Published online Nov 7, 2012. doi: 10.3748/wjg.v18.i41.5948
Revised: June 7, 2012
Accepted: June 28, 2012
Published online: November 7, 2012
AIM: To evaluate the effect of prokinetic drugs on electrogastrography (EGG) parameters according to symptomatic changes in patients with functional dyspepsia (FD).
METHODS: Seventy-four patients with FD were prospectively enrolled in this study between December 2006 and December 2010. We surveyed the patients using a questionnaire on dyspeptic symptoms before and after an 8-wk course of prokinetic drug treatment. We also measured cutaneous pre-prandial and post-prandial EGG recordings including percentage of gastric waves (normogastria, bradygastria, tachygastria), dominant frequency (DF), dominant power (DP), dominant frequency instability coefficient (DFIC), dominant power instability coefficient (DPIC), and the ratio of post-prandial to fasting in DP before and after the 8-wk course of prokinetic drug treatment.
RESULTS: Fifty-two patients (70%) achieved symptomatic improvement after prokinetic drug treatment. Patients who had normal gastric slow waves showed symptom improvement group after treatment. Post-prandial DF showed a downward trend in the symptom improvement group, especially in the itopride group. Post-prandial DP was increased regardless of symptom improvement, especially in the itopride group and mosapride group. Post-prandial DFIC and DPIC in the symptom improvement group were significantly increased after the treatment. The EGG power ratio was increased after treatment in the symptom improvement group (0.50 ± 0.70 vs 0.93 ± 1.77, P = 0.002), especially in the itopride and levosulpiride groups.
CONCLUSION: Prokinetics could improve the symptoms of FD by regulating gastric myoelectrical activity, and EGG could be a useful tool in evaluating the effects of various prokinetics.
- Citation: Lim HC, Lee SI, Chen JD, Park H. Electrogastrography associated with symptomatic changes after prokinetic drug treatment for functional dyspepsia. World J Gastroenterol 2012; 18(41): 5948-5956
- URL: https://www.wjgnet.com/1007-9327/full/v18/i41/5948.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i41.5948
Electrogastrography (EGG) is a noninvasive technique for recording gastric myoelectrical activity using electrodes on the abdominal wall overlying the stomach. EGG has been used as a diagnostic tool to determine the mechanism of symptom generation in patients who have dyspeptic symptoms, including nausea, vomiting, post-prandial fullness, bloating, and early satiety, due to gastric motility disorders and abnormal gastric myoelectrical activity[1]. The EGG records the rhythms of gastric slow waves, which provide information on the velocity and propagations of gastric contractions. The previous studies showed the associations of tachyarrhythmia with absent antral contractions, and bradyarrhythmia with strong or absent antral contractions[2]. Gastric dysrythmia including bradygastria and tachygastria is observed in 31%-69% of patients with functional dyspepsia (FD), and several gastric rhythm abnormalities were described in patients with diabetic gastroparesis and motion sickness[3-6]. EGG also records the gastric myoelectrical activities which show the amplitude of gastric contraction. The amplitude increases in the post-prandial state in healthy populations (90%-95%) and a lack of an increase is believed to reflect decreased gastric motor activity[7].
Prokinetic drugs are used to treat FD by potentially enhancing gastrointestinal motility and accelerating gastric emptying. Several prokinetic drugs, such as cisapride and domperidone, are known to correct dysrhythmias and symptoms in patients with gastroparesis and dyspepsia[8,9]. Recently, prokintics drugs, such as itopride hydrochloride, mosapride citrate, and levosulpiride, were used widely for treatment of upper gastrointestinal motility disease, but the clinical utility of changes in EGG parameters after treatment with these prokinetics in patients with FD symptoms has not been well established[10].
This prospective study was conducted to evaluate the effect of itopride hydrochloride, mosapride citrate, and levosulpiride on EGG parameters according to symptomatic changes in patients with FD.
This study was a prospective study approved by the Institutional Review Committee of Yonsei University Health System and was conducted in compliance with the Declaration of Helsinki. All patients were fully informed of the purposes of the study and written informed consent was obtained from all patients prior to participation.
We reviewed patients who visited the Gangnam Severance Hospital, Yonsei University, South Korea for dyspeptic symptoms between December 2006 and December 2010. Patients with symptoms meeting the Rome III criteria for FD underwent the following procedures[11]: an interview on medical history, physical examination, hematologic and chemical evaluations, upper esophagogastro-duodenoscopy or an upper gastrointestinal series, before taking prokinetic drugs. Exclusion criteria included patients (1) who had organic or metabolic diseases (i.e., diabetes mellitus, liver cirrhosis); (2) who had gastrointestinal diseases which had associated dyspeptic symptoms such as inflammatory bowel disease, cancer and ulcers; (3) who had a history of abdominal surgery; and (4) who were taking drugs which could affect gastrointestinal motility, including other prokinetics, cholinergic/anticholinergic agents, and antidepressive agents, for at least 4 wk prior to study start.
