Published online Apr 14, 2012. doi: 10.3748/wjg.v18.i14.1700
Revised: February 20, 2012
Accepted: February 26, 2012
Published online: April 14, 2012
According to a review article by Biecker et al published in a previous issue of World Journal of Gastroenterology in March 2011, intestinal decontamination with norfloxacin remains the mainstay of primary prophylaxis of spontaneous bacterial peritonitis (SBP) at the expense of development of quinolone-resistant bacteria after long-term use. In our research, the administration of a 4-wk regimen with rifaximin 1200 mg/d reduced significantly the ascitic neutrophil count in cirrhotic patients with sterile ascites in line with a significant decrease in plasma endotoxin levels. Our observations concur with recent findings, showing a significantly reduced 5-year probability of SBP in cirrhotic patients taking rifaximin.
- Citation: Kalambokis GN, Mouzaki A, Rodi M, Tsianos EV. Rifaximin for the prevention of spontaneous bacterial peritonitis. World J Gastroenterol 2012; 18(14): 1700-1702
- URL: https://www.wjgnet.com/1007-9327/full/v18/i14/1700.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i14.1700
We read with great interest the article by Biecker et al[1] regarding management of ascites published on World J Gastroenterol 2011; 17: 1237-1248. Development of spontaneous bacterial peritonitis (SBP) is a major complication of ascites and possibly the final step in a series of events, including intestinal bacterial overgrowth (IBO), bacterial translocation (BT) resulting in bacteremia, endotoxemia, and colonization of mesenteric lymph nodes, and finally seeding of bacteria into the ascitic fluid (AF). Indeed, SBP in non-hospitalized patients is mostly caused by gram-negative bacteria of intestinal origin. It has been hypothesized that patients at risk of SBP have probably sustained multiple episodes of colonization and resolution before they present with the first clinically apparent infection. In this respect, a previous study showed that higher neutrophil counts in sterile AF are associated with higher risk of subsequent development of SBP[2]. The high mortality of SBP warrants its prevention with administration of antibiotics aimed at decreasing the burden of gut bacteria, thus interrupting the sequence of events leading to AF infection. Norfloxacin is widely used for primary prophylaxis of SBP; however its extensive long-term use has increased the incidence of quinolone-resistant and gram-positive SBP[1].
Rifaximin is an antibiotic with a broad-spectrum activity against gram-positive and gram-negative micro-organisms within the gastrointestinal tract. The main advantage of rifaximin is that it is virtually unabsorbable, which minimizes the antimicrobial resistance and adverse events and renders the drug safe in all patient populations. In addition, rifaximin has a better activity against gram-positive organisms than norfloxacin[3].
We investigated whether rifaximin can reduce the burden of gut flora and BT, which are the requisite effects of a drug used for SBP prophylaxis, by studying its effects on circulating endotoxin levels and AF neutrophil counts in cirrhotic patients with sterile ascites.
Sixteen cirrhotic patients with ascites with no history of previous SBP episodes who required regularly a large-volume paracentesis were included in our study. Cirrhosis was established by non-invasive and/or histological criteria; all patients were Child Pugh class C. The patients were studied at baseline and after a 4-wk regimen with rifaximin 1200 mg/d (Group 1, n = 9; alcohol/viral etiology: 7/2) or an observational period (Group 2, n = 7; alcohol/viral etiology: 5/2). Exploratory paracentesis was performed in association with each therapeutic paracentesis to exclude ascitic fluid infection. All patients were included after written informed consent was obtained from them and the local scientific-ethical committee approved the study. Criteria for inclusion were: (1) Abstinence from alcohol for at least 6 mo before inclusion; (2) Absence of clinical and laboratory signs of bacterial infections; (3) No history of variceal bleeding within the 2 wk preceding the study; and (4) No treatment with antibiotics during the last 8 wk before inclusion. For ethical reasons, only patients with AF total protein concentration >1 g/dL were studied. AF white blood cell (WBC) and neutrophil count, the proportion of neutrophils in AF (AF% neutrophils), and plasma endotoxin levels were measured at baseline and at the end of observational or treatment period. For the detection of plasma endotoxin levels, the Limulus amebocyte lusate chromogenic endpoint assay (Hycult biotech, Uden, The Netherlands) was used as instructed by the manufacturer. The Wilcoxon matched pairs test was used for comparing variations within the same group. Results were expressed as mean ± SE. Statistical significance was designated as P < 0.05.
