Published online Oct 28, 2012. doi: 10.3748/wjg.v18.i40.5719
Revised: September 4, 2012
Accepted: September 12, 2012
Published online: October 28, 2012
AIM: To determine whether the carbon monoxide (CO)-releasing molecules (CORM)-liberated CO suppress inflammatory responses in the small intestine of septic mice.
METHODS: The C57BL/6 mice (male, n = 36; weight 20 ± 2 g) were assigned to four groups in three respective experiments. Sepsis in mice was induced by cecal ligation and puncture (CLP) (24 h). Tricarbonyldichlororuthenium (II) dimer (CORM-2) (8 mg/kg, i.v.) was administrated immediately after induction of CLP. The levels of inflammatory cytokines [interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α)] in tissue homogenates were measured with enzyme-linked immunosorbent assay. The levels of malondialdehyde (MDA) in the tissues were determined. The levels of nitric oxide (NO) in tissue homogenate were measured and the expression levels of intercellular adhesion molecule 1 (ICAM-1) and inducible nitric oxide synthase (iNOS) in the small intestine were also assessed. NO and IL-8 levels in the supernatants were determined after the human adenocarcinoma cell line Caco-2 was stimulated by lipopolysaccharide (LPS) (10 g/mL) for 4 h in vitro.
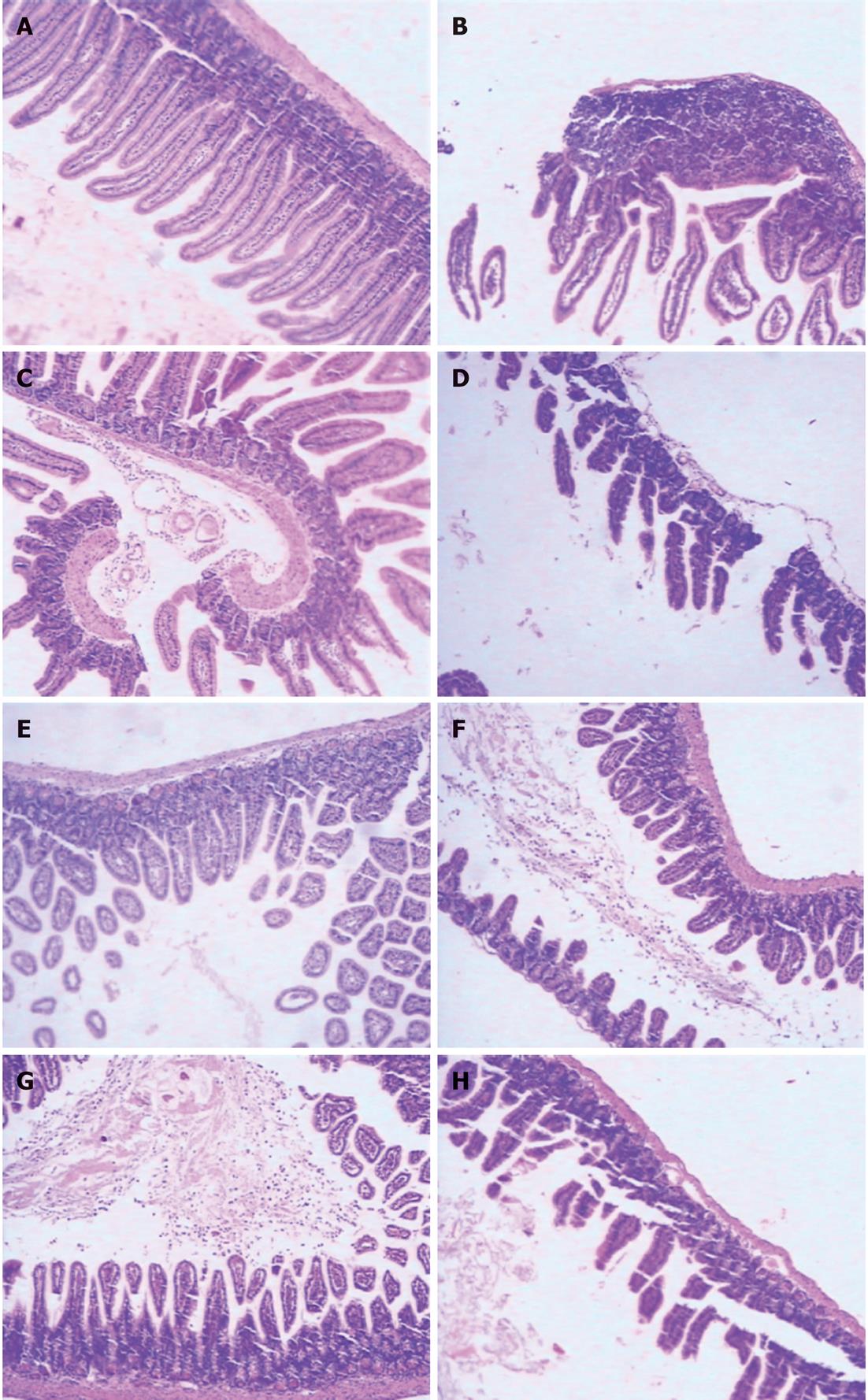
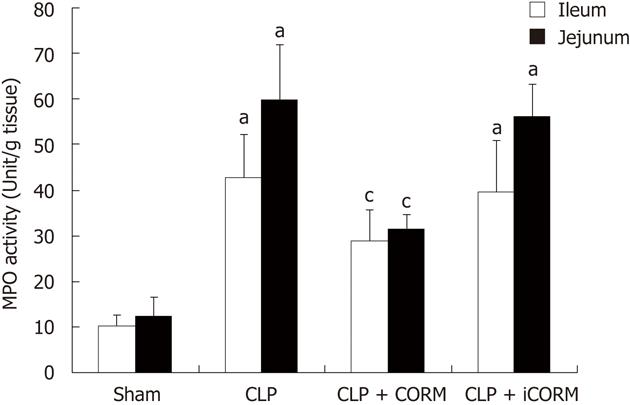
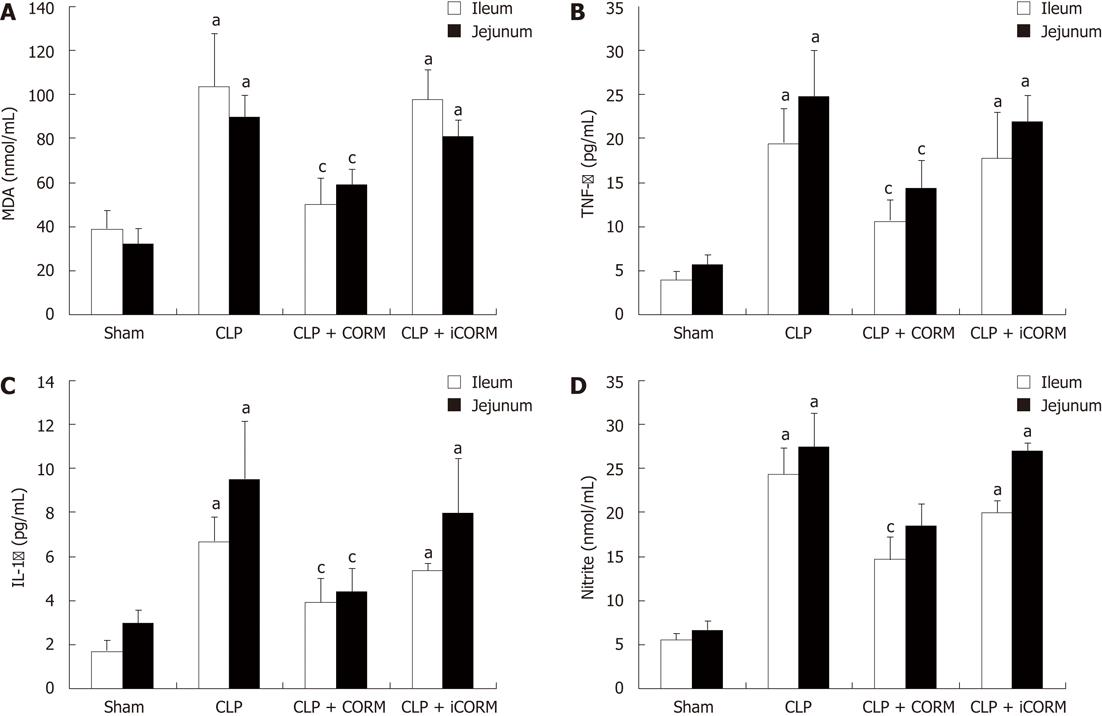
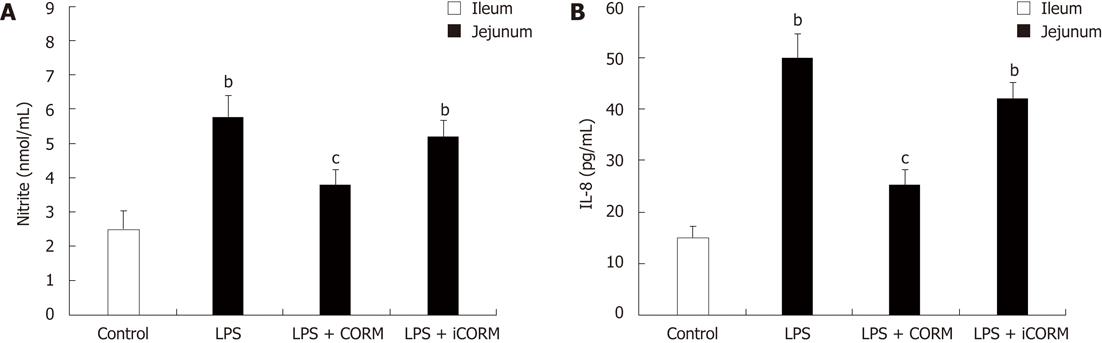
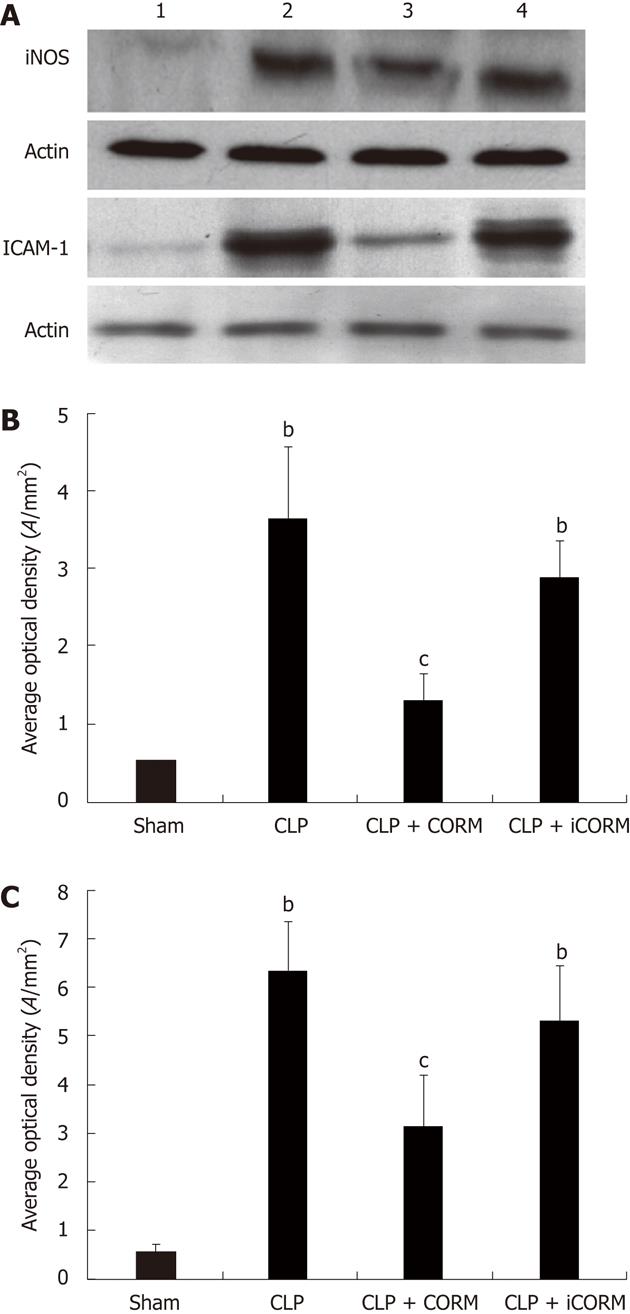
RESULTS: At 24 h after CLP, histological analysis showed that the ileum and jejunum from CLP mice induced severe edema and sloughing of the villous tips, as well as infiltration of inflammatory cells into the mucosa. Semi-quantitative analysis of histological samples of ileum and jejunum showed that granulocyte infiltration in the septic mice was significantly increased compared to that in the sham group. Administration of CORM-2 significantly decreased granulocyte infiltration. At 24 h after CLP, the tissue MDA levels in the mid-ileum and mid-jejunum significantly increased compared to the sham animals (103.68 ± 23.88 nmol/mL vs 39.66 ± 8.23 nmol/mL, 89.66 ± 9.98 nmol/mL vs 32.32 ± 7.43 nmol/mL, P < 0.01). In vitro administration of CORM-2, tissue