Published online Nov 21, 2012. doi: 10.3748/wjg.v18.i43.6263
Revised: September 13, 2012
Accepted: September 22, 2012
Published online: November 21, 2012
AIM: To compare the clinicopathological characteristics of human epidermal growth factor receptor 2 (HER2)-positive and HER2-negative Barrett’s adenocarcinoma in Japan.
METHODS: We performed immunohistochemical analysis of HER2 in 30 samples taken from patients with Barrett’s adenocarcinoma and dual color in situ hybridization in cases showing 2+ reactions. We compared the clinicopathological characteristics of HER2-positive and HER2-negative patients.
RESULTS: HER2 positivity was identified in 8 (27%) carcinoma samples. We found that HER2 expression was associated with p53 overexpression (100% vs 52.6% in pT1 tumor; 100% vs 54.5% in all stage tumor, P < 0.05) and protruding lesions at the early disease stage. There was no association between the mucin phenotype of the carcinomas and prognosis. HER2 expression and low clinical stage were unexpectedly different between Barrett’s adenocarcinoma patients and gastric cancer patients, but the macroscopic features may be associated with earlier diagnosis in these patients.
CONCLUSION: Our results suggest that HER2-positive Barrett’s adenocarcinomas are associated with p53 overexpression and lesion protrusion at the early disease stage.
- Citation: Tanaka T, Fujimura A, Ichimura K, Yanai H, Sato Y, Takata K, Okada H, Kawano S, Tanabe S, Yoshino T. Clinicopathological characteristics of human epidermal growth factor receptor 2-positive Barrett's adenocarcinoma. World J Gastroenterol 2012; 18(43): 6263-6268
- URL: https://www.wjgnet.com/1007-9327/full/v18/i43/6263.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i43.6263
Recent studies have shown that the incidence of Barrett’s adenocarcinoma has been increasing in Japan. Although human epidermal growth factor receptor 2 (HER2) was reported to be amplified and overexpressed in some Barrett’s adenocarcinomas, the relationship between HER2 expression and patient clinicopathological characteristics has not yet been clarified.
The HER2 gene, a proto-oncogene, is located on chromosome 17q11.2-12 and encodes the transmembrane glycoprotein receptor p185HER2 (or HER2), which is targeted by the humanized monoclonal antibody trastuzumab (Herceptin®)[1]. HER2 is amplified and overexpressed in approximately 25% of breast cancer patients, and is associated with an aggressive clinical course and poor prognosis[2]. Recently, HER2 overexpression and amplification was detected in approximately 22% of advanced gastric cancers, and targeting of the extracellular domain of HER2 in these patients was associated with improved clinical benefits compared with chemotherapy alone in a phase III trial[3]. Several recent studies have reported HER2 status in Barrett’s adenocarcinoma; they observed a prevalence of HER2 protein overexpression or gene amplification in Barrett’s adenocarcinomas ranging from 11% to 72%[4-8]. Lack of agreement among these studies may be related to the differing sensitivities of the assay methods used to assess HER2[9-13].
HER2 has been recognized as an important prognostic factor in breast cancer[2]. However, the clinicopathological characteristics of HER-positive Barrett’s adenocarcinoma are controversial. In this study, we used the criteria of the trastuzumab for gastric cancer (ToGA) trial to evaluate the HER2 status of Barrett’s adenocarcinomas in Japan by clarifying the clinicopathological characteristics of HER2-positive Barrett’s esophageal adenocarcinomas and examining their morphological immunohistochemical characteristics.
Samples were collected from 30 patients who visited the Okayama University Hospital between May 1998 and March 2011. Histological sections and immunohistochemical results were reviewed to confirm the diagnosis. The definition of Barrett’s esophagus and Barrett’s adenocarcinoma is controversial[14,15]. In this study, the criterion for the clinical diagnosis of Barrett’s adenocarcinoma was that the tumor foci were located in Barrett’s mucosa, which is also referred to as the columnar-lined esophagus. The histological criterion was carcinoma that presented or was in contact with Barrett’s mucosa, defined as columnar-lined mucosa with or without intestinal-type epithelium. Clinical information was obtained from the medical records of patients at Okayama University Hospital. The patients underwent a standardized informed consent procedure.