Protocol for drug administration: A prokinetic drug was administered after patients completed the questionnaires on FD and baseline EGG recordings were completed. The patients were assigned to one of 3 groups based on the type of treatment drug: itopride hydrochloride (Ganaton®, Choogwae Pharma, South Korea) (n = 24), mosapride citrate (Gasmotin®, Daewoong Pharma, South Korea) (n = 28), and levosulpiride (Levopride®, SK Chemical Life Science, South Korea) (n = 22). Itopride hydrochloride (50 mg tablet), mosapride citrate (5 mg tablet), and levosulpiride (25 mg tablet) were administered to patients in each group 3 times a day in the post-prandial state for 8 wk, and drugs which could affect gastrointestinal function were not allowed to be used throughout the study.
Questionnaires for functional dyspepsia: Symptoms of epigastric pain, epigastric burning, post-prandial fullness, early satiety, post-prandial bloating, and post-prandial nausea or excessive belching were scored in accordance with the following scheme: 0 = none, 1 = mild (symptoms could be ignored if the patient did not think about it), 2 = moderate (symptoms could not be ignored but did not influence daily activities), 3 = severe (symptoms influenced daily activities)[12]. For each patient, the total symptom severity score was the sum of the 6 symptom scores (minimum 0 to maximum 18). The frequency of dyspeptic symptoms also described above was scored in accordance with the following scheme: 0 = none, 1 = once or twice a month 2 = once or twice a week, 3 = more than 3 times a week. These scores were added to yield the total symptom frequency score (minimum 0 to maximum 18). The questionnaires were completed again after 8-wk treatment.
Electrogastrography: EGG (Digitrapper EGG; Synetics Medical Inc, Stockholm, Sweden) was used to record gastric myoelectrical activity with low and high cutoff frequencies of 1 and 10 cpm, respectively. After an overnight fast, EGG recordings were obtained in the morning for 30 min in the fasting state and for another 30 min after a test meal at baseline before treatment. This procedure was repeated after 8-wk treatment. To reduce the resistance between electrode and skin, hair was shaved and skin abraded with prepping paste (OMNI PREP®, D.O. Weaver & Co. United States) on the abdomen, and conductive cream (Signa Creme®, Parker Laboratories, United States) was applied to the skin. Two electrodes were placed on the abdomen, one midway between the xyphoid process and umbilicus, and the other 5 cm to the left, just below the costal margin. A reference electrode was placed on the right side of the abdomen. These electrodes were connected to a Digitrapper EGG recording devices. The patients were in a sitting position leaning 45° in a comfortable chair. The test meal was composed of solid food (rice rolled up in dried seaweed with orange juice, 500 kcal). The EGG data were uploaded into a personal computer and analyzed by a software program (Polygram for Windows, version 6.40, Synetics Medical Inc, Stockholm, Sweden).
EGG recordings were analyzed to derive the following parameters: (1) percentage of normal gastric waves (2.0-4.0 cpm), bradygastric waves (1.0-2.0 cpm), and tachygastric waves (4.0-10.0 cpm); (2) dominant frequency (DF); (3) dominant power (DP); (4) dominant frequency instability coefficient (DFIC, %); (5) dominant power instability coefficient (DPIC, %); and (6) the ratio of post-prandial to fasting in DP. A percentage of normal slow wave frequency of more than 70% was defined as normal.
The patients were classified into 2 groups: a symptom improvement group if symptom severity and frequency scores decreased after treatment with prokinetic drugs; and a symptom resistance group if symptom severity and frequency scores increased or were unchanged after treatment. EGG parameters at baseline were compared with post-treatment EGG parameters, according to symptomatic improvement and types of prokinetic drugs used in this study.
Demographic data, questionnaire scores and parameters recorded in EGG were statistically analyzed by the paired Student t test and Fisher’s exact test using SPSS 17.0. Data are expressed as the mean ± SE and a P-value < 0.05 was considered significant.
This study included 74 patients (26 men, 48 women: median age 51.7 years, range: 19-70 years). After 8 wk of prokinetic drug treatment, 52 patients (70%) showed symptomatic improvement, while 22 patients (30%) had no improvement or aggravated symptoms. There were no significant demographic differences between patients with improved symptoms and those without improvement (Table 1). There were no significant differences in demographics, and symptom improvement rate among the itopride hydrochloride group, the mosapride citrate group, and the levosulpiride group (Table 2).