MDA levels were significantly decreased (50.65 ± 11.46 nmol/mL, 59.32 ± 6.62 nmol/mL, P < 0.05). Meanwhile, the tissue IL-1β and TNF-α levels in the mid-ileum significantly increased compared to the sham animals (6.66 ± 1.09 pg/mL vs 1.67 ± 0.45 pg/mL, 19.34 ± 3.99 pg/mL vs 3.98 ± 0.87 pg/mL, P < 0.01). In vitro administration of CORM-2, tissue IL-1β and TNF-α levels were significantly decreased (3.87 ± 1.08 pg/mL, 10.45 ± 2.48 pg/mL, P < 0.05). The levels of NO in mid-ileum and mid-jejunum tissue homogenate were also decreased (14.69 ± 2.45 nmol/mL vs 24.36 ± 2.97 nmol/mL, 18.47 ± 2.47 nmol/mL vs 27.33 ± 3.87 nmol/mL, P < 0.05). The expression of iNOS and ICAM-1 in the mid-ileum of septic mice at 24 h after CLP induction significantly increased compared to the sham animals. In vitro administration of CORM-2, expression of iNOS and ICAM-1 were significantly decreased. In parallel, the levels of NO and IL-8 in the supernatants of Caco-2 stimulated by LPS was markedly decreased in CORM-2-treated Caco-2 cells (2.22 ± 0.12 nmol/mL vs 6.25 ± 1.69 nmol/mL, 24.97 ± 3.01 pg/mL vs 49.45 ± 5.11 pg/mL, P < 0.05).
CONCLUSION: CORM-released CO attenuates the inflammatory cytokine production (IL-1β and TNF-α), and suppress the oxidative stress in the small intestine during sepsis by interfering with protein expression of ICAM-1 and iNOS.
- Citation: Wang X, Cao J, Sun BW, Liu DD, Liang F, Gao L. Exogenous carbon monoxide attenuates inflammatory responses in the small intestine of septic mice. World J Gastroenterol 2012; 18(40): 5719-5728
- URL: https://www.wjgnet.com/1007-9327/full/v18/i40/5719.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i40.5719
Sepsis is a complex clinical syndrome resulting from a harmful host inflammatory response to infection. Despite advancements in understanding the pathophysiology of sepsis, clinical outcomes are variable and the mortality rate remains high among the patients[1]. Cecal ligation and puncture (CLP) may induce the activation of an inflammatory cascade, leading to sepsis and multiple organ failure[2]. Some reports have indicated that the inflammatory response syndrome, which contributes to oxidative cell/tissue damage, might frequently be accompanied by leukocyte