An automated immunostainer (Ventana Medical Systems, Tucson, AZ, United States) was used to perform all immunohistochemical analyses. The following monoclonal antibodies were used: p53 (DO-7; Dako, Glostrup, Denmark), MUC2 (Ccp58; Novocastra Laboratories, Newcastle upon Tyne, United Kingdom), MUC5AC (CLH2, Novocastra Laboratories, Newcastle upon Tyne, United Kingdom), MUC6 (CLH5; Novocastra Laboratories), and CD10 (56C6; Dako). For the evaluation of p53 staining, only cells with nuclear immunostaining significantly more pronounced than that of the control cells of the normal esophageal mucosa were considered positive. MUC5AC and MUC6 are markers of gastric epithelial cells, and MUC2 and CD10 are typical of the intestinal epithelial cell phenotype[16]. Barrett’s adenocarcinoma, in which more than 10% of the section area consisted of at least 1 gastric or intestinal epithelial cell phenotype, were classified as gastric (G type) or intestinal (I type) phenotypic cancers. Those which showed both gastric and intestinal phenotypes were classified as gastric and intestinal mixed phenotypic (GI type) cancers, whereas those showing neither gastric nor intestinal phenotypic expression were grouped as unclassified (N type).
HER2 protein expression was assessed in carcinoma cells by immunohistochemistry (IHC) in paraffin-embedded 5-μm tissue sections according to the manufacturer’s instructions (Ventana I-VIEW pathway HER2/neu kit; Ventana Medical Systems). Only cell membrane staining was considered positive. Each case was analyzed by a pathologist blinded to the clinical outcome who used criteria specific to upper gastrointestinal cancer that included 2 parameters: (1) the intensity of complete, basolateral, or lateral membrane staining (0, none; 1, faint; 2, weak to moderate; and 3, strong); and (2) the percentage of cancer cells with a given staining intensity. These parameters were used to determine the IHC score according to the ToGA criteria: high (IHC 3+), strong intensity in 10% or more of the cancer cells; medium (IHC 2+), weak to moderate intensity in 10% or more; low (IHC 1+), faint intensity in 10% or more; absent (IHC 0). Dual in situ hybridization (DISH) using a Ventana INFORM HER2 ISH kit (Ventana Medical Systems) was used to assess HER2 gene amplification in all IHC 2+ cases by preparing the carcinoma cells in 5 µm tissue sections. Briefly, for each case, a parallel hematoxylin and eosin-stained slide was examined for regions of carcinoma by a pathologist. The complete tissue section was scanned by the pathologist to detect any subpopulation of amplified cells. A total of 20 representative nuclei from the invasive tumor were scored. A specimen with a HER2/centromeric enumeration probe 17 (CEP17) ratio of 2.0 or more in tumor cells was classified as HER2 amplified according to the ToGA guidelines[17].
A case was considered HER2 positive if it was (1) IHC 3+ or (2) IHC 2+ plus after gene amplification. The remaining cases (i.e., non-amplified IHC 2+ or IHC 0-1+) were considered HER2 negative.
A χ2 test or Fisher’s exact test, depending on the sample size, was used to examine categorical variables to compare the clinical characteristics of the different groups of patients. The t-test was used to compare mean values. SPSS Version 14.0 (SPSS, Chicago, IL, United States) was used to analyze the data.
The studied population consisted of 20 men and 10 women (male to female ratio = 2:1). Their ages ranged from 42 to 87 years old. Sixteen lesions were identified as protruded or superficial elevated types (type 0-I or type 0-IIa), 1 lesion was identified as flat type (type 0-IIb), and 11 lesions were identified as superficial depressed type (type 0-IIc). Three lesions were identified as Borrmann type 3. In accordance with the World Health Organization’s classification standards, 21 patients (70%) had well-differentiated tumors and 9 had moderately differentiated tumors (30%). According to the tumor-node-metastasis classification, 13 patients were pT1a, 13 were pT1b, 1 was pT2, 2 were pT3, and 1 was pT4a. All stage pT3 and pT4 patients died from the disease, but the other patients remained alive without suffering from the disease. Taking into account the combination of expression of 4 markers, the 30 cancers were divided phenotypically into 7 G, 12 GI, 8 I, and 3 N types, independent of the histological classification. Expression of p53 was demonstrated in 20 (67%) of 30 cancers. The available data for each patient are summarized in Table 1.