| Total patients (n = 74) | Symptom improvement (n = 52) | Symptom resistance (n = 22) | ||||
| Male:female (n) | 26:48 | 18:34 | 8:14 | |||
| Age (range), yr | 51.7 (19-70) | 53.5 (27-70) | 47.6 (19-70) | |||
| Height | 165.2 ± 12.4 | 166.3 ± 11.6 | 164.9 ± 10.1 | |||
| Weight | 61.3 ± 9.7 | 62.3 ± 9.1 | 58.8 ± 10.2 | |||
| Pre-treatment | Post-treatment | Pre-treatment | Post-treatment | Pre-treatment | Post-treatment | |
| Symptom severity score | 8.09 ± 0.43 | 5.51 ± 0.46a | 8.90 ± 0.46 | 4.52 ± 0.47a | 6.18 ± 0.85 | 7.86 ± 0.92 |
| Symptom frequency score | 9.27 ± 0.49 | 6.81 ± 0.54a | 9.96 ± 0.54 | 5.79 ± 0.60a | 7.64 ± 1.01 | 9.23 ± 0.97 |
| Gastric dysrhythmia (pre-prandial) | ||||||
| Bradygastria | 16 (21.6) | 11 (14.9) | 9 (17.3) | 7 (13.5) | 6 (27.3) | 4 (18.2) |
| Normogastria | 33 (44.6) | 52 (70.3)a | 26 (50.0) | 37 (71.2)a | 9 (40.9) | 15 (68.2) |
| Tachygastria | 25 (33.8) | 11 (14.9)a | 17 (32.7) | 8 (15.4)a | 7 (31.8) | 3 (13.6) |
| Gastric dysrhythmia (post-prandial) | ||||||
| Bradygastria | 17 (23.0) | 10 (13.5) | 10 (19.2) | 6 (11.5) | 4 (18.2) | 4 (18.2) |
| Normogastria | 33 (44.6) | 45 (60.8) | 26 (50.0) | 37 (71.2)a | 11 (50) | 13 (59.1) |
| Tachygastria | 24 (32.4) | 19 (25.7) | 16 (30.8) | 14 (26.9) | 7 (31.8) | 5 (22.7) |
| Itopide (n = 24) | Mosapride (n = 28) | Levosulpiride (n = 22) | ||||
| Male:female (n) | 5:19 | 10:18 | 11:11 | |||
| Age (range), yr | 49.8 (30-64) | 49.6 (19-70) | 56.6 (39-70) | |||
| Height | 166.1 ± 10.1 | 163.0 ± 11.0 | 167.1 ± 13.2 | |||
| Weight | 60.8 ± 7.7 | 62.7 ± 11.5 | 61.3 ± 10.5 | |||
| Symptom improvement | 18 (75) | 17 (61) | 17 (77) | |||
| Pre-treatment | Post-treatment | Pre-treatment | Post-treatment | Pre-treatment | Post-treatment | |
| Symptom severity score | 7.96 ± 0.84 | 5.00 ± 0.86a | 7.61 ± 0.64 | 5.46 ± 0.79a | 8.86 ± 0.76 | 6.14 ± 0.76a |
| Symptom frequency score | 9.21 ± 0.92 | 6.08 ± 0.99a | 8.86 ± 0.78 | 6.86 ± 0.89 | 9.86 ± 0.83 | 7.55 ± 0.96 |
| Gastric dysrhythmia (pre-prandial) | ||||||
| Bradygastri | 5 (20.8) | 4 (16.7) | 6 (21.4) | 4 (14.3) | 4 (18.2) | 3 (13.6) |
| Normogastria | 8 (33.3) | 17 (70.8)a | 15 (53.6) | 18 (64.3) | 12 (54.5) | 15 (68.2) |
| Tachygastria | 11 (45.8) | 3 (12.6)a | 7 (25) | 6 (21.4) | 6 (27.3) | 4 (18.2 |
| Gastric dysrhythmia (post-prandial) | ||||||
| Bradygastria | 5 (20.8) | 3 (12.5) | 6 (21.4) | 6 (21.4) | 3 (13.6) | 1 (4.5) |
| Normogastria | 11 (45.8) | 14 (58.3) | 12 (42.9) | 16 (57.1) | 14 (63.6) | 15 (68.2) |
| Tachygastria | 8 (33.3) | 7 (29.2) | 10 (35.7) | 6 (21.4) | 5 (22.7) | 6 (27.3) |
The mean symptom severity score for all patients was 8.09 ± 0.43 at baseline vs 5.51 ± 0.46 post-treatment (P < 0.05). Symptom severity scores were significantly decreased in the symptom improvement group, while there were no significant changes in the symptom resistance group. Symptom severity scores were significantly decreased after all prokinetic drugs (Table 2).
The mean symptom frequency score of all patients was 9.27 ± 0.49 at baseline and 6.81 ± 0.54 after treatment (P < 0.05). Symptom frequency scores were significantly decreased in the symptom improvement group, while there were no significant changes in the symptom resistance group. Symptom severity scores were decreased after all prokinetic drugs, but significant differences were shown only in the itopride hydrochloride group (Table 2).
