INTRODUCTION
Acute-on-chronic liver failure (ACLF) is a severe life-threatening condition with a high fatality, which still lacks effective treatment options[1]. Evidences have indicated that intestinal endotoxemia plays a significant role in the pathogenesis and progression of liver failure[2,3], which results from overgrowth of gram-negative bacteria in gut, and/or bacteria translocation into the peritoneal cavity due to the high permeability of intestinal wall[4,5].
Recent studies have shown that high mobility group box-1 (HMGB1) protein is involved in the progression of endotoxemia[6-8]. HMGB1 can be released into the extracellular milieu by activated macrophages, immuno-stimulated gut epithelial cells and necrotic or damaged cells in a delayed manner relative to the secretion of tumor necrosis factor-α (TNF-α) and interleukin (IL)-1[9-11]. HMGB1 contributes to the pathogenesis of many diverse inflammatory disorders[12-14]. A growing number of studies have indicated that HMGB1 is a successful therapeutic target in numerous experimental models, such as ischemia/reperfusion[15], acute respiratory distress syndrome[16], rheumatoid arthritis[17], sepsis[18], and cancer[19]. Therefore, we proposed that HMGB1 as an important inflammatory mediator of endotoxemia should also play a significant role in the pathogenesis of liver failure[20].
Ethyl pyruvate (EP), a simple aliphatic ester derived from pyruvic acid, has been shown to improve survival and ameliorate organ dysfunction in a variety of animal models of critical illness, including acute endotoxemia[21], polymicrobial bacterial sepsis[22], postsurgical ileus[23], burn injury[24], acute pancreatitis[25], organ ischemia and reperfusion injury[26,27], and hemorrhagic shock[28]. As an effective anti-inflammatory agent, EP inhibits the activation of key inflammatory signaling pathways[29,30], and down-regulates the secretion of multiple inflammatory proteins, including IL-6, TNF-α and HMGB1[31-33]. These findings suggest that by reducing the levels of proinflammatory mediators, EP could also be a good candidate for treating or protecting against liver failure.
In the present study, we established an ACLF rat model, and investigated whether EP had protective effects on ACLF.
MATERIALS AND METHODS
Experimental animals
Female specific pathogen free Wistar rats, weighing 180-220 g, were purchased from the Experimental Animal Center of Wuhan University. All animals were acclimated to the laboratory for 5 d before experiment and were maintained in a light-controlled room (12-h light/dark cycle) at an ambient temperature of 25 °C with free access to water and standard chow. All animals were given humane care in accordance with the institutional guidelines.
Establishment of animal models
The ACLF rat models induced by human serum albumin (HSA), D-galactosamine (D-Gal) and lipopolysaccharide (LPS) were established as described elsewhere[34]. In brief, an immune liver fibrosis rat model was firstly induced by HSA (Octapharma GmbH, Austria)[35,36]. HSA was diluted to a concentration of 8 g/L with physiological saline and emulsified with an equal amount of incomplete Freund’s adjuvant. The rats were injected subcutaneously at multipoints with 0.5 mL above solutions containing 4 mg HSA for a total of four times (a 14-d interval between the first and second time; a 10-d interval between the third and fourth time). Then, 4 mg HSA was injected into rat tail vein twice a week for 6 wk. Next, the rats with immune liver fibrosis were injected intraperitoneally with D-Gal (a purity of 98%, Sigma-Aldrich Co., United States) at a dose of 400 mg/kg combined with LPS (a purity of 99%, Sigma-Aldrich Co., United States) at a dose of 100 μg/kg to induce acute liver failure on the basis of chronic liver injury[37,38]. The normal rats were administrated with physiological saline (i.e., the vehicle solution of HSA, D-gal and LPS) at the same time points as did in the model rats, which served as a control.
