Published online Apr 14, 2012. doi: 10.3748/wjg.v18.i14.1695
Revised: December 5, 2011
Accepted: December 31, 2011
Published online: April 14, 2012
Conventional manometry presents significant challenges, especially in assessment of pharyngeal swallowing, because of the asymmetry and deglutitive movements of oropharyngeal structures. It only provides information about intraluminal pressure and thus it is difficult to study functional details of esophageal motility disorders. New technology of solid high resolution impedance manometry (HRIM), with 32 pressure sensors and 6 impedance sensors, is likely to provide better assessment of pharyngeal swallowing as well as more information about esophageal motility disorders. However, the clinical usefulness of application of HRIM in patients with oropharyngeal dysphagia or esophageal dysphagia is not known. We experienced a case of Huntington’s disease presenting with both oropharyngeal and esophageal dysphagia, in which HRIM revealed the mechanism of oropharyngeal dysphagia and provided comprehensive information about esophageal dysphagia.
- Citation: Lee TH, Lee JS, Kim WJ. High resolution impedance manometric findings in dysphagia of Huntington’s disease. World J Gastroenterol 2012; 18(14): 1695-1699
- URL: https://www.wjgnet.com/1007-9327/full/v18/i14/1695.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i14.1695
Conventional manometry presents considerable challenges in evaluation of oropharyngeal dysphagia because of the asymmetry and deglutitive movements of oropharyngeal structures[1]. Introduction of high resolution manometry (HRM), which employs pressure sensors at 1 cm intervals across the entire anatomic region from the oropharynx to the stomach represented a significant improvement in data recording and diagnostic yield, especially in cases of functional dysphagia over conventional manometry[1,2]. However identifying a manometric abnormality does not equate to identifying a disease and thus achalasia and (perhaps) diffuse esophageal spasm are relevant to manometric findings, which have a functional correlate and can cause dysphagia[3]. Most recently, high resolution impedance manometry (HRIM) has been introduced to combine the benefits of HRM and impedance-based bolus transit assessment. Actually patients with normal manometry can have abnormal bolus transit, and patients with abnormal manometry can have normal bolus transit[4]. Koya et al[5] reported that abnormal impedance even in patients with normal manometry may be a sensitive indicator of esophageal functional abnormality as represented by the symptom of dysphagia in these patients.
To our knowledge, little is known with regard to investigation of dysphagia using the HRIM technique. We experienced a case of Huntington’s disease presenting with both oropharyngeal and esophageal dysphagia, in which HRIM revealed the mechanism of oropharyngeal dysphagia, and provided comprehensive information about esophageal dysphagia.
A 65-year-old male was admitted to the hospital because of a 5-year history of progressive dysphagia. Five years before admission, he began to have difficulty in swallowing liquids such as tea and thin soup. Three years later he complained of difficulty in eating liquids as well as solid foods, pain extended up to the manubrium, and he had lost 12 kg in weight. Ten years before admission, the patient became aware of involuntary movements of all his limbs and face. In his family history, his mother and younger brother complained of the same symptoms.
The patient had a history of pulmonary tuberculosis, which had been cured 30 years previously. He had smoked cigarettes for 40 years. He had drunk moderate amounts of alcohol for 40 years, but he had stopped drinking 3 years before admission. His body temperature was 36.5 °C, pulse was 85 bpm, and respirations were 18 breaths/min. Blood pressure was 125/85 mmHg. Physical examination revealed mild dysarthria, chorea, and limitation of down gaze in eye movement. No muscle rigidity, muscle atrophy or pathologic reflexes were noted. Brain magnetic resonance imaging exhibited ventriculomegaly and mild atrophy in all regions of his brain. The patient was finally diagnosed with Huntington’s disease by the result that the CAG repeat numbers in the Huntington gene were 44 in comparison with the normal numbers of 10-35.
The whole mucosa of the hypopharynx and esophagus was normal except for a mucosal break less than 5 mm from the esophagogastric junction. Endoscopy revealed relatively normal opening of the upper esophageal sphincter (UES) and low esophageal sphincter, and no presence of residual food in the esophagus. However, spastic contraction of the mid esophagus was noticed.
Esophagography demonstrated barium retention with significant delay of contrast passage into the stomach. There were frequent irregular contractions between the mid and distal esophagus. There were no typical signs of primary esophageal motility disorder such as bird-beak appearance or corkscrew appearance.
