological basis. Chin Med J (Engl). 1992;105:771-774. [PubMed] |
| 24. | Honda H, Matsuura Y, Onitsuka H, Murakami J, Kaneko K, Murayama S, Kanematsu T, Masuda K. Differential diagnosis of hepatic tumors (hepatoma, hemangioma, and metastasis) with CT: value of two-phase incremental imaging. AJR Am J Roentgenol. 1992;159:735-740. [PubMed] |
| 25. | Hanafusa K, Ohashi I, Gomi N, Himeno Y, Wakita T, Shibuya H. Differential diagnosis of early homogeneously enhancing hepatocellular carcinoma and hemangioma by two-phase CT. J Comput Assist Tomogr. 1997;21:361-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
Peer reviewer: Paul E Sijens, PhD, Associate Professor, Radiology Department, University Medical Center Groningen, Hanzeplein 1, 9713GZ Groningen, The Netherlands
S- Editor Gou SX L- Editor Kerr C E- Editor Xiong L