| Characteristics | Values |
| Male/female | 20/10 |
| Age, yr (mean ± SD) | 71.1 ± 9.7 |
| Surgical resection/endoscopic resection | 14/16 |
| Location: Siewert I/II | 12/18 |
| Tumor size, mm (mean ± SD) | 27.1 ± 19.9 |
| Depth of primary tumor: T1a/T1b/T2/T3/T4 | 13/13/1/2/1 |
| Macroscopic appearance: 0-I/0-IIa/0-IIb/0-IIc/3 | 8/8/1/11/3 |
| Histology: tub1/tub2 | 21/9 |
| Histology tub1/tub2: mucin phenotype G/GI/I/N | 7/12/8/3 |
| p53: positive/negative | 20/10 |
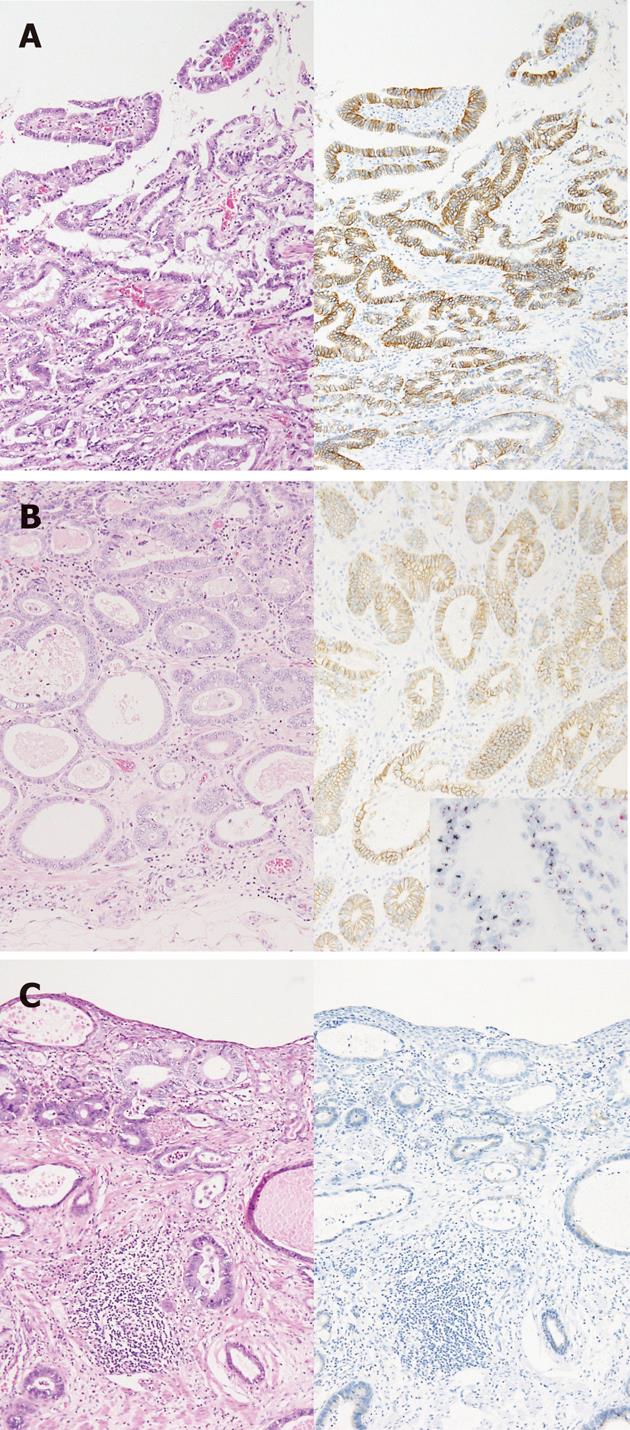
Of the 30 patients diagnosed with Barrett’s adenocarcinoma, stained sections in 4 cases (13%) were classified as IHC 3+ (Figure 1A), 6 cases (20%) were IHC 2+ (Figure 1B), 4 cases (13%) were IHC 1+, and 16 cases (53%) were IHC 0 (Figure 1C). Criteria developed for the evaluation of upper gastrointestinal tract carcinoma were used to score the HER2-stained samples. DISH was used to assess HER2 gene amplification in 6 IHC 2+ cases. A specimen with a HER2/CEP17 ratio of 2.0 or more in cancer cells was classified as HER2 amplified (Figure 1B), consistent with the eligibility criteria for the ToGA study. HER2 amplification was detected in 4 of IHC 2+ cases.