On swallowing of a spoonful of pudding mixed with barium powder, the patient had a tendency to eat rapidly. Labial closure was normal but disorganized tongue movement, as well as postural instability induced by chorea resulted in residual bolus in the vallecula and pyriform sinuses.
A solid-state HRIM manometry assembly (Sandhill Scientific Instruments Inc. United States) was used to evaluate dysphagia in the patient. A HRIM study was also performed in 26 healthy persons to compare the characteristics of pharyngeal motility. The HRIM catheter was 4.0 mm diameter with 32 solid pressure sensors and 6 impedance sensors. The 4 active impedance channels were located in the traditional locations for analysis, i.e., 5, 10, 15 and 20 cm above the high pressure zone of the lower esophageal sphincter (LES). There were 32 pressure sensors which spanned the esophagus from UES to the LES to allow for evaluation of swallows from initiation of the swallow to closure of the LES. The zero mark on the probe was located at the channel used for LES analysis. The study was performed in a sitting position after at least 6-h fasting. The HRIM assembly was passed transnasally and positioned to record from the hypopharynx to the stomach with about 3-5 intragastric sensors. The catheter was fixed in place by taping it to the nose. The manometric protocol included a 5-min period to assess basal sphincter pressure, 10 5-mL saline swallows and 10 5-mL viscous swallows (so called standard method). Manometric data were acquired and stored using software (Sandhill Scientific Instruments Inc. United States). The HRIM catheter was pulled back by 10 cm and the same sessions were repeated because of inability to assess all the pharyngeal manometric information and bolus transit of pharyngoesophageal segment (so called modified method). Takasaki et al[6] reported that vocalizing “kakakaka” in investigation of pharyngeal swallow using high resolution manometry easily identified the locations of the velopharyngeal swallowing pressure. Therefore vocalizing “kakakaka” was added to the HRIM study using modified method.
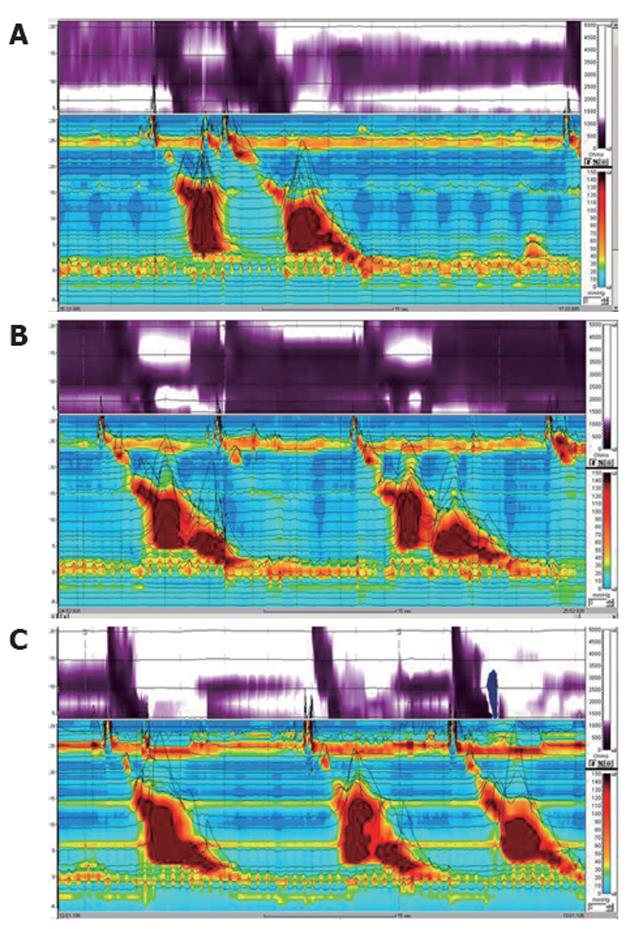
The results of HRIM by the standard method are summarized in Table 1. Impedance results demonstrated 100% swallowing with incomplete bolus transit of both the liquid and the viscous solution. Manometric results revealed high LES pressure with incomplete relaxation (Figure 1A). For liquid bolus, distal esophageal high pressure simultaneous repetitive contraction was observed in 70% of swallows on the manometric topographic view and abnormal liquid transit was noted on the impedance contour view. For viscous bolus, contractile pressures were higher, and simultaneous repetitive contractions were noted on the manometric topographic view, which was associated with the sensation of retrosternal bolus hold-up, chest pain, and abnormal viscous transit (Figure 1B).