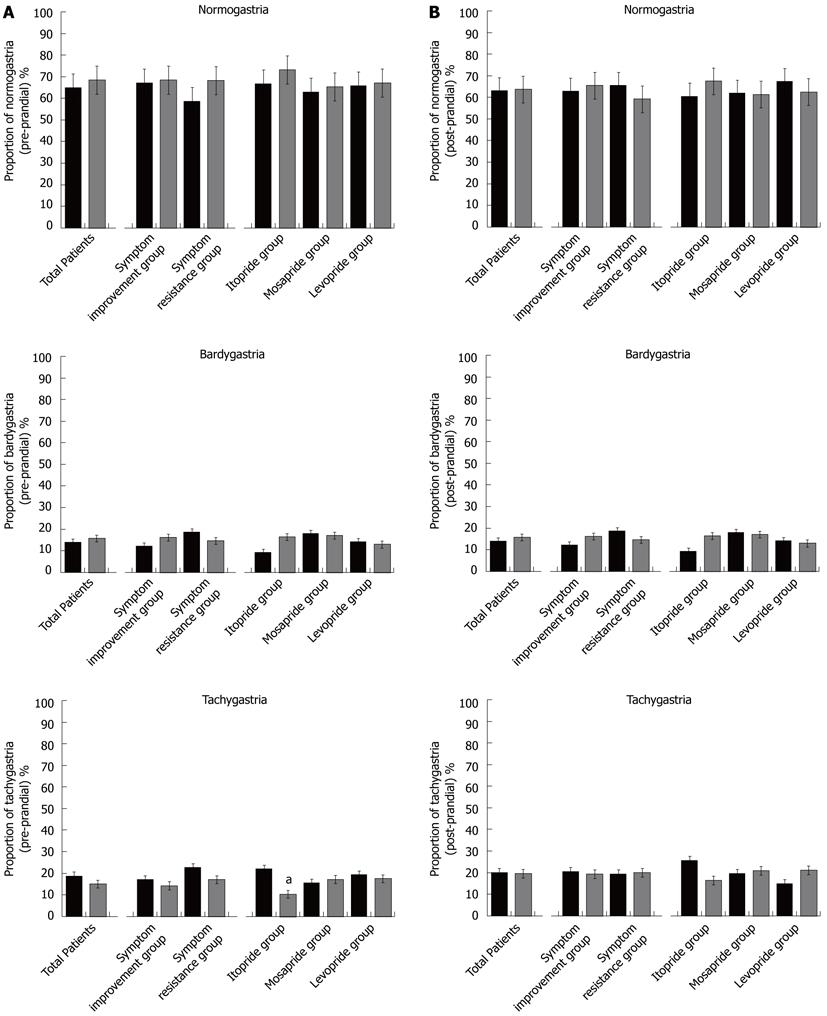
Patients who had gastric dysrhythmia: After prokinetic treatment, the number of patients who had normal gastric slow waves was increased in the symptom improvement group and in the itopride treatment group. In particular, the number of patients who had tachygastria were decreased in the symptom improvement group and in the itopride treatment group (Tables 1 and 2).
Percentage of gastric slow waves: The pre-prandial percentage of gastric slow waves was 64.99% ± 2.93% for normal, 14.01% ± 1.93% for bradygastria, and 18.73% ± 2.24% for tachygastria at pre-treatment (Figure 1A). At the end of the 8-wk treatment, the percentage of pre-prandial gastric slow waves was 68.47% ± 2.54% for normal, 16.12% ± 2.94% for bradygastria, and 15.09% ± 1.71% for tachygastria. Dysrythmia did not show significant changes regardless of symptom improvement. The itopride treatment group showed significant decreases in pre-prandial tachygastria, but there were no significant changes in the mosapride and levosulpiride treatment groups. The percentage of post-prandial gastric slow waves was 63.08% ± 2.25% for normal, 16.52% ± 2.22% for bradygastria and 20.09% ± 2.05% for tachygastria at pre-treatment. At the end of prokinetic treatment, the percentage of post-prandial gastric slow waves was 63.87% ± 2.25% for normal, 16.52% ± 1.92% for bradygastria, and 20.09% ± 19.48% for tachygastria (Figure 1B). There were no significant changes regardless of symptom improvement, nor were there any significant changes among the itopride, mosapride, and levosulpiride treatment groups.