Experimental design
Animals were randomly divided into normal group (n = 15), model group (n = 15) and EP treatment group (n = 60). The rats of EP treatment group were further divided into four subgroups (each subgroup n = 15), which were injected intravenously with Ringer’s EP solution, containing Na+ 130 mmol/L, K+ 4 mmol/L, Ca2+ 2.7 mmol/L, Cl- 139 mmol/L, and EP 28 mmol/L, pH = 7.02 (EP with a purity of 98% was purchased from Sigma-Aldrich Co., United States), at a dose of 40 mg/kg[39,40] at 3 h, 6 h, 12 h and 24 h. The time of combined administration of D-Gal and LPS was accepted as baseline (time point 0). EP was administrated subsequently at different time points. The rats of normal and model groups were injected intravenously with the same volume of Ringer’s solution at the same time points. Five rats were sacrificed randomly for blood and hepatic tissue sampling at 48 h in each group. The remaining ten rats were observed for survival every 12 h for 7 d after induction of ACLF.
Blood sample examination
The serum levels of endotoxin were examined with Limulus Amebocyte Lysate (LAL, QCL-1000™, BioWhittaker, United States) according to the manufacturer’s protocols. The serum levels of HMGB1, TNF-α, IFN-γ, IL-10 and IL-18 were measured using a rat HMGB1 enzyme linked immunosorbent assay (ELISA) Kit (Wuhan EIAab Science Co., Ltd, China) and commercial ELISA kits (Jingmei Biotech Co., Ltd, China) following the manufacturer’s instructions, respectively. The alanine transaminase (ALT) level was determined by routine biochemical methods using a Hitachi Automatic Analyzer (Hitachi, Inc., Japan).
Histological examination
Liver samples were fixed in 10% buffered formalin for 24 h, embedded in paraffin, sliced into 5-μm thick sections and stained with hematoxylin and eosin (HE) to evaluate the pathological changes of the liver. The other portions of liver specimens were fixed in 2.5% glutaraldehyde, embedded in epoxy resin, sliced into ultrathin sections (60 nm in thickness) and stained with uranyl acetate and lead citrate to observe the ultrastructural changes of liver tissues under transmission electron microscope.
Real-time reverse transcription-polymerase chain reaction of HMGB1 mRNA
Total RNA was extracted from approximately 100 mg frozen liver tissues using TRIzol reagent (Invitrogen Co., United States). Two μg total RNA was reverse-transcribed into cDNA using a random primer hexamer (Promega Corporation, United States) and a MultiScribe™ Reverse Transcriptase (Applied Biosystems, United States). Gene expression was measured in real-time using primers and QuantiTect SYBR green polymerase chain reaction (PCR) Kit (QIAGEN Co., Ltd., China). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal control. The primers for rat HMGB1 were 5’-CGGATGCTTCTGTCAACTTCT-3’ and 5’-AGTTTCTTCGCAACATCACCA-3’; for rat GAPDH, were 5’-TGCACCACCAACTGCTTA-3’ and 5’-GGATGCAGGGATGATGTT-3’. Thermal cycling conditions included pre-incubation at 50 °C for 2 min, 95 °C for 10 min followed by 40 cycles of 95 °C for 30 s, 58 °C for 30 s and 72 °C for 45 s. LightCycler (Roche, Switzerland) was used to collect data automatically and analyze the value of threshold cycle (Ct). The fold change of HMGB1 mRNA expression in the model group or EP treatment group relative to the control group was detected using 2-ΔΔCt method[41].
Western blot analysis of HMGB1 protein
In brief, 50 μg protein extracts from 100 mg liver tissue samples were subjected to 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis and then transferred to a Protran® nitrocellulose membrane (Schleicher and Schuell BioScience GmbH, Germany). The membrane was incubated with primary rabbit anti-rat HMGB1 antibodies (Boisynthesis Biotechnology Co., Ltd., China), and then with horseradish peroxidase-conjugated goat anti-rabbit secondary antibodies (Golden Bridge Biotechnology Co., Ltd., China), and finally detected by chemiluminescence using Enhanced NuGlo™ Chemiluminescent Substrate Kit (Alpha Diagnostic Intl. Inc., United States) followed by autoradiography and densitometric analysis. Membranes were also probed for β-actin as an additional loading control.
Immunofluorescence and confocal microscopy of HMGB1
Briefly, tissue sections were deparaffinized in xylene and rehydrated in a series of alcohol dilutions. The tissue sections underwent antigen retrieval using a commercial regent (Boster Biotechnology Co., Ltd., Wuhan, China), blocked with 10% normal goat serum in a humid chamber at 37 °C for 20 min to reduce non-specific binding, and then incubated with primary rabbit anti-rat HMGB1 polyclonal antibodies at 1:200 dilution at 4 °C overnight. The sections were then incubated away from light with a secondary fluorescein isothiocyanate-conjugated goat anti-rabbit IgG (Boster Biotechnology Co., Ltd., China) for 60 min at 37 °C, counterstained with 2 μg/mL propidium iodide (Promoter Bio Co., Ltd., China) for 10 min, and observed under a Leika DM-IRE2 confocal microscope.