We defined HER2 positivity as IHC 3+ or IHC 2+ with gene amplification, which were the characteristics of the group that derived the greatest benefit from trastuzumab in the ToGA study. The positive rate of HER2 was 27% (Table 2).
| pT1 tumor | All stage tumor | |||
| HER2+ | HER2- | HER2+ (27%) | HER2- (73%) | |
| Male/female | 3/4 | 13/6 | 4/4 | 16/6 |
| Age, yr (mean ± SD) | 70.6 ± 9.9 | 71.4 ± 10.8 | 70.3 ± 9.2 | 71.4 ± 10.0 |
| Location: Siewert I/II | 3/4 | 11/18 | 3/5 | 9/13 |
| Tumor size, mm (mean ± SD) | 20.6 ± 17.7 | 23.9 ± 16.6 | 23.6 ± 18.5 | 28.3 ± 20.7 |
| Depth of primary tumor: T1a/T1b | 3/4 | 10/9 | 3/4/0/0/1 | 10/9/1/2/0 |
| Macroscopic appearance: 0-I/0-IIa/0-IIb/0-IIc | 3/3/0/1 | 4/4/1/10 | 3/3/0/1/1 | 4/5/1/10/2 |
| Histology: tub1/tub2 | 7/1 | 14/5 | 7/1 | 14/8 |
| Mucin phenotype: G/GI/I/N | 4/2/1/0 | 3/9/6/1 | 4/2/2/0 | 3/10/6/3 |
| p53: positive/negative | 7/0 | 10/9 | 8/0 | 12/10 |
HER2-positive adenocarcinomas were present in 4 men and 4 women. The patients ranged in age from 52 to 80 years. Six cases were the protruded or superficially elevated types (0-I or 0-IIa). Three tumors were located in the Siewert I region and 5 in the Siewert II region. The mean tumor size was 23.6 mm (11-60 mm). Of the pT1 cases, HER2-positive cases were significantly more associated with protruding lesions compared with HER2-negative cases (P < 0.05) (Table 2). Seven cases had well-differentiated tumors, and 1 case had a moderately-differentiated tumor. In the mucin phenotypical analysis, 4 cases were G type, 2 cases GI type, and 2 cases were I type; there were no significant differences in mucin phenotypes between the HER2-positive and HER2-negative cases. The p53 positivity rate was higher in the HER2-positive tumors than in the HER2-negative tumors (P < 0.05) (Table 2). There were no prognostic differences between the HER2-positive and HER2-negative cases.
In this study, we confirmed that 27% Barrett’s adenocarcinomas in Japanese patients were HER2 positive. We also found that early-stage HER2-positive Barrett’s adenocarcinomas were significantly associated with protruding lesions and had a high rate of p53 positivity.
Previous studies that have examined HER2 status in Barrett’s adenocarcinoma observed a prevalence of HER2 protein overexpression or gene amplification ranging from 11% to 72%[4-8]. Except for 1 study, the lack of agreement among these studies may have been related to the differing sensitivities of the assay methods used to assess HER2. Various antibodies and probes for in situ hybridization were used, and the test conditions also were not standardized. In this study, the HER2 tests were performed according to the criteria of the ToGA trial, which is the standard test for HER2 status of gastric cancer. Brien et al[18] used fluorescence in situ hybridization to evaluate HER2 amplification and reported a significant association between amplification and poorer survival. However, in the present study, a low threshold of 4 or more signals per nucleus was used to determine HER2 amplification. On the other hand, according to the ToGA criteria, HER2-positive esophageal adenocarcinomas with Barrett’s esophagus had favorable prognoses[19]. The influence and criteria of HER2 expression may still be controversial. In this study, there were no prognostic differences between the HER2-positive and HER2-negative cases. Except for the pT3 and pT4 patients, most patients were early stage and remained alive without disease. Thus, the prognostic significance of HER2 expression was not clarified in this study.