sequestration in many important organ systems in the body[3]. The increase of production of pro-inflammatory mediators such as interleukin (IL)-1β and tumor necrosis factor-α (TNF-α) is closely associated with activation of leukocytes and macrophages which were sequestrated in the tissue[4,5]. Leukocyte sequestration and its subsequent infiltration in organ tissue can cause leukocyte activation and contribute to vascular damage and the development of systemic inflammatory reaction. As the prerequisite, activation of leukocytes and endothelial cells results in aggregation of leukocytes, platelets and erythrocytes in vitro. This may favor disseminated intravasal coagulation and further multiple organ failure.
It has been shown that endogenous carbon monoxide (CO), a bi-product of inducible heme oxygenase (HO-1) can modulates inflammation. Recent studies suggest that exogenously administered CO inhibits lipopolysaccharide (LPS)-induced production of cytokines both in vivo and in vitro, and consequently exhibits an important cytoprotective function and anti-inflammatory properties which benefit for the resolution of acute inflammation[6].
Recently, transitional metal carbonyls have been identified as potential CO-releasing molecules (CORMs) with the potential to facilitate the pharmaceutical use of CO by delivering it to tissues and organs[7]. Our previous studies[8-14] firstly confirmed that CORM-released CO attenuated leukocytes sequestration in the liver, lung and small intestine of burned mice by interfering with nuclear factor-κB (NF-κB) activation, protein expression of intercellular adhesion molecule-1 (ICAM-1) and therefore suppressing endothelial cells pro-adhesive phenotype. However, to date, little is known if CORM-released CO can down-regulate the inflammatory production and oxidative stress in the small intestine of the septic mice. This study aims to investigate the protective effects and the underlying mechanisms of tricarbonyldichlororuthenium (II) dimer (CORM-2), one of the novel groups of CORMs, on the suppression of inflammatory cytokine production and malondialdehyde (MDA) levels as an indicator of oxidative stress index in the small intestine of septic mice induced by CLP.