Statistical analysis
Results were expressed as mean ± SD. Differences among groups were assessed using unpaired Student t test and one-way analysis of variance. Animal survival data were evaluated using the Kaplan-Meier method and compared with the log-rank test. P < 0.05 was considered statistically significant.
RESULTS
Effect of EP on serum endotoxin, HMGB1, ALT and inflammatory cytokines
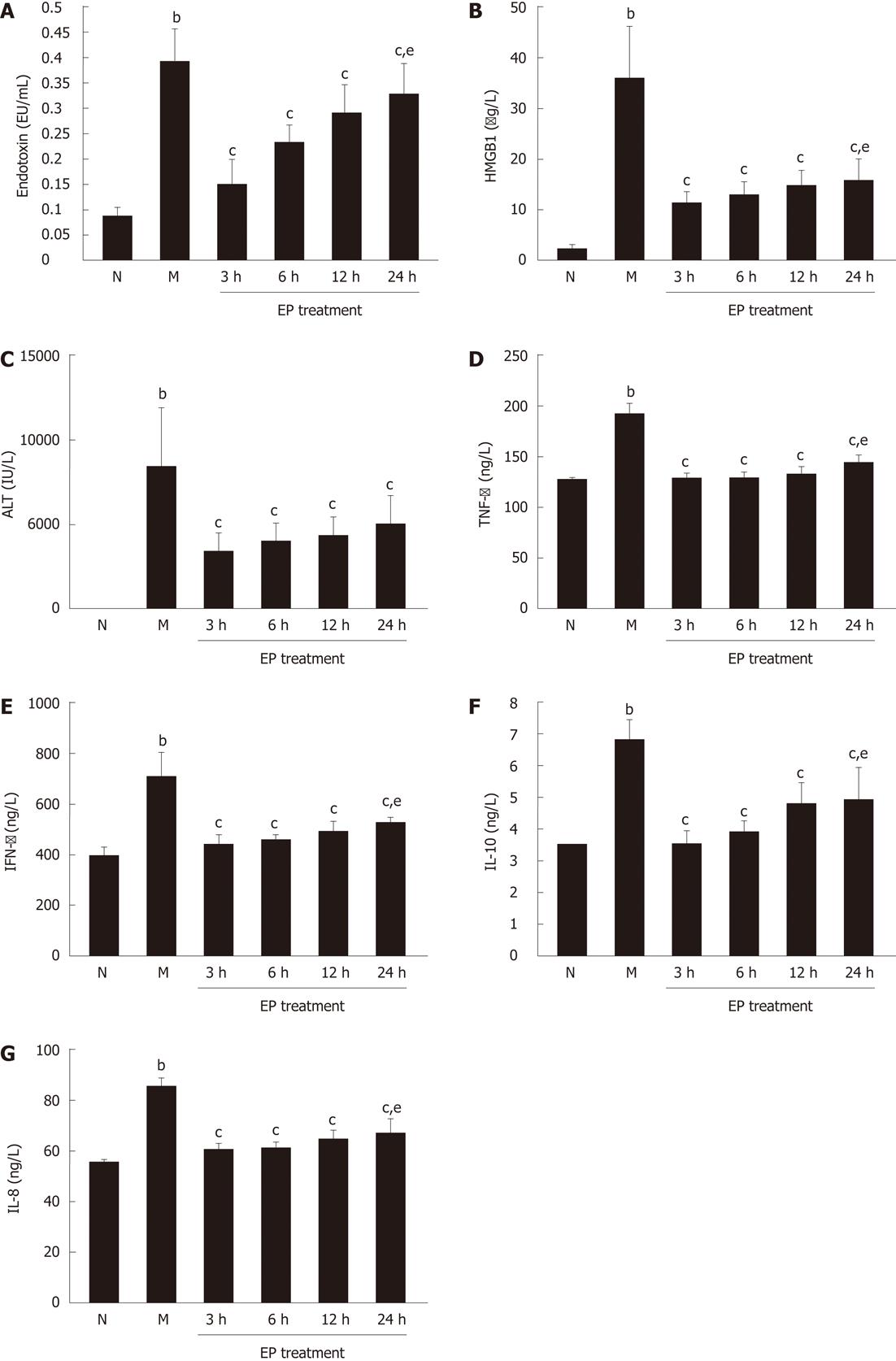
As shown in Figure 1, serum levels of endotoxin, HMGB1, ALT, TNF-α, IFN-γ, IL-10 and IL-18 were all significantly increased in rats of model group (0.394 ± 0.066 EU/mL, 35.42 ± 10.86 μg/L, 8415.87 ± 3567.54 IU/L, 190.77 ± 12.34 ng/L, 715.38 ± 86.03 ng/L, 6.85 ± 0.64 ng/L and 85.19 ± 3.49 ng/L, respectively) at 48 h after induction of ACLF compared with normal group (0.086 ± 0.017 EU/mL, 2.14 ± 0.27 μg/L, 38.64 ± 8.82 IU/L, 124.40 ± 4.12 ng/L, 398.66 ± 32.91 ng/L, 3.49 ± 0.24 ng/L and 55.38 ± 1.25 ng/L, P < 0.01). However, compared with model group, the serum levels of endotoxin, HMGB1, ALT, TNF-α, IFN-γ, IL-10 and IL-18 were all markedly lowered in EP treatment group at each time point, even at 24 h (0.327 ± 0.064 EU/mL, 15.73 ± 4.18 μg/L, 4863.78 ± 1864.13 IU/L, 143.24 ± 6.45 ng/L, 526.00 ± 28.68 ng/L, 4.96 ± 0.96 ng/L and 66.66 ± 5.59 ng/L, respectively, P < 0.01). There were significant differences in serum levels of endotoxin, HMGB1, TNF-α, IFN-γ, IL-10 and IL-18 in the EP treatment group at 24 h and at 3 h (0.155 ± 0.045 EU/mL, 11.13 ± 2.58 μg/L, 128.55 ± 5.76 ng/L, 438.16 ± 38.10 ng/L, 3.55 ± 0.36 ng/L, 60.35 ± 1.63 ng/L, P < 0.05).
Figure 1 Effect of ethyl pyruvate on serum endotoxin, high mobility group box-1, hepatic enzyme and inflammatory cytokines in rat acute-on-chronic liver failure model.