| Initial finding | After botulinum toxin injection | |
| Manometry | ||
| LES pressure (mmHg) | 44.2 | 37.2 |
| Relaxation of LES | ||
| Residual pressure (mmHg) | 12.1 | 10.8 |
| Duration (s) | 10.1 | 9.3 |
| Relaxation percent (%) | 74 | 71 |
| Body peristalsis | ||
| Simultaneous contraction (%) | 70 | 60 |
| Peristaltic contraction (%) | 30 | 40 |
| Aperistalsis (%) | 0 | 0 |
| Amplitude of lower esophagus (mean, mmHg) | 441 | 382 |
| Impedance | ||
| Liquid swallow | ||
| Incomplete bolus transit (%) | 100 | 70 |
| Complete bolus transit (%) | 0 | 30 |
| Viscous swallow | ||
| Incomplete bolus transit (%) | 100 | 70 |
| Complete bolus transit (%) | 0 | 30 |
Given the esophageal dysmotility with a spastic component, subsequent trials of a proton pump inhibitor, a nitrate, a calcium channel blocker, and a phosphodiesterase inhibitor were made for 1 mo. There was no response to these drugs in the patient. Treatment with 100 U botulinum toxin injected around the lower esophagus showed considerable improvement in the impedance results and in symptoms such as retrosternal bolus hold-up and chest pain despite no change in the manometry results (Figure 1C).
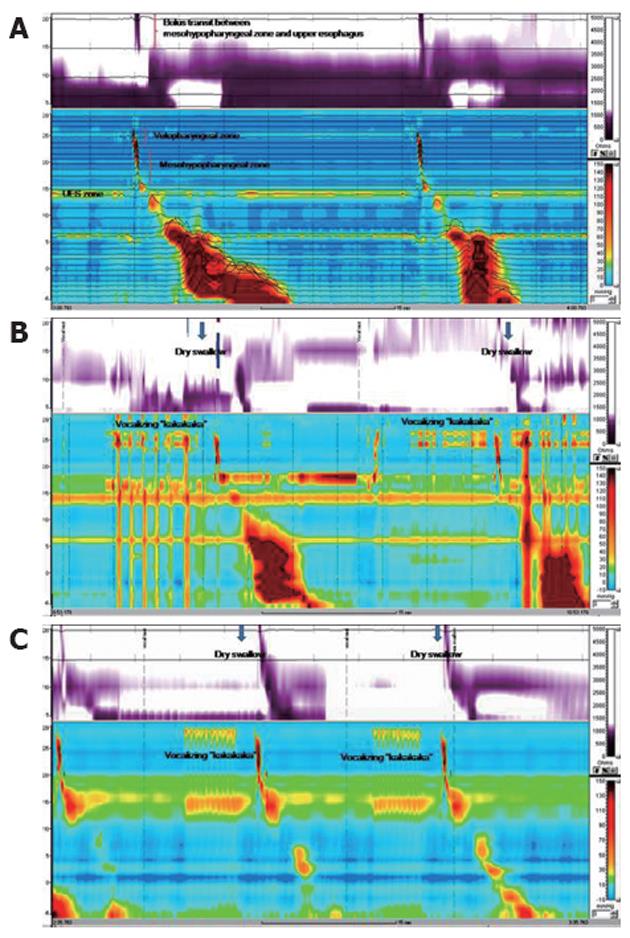
HRIM using the standard method demonstrated normal UES relaxation and peristaltic pharyngeal pressure (UES pressure 41.1 mmHg, residual pressure -1.1 mmHg, duration 0.6 s, 100% relaxation). After withdrawal of the catheter by 10 cm, HRIM revealed unremarkable bolus transit and peristalsis between the meso hypopharynx and upper esophagus (Figure 2A). However, HRIM using the modified method and vocalizing “kakakaka” revealed irregular contractions of the velopharyngeal zone, simultaneous contraction between the velopharyngeal and mesohypopharyngeal zone, and impaired bolus transit of the pharyngo-upper esophageal segment (Figure 2B). In contrast to the HRIM findings of our case, HRIM findings using the modified method from all healthy persons showed regular contractions of the velopharyngeal zone and normal bolus transit between mesohypopharyngeal zone and the upper esophagus (Figure 2C).