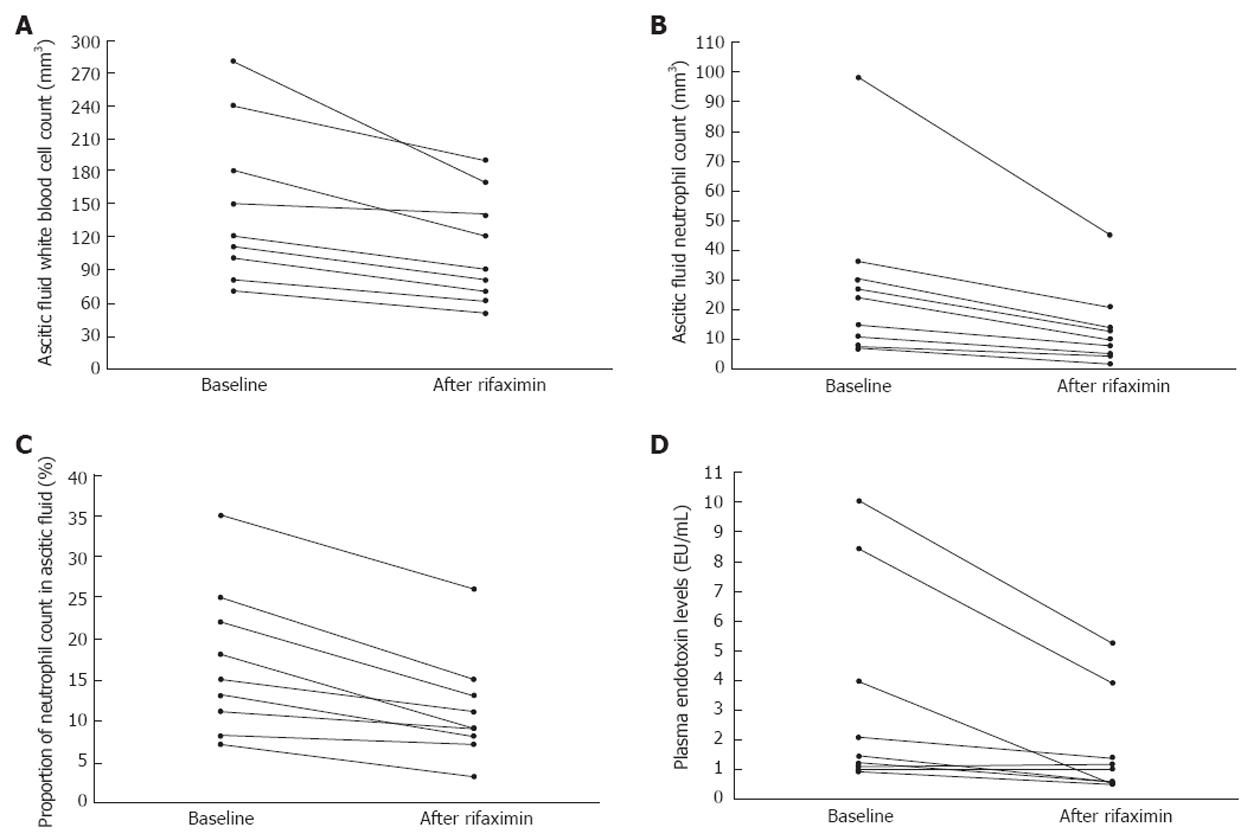
Rifaximin caused significant reductions in AF WBC, neutrophil count, AF% neutrophil count, and plasma endotoxin levels (Table 1); the values of the abovementioned parameters decreased uniformly in all patients (Figure 1). No significant changes in the AF cytological characteristics or plasma endotoxin levels were noted in Group 2. No patient developed AF infection during the study period and no side-effects were noted by the use of rifaximin.
| Group 1 (n = 9) | Group 2 (n = 7) | |||||
| Baseline | 4 wk | P value | Baseline | 4 wk | P value | |
| WBC count (per mm3) | 147.7 ± 24.1 | 107.7 ± 16.6 | 0.004 | 164.5 ± 30.2 | 175 ± 20.8 | NS |
| Neutrophil count (per mm3) | 28.4 ± 9.3 | 13.5 ± 4.3 | 0.01 | 34.6 ± 6.4 | 37.9 ± 7.2 | NS |
| AF% neutrophils | 17.1 ± 3 | 11.2 ± 2.1 | 0.0008 | 21.6 ± 3.5 | 22.1 ± 2.5 | NS |
| Plasma endotoxin (EU/mL) | 3.3 ± 1.1 | 1.6 ± 0.5 | 0.03 | 2.9 ± 0.9 | 3 ± 0.8 | NS |
Our findings strongly suggest that rifaximin suppresses IBO, which in turn reduces BT and the subclinical activation of AF defence mechanisms from prior silent colonisations with bacteria in cirrhotic patients with sterile ascites. The reduction of endotoxemia by rifaximin may further reduce BT by causing a fall in portal pressures[4] considering that portal hypertension induces structural abnormalities in intestinal mucosa leading to an enhanced permeability[1]. Overall, the effects of rifaximin on IBO and BT in our study are consistent with recent findings, showing a significantly reduced 5-year probability of SBP in cirrhotic patients taking rifaximin[5]. In conclusion, the role of rifaximin as an alternative mean of preventing SBP deserves further attention in prospective studies.
Peer reviewer: Erwin Biecker, MD, PhD, Helios Klinikum, Ringstr. 49, 53343 Siegburg, Germany
S- Editor Gou SX L- Editor Ma JY E- Editor Li JY
| 1. | Biecker E. Diagnosis and therapy of ascites in liver cirrhosis. World J Gastroenterol. 2011;17:1237-1248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 2. | Such J, Hillebrand DJ, Guarner C, Berk L, Zapater P, Westengard J, Peralta C, Soriano G, Pappas J, Runyon BA. Tumor necrosis factor-alpha, interleukin-6, and nitric oxide in sterile ascitic fluid and serum from patients with cirrhosis who subsequently develop ascitic fluid infection. Dig Dis Sci. 2001;46:2360-2366. [PubMed] |
| 3. | Koo HL, DuPont HL. Rifaximin: a unique gastrointestinal-selective antibiotic for enteric diseases. Curr Opin Gastroenterol. 2010;26:17-25. [PubMed] |
| 4. | Vlachogiannakos J, Saveriadis AS, Viazis N, Theodoropoulos I, Foudoulis K, Manolakopoulos S, Raptis S, Karamanolis DG. Intestinal decontamination improves liver haemodynamics in patients with alcohol-related decompensated cirrhosis. Aliment Pharmacol Ther. 2009;29:992-999. [PubMed] |
| 5. | Vlachogiannakos J, Viazis N, Vasianopoulou P, Vafiadis-Zouboulis I, Karamanolis DG, Ladas SD. Long-term administration of rifaximin improves the prognosis of patients with alcohol-related decompensated cirrhosis: a case control study. Hepatology. 2010;328A. |