A: Endotoxin; B: High mobility group box-1 (HMGB1); C: Alanine transaminase (ALT); D: Tumor necrosis factor-α (TNF-α); E: Interferon-γ (IFN-γ); F: Interleukin (IL)-10; G: IL-18. bP < 0.01 vs normal; cP < 0.05 vs model; eP < 0.05 vs ethyl pyruvate (EP) treatment at 3 h. Results are expressed as mean ± SD (n = 5). N: Normal group; M: Model group.
Effect of EP on liver histology
HE staining showed a normal structure of hepatic lobule in the rat liver tissues of normal group. Neither degeneration nor necrosis was presented. Hepatocytes were arrayed radiatively around the central vein (Figure 2A1). In the rat liver tissues of model group, the structure of hepatic lobules was in disorder with infiltration of many periportal inflammatory cells, hepatic plate was dissociated, and hepatocytes were swelling or with lytic necrosis. Massive or submassive necrosis of the regenerated nodules was revealed in some areas, while fibrosis septa were intact (Figure 2B1). However, liver histology was significantly improved in rats of EP treatment group as compared with the model group regardless of different time points of treatment. There was an obvious decrease of inflammatory cell infiltration, well-arranged hepatic cords, alleviation of hepatocyte swelling and less pyrenolysis (Figure 2C1, D1, E1 and F1). Under electronic microscope, the hepatocyte ultrastructure of rats in the normal group appeared normal (Figure 2A2), while in the model group, the glycogen granules decreased obviously in cytoplasm, endoplasmic reticulum was dilated and broken, mitochondrion was swollen, mitochondrial cristae were lysed, chromatin appeared vanishing, and even vacuolization occurred in the nuclei (Figure 2B2). Compared with the model group, hepatocyte ultrastructure was significantly improved in rats of EP treatment group, which showed that both endoplasmic reticulum dilation and mitochondrion swelling were alleviated obviously. Mitochondrial cristae and membrane remained clear, chromatin was symmetrical, and nucleoli and nuclear membrane were also legible (Figure 2C2, D2, E2 and F2).