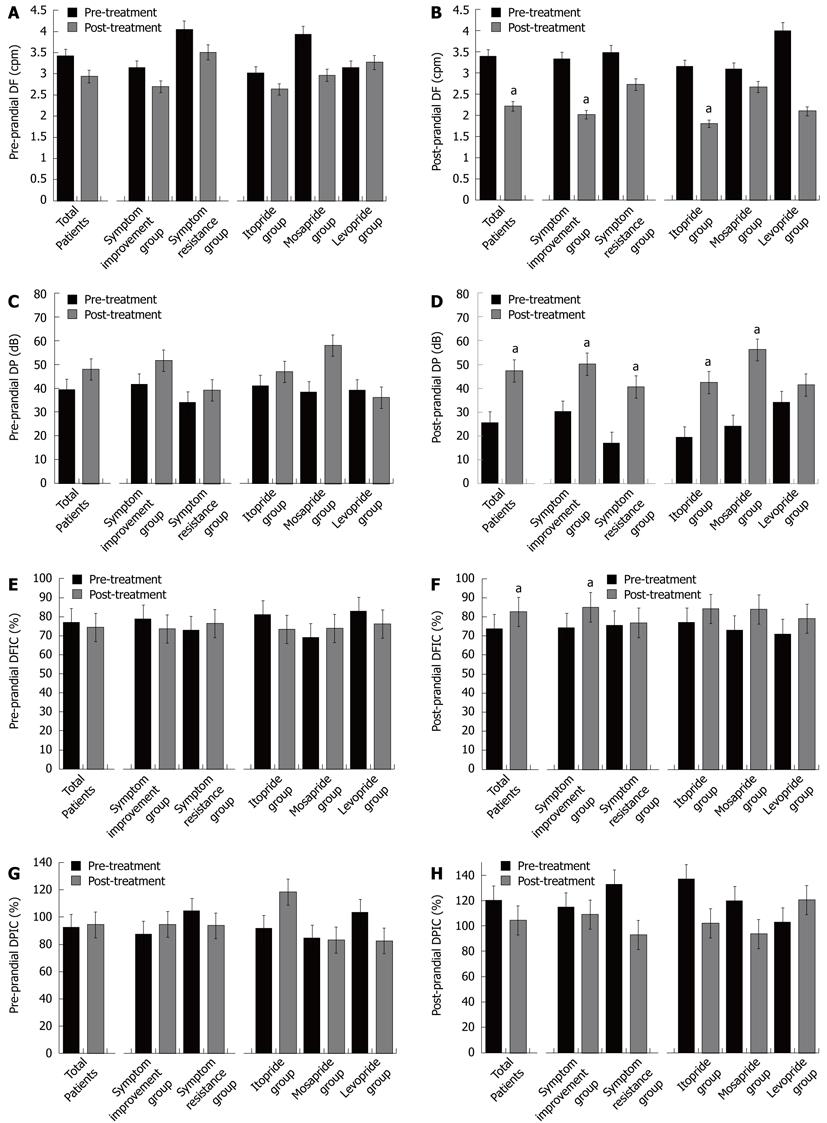
Dominant frequency and dominant power: Pre-prandial DF showed no significant changes regardless of symptom improvement or type of prokinetic drug (Figure 2). Post-prandial DF was decreased after treatment in the symptom improvement group and especially in the itopride treatment group. Pre-prandial DP showed no significant changes regardless of symptom improvement or prokinetic drug after treatment. Post-prandial DP was increased regardless of symptom improvement especially in the itopride group (19.34 ± 6.08 at baseline vs 42.49 ± 6.13 after treatment, P = 0.010) and mosapride group (24.04 ± 6.47 at baseline vs 56.24 ± 11.83 after treatment, P = 0.020).
Dominant frequency instability coefficient and dominant power instability coefficient: Pre-prandial DFIC and DPIC after treatment were not changed regardless of symptom improvement and type of prokinetic drug (Figure 2). Post-prandial DFIC and DPIC were significantly increased after treatment (74.29% ± 24.45% vs 82.69% ± 27.05%, P = 0.035) in the symptom improvement group, but there was no significant differences between the prokinetics.
Power ratio: After treatment, the EGG power ratio was increased in the symptom improvement group (0.64 ± 0.07 vs 1.23 ± 0.16, P = 0.002), especially in the levosulpiride treatment group (Figure 3).
FD is a common clinical syndrome characterized by pain or discomfort in the upper abdomen without any identifiable structural or biochemical abnormality. The pathophysiology of FD involves various mechanisms, including delayed gastric emptying, impaired accommodation in the proximal stomach, and increase duodenal sensitivity to lipid or acid, and pathologic factors include genetic susceptibility, Helicobacter pylori (H. pylori) infection, and psychological factors[13]. There has been no single available therapy for FD due to the heterogeneity of the symptoms and various mechanisms and pathologic factors. Accordingly, a wide variety of treatment methods have been used for FD such as dietary and lifestyle modification, H. pylori eradication, antacids, mucosal protectants, prokinetics, and psychological and complementary therapy[13].
Abnormal gastric motility such as delayed gastric emptying or uncoordinated antral contraction is common in functional dyspepsia[14,15]. Gastrointestinal motor dysfunctions can be assessed by gastric emptying scan and/or manometry, and gastric myoelectrical abnormalities can be detected by noninvasive cutaneous EGG. EGG as a diagnostic technique has been frequently used for the detection of gastric dysrhythmia in patients with nausea, vomiting and other dyspeptic symptoms. Several previous studies have shown a positive correlation between abnormal EGG and delayed gastric emptying[16-18].