Barrett’s adenocarcinoma is thought to develop as a result of gastroesophageal reflux that initiates a metaplastic change in the lower esophageal epithelium. Barrett’s esophagus is significant because the condition has a risk for neoplastic transformation through a metaplasia-dysplasia-carcinoma sequence. In Western populations, there has been an increase in the incidence of adenocarcinoma of the esophagus and esophagogastric junction region. In Japan, esophageal Barrett’s adenocarcinomas are less common than in Western countries. However, in recent years there has been a gradual increase in the detection of both Barrett’s esophagus and Barrett’s adenocarcinoma[12]. Traditionally, Barrett’s adenocarcinoma has been believed to be preceded by the development of dysplasia with intestinal characteristics. Recently, Brown et al[13] and Park et al[20] validated the existence of 2 main types of dysplasia (i.e., foveolar and adenomatous) which were significantly associated with gastric and intestinal immunophenotypic markers. Khor et al[21] suggested that non-intestinal columnar metaplasia may be an unstable intermediate state at risk for neoplastic progression. In this study, we evaluated the mucin phenotype of Barrett’s adenocarcinoma and found that more than half of the cases were grouped as gastric and mixed phenotypes. This result suggests the presence of a gastric pathway of carcinogenesis in Barrett’s esophagus. Heterogeneity of HER2 status was seen in approximately 80% of samples with moderate or strong HER2 IHC reactivity, which was higher than that observed in breast cancer. There were only 2 diffusely strong positive cases. In our study, heterogeneity of HER2 overexpression and gene amplification appeared to represent clusters, and the intensity of IHC staining within clusters was relatively uniform. The IHC staining patterns and gene amplification appeared correlated.
Seven (23%) of the 30 studied cases were the pure gastric mucin phenotype, and 12 cases were the mixed phenotype. In these cases, the background Barrett’s mucosa also showed the foveolar type with or without specialized columnar epithelium (intestinal type mucosa). Carcinogenesis of complete gastric type adenocarcinomas is derived from foveolar type dysplasia with aneuploidy[22], and intestinal type carcinomas were regarded as progressing through the metaplasia-dysplasia-carcinoma sequence, with p53 alteration[23]. In this study, HER2-positive carcinomas showed both gastric and intestinal phenotypes. It is believed that p53 has an important role in intestinal-type Barrett’s adenocarcinoma; however, p53 overexpression was observed in all HER2-positive cases regardless of mucin phenotype. These results suggest a third pathway involving abnormalities of both HER2 and p53. Gastric adenocarcinoma is also significantly correlated with HER2 positivity[24-26]. These data suggest a possible role of p53 abnormality in the development of HER2-positive adenocarcinoma of the upper gastrointestinal tract.
The macroscopic appearance of HER2-positive Barrett’s adenocarcinomas was different from that of the HER2-negative cases in early-stage disease (pT1). The frequency of protruding lesions was significantly higher in the HER2-positive cases than in HER2-negative cases. Ten cases of HER2-negative adenocarcinomas were the protruded type, 9 cases were mixed or intestinal mucin phenotypes, and only 1 case was the gastric phenotype. Thus, protruded-type lesions of the complete gastric phenotype can indicate HER2-positive status.
In Japan, the incidence of esophageal adenocarcinoma has been increasing, but is still very low compared with squamous cell carcinoma. The incidence of Barrett’s adenocarcinoma is much higher in Western countries than in Japan. The relationship between human epidermal growth factor receptor 2 (HER2) expression and patient clinicopathological characteristics has not yet been clarified.
HER2 was reported to be amplified and overexpressed in some Barrett’s adenocarcinomas. The reported expression rates have varied widely due to various different methods and criteria being applied to determine HER2 expression. A practical method for determining HER2 expression has been established on the basis of the results of the trastuzumab for gastric cancer trial.