Effect of EP on HMGB1 expression in liver tissues
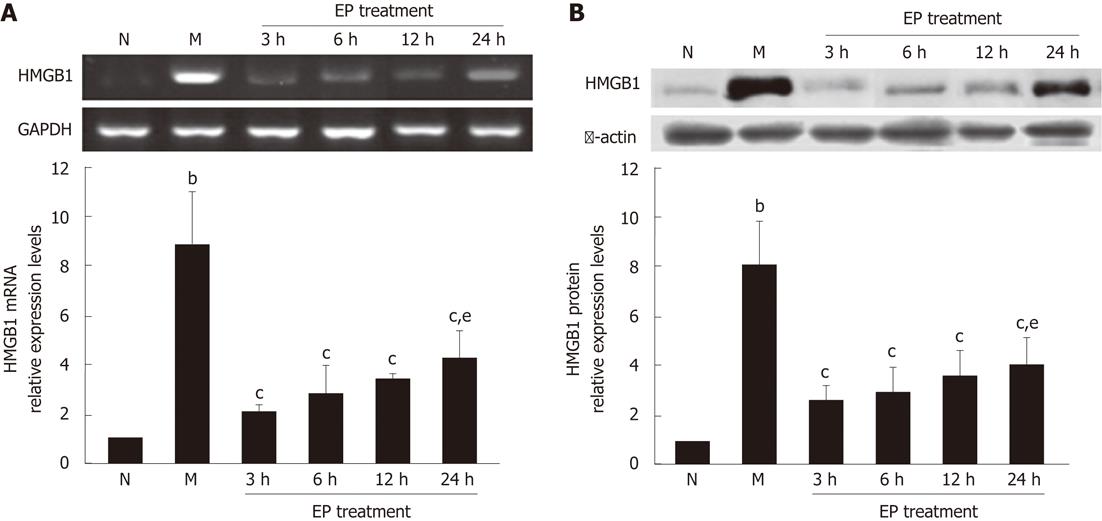
The real-time reverse transcription-PCR showed that the expression levels of HMGB1 mRNA in the liver tissues of rats in the model group were significantly higher than in the normal group. However, the expression levels of HMGB1 mRNA in EP treatment group were all significantly decreased at 3 h, 6 h, 12 h and 24 h (2.02 ± 0.27, 2.78 ± 1.16, 3.34 ± 0.31 and 4.21 ± 1.22) as compared with the model group (8.84 ± 2.13) (Figure 3A). Western blot assay further found that the expression of HMGB1 protein was significantly increased in the model group compared with the normal group, but significantly decreased in the EP treatment group at 3 h, 6 h, 12 h and 24 h (2.33 ± 0.67, 2.83 ± 1.07, 3.39 ± 1.01 and 4.02 ± 1.02) compared with the models (7.97 ± 1.85) (Figure 3B). There was no significant difference of HMGB1 expression levels in the EP treatment group at 24 h and 3 h (4.21 ± 1.22 vs 2.02 ± 0.27 for HMGB1 mRNA; 4.02 ± 1.02 vs 2.33 ± 0.67 for HMGB1 protein) (Figure 3).
Figure 3 Effect of ethyl pyruvate on high mobility group box-1 expression in liver tissues of rat acute-on-chronic liver failure model.
A: A representative reverse transcription-polymerase chain reaction specific for high mobility group box-1 (HMGB1) mRNA and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA (internal control) in each group shown in the electrophotogram; B: Western blot assay showing the expression of HMGB1 protein in the liver tissues of rats in each group. bP < 0.01 vs normal; cP < 0.05 vs model; eP < 0.05 vs ethyl pyruvate (EP) treatment at 3 h. The time points of 3 h, 6 h, 12 h and 24 h represent four subgroups of EP treatment group. Results are expressed as mean ± SD (n = 5). N: Normal group; M: Model group.