The most common abnormal EGG finding is dysrhythmia, low EGG power ratio and high instability coefficient[19-22]. The percentage of patients who had gastric dysrhythmia (percent of normal slow waves < 70%) were 55.4% at pre-prandial and post-prandial periods in our study. This data was similar to previous studies which reported dysrhythmias in 31%-69% of functional dyspepsia cases[4]. However, we did not find any significant difference in the percentage of gastric slow waves between the symptom improvement group and the symptom resistance group after treatment and there were no correlations between gastric dysrhythmia and symptom severity or symptom frequency either. This could be because FD symptoms are caused by different abnormalities, for example, impaired gastric accommodation (vagally and nitrergically mediated mechanisms) may cause symptoms but this has little to do with gastric slow waves[23].
Prokinetics such as cisapride (5-HT4 agonist/weak 5-HT3 antagonist) and domperidone (D2 antagonist) have been shown to improve gastric dysrhythmia in patients with diabetic gastroparesis, whereas low dose erythromycin was reported to have no effects on dysrhythmia[24-27]. Few studies showed that mosapride improved the gastric dysrhythmia and power ratio. In our study, itopride, mosapride and levopride showed improvements in gastric dysrhythmia in the pre-prandial state, but significant differences were shown only with itopride[28,29].
The DF reflects the regularity of gastric slow waves and the DP reflects the amplitude of gastric slow waves. However, the relationship of DF and DP with functional dyspepsia was not clear[30]. Our data showed a decrease in post-prandial DF in the symptom improvement group, and post-prandial DP was high regardless of symptom improvement. Itopride significantly decreased post-prandial DF, and both itopride and mosapride increased post-prandial DP. According to this study, prokinetics might improve the symptoms of FD by improvement in dysrhythmic gastric movement which is represented by decreased DF, and by activating gastric movement which is represented by increased DP.
DPIC increases during antral contractions, and DFIC increases during pregnancy and in patients with gastroesophageal reflux disease. Previous studies showed that pediatric patients who have dyspeptic symptoms reported a high instability coefficient, however, there was not enough data showing the relationship between the DPIC/DFIC and clinical symptoms in FD patients clearly[31-33]. Our data showed increased DPIC/DFIC in the symptom improvement group after prokinetic drug treatment[34]. Increased DPIC/DFIC might be due to the increased variability of changes in gastric movement activated by prokinetics.
The EGG power ratio increases after an appropriate test meal in normal subjects, and decreases in gastroparesis and FD patients[1]. The EGG power ratio increased in responders after prokinetic treatment with itopride and levosulpiride, but not with mosapride in our study. The EGG power ratio is believed to be associated with gastric contractility; the increase in the EGG power ratio observed in this study reflected an increase in gastric contractions. This data is in agreement with previous studies in that prokinetics, especially levosulpiride, increased gastric contractions or gastric emptying[35].
In summary, dysrhythmia was recorded about half of the time in FD patients, and prokinetic treatment successfully improved symptoms. The symptom improvement group showed decreased post-prandial DF and increased post-prandial DP, DFIC/DPIC and power ratio after treatment with prokinetics. Itopride improved gastric dysrhythmia, decreased post-prandial DF, and increased post-prandial DP; mosapride increased post-prandial DP and levosulpiride increased the EGG power ratio.
The mechanism of prokinetics on gastric electrical activity could be (1) to stabilize the gastric slow waves which is represented by an improvement in gastric dysrhythmia and a decrease in post-prandial DF; and (2) to increase gastric motility which is represented by an increase in post-prandial DP and in the EGG power ratio by activating gastric movements which is represented by increased DPIC/DFIC.
In conclusion, the findings of this study suggest that prokinetics could improve the symptoms of FD by regulating gastric myoelectrical activity, and the EGG could be a useful tool to evaluate the effects of various prokinetics.
Electrogastrography (EGG) abnormalities are frequently observed in patients with functional dyspepsia (FD). However, changes in EGG parameters after treatment with prokinetics according to symptom improvement have not been well investigated.
Prokinetic drugs are used in functional dyspepsia to enhance gastrointestinal motility and correct dysrhythmias in FD patients. In this study, the authors observed that prokinetics could improve the symptoms of FD by regulating gastric myoelectrical activity.
Prokinetics successfully improved symptoms of FD, but the improvement did not seem to be correlated with any of the EGG parameters. Instead, there were some unique changes in EGG parameters according to the prokinetic drug. This study suggests that different prokinetics may have different mechanisms of action in regulating gastric myoelectrical activity, and the EGG could be a useful tool in evaluating the effects of various prokinetics.