To our knowledge, this report is the first study of Huntington’s disease using HRIM, which indicated the combined oropharyngeal and esophageal dysphagia. The patient had difficulty in initiating swallowing and had retrosternal bolus hold-up. Insidious onset of dysphagia associated with some neurologic symptoms such as chorea and dysarthria suggested oropharyngeal dysphagia from a neurologic basis. A video fluoroscopic swallowing study provided the diagnosis of oropharyngeal dysphagia which resulted from a lack of coordination between the oral and pharyngeal stage. Unexpected involuntary movement in the oral cavity induced oropharyngeal incoordination. Oropharyngeal dysphagia in Huntington’s disease results from tachyphagia, or rapid uncontrolled swallowing, secondary to impaired sensory and cognitive function[7]. Furthermore, it is caused by buccolingual chorea resulting in food being transferred impulsively[7]. Respiratory chorea, marked by involuntary respiratory movements, occurs in approximately 40% of patients, and interrupts the normal respiratory-deglutition cycle[8]. Given the anatomical structures involved in vocalization, the HRIM findings using the modified method may reflect the mechanism of oropharyngeal dysphagia in the Huntington’s disease patient. In other words, the modified test may disclose oropharyngeal incoordination related to buccolingual chorea, which cannot be detected even with HRM or HRIM using the usual protocol such as the liquid and viscous swallow test. Further investigation should be carried out to confirm the role of the vocal test in either HRM or HRIM studies for assessing oropharyngeal dysphagia related to chorea.
HRIM results concerning esophageal dysphagia appeared to indicate diffuse esophageal spasm (DES) such as simultaneous contraction associated with > 10% of wet swallows, mean simultaneous contraction amplitude > 30 mmHg, and repetitive contractions. Esophageal dysmotility of the patient, however, can be better classified as an atypical disorder of LES relaxation because there was incomplete relaxation of the LES[9]. The esophageal dysmotility in this case may be an intermediate form between DES and achalasia because a few case reports have suggested a transition from DES to achalasia in some patients[10-12].
Kagel et al[13] reported esophageal dysphagia was relatively uncommon in Huntington’s disease. It was most likely secondary to the disruptive effects of chorea in the aerodigestive tract. To our knowledge there has been no report regarding spastic esophageal dysmotility in Huntington’s disease. The cause of the spastic esophageal motility disorder in the patient was unknown. There are a few studies documenting acid reflux-induced esophageal spasm[12,14,15]. Simultaneous contractions from gastroesophageal reflux should be treated first by a proton pump inhibitor[16]. Reflux esophagitis in this case may be considered the cause of the esophageal dysmotility with a spastic component. However, there was no improvement under therapy with a proton pump inhibitor.
Considering dysphagia associated with chest pain attributable to esophageal spasm, subsequent trials of a nitrate, a calcium channel blocker, and a phosphodiesterase inhibitor were performed. There was no response to these drugs in the patient. Actually current treatments for esophageal spasm, including calcium channel blockers and nitrate donors, are limited by poor efficacy and side effects[17]. Recently botulinum toxin injections in the lower esophagus and at the level of the gastroesophageal junction have been reported to have a beneficial effect in patients suffering from DES[18-20]. These beneficial effects of botulinum toxin are perhaps related to the improved manometric results. Interestingly, injection of botulinum toxin in this case showed improvement in impedance results and symptoms despite no improved manometry results. It suggests that the esophageal symptoms are more closely related to disturbed bolus transport and impaired clearance than esophageal dysmotility per se[4]. It may also suggest that esophageal dysmotility in terms of abnormal contractility and impaired bolus transit is limited in explaining the dysphagia. Dysphagia symptoms are related to factors other than esophageal dysmotility, e.g., esophageal sensitivity disorder.