Effect of EP on survival
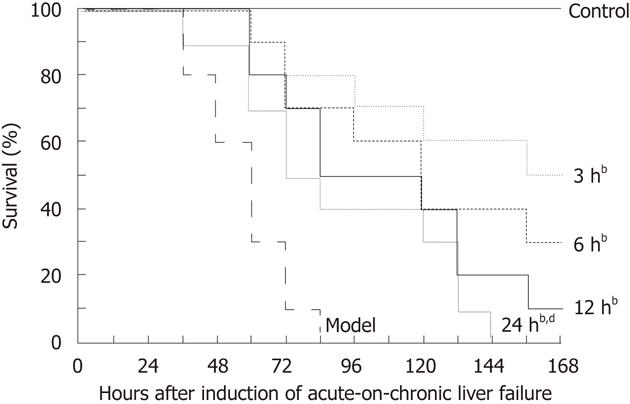
Survival curves of each group are shown in Figure 4. The ACLF rats all died within 3 d without EP treatment, and their median survival time was 60 h. EP treatment at 3 h, 6 h, 12 h and 24 h significantly prolonged the median survival time of the ACLF rats to 162 h, 120 h, 102 h and 78 h, respectively. By log-rank test, there was a significant difference among the curves (χ2 = 41.17, P < 0.0001).
Figure 4 Effect of ethyl pyruvate on survival in rat acute-on-chronic liver failure model.
Kaplan-Meier survival curves showed that the median survival time of the model group was 60 h, and the median survival time in ethyl pyruvate treatment group was 162 h, 120 h, 102 h and 78 h, respectively, after treatment at 3 h, 6 h, 12 h and 24 h. By log-rank test, bP < 0.01 vs model; dP < 0.01 vs 3 h.
Subcellular localization of HMGB1 expression
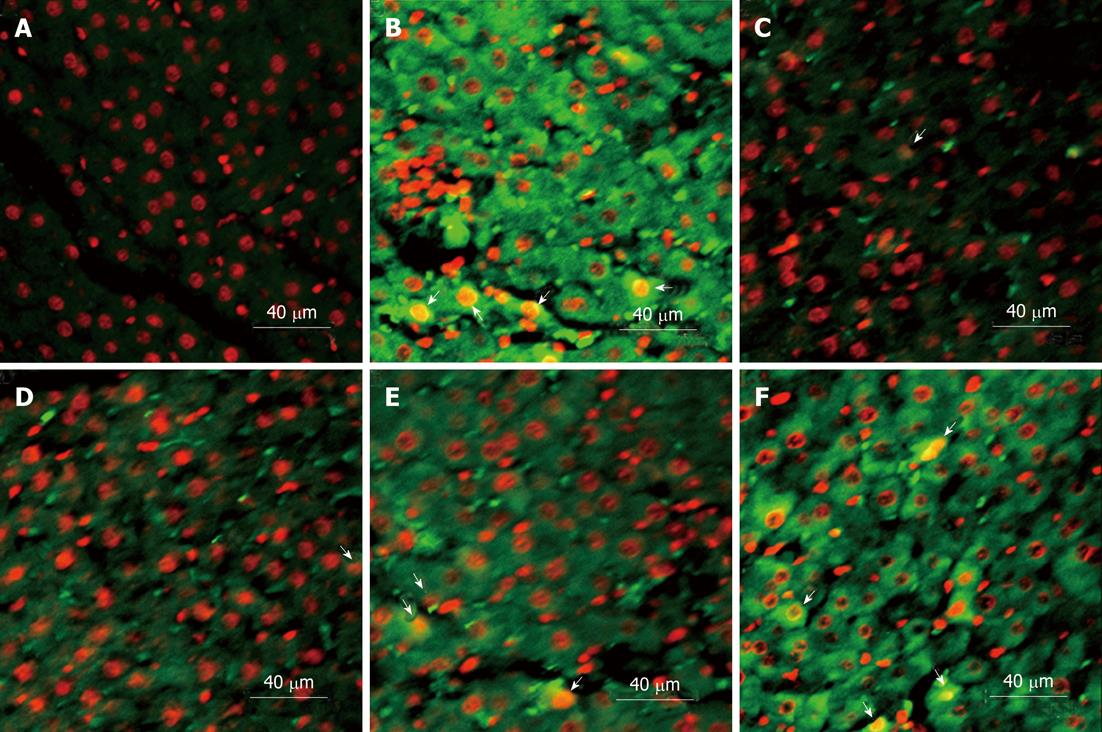
Under confocal laser scanning microscope, HMGB1 expression in liver cells (green fluorescence) was mainly localized in the cytoplasm. A small number of HMGB1 was also observed in the nuclei (Figure 5).