CORM-2 was obtained from Sigma Aldrich and solubilized in dimethyl sulfoxide (DMSO) to obtain a 10 mmol/L stock. Inactive form of the compound (negative control) was also used in some experiments and it was prepared as follows: CORM-2 was "inactivated" (iCORM-2) by adding the compound to DMSO and kept for 18 h at 37 °C in a 5% CO2 humidified atmosphere to liberate CO. The iCORM-2 solution was finally bubbled with nitrogen to remove the residual CO present in the solution. Cell culture reagents were obtained from Gibco (Grand Island, NY) and culture supplies from Corning (Corning, NY) and Falcon (Lincoln Park, NJ). Polyclonal antibody against ICAM-1 and inducible nitric oxide synthase (iNOS) was purchased from Santa Cruz Biotechnology Inc., United States. All the other chemicals were of reagent grade and obtained from Sigma unless otherwise stated.
The C57BL/6 mice (male, n = 36; body weight 20 ± 2 g) were fed a standard laboratory diet and water ad libitum. Mice were assigned to four groups in three respective experiments. In each experiment, mice in sham group (n = 9) were underwent sham procedure, whereas mice in CLP group (n = 9) received cecal ligation and puncture, mice in CORM-2 group (n = 9) and iCORM group (n = 9) subjected to the same injury with immediate administration of CORM-2 (8 mg/kg, i.v.) and iCORM-2 (8 mg/kg, i.v.), respectively. The concentration of CORM-2 used in the present study was based on a previous report in of the use of this compound in mice[15] and the preliminary experiments in our lab[8,9]. The experimental protocol was approved by the Council on Animal Care at Jiangsu University on the Protection and the Welfare of Animals and followed the National Institutes of Health guidelines for the care and use of experimental animals.
Mice were anesthetized with 2% isoflurane in oxygen via a facemask. A 2-cm midline incision was made through the abdominal wall; the cecum was identified and ligated with a 3-0 silk tie 1 cm from the tip. Care was taken not to cause bowel obstruction. A single puncture of the cecal wall was performed with a 20-gauge needle. The cecum was lightly squeezed to express a small amount of stool from the puncture site to assure a full-thickness perforation. Great care was taken to preserve the continuity of flow between the small and large bowels. The cecum was returned to the abdominal cavity, and the incision was closed with surgiclips. Sham mice underwent midline laparotomy under anesthesia; the cecum was exteriorized and returned to the abdomen, and the incision was closed with surgiclips. The animals were sacrificed at 24 h after experimental manipulation.
The mid-ileum and mid-jejunum specimens harvested from different groups of animals were immersed in 4% formaldehyde solution at 24 h after CLP. The tissue was embedded in paraffin wax, serially sectioned, and stained with hematoxylin-eosin. Tissue morphologic characteristics were evaluated under light microscope. Ileum and jejunum tissues were evaluated for density of granulocytes and degree of hydropic degeneration. Tissues were evaluated in a semi-quantitatively manner by two experienced independent examiners who were blinded to the experimental groups. A scoring system was used for each item using 0 up to 2 points for the different states of organ damage (with 2 being most granulocytes, edema and degeneration). Afterwards, the mean ± SE of each item was calculated.
The intestine was exposed at 24 h after CLP. Retaining approximately the first 5 cm-long proximal segment of intestine, 3 cm-long segments of jejunum and ileum were removed, cleaned, and snap-frozen in liquid nitrogen. The samples were stored at -70 °C. Equal weights (100 mg wet weight) of intestine from various groups were suspended in 1 mL phosphate buffered saline and sonicated on ice (30 cycles, twice for 30 s)[8,9]. Homogenates were cleared by centrifuging at 12 000 r/min at 4 °C, and the supernatants were stored at -70 °C. Protein levels in the homogenates were determined using the Bio-Rad (Hercules, CA) assay kit.
The levels of the inflammatory cytokines IL-1β and TNF-α in ileum and jejunum tissue homogenates were measured using enzyme-linked immunosorbent assay kits (R and D Systems, Minneapolis, MN) following the manufacturers instructions.
Caco-2, the human adenocarcinoma cell line, was obtained from American Tissue Culture Collection (ATCC, Rockville, MD, United States). The cells were passaged and grown in Dulbecco’s modified Egle’s medium (DMEM) supplemented with 1% nonessential amino acids, 10% fetal calf serum, 1 mg/L streptomycin, and 1000 U/L penicillin (Complete DMEM). Experiments were performed with cell monolayers that were in culture for 21-25 d. Cells were seeded in the collagen-coated 48-well plates with the same cell density. Medium was replaced every day when each experiment began. Cells were cultured at 37 °C, 5% CO2, and 90% related humidity. The supernatants of Caco-2 cell stimulated by LPS (10 g/mL) for 4 h were collected for nitric oxide (NO) and IL-8 levels measurement.
NO was measured as described previously[11]. Briefly, 100 L of supernatants was removed and incubated with 100 L of Griess reagent (10 g/L sulfanilamide and 1 g/L N-1-naphthylethylenediamine dihydrochloride in 25 mL/L phosphoric acid) in a 96-well plate. The plate was incubated for 10 min at room temperature. Nitrite production was quantified spectrophotometrically using an automated colorimetric procedure. Absorbance at 540 nm was measured using a microplate reader (Bio-Tek, United States). The nitrite concentration was calculated by comparing samples with standard solutions of sodium nitrite produced in the culture medium. All samples were assayed in triplicate.
MDA formation was utilized as a lipoperoxidation index. The levels of MDA in the small intestines were determined as described by Sun et al[10]. Briefly, tissue was homogenized in 1.15% KCl solution (1:10 volume). An aliquot (0.1 mL) of the homogenate was added to a reaction mixture [200 L of 8.1% SDS, 1500 L of 20% acetic acid (pH 3.5), 1500 L of 0.8% thiobarbituric acid, and 700 L distilled water]. Samples were then boiled for 1 h at 95 °C and centrifuged at 3000 ×g for 10 min. The absorbance of the supernatant was measured by spectrophotometry at 532 nm (Jenway, Mod. 6300, Dunmow, Essex, United Kingdom). Data were expressed in nmol per milligram of wet tissue.