There was controversy in the significance of EGG as diagnostic tool in FD due to the lack of data and standardized methodology. By understanding the changes in EGG parameters, this study might indicate a future strategy for EGG in evaluating the improvement in FD after prokinetic drug treatment. This study is an important basis for future experiments using EGG in pharmacology.
EGG represents gastric myoelectrical activity. Dysrhythmia (bradygastria, tachygastria) reflect uncoordinated antral contraction, and the power ratio reflects gastric contractions. Dominant frequency reflects the regularity of gastric slow waves and dominant power reflects the amplitude of gastric slow waves. Dominant power instability coefficient increases during antral contractions and, dominant frequency instability coefficient increases during pregnancy.
The authors tried to clarify the relation of EGG and FD symptoms and found the symptom improvement group after prokinetics therapy showed decreased post-prandial dominant frequency and increased dominant frequency instability coefficient/dominant power instability coefficient and increased power ratio.
| 1. | Parkman HP, Hasler WL, Barnett JL, Eaker EY. Electrogastrography: a document prepared by the gastric section of the American Motility Society Clinical GI Motility Testing Task Force. Neurogastroenterol Motil. 2003;15:89-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 211] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 2. | Abell TL, Malagelada JR. Glucagon-evoked gastric dysrhythmias in humans shown by an improved electrogastrographic technique. Gastroenterology. 1985;88:1932-1940. [PubMed] |
| 3. | Brzana RJ, Koch KL, Bingaman S. Gastric myoelectrical activity in patients with gastric outlet obstruction and idiopathic gastroparesis. Am J Gastroenterol. 1998;93:1803-1809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 63] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 4. | Chen J, McCallum RW. Gastric slow wave abnormalities in patients with gastroparesis. Am J Gastroenterol. 1992;87:477-482. [PubMed] |
| 5. | Koch KL. Electrogastrography: physiological basis and clinical application in diabetic gastropathy. Diabetes Technol Ther. 2001;3:51-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 63] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Stern RM, Koch KL, Stewart WR, Lindblad IM. Spectral analysis of tachygastria recorded during motion sickness. Gastroenterology. 1987;92:92-97. [PubMed] |
| 7. | Parkman HP, Harris AD, Miller MA, Fisher RS. Influence of age, gender, and menstrual cycle on the normal electrogastrogram. Am J Gastroenterol. 1996;91:127-133. [PubMed] |
| 8. | Saad RJ, Chey WD. Review article: current and emerging therapies for functional dyspepsia. Aliment Pharmacol Ther. 2006;24:475-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 48] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | Jung IS, Kim JH, Lee HY, Park H, Lee SI. Endoscopic evaluation of gastric emptying and effect of mosapride citrate on gastric emptying. Yonsei Med J. 2010;51:33-38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Pohle T, Domschke W. Gastric function measurements in drug development. Br J Clin Pharmacol. 2003;56:156-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Drossman DA. The functional gastrointestinal disorders and the Rome III process. Gastroenterology. 2006;130:1377-1390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1467] [Cited by in RCA: 1493] [Article Influence: 74.7] [Reference Citation Analysis (1)] |
| 12. | Svedlund J, Sjödin I, Dotevall G. GSRS--a clinical rating scale for gastrointestinal symptoms in patients with irritable bowel syndrome and peptic ulcer disease. Dig Dis Sci. 1988;33:129-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 884] [Cited by in RCA: 1084] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 13. | El-Serag HB, Talley NJ. Systemic review: the prevalence and clinical course of functional dyspepsia. Aliment Pharmacol Ther. 2004;19:643-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 222] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 14. | Tack J, Bisschops R, Sarnelli G. Pathophysiology and treatment of functional dyspepsia. Gastroenterology. 2004;127:1239-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 342] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 15. | Camilleri M, Brown ML, Malagelada JR. Relationship between impaired gastric emptying and abnormal gastrointestinal motility. Gastroenterology. 1986;91:94-99. [PubMed] |