Figure 5 Immunofluorescence and confocal microscopy of high mobility group box-1 expression.
Subcellular high mobility group box-1 expression (green fluorescence) was mainly localized in the cytoplasm, and also observed in a small number of nuclei (arrows). A: Normal group; B: Model group; C-F: Ethyl pyruvate treatment at 3 h, 6 h, 12 h and 24 h.
DISCUSSION
The ACLF model has been widely accepted as a good mimic for pathophysiological process and histological characteristics of ACLF[37,38]. In the present study, an ACLF rat model was established firstly by inducing immune liver injury with HSA, and secondly by inducing acute liver failure with D-Gal and LPS treatment. Most of the rats treated with HSA developed cirrhosis and fibrosis, and 90% of them died of acute liver failure after administration of D-Gal and LPS, with a mean survival time of 60 h. Liver histopathology showed massive or submassive necrosis of the regenerated nodules in some areas, where fibrosis septa were intact. Liver function tests were consistent with massive necrosis of hepatocytes. Serum levels of TNF-α and IL-10 increased significantly, a similar result as seen in the patients with ACLF. These results verified that an ACLF model was successfully established, which was further confirmed by pathological characteristics, biochemical data and inflammatory profiles.
HMGB1 protein is involved in the processes of endotoxemia and multiple inflammatory cytokines actively secreted from monocytes/macrophages in the patients and animal models with liver failure[42,43]. Many studies have shown that HMGB1 stimulates the release of inflammatory cytokines in different cell-lines[44-46]. HMGB1 has been also identified as a successful therapeutic target in diverse inflammatory disorders[7,8]. Previously, we demonstrated that the high expression of HMGB1 and the low percentage of Treg cells play an important role in the pathogenesis of liver failure[20]. In the present study, the serum levels of TNF-α, IFN-γ, IL-10, IL-18 and HMGB1 were significantly increased in the ACLF model compared with the normal group. The tissue levels of HMGB1 were also significantly increased and translocations of HMGB1 from nuclei into cytoplasm were presented in ACLF rats. These findings suggested that the increased expression of HMGB1 and/or translocations of HMGB1 were associated with severe liver injury in ACLF, and blocking the release of HMGB1 may represent one of the novel therapeutic strategies for liver failure.
EP has been shown to be beneficial in a variety of animal models of acute and chronic inflammatory conditions[21-28,47,48]. This study investigated whether EP had protective effects on the ACLF model. The histological examinations of liver tissues in ACLF rats showed severe pathological changes at 48 h after induction of ACLF. However, the hepatic histopathological changes were significantly improved in EP treatment group regardless of treatment time after induction of ACLF. Moreover, serum levels of endotoxin, HMGB1, ALT, TNF-α, IFN-γ, IL-10 and IL-18 were all markedly reduced with EP treatment at different time points. These findings suggested that EP exhibited a protective effect against ACLF rat model through inhibiting the production of inflammatory mediators. Since these inflammatory cytokines, especially the HMGB1, are produced actively by the hepatic macrophage, a major population of monocytes/macrophage lineage, whether EP exerts its inhibitory effects on these inflammatory cytokines through suppressing the hepatic macrophage remains to be further clarified.