Myeloperoxidase (MPO) activity was measured according to the established method[8,9]. Briefly, tissue was homogenized in 0.5 mL of 50 mmol/L potassium phosphate buffer (pH 7.4) and centrifuged at 10 000 ×g at 4 °C for 30 min. The remaining pellet was resuspended in 0.5 mL of 50 mmol/L potassium buffer at pH 6.0 with 0.5% hexadecyltrimethylammonium bromide, sonicated on ice, and then centrifuged at 12 000 ×g at 4 °C for 10 min. Supernatants were then assayed at a 1:20 dilution in reaction buffer containing 50 mmol/L phosphate buffer, 530 mmol/L o-dianisidine, and 20 mmol/L H2O2 solution. One unit of enzyme activity was defined as the amount of MPO that caused a change in absorbance measured at 460 nm for 3 min. MPO activity was expressed as U/g tissue.
Tissues were homogenized for extract preparations in ice-cold mild lysis buffer, containing 1% Nonidet P-40, 0.15 mol/L NaCl, 0.01 mol/L sodium phosphate (pH 7.2), 2 mmol/L ethylene diamine tetraacetic acid, 50 mmol/L sodium fluoride, 0.2 mmol/L sodium vanadate, and 1 g/mL of aprotinin. The tissue homogenates were centrifuged at 20 000 ×g for 15 min and supernatants were collected. Sodium dodecyl sulphate-polyacrylamide gel electrophoresis was performed on an equivalent amount of protein samples using precast 7% resolving/4% stacking Tris-HCl gels (Bio-Rad, Hercules, CA). Separated proteins were then transferred to polyvinylidene difluoride membranes (Amersham Pharmacia Biotech, Inc., Piscataway, NJ). Membranes were blocked in 5% nonfat milk in TBS buffer containing 0.1% Tween 20 (TBST) for 1 h at room temperature. Blocked membranes were incubated in primary antibody specific for mouse iNOS and ICAM-1 at a concentration of 1:2000 and 1:5000, respectively in TBST overnight at 4 °C. Then the membranes were washed and probed with horseradish peroxidase-conjugated secondary antibody (Amersham Pharmacia Biotech, Inc., Piscataway, NJ) for 1 h at room temperature. Chemiluminescence detection was performed with the Amersham enhanced chemiluminescence detection kit according to the manufacturer’s instructions. To ensure a similar amount of protein in each sample, the membranes were "stripped off", reprobed with actin, developed with horseradish peroxidase-conjugated secondary antibody, and visualized by enhanced chemiluminescence.
All the values were presented as mean ± SE. Statistical analysis was performed using the analysis of variance and Student’s t-test for the comparisons. P < 0.05 was considered to be statistically significant.
Histological analysis showed that the ileum and jejunum from sham mice had the normal architecture of the intestinal epithelium and wall, while CLP induced severe edema and sloughing of the villous tips, as well as infiltration of inflammatory cells into the mucosa. Semi-quantitative analysis of histological samples of ileum and jejunum showed that the granulocyte infiltration in the septic mice was significantly increased compared with that in the sham group. Administration of CORM-2 (8 mg/kg, i.v.), significantly decreased granulocyte infiltration. However, CORM-2 did not improve the hydropic degeneration induced by sepsis in either the ileum or jejunum (Figure 1 and Table 1).
To determine whether the CLP-induced increase in polymorphonuclear neutrophil (PMN) accumulation in the small intestine was effectively prevented by CORM-2, the activity of MPO, an enzyme in azurophilic granules of neutrophils, was assessed. Extracts of the ileum and jejunum samples were examined for content of MPO 24 h after sepsis. The mean MPO levels are shown in Figure 2. MPO activity in organs obtained from septic mice was markedly increased compared with that in the sham group (P < 0.01), while it was significantly decreased by treatment with CORM-2 (P < 0.05).
Tissue MDA levels are considered important markers of lipoperoxidation associated to oxidative stress. The mean MDA levels detected in the mid-ileum and mid-jejunum of mice were significantly affected by CLP injury. At 24 h after CLP, the tissue MDA levels in the mid-ileum and mid-jejunum significantly increased compared with the sham animals. After in vitro administration of CORM-2 (8 mg/kg, i.v.), tissue MDA levels were significantly decreased (Figure 3A).
As shown in Figure 3A, at 24 h after CLP, the expression of TNF-α and IL-1 in mid-ileum and mid-jejunum homogenates of CLP-challenged mice was markedly increased compared with the sham mice. After administration of CORM-2, the elevation levels of TNF-α and IL-1β in tissue homogenates were significantly diminished (Figure 3B and C).
As shown in Figure 3D, production of nitrite was low in sham group. After CLP challenge, nitrite levels in tissue homogenates were significantly increased (P < 0.05 vs sham group). However, nitrite levels were markedly decreased in the CORM-2 group compared with the CLP group (P < 0.05).
Production of IL-8 and nitrite was low in the control Caco-2 cells. After stimulation of LPS, IL-8 and nitrite levels were significantly increased (P < 0.05 vs control group). CORM-2 significantly reduced IL-8 and nitrite generation in the LPS-stimulated Caco-2 cells (P < 0.05 vs CLP group, Figure 4).
The expression of iNOS and ICAM-1 in the mid-ileum of septic mice at 24 h after CLP induction was significantly enhanced compared with the sham animals. After in vitro administration of CORM-2 (8 mg/kg, i.v.), the expression of iNOS and ICAM-1 was significantly decreased (Figure 5).
Sepsis, which frequently occurs after hemorrhage, trauma, burn, or abdominal surgery, continues to be a clinical challenge in clinic as a leading cause of morbidity and mortality in severely ill patients[1,16-19]. Despite the therapeutic strategies focusing on local host defenses and the inhibition of overwhelming inflammation response, little progress has been achieved. CLP may induce the activation of an inflammatory cascade, and cause damage to multiple organs, leading to sepsis and multiple organ failure. This model seems to resemble qualitatively as well as quantitatively the clinical observations of vascular reactivity and inflammation in the course of polymicrobial peritonitis, bacteremia, and systemic sepsis[20-23]. Therefore, this study aims to evaluate the possible role of CORM-derived CO in CLP-induced sepsis.