| 16. | Agréus L, Svärdsudd K, Nyrén O, Tibblin G. Irritable bowel syndrome and dyspepsia in the general population: overlap and lack of stability over time. Gastroenterology. 1995;109:671-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 459] [Cited by in RCA: 451] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 17. | Chen JD, Lin Z, Pan J, McCallum RW. Abnormal gastric myoelectrical activity and delayed gastric emptying in patients with symptoms suggestive of gastroparesis. Dig Dis Sci. 1996;41:1538-1545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 194] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 18. | Parkman HP, Miller MA, Trate D, Knight LC, Urbain JL, Maurer AH, Fisher RS. Electrogastrography and gastric emptying scintigraphy are complementary for assessment of dyspepsia. J Clin Gastroenterol. 1997;24:214-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 70] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 19. | Levanon D, Zhang M, Chen JD. Efficiency and efficacy of the electrogastrogram. Dig Dis Sci. 1998;43:1023-1030. [PubMed] |
| 20. | Lin X, Chen JZ. Abnormal gastric slow waves in patients with functional dyspepsia assessed by multichannel electrogastrography. Am J Physiol Gastrointest Liver Physiol. 2001;280:G1370-G1375. [PubMed] |
| 21. | Koch KL, Hong SP, Xu L. Reproducibility of gastric myoelectrical activity and the water load test in patients with dysmotility-like dyspepsia symptoms and in control subjects. J Clin Gastroenterol. 2000;31:125-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 110] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 22. | Lu CL, Chen CY, Chang FY, Kang LJ, Lee SD, Wu HC, Kuo TS. Impaired postprandial gastric myoelectrical activity in Chinese patients with nonulcer dyspepsia. Dig Dis Sci. 2001;46:242-249. [PubMed] |
| 23. | Kindt S, Tack J. Impaired gastric accommodation and its role in dyspepsia. Gut. 2006;55:1685-1691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 168] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 24. | Chang CS, Lien HC, Yeh HZ, Poon SK, Tung CF, Chen GH. Effect of cisapride on gastric dysrhythmia and emptying of indigestible solids in type-II diabetic patients. Scand J Gastroenterol. 1998;33:600-604. [PubMed] |
| 25. | Orr WC, Zhang M, McClanahan J, Sloan S, Chen JD. Gastric myoelectric activity in older adults treated with cisapride for gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2000;14:337-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Koch KL, Stern RM, Stewart WR, Vasey MW. Gastric emptying and gastric myoelectrical activity in patients with diabetic gastroparesis: effect of long-term domperidone treatment. Am J Gastroenterol. 1989;84:1069-1075. [PubMed] |
| 27. | Chen JD, Lin ZY, Edmunds MC, McCallum RW. Effects of octreotide and erythromycin on gastric myoelectrical and motor activities in patients with gastroparesis. Dig Dis Sci. 1998;43:80-89. [PubMed] |
| 28. | Kamiya T, Adachi H, Hirako M, Shikano M, Matsuhisa E, Wada T, Ogasawara N, Nojiri S, Kataoka H, Sasaki M. Impaired gastric motility and its relationship to reflux symptoms in patients with nonerosive gastroesophageal reflux disease. J Gastroenterol. 2009;44:183-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 29. | Endo J, Nomura M, Morishita S, Uemura N, Inoue S, Kishi S, Kawaguchi R, Iga A, Ito S, Nakaya Y. Influence of mosapride citrate on gastric motility and autonomic nervous function: evaluation by spectral analyses of heart rate and blood pressure variabilities, and by electrogastrography. J Gastroenterol. 2002;37:888-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 30. | Stevens JE, Russo A, Maddox AF, Rayner CK, Phillips L, Talley NJ, Giguère M, Horowitz M, Jones KL. Effect of itopride on gastric emptying in longstanding diabetes mellitus. Neurogastroenterol Motil. 2008;20:456-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 31. | Chen JD, Richards RD, McCallum RW. Identification of gastric contractions from the cutaneous electrogastrogram. Am J Gastroenterol. 1994;89:79-85. [PubMed] |
| 32. | Riezzo G, Pezzolla F, Darconza G, Giorgio I. Gastric myoelectrical activity in the first trimester of pregnancy: a cutaneous electrogastrographic study. Am J Gastroenterol. 1992;87:702-707. [PubMed] |
| 33. | Janssens J, Peeters TL, Vantrappen G, Tack J, Urbain JL, De Roo M, Muls E, Bouillon R. Improvement of gastric emptying in diabetic gastroparesis by erythromycin. Preliminary studies. N Engl J Med. 1990;322:1028-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 566] [Cited by in RCA: 503] [Article Influence: 14.0] [Reference Citation Analysis (2)] |
| 34. | Chen JD, Lin X, Zhang M, Torres-Pinedo RB, Orr WC. Gastric myoelectrical activity in healthy children and children with functional dyspepsia. Dig Dis Sci. 1998;43:2384-2391. [PubMed] |
| 35. | Levanon D, Zhang M, Orr WC, Chen JD. Effects of meal volume and composition on gastric myoelectrical activity. Am J Physiol. 1998;274:G430-G434. [PubMed] |
Peer reviewers: Julio Horacio Carri, Professor, Department of Gastroenterology, Internal Medicine, Universidad Nacional de Córdoba, Av. Estrada 160, 5000 Córdoba, Argentina; Nageshwar Duvvuru Reddy, Professor, Department of Gastroenterology, Asian Institute of Gastroenterology, A-27, Journalist Colony, Jubilee Hillshyderabad, Hyderabad 500033, India; Dr. Tomoyuki Shibata, Department of Gastroenterology, Fujita Health University, 1-98 Dengakugakubo, Kutsukake-cho, Toyoake 470-1192, Japan
S- Editor Gou SX L- Editor Cant MR E- Editor Zhang DN