This was a retrospective study that assessed the incidence of HER2 positivity according to newly-established methods and criteria, and investigated the clinicopathological characteristics according to the new diagnostic criteria.
Since the sample size of the study was not sufficiently large, the evidence may not be robust. Even so, the authors believe that this study provides valuable data from the evaluation of HER2-positive Barrett’s adenocarcinoma.
HER2 is an important member of the epidermal growth factor receptor family that has been shown to act as an oncogene in many types of cancers.
This is a good descriptive study in which authors analyzed the clinicopathological characteristics of HER-2 positive Barrett’s adenocarcinoma. The results suggest that HER2-positive Barrett’s adenocarcinomas are associated with p53 overexpression and lesion protrusion at the early disease stage.
| 1. | Baselga J, Tripathy D, Mendelsohn J, Baughman S, Benz CC, Dantis L, Sklarin NT, Seidman AD, Hudis CA, Moore J. Phase II study of weekly intravenous recombinant humanized anti-p185HER2 monoclonal antibody in patients with HER2/ neu- overexpressing metastatic breast cancer. J Clin Oncol. 1996;14:737-744. [PubMed] |
| 2. | Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8336] [Cited by in RCA: 8622] [Article Influence: 221.1] [Reference Citation Analysis (0)] |
| 3. | Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687-697. [PubMed] [DOI] [Full Text] |
| 4. | Jankowski J, Coghill G, Hopwood D, Wormsley KG. Oncogenes and onco-suppressor gene in adenocarcinoma of the oesophagus. Gut. 1992;33:1033-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 106] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 5. | al-Kasspooles M, Moore JH, Orringer MB, Beer DG. Amplification and over-expression of the EGFR and erbB-2 genes in human esophageal adenocarcinomas. Int J Cancer. 1993;54:213-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 165] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 6. | Fléjou JF, Paraf F, Muzeau F, Fékété F, Hénin D, Jothy S, Potet F. Expression of c-erbB-2 oncogene product in Barrett’s adenocarcinoma: pathological and prognostic correlations. J Clin Pathol. 1994;47:23-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 79] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 7. | Hardwick RH, Shepherd NA, Moorghen M, Newcomb PV, Alderson D. c-erbB-2 overexpression in the dysplasia/carcinoma sequence of Barrett’s oesophagus. J Clin Pathol. 1995;48:129-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Polkowski W, van Sandick JW, Offerhaus GJ, ten Kate FJ, Mulder J, Obertop H, van Lanschot JJ. Prognostic value of Laurén classification and c-erbB-2 oncogene overexpression in adenocarcinoma of the esophagus and gastroesophageal junction. Ann Surg Oncol. 1999;6:290-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 86] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 9. | Walch A, Specht K, Bink K, Zitzelsberger H, Braselmann H, Bauer M, Aubele M, Stein H, Siewert JR, Höfler H. Her-2/neu gene amplification, elevated mRNA expression, and protein overexpression in the metaplasia-dysplasia-adenocarcinoma sequence of Barrett’s esophagus. Lab Invest. 2001;81:791-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Rossi E, Villanacci V, Bassotti G, Casa DD, Missale G, Minelli L, Cestari R. Her-2/neu in Barrett esophagus: a comparative study between histology, immunohistochemistry, and fluorescence in situ hybridization. Diagn Mol Pathol. 2006;15:125-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Thompson SK, Sullivan TR, Davies R, Ruszkiewicz AR. Her-2/neu gene amplification in esophageal adenocarcinoma and its influence on survival. Ann Surg Oncol. 2011;18:2010-2017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 12. | Hongo M. Review article: Barrett’s oesophagus and carcinoma in Japan. Aliment Pharmacol Ther. 2004;20 Suppl 8:50-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 64] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 13. | Brown IS, Whiteman DC, Lauwers GY. Foveolar type dysplasia in Barrett esophagus. Mod Pathol. 