During sepsis, the most frequent complications within the gastrointestinal tract are small intestinal and mucosal barrier dysfunctions. Our previous data indicated that the intestine is one of the tissues most sensitive to the ischemia and reperfusion (I/R) induced by thermal injury[8-10], and a novel metal carbonyl-based compound (CORM-2) which exerts a protective effect against the pathological changes caused by thermal injury of the small intestine. In this study, we employed the same compound to determine whether it suppresses inflammatory cytokine production and oxidative stress in the small intestine of the septic mice.
Leukocyte sequestration and its subsequent infiltration in tissues can cause leukocyte activation and contribute to vascular damage and the development of systemic inflammatory reaction. MPO is an enzyme that is found predominantly in the azurophilic granules of PMN. Tissue MPO activity is frequently utilized to estimate tissue PMN accumulation in damaged tissues and correlates significantly with the number of PMN determined histochemically in tissues[24-26]. In the present study, we found that MPO activities in small intestine were markedly enhanced after CLP and in vitro administration of CORM-2 resulted in significantly downregulation of MPO activity. The direct cause of leukocytes sequestration after CLP is considered to be the higher expression of adhesion molecules (ICAM-1). ICAM-1 activates leukocytes and endothelial cells, which in turn prompt the release of various inflammatory mediators, resulting in systemic inflammatory response syndrome, acute respiratory distress syndrome and multiorgan dysfunction syndrome[27]. At 24 h after CLP, the expression of ICAM-1 in small intestine was markedly upregulated. In vitro administration of CORM-2 inhibited the upregulation of ICAM-1 induced by CLP. These findings indicated that CORM-2 can effectively prevent PMN chemotaxis and infiltration in the tissues after CLP, consequently decreasing the production of oxidants and reducing tissue oxidative injury.
Pro-inflammatory cytokines, such as TNF-α and IL-1 have been shown to be released early after an inflammatory stimulus. TNF-α is a pleiotropic cytokine with strong proinflammatory and immunomodulatory properties, and plays a critical role in inflammation and inflammatory bowel disease[28,29]. Various strategies have been explored to inhibit TNF-α. To confirm if prevention of sepsis-induced intestinal dysmotility by CORM-2 was partly through interruption of the cycle of inflammatory events in the local intestine, we investigated the expression of inflammatory cytokines TNF-α in the small intestine of the septic mice. We observed marked increases in TNF-α levels in the tissue homogenates of ileum and jejunum after CLP injury. In vitro administration of CORM-2 was able to inhibit the inflammatory production in enteric tissue induced by CLP. Our findings strongly indicated that CORM-2 appears to inhibit upregulation of inflammatory production, and consequently might effectively decrease inflammatory response in the small intestine induced by CLP. Similarly, another important cytokine, IL-1, was also found to be markedly upregulated in ileum and jejunum of septic mice. Downregulation of IL-1 levels was most remarkable in the small intestine of septic mice treated with CORM-2. These findings demonstrate that treatment with CORM-2 suppresses the production of IL-1 and TNF-α, and subsequently attenuates the inflammatory response induced by CLP in the small intestine.
Tissue MDA content, the last product of lipid breakdown caused by oxidative stress, is considered to be a good indicator of radical-induced lipid peroxidation[29]. Increased lipid peroxidation in the small intestine of animals with intestinal I/R, was evidenced by significantly increased MDA levels[30]. In the present study, intestinal MDA levels in CLP groups increased markedly, suggesting that significant increase of oxidative stress occurs at 24 h after experimental manipulation. In vitro administration of CORM-2 led to the significantly downregulation of the mean MDA levels in the small intestine of septic mice. This indicated that CORM-2 effectively prevents lipid peroxidation in the small intestine after CLP, consequently decreasing the production of oxidants and reducing the tissue oxidative injury, which contributes to the bowel functional damage to the bowel.
Sepsis alters the concentrations of NO, an inflammatory factor, in plasma and endothelial cells[31]. NO is produced by various types of cells such as macrophages, cardiac myocytes, and vascular smooth muscle and glial cells in response to endotoxin and other inflammatory stimuli. Although it is suggested that NO has a counter-inflammatory activity acting on the cells of the adaptive immune system, in particular, T cells, many studies have well established that NO is indeed a proinflammatory mediator, overproduction of NO plays a major role in the pathophysiology of septic shock, and induction of NOS with consequent excessive NO formation has been proposed as a major factor in pathologic vasodilatation and tissue damage. As an intercellular signaling factor, NO potentially leads to the continuous formation of peroxynitrite. Peroxynitrite damages the organs possibly through lipid peroxidation and/or nitration of cell membrane proteins.
Previously, we demonstrated that thermal injury induced lung neutrophil deposition, lung iNOS expression, and lung damage[9]. We have also shown that NO from iNOS regulated proinflammatory activation, gene expression, and tissue injury in the liver after thermal injury, and CORM-2 inhibited the expression of iNOS in liver tissues, reducing liver injury and tissue PMN infiltration in thermally injured mice[8]. In this study, we measured NO production and expression levels of iNOS in tissue homogenates of the ileum and jejunum of CLP mice following resuscitation to determine whether iNOS was also generated at this site. There was a significant increase of intestinal mucosal iNOS activity within the base of intestinal villi following CLP, while NO production also markedly increased, suggesting that peroxynitrite plays a vital role in CLP-induced intestinal damage. The production of NO and expression of iNOS were significantly inhibited by in vitro administration of CORM-2. In parallel, the in vitro experiments showed that LPS caused a significant increase of NO production in Caco-2 cells, which was prevented effectively with CORM-2 treatment. These data showed that CORM-2 exhibits, at least partly, an important role in inhibiting iNOS expression, subsequently downregulating the NO production, and attenuating the oxidative stress and tissue damage. NF-κB family members control transcriptional activity of various promoters of proinflammatory cytokines, cellsurface receptors, transcription factors, and adhesion molecules that are involved in intestinal inflammation. Previously, using a thermal injury model in mice, we have shown that CORM-2 plays a pivotal role in inhibition of NF-κB activity in the liver, which subsequently decreases hepatocellular secretion of inflammatory cytokines and burn-related hepatic dysfunction[11]. In this study, NF-κB activity in mid-ileum was elevated by CLP, while it was markedly inhibited by administration of CORM-2 (data not shown). These results show that CORM-2 plays, at least partly, an important role in inhibition of NF-κB activity in the small intestine.