A recent study has shown that treatment with anti-HMGB1 antibodies in mice attenuates LPS-induced lethality, even when antibody administration is delayed until after the onset of the early proinflammatory cytokine response[6]. In this study, the median survival time of the ACLF rats was 60 h. But EP treatment at 3 h, 6 h, 12 h and 24 h significantly prolonged the median survival time of the model rats to 162 h, 120 h, 102 h and 78 h, respectively, after induction of ACLF. The results showed that HMGB1 expressions in both serum and liver tissues were effectively inhibited in EP treatment group, accompanied with a significant decrease of endotoxin and improvement of hepatic histopathology. In EP treatment group, translocations of HMGB1 from nuclei into cytoplasm were markedly reduced depending on the treatment time points. The inhibitory effect of EP on HMGB1 at 3 h was better than at 24 h after induction of ACLF. It may indicate that early EP treatment could result in more obvious improvement in ACLF rats. However, EP treatment at 24 h after induction of ACLF also improved the liver histological changes and prolonged the survival of ACLF rats. It suggests that EP, as a HMGB1 inhibitor, could improve the ACLF outcome, even when HMGB1 inhibitor administration was delayed until induction of ACLF and after the onset of the early proinflammatory factor response. This indicates that targeted therapy against HMGB1 could be initiated even in the later stage of acute inflammation. It would provide a wider therapeutic time window for the treatment of endotoxemia in ACLF.
In summary, the present study demonstrates that EP administration can inhibit HMGB1 expression, decrease endotoxin level, reduce secretion of inflammatory cytokines, ameliorate hepatocellular injury, improve survival, and protect against ACLF in rats. These findings suggest that EP may be a potential and novel therapeutic agent for severe liver inflammatory injury.
COMMENTS
Background
Acute-on-chronic liver failure (ACLF) is a severe life-threatening condition, for which no effective treatment is available. More and more evidences have indicated that intestinal endotoxemia plays a significant role in the pathogenesis and progression of liver failure. A growing number of studies have also shown that high mobility group box-1 (HMGB1), as an important inflammatory mediator of endotoxemia, is a successful therapeutic target in experimental models of severe inflammatory diseases.
Research frontiers
Ethyl pyruvate (EP), an inhibitor of HMGB1, has been shown to improve survival and ameliorate organ dysfunction in a variety of animal models of critical illness. However, whether EP could also be a good candidate for the treatment of liver failure remains unknown. In this study, an ACLF rat model was treated with EP at different time points. The effects of EP on liver failure were investigated by observing the changes of various parameters, including endotoxin, HMGB1, liver function, cytokines, liver histology and animal survival.
Innovations and breakthroughs
The most common clinical cause of liver failure in Asia, especially in China, is an acute episode of liver function decompensation on the basis of an already impaired liver function caused mostly by chronic hepatitis B virus infection, which is defined as ACLF. Therefore, the traditional animal models of ACLF induced directly by D-galactosamine (D-Gal) or lipopolysaccharide (LPS) in previous studies can not simulate the pathophysiological process of ACLF. In this study, the authors employed an ACLF rat model which was established firstly by human serum albumin treatment to induce immune liver injury, and secondly by administration of D-Gal and LPS to induce acute liver failure on the basis of chronic liver injury. Since this animal model can well simulate part of the pathophysiological processes of acute liver failure on the basis of chronic liver diseases, the experimental results of treatment with EP turned out to be more persuasive. This study showed that EP administration can inhibit HMGB1 expression, decrease endotoxin level, reduce secretion of inflammatory cytokines, ameliorate hepatocellular injury, and improve the survival of the ACLF rats.
Applications
The study results suggest that EP may be a potential and novel therapeutic agent for severe liver inflammatory injury.
Terminology
ACLF is defined as acute hepatic insult manifesting as jaundice and coagulopathy, complicated within 4 wk with ascites and/or encephalopathy in a patient with previously diagnosed or undiagnosed chronic liver disease according to the consensus recommendations of the Asian Pacific Association for the Study of the Liver; HMGB1 protein, as a late inflammatory mediator, can be released into the extracellular milieu from activated macrophages, immuno-stimulated gut epithelial cells and necrotic or damaged cells in a delayed manner relative to the secretion of other proinflammatory mediators, such as tumor necrosis factor-α and interleukin-1, and contributes to the pathogenesis of diverse inflammatory and infectious disorders; EP, which is a simple aliphatic ester derived from pyruvic acid and is synthesized from pyruvate and ethanol, is stable in Ringer’s EP solution through interactions with calcium and potassium ions. Treatment with EP has been shown to be beneficial in animal models of a variety of acute and chronic inflammatory conditions.
Peer review
This is a very well designed study and the literature relating to the rationale for using this compound in this animal model to determine its potential effects has been thoroughly explored. The study is well presented in terms of its goals and purposes. There is convincing data to suggest that EP may be explored as a potential therapeutic agent for severe liver diseases.