In conclusion, HRIM enabled us to obtain more detailed and important information in the Huntington’s disease patient with both oropharyngeal dysphasia and esophageal spastic dysmotility. Further investigation of HRIM will be needed to assess its role in either oropharyngeal or esophageal dysphagia.
| 1. | Kahrilas PJ, Sifrim D. High-resolution manometry and impedance-pH/manometry: valuable tools in clinical and investigational esophagology. Gastroenterology. 2008;135:756-769. [PubMed] |
| 2. | Fox MR, Bredenoord AJ. Oesophageal high-resolution manometry: moving from research into clinical practice. Gut. 2008;57:405-423. [PubMed] |
| 3. | Pandolfino JE, Kahrilas PJ. AGA technical review on the clinical use of esophageal manometry. Gastroenterology. 2005;128:209-224. [PubMed] |
| 4. | Tutuian R, Castell DO. Combined multichannel intraluminal impedance and manometry clarifies esophageal function abnormalities: study in 350 patients. Am J Gastroenterol. 2004;99:1011-1019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 199] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 5. | Koya DL, Agrawal A, Freeman JE, Castell DO. Impedance detected abnormal bolus transit in patients with normal esophageal manometry. Sensitive indicator of esophageal functional abnormality? Dis Esophagus. 2008;21:563-569. [PubMed] |
| 6. | Takasaki K, Umeki H, Enatsu K, Tanaka F, Sakihama N, Kumagami H, Takahashi H. Investigation of pharyngeal swallowing function using high-resolution manometry. Laryngoscope. 2008;118:1729-1732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 75] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 7. | Shoulson I, Fahn S. Huntington disease: clinical care and evaluation. Neurology. 1979;29:1-3. [PubMed] |
| 8. | Yorkston KMR, Strand E. Management of speech and swallowing in degenerative disease. Tucson, AZ: Communication Skill Builders 1995; . |
| 9. | Spechler SJ, Castell DO. Classification of oesophageal motility abnormalities. Gut. 2001;49:145-151. [PubMed] |
| 10. | Khatami SS, Khandwala F, Shay SS, Vaezi MF. Does diffuse esophageal spasm progress to achalasia? A prospective cohort study. Dig Dis Sci. 2005;50:1605-1610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 29] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Millan MS, Bourdages R, Beck IT, DaCosta LR. Transition from diffuse esophageal spasm to achalasia. J Clin Gastroenterol. 1979;1:107-117. [PubMed] |
| 12. | Robson K, Rosenberg S, Lembo T. GERD progressing to diffuse esophageal spasm and then to achalasia. Dig Dis Sci. 2000;45:110-113. [PubMed] |
| 13. | Kagel MC, Leopold NA. Dysphagia in Huntington's disease: a 16-year retrospective. Dysphagia. 1992;7:106-114. [PubMed] |
| 14. | Campo S, Traube M. Manometric characteristics in idiopathic and reflux-associated esophageal spasm. Am J Gastroenterol. 1992;87:187-189. [PubMed] |
| 15. | Crozier RE, Glick ME, Gibb SP, Ellis FH, Veerman JM. Acid-provoked esophageal spasm as a cause of noncardiac chest pain. Am J Gastroenterol. 1991;86:1576-1580. [PubMed] |
| 16. | Tutuian R, Castell DO. Review article: oesophageal spasm - diagnosis and management. Aliment Pharmacol Ther. 2006;23:1393-1402. [PubMed] |
| 17. | Grübel C, Borovicka J, Schwizer W, Fox M, Hebbard G. Diffuse esophageal spasm. Am J Gastroenterol. 2008;103:450-457. [PubMed] |
| 18. | Miller LS, Pullela SV, Parkman HP, Schiano TD, Cassidy MJ, Cohen S, Fisher RS. Treatment of chest pain in patients with noncardiac, nonreflux, nonachalasia spastic esophageal motor disorders using botulinum toxin injection into the gastroesophageal junction. Am J Gastroenterol. 2002;97:1640-1646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 74] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 19. | Storr M, Allescher HD, Rösch T, Born P, Weigert N, Classen M. Treatment of symptomatic diffuse esophageal spasm by endoscopic injections of botulinum toxin: a prospective study with long-term follow-up. Gastrointest Endosc. 2001;54:754-759. [PubMed] |
| 20. | Storr M, Linke R, Nicolaus M, Göke B, Schirra J. [Injection of botulinum toxin for diffuse esophageal spasm]. Dtsch Med Wochenschr. 2005;130:266-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
Peer reviewers: Piero Marco Fisichella, MD, Department of Surgery, Loyola University Medical Center, 2160 S. 1st Ave, Maywood, IL 60153, United States; Dr. Wojciech Blonski, MD, PhD, Gastrointestinal Research Ground Centrex, University of Pennsylvania, 3400 Spruce St, Philadelphia, PA 19104, United States
S- Editor Gou SX L- Editor Cant MR E- Editor Li JY