In conclusion, the data presented in this study suggest a protective role of CORM-2, one of the novel CORMs, in the small intestine of the septic mice. The potential mechanism of this beneficial effect of CORM-2 appears to suppress oxidative stress, and decrease the production of IL-1 and TNF-α. This was accompanied by a decrease of NO production and protein expression of ICAM-1 and iNOS, thus suppressing the tissue damage in the small intestine. However, the therapeutic potential of anti-inflammation or anti-oxidative stress strategies in this setting should be further validated by future studies.
Sepsis is a complex clinical syndrome resulting from a harmful host inflammatory response to infection. Cecal ligation and puncture (CLP) may induce the activation of an inflammatory cascade, and may lead to sepsis and multiple organ failure. There have been several reports indicating that the inflammatory response syndrome, which contributes to oxidative cell/tissue damage, might frequently be accompanied by leukocyte sequestration in many important organ systems in the body. The increase of production of pro-inflammatory mediators such as interleukin (IL)-1β and tumor necrosis factor-α (TNF-α) is closely associated with activation of leukocytes and macrophages which were sequestrated in the tissue. During sepsis, the most frequent complications within the gastrointestinal tract are small intestine and mucosal barrier dysfunction. Their previous data indicated that the intestine is one of the most sensitive tissues to ischemia and reperfusion induced by thermal injury.
Sepsis, which frequently occurs after hemorrhage, trauma, burn, or abdominal surgery, continues to be a challenge in clinic as a leading cause of morbidity and mortality in severely ill patients. Despite therapeutic strategies focus on local host defenses and the inhibition of overwhelming inflammation response, little progress has been achieved. Their previous studies firstly confirmed that carbon monoxide (CO)-releasing molecules (CORM)-released CO attenuated leukocytes sequestration in the liver, lung and small intestine of burned mice by interfering with nuclear factor κB activation, protein expression of intercellular adhesion molecule 1 (ICAM-1) and therefore suppressing endothelial cells pro-adhesive phenotype. However, to date, little is known if CORM-released CO can down-regulate the inflammatory production and oxidative stress in the small intestine in septic mice.
In the present study, the authors found that a protective role of tricarbonyldichlororuthenium (II) dimer (CORM-2), one of the novel CORMs, on the small intestine during sepsis. The potential mechanism of this beneficial effect of CORM-2 appears to suppress oxidative stress, decrease the myeloperoxidase activities and production of IL-1 and TNF-α. This was accompanied by a decrease of nitric oxide (NO) production and protein expression of ICAM-1 and inducible NO synthase, and therefore suppress tissue damage in the small intestine. These data indicated that CORM-2 effectively prevents polymorphonuclear neutrophil chemotaxis and infiltration in the tissue after CLP, consequently decreased the production of oxidants, reduced tissue oxidative injury.
The protective role of CORM-2, one of the CORMs, on the organs during sepsis, and its therapeutic potential in anti-inflammation or anti-oxidative stress will be the novel strategies in the future.
The authors report the protective role of CORM-2, one of the CORMs, on the organs during sepsis, and its therapeutic potential in anti-inflammation or anti-oxidative stress. This is a well-written article. The study is interesting and goes along with previous data on modulation of inflammation by CO.
| 1. | Brunkhorst FM, Oppert M, Marx G, Bloos F, Ludewig K, Putensen C, Nierhaus A, Jaschinski U, Meier-Hellmann A, Weyland A. Effect of empirical treatment with moxifloxacin and meropenem vs meropenem on sepsis-related organ dysfunction in patients with severe sepsis: a randomized trial. JAMA. 2012;307:2390-2399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 160] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 2. | Cakir B, Cevik H, Contuk G, Ercan F, Ekşioğlu-Demiralp E, Yeğen BC. Leptin ameliorates burn-induced multiple organ damage and modulates postburn immune response in rats. Regul Pept. 2005;125:135-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 3. | Chen LW, Chang WJ, Wang JS, Hsu CM. Thermal injury-induced peroxynitrite production and pulmonary inducible nitric oxide synthase expression depend on JNK/AP-1 signaling. Crit Care Med. 2006;34:142-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Mileski W, Borgstrom D, Lightfoot E, Rothlein R, Faanes R, Lipsky P, Baxter C. Inhibition of leukocyte-endothelial adherence following thermal injury. J Surg Res. 1992;52:334-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 65] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 5. | Hansbrough JF, Wikström T, Braide M, Tenenhaus M, Rennekampff OH, Kiessig V, Bjursten LM. Neutrophil activation and tissue neutrophil sequestration in a rat model of thermal injury. J Surg Res. 1996;61:17-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 69] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Piantadosi CA. Biological chemistry of carbon monoxide. Antioxid Redox Signal. 2002;4:259-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 144] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 7. | Hayashi S, Takamiya R, Yamaguchi T, Matsumoto K, Tojo SJ, Tamatani T, Kitajima M, Makino N, Ishimura Y, Suematsu M. Induction of heme oxygenase-1 suppresses venular leukocyte adhesion elicited by oxidative stress: role of bilirubin generated by the enzyme. Circ Res. 1999;85:663-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 280] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 8. | Sun BW, Chen ZY, Chen X, Liu C. Attenuation of leukocytes sequestration by carbon monoxide-releasing molecules: liberated carbon monoxide in the liver of thermally injured mice. J Burn Care Res. 2007;28:173-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Sun B, Sun H, Liu C, Shen J, Chen Z, Chen X. Role of CO-releasing molecules liberated CO in attenuating leukocytes sequestration and inflammatory responses in the lung of thermally injured mice. J Surg Res. 2007;139:128-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 10. | Sun BW, Jin Q, Sun Y, Sun ZW, Chen X, Chen ZY, Cepinskas G. Carbon liberated from CO-releasing molecules attenuates leukocyte infiltration in the small intestine of thermally injured mice. World J Gastroenterol. 2007;13:6183-6190. [PubMed] [DOI] [Full Text] |