2010;23:834-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 51] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 15. | Takubo K, Vieth M, Aida J, Sawabe M, Kumagai Y, Hoshihara Y, Arai T. Differences in the definitions used for esophageal and gastric diseases in different countries: endoscopic definition of the esophagogastric junction, the precursor of Barrett’s adenocarcinoma, the definition of Barrett’s esophagus, and histologic criteria for mucosal adenocarcinoma or high-grade dysplasia. Digestion. 2009;80:248-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 16. | Tatematsu M, Tsukamoto T, Inada K. Stem cells and gastric cancer: role of gastric and intestinal mixed intestinal metaplasia. Cancer Sci. 2003;94:135-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 142] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 17. | Rüschoff J, Hanna W, Bilous M, Hofmann M, Osamura RY, Penault-Llorca F, van de Vijver M, Viale G. HER2 testing in gastric cancer: a practical approach. Mod Pathol. 2012;25:637-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 379] [Cited by in RCA: 450] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 18. | Brien TP, Odze RD, Sheehan CE, McKenna BJ, Ross JS. HER-2/neu gene amplification by FISH predicts poor survival in Barrett’s esophagus-associated adenocarcinoma. Hum Pathol. 2000;31:35-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 19. | Park do Y, Srivastava A, Kim GH, Mino-Kenudson M, Deshpande V, Zukerberg LR, Song GA, Lauwers GY. Adenomatous and foveolar gastric dysplasia: distinct patterns of mucin expression and background intestinal metaplasia. Am J Surg Pathol. 2008;32:524-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 79] [Article Influence: 4.4] [Reference Citation Analysis (1)] |
| 20. | Yoon HH, Shi Q, Sukov WR, Wiktor AE, Khan M, Sattler CA, Grothey A, Wu TT, Diasio RB, Jenkins RB. Association of HER2/ErbB2 expression and gene amplification with pathologic features and prognosis in esophageal adenocarcinomas. Clin Cancer Res. 2012;18:546-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 473] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 21. | Khor TS, Alfaro EE, Ooi EM, Li Y, Srivastava A, Fujita H, Park Y, Kumarasinghe MP, Lauwers GY. Divergent expression of MUC5AC, MUC6, MUC2, CD10, and CDX-2 in dysplasia and intramucosal adenocarcinomas with intestinal and foveolar morphology: is this evidence of distinct gastric and intestinal pathways to carcinogenesis in Barrett Esophagus? Am J Surg Pathol. 2012;36:331-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 22. | Rucker-Schmidt RL, Sanchez CA, Blount PL, Ayub K, Li X, Rabinovitch PS, Reid BJ, Odze RD. Non-adenomatous dysplasia in Barrett esophagus: a clinical, pathologic, and DNA content flow cytometric study. Am J Surg Pathol. 2009;33:886-893. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 23. | Moore JH, Lesser EJ, Erdody DH, Natale RB, Orringer MB, Beer DG. Intestinal differentiation and p53 gene alterations in Barrett’s esophagus and esophageal adenocarcinoma. Int J Cancer. 1994;56:487-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 24. | Lee KE, Lee HJ, Kim YH, Yu HJ, Yang HK, Kim WH, Lee KU, Choe KJ, Kim JP. Prognostic significance of p53, nm23, PCNA and c-erbB-2 in gastric cancer. Jpn J Clin Oncol. 2003;33:173-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 79] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 25. | Al-Moundhri MS, Nirmala V, Al-Hadabi I, Al-Mawaly K, Burney I, Al-Nabhani M, Thomas V, Ganguly SS, Grant C. The prognostic significance of p53, p27 kip1, p21 waf1, HER-2/neu, and Ki67 proteins expression in gastric cancer: a clinicopathological and immunohistochemical study of 121 Arab patients. J Surg Oncol. 2005;91:243-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | Kataoka Y, Okabe H, Yoshizawa A, Minamiguchi S, Yoshimura K, Haga H, Sakai Y. HER2 expression and its clinicopathological features in resectable gastric cancer. Gastric Cancer. 2012;Mar 14 [Epub ahead of print]. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
Peer reviewer: Elfriede Bollschweiler, Professor, Department of Surgery, University of Cologne, Kerpener Strabe 62, 50935 Köln, Germany
S- Editor Gou SX L- Editor Rutherford A E- Editor Xiong L