| 11. | Sun BW, Sun Y, Sun ZW, Chen X. CO liberated from CORM-2 modulates the inflammatory response in the liver of thermally injured mice. World J Gastroenterol. 2008;14:547-553. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Liu DM, Sun BW, Sun ZW, Jin Q, Sun Y, Chen X. Suppression of inflammatory cytokine production and oxidative stress by CO-releasing molecules-liberated CO in the small intestine of thermally-injured mice. Acta Pharmacol Sin. 2008;29:838-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 13. | Sun B, Sun Z, Jin Q, Chen X. CO-releasing molecules (CORM-2)-liberated CO attenuates leukocytes infiltration in the renal tissue of thermally injured mice. Int J Biol Sci. 2008;4:176-183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 51] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 14. | Sun B, Zou X, Chen Y, Zhang P, Shi G. Preconditioning of carbon monoxide releasing molecule-derived CO attenuates LPS-induced activation of HUVEC. Int J Biol Sci. 2008;4:270-278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 57] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 15. | Stein AB, Guo Y, Tan W, Wu WJ, Zhu X, Li Q, Luo C, Dawn B, Johnson TR, Motterlini R. Administration of a CO-releasing molecule induces late preconditioning against myocardial infarction. J Mol Cell Cardiol. 2005;38:127-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 103] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 16. | Leal YA, Alvarez-Nemegyei J, Velázquez JR, Rosado-Quiab U, Diego-Rodríguez N, Paz-Baeza E, Dávila-Velázquez J. Risk factors and prognosis for neonatal sepsis in southeastern Mexico: analysis of a four-year historic cohort follow-up. BMC Pregnancy Childbirth. 2012;12:48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 82] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 17. | Velissaris D, Karanikolas M, Flaris N, Fligou F, Marangos M, Filos KS. Commonly used severity scores are not good predictors of mortality in sepsis from severe leptospirosis: a series of ten patients. Crit Care Res Pract. 2012;2012:532376. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Ogawa Y, Yamakawa K, Ogura H, Kiguchi T, Mohri T, Nakamori Y, Kuwagata Y, Shimazu T, Hamasaki T, Fujimi S. Recombinant human soluble thrombomodulin improves mortality and respiratory dysfunction in patients with severe sepsis. J Trauma Acute Care Surg. 2012;72:1150-1157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 19. | Biolatti C, Bellino C, Borrelli A, Capucchio M, Gianella P, Maurella C, Miniscalco B, Nebbia P, Zoppi S, Cagnasso A. Sepsis and bacterial suppurative meningitis-meningoencephalitis in critically ill neonatal Piedmontese calves: clinical approach and laboratory findings. Schweiz Arch Tierheilkd. 2012;154:239-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Metz M, Doyle E, Bindslev-Jensen C, Watanabe T, Zuberbier T, Maurer M. Effects of antihistamines on innate immune responses to severe bacterial infection in mice. Int Arch Allergy Immunol. 2011;155:355-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 21. | Dejager L, Pinheiro I, Dejonckheere E, Libert C. Cecal ligation and puncture: the gold standard model for polymicrobial sepsis? Trends Microbiol. 2011;19:198-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 571] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 22. | Hasegawa A, Iwasaka H, Hagiwara S, Asai N, Nishida T, Noguchi T. Alternate day calorie restriction improves systemic inflammation in a mouse model of sepsis induced by cecal ligation and puncture. J Surg Res. 2012;174:136-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 23. | Medina E. Murine model of polymicrobial septic peritonitis using cecal ligation and puncture (CLP). Methods Mol Biol. 2010;602:411-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 24. | Fei D, Meng X, Kang K, Nan C, Zhao M, Pan S, Gao M, Yang S, Zhao M. Heme oxygenase-1 modulates thrombomodulin and activated protein C levels to attenuate lung injury in cecal ligation and puncture-induced acute lung injury mice. Exp Lung Res. 2011;38:173-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | Palani K, Rahman M, Hasan Z, Zhang S, Qi Z, Jeppsson B, Thorlacius H. Rho-kinase regulates adhesive and mechanical mechanisms of pulmonary recruitment of neutrophils in abdominal sepsis. Eur J Pharmacol. 2012;682:181-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 26. | Zhan J, Liu Y, Zhang Z, Chen C, Chen K, Wang Y. Effect of penehyclidine hydrochloride on expressions of MAPK in mice with CLP-induced acute lung injury. Mol Biol Rep. 2011;38:1909-1914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 27. | Cuschieri J, Gourlay D, Garcia I, Jelacic S, Maier RV. Modulation of endotoxin-induced endothelial activity by microtubule depolymerization. J Trauma. 2003;54:104-112; discussion 112-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 28. | Peña G, Cai B, Deitch EA, Ulloa L. JAK2 inhibition prevents innate immune responses and rescues animals from sepsis. J Mol Med (Berl). 2010;88:851-859. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 29. | Gül M, Ayan M, Seydanoğlu A, Cander B, Girişgin S, Erayman I, Erdem S. The effect of N-acetyl cysteine on serum glutathione, TNF-alpha and tissue malondialdehyde levels in the treatment of sepsis. Ulus Travma Acil Cerrahi Derg. 2011;17:293-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 30. | Hacioglu A, Algin C, Pasaoglu O, Pasaoglu E, Kanbak G. Protective effect of leptin against ischemia-reperfusion injury in the rat small intestine. BMC Gastroenterol. 2005;5:37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 31. | Carnio EC, Stabile AM, Batalhão ME, Silva JS, Antunes-Rodrigues J, Branco LG, Magder S. Vasopressin release during endotoxaemic shock in mice lacking inducible nitric oxide synthase. Pflugers Arch. 2005;450:390-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
Peer reviewer: Alireza Mani, MD, PhD, Department of Physiology, Faculty of Medical Sciences, Tarbiat Modares University, Tehran 14115-331, Iran
S- Editor Gou SX L- Editor A E- Editor Xiong L