Published online Sep 28, 2011. doi: 10.3748/wjg.v17.i36.4076
Revised: June 15, 2011
Accepted: June 22, 2011
Published online: September 28, 2011
AIM: To determine the effect of non-selective cyclooxygenase (COX) inhibitors, selective COX-2 inhibitors and nitric oxide (NO)-releasing aspirin in the healing of ulcerative colitis.
METHODS: Rats with 2,4,6 trinitrobenzenesulfon-ic acid (TNBS)-induced colitis received intragastric (ig) treatment with vehicle, aspirin (ASA) (a non-selective COX inhibitor), celecoxib (a selective COX-2 inhibitor) or NO-releasing ASA for a period of ten days. The area of colonic lesions, colonic blood flow (CBF), myeloperoxidase (MPO) activity and expression of proinflammatory markers COX-2, inducible form of nitric oxide synthase (iNOS), IL-1β and tumor necrosis factor (TNF)-α were assessed. The effects of glyceryl trinitrate (GTN), a NO donor, and 2-(4-carboxyphenyl)-4,5-dihydro-4,4,5,5-tetramethyl-1H-imidazolyl-1-oxy-3-oxide, onopotassium salt (carboxy-PTIO), a NO scavenger, administered without and with ASA or NO-ASA, and the involvement of capsaicin-sensitive afferent nerves in the mechanism of healing the experimental colitis was also determined.
RESULTS: Rats with colitis developed macroscopic and microscopic colonic lesions accompanied by a significant decrease in the CBF, a significant rise in colonic weight, MPO activity and plasma IL-1β and TNF-α levels. These effects were aggravated by ASA and 5-(4-chlorophenyl)-1-(4-methoxyphenyl)-3-(trifluoromethyl)-1H-pyrazole (SC-560), but not celecoxib and counteracted by concurrent treatment with a synthetic prostaglandin E2 (PGE2) analog. Treatment with NO-ASA dose-dependently accelerated colonic healing followed by a rise in plasma NOx content and CBF, suppression of MPO and downregulation of COX-2, iNOS, IL-1β and TNF-α mRNAs. Treatment with GTN, the NO donor, significantly inhibited the ASA-induced colonic lesions and increased CBF, while carboxy-PTIO or capsaicin-denervation counteracted the NO-ASA-induced improvement of colonic healing and the accompanying increase in the CBF. These effects were restored by co-treatment with calcitonin gene related peptide (CGRP) and NO-ASA in capsaicin-denervated animals.
CONCLUSION: NO-releasing ASA, in contrast to ASA, COX-1 inhibitors, and SC-560, accelerated the healing of colitis via a mechanism involving NO mediated improvement of microcirculation and activation of sensory nerves releasing CGRP.
- Citation: Zwolinska-Wcislo M, Brzozowski T, Ptak-Belowska A, Targosz A, Urbanczyk K, Kwiecien S, Sliwowski Z. Nitric oxide-releasing aspirin but not conventional aspirin improves healing of experimental colitis. World J Gastroenterol 2011; 17(36): 4076-4089
- URL: https://www.wjgnet.com/1007-9327/full/v17/i36/4076.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i36.4076
In humans inflammatory bowel disease (IBD) includes ulcerative colitis (UC) and Crohn’s disease, considered as chronically relapsing disorders. The pathogenesis of IBD is complex, with individuals displaying a genetic predisposition, specific immunological properties of the gastrointestinal (GI) mucosa and the type of GI microflora all being involved[1-4]. The features of animal models of colitis do not necessary mimic the human scenario of UC with the use of chemical stimuli and the magnitude of inflammatory changes. However, it is believed that an increase in mucosal prostaglandin (PG) synthesis, mainly cyclooxygenase (COX)-2 derived, correlates with the disease activity of human IBD and experimental colitis in rats[5]. Nonsteroidal anti-inflammatory drugs (NSAIDs) are commonly used in the treatment of pain and inflammation, but are also recommended prophylactically and used as therapeutic strategies against both neurological and cardiological disorders. The therapeutic effects and side effects of NSAIDs in the gut are the consequence of the inhibition of COX activity, which is the key enzyme in the biosynthesis of prostanoids from arachidonate[6]. It was reported that COX-2 is undetectable or expressed at very low levels in the healthy GI mucosa of humans and animals but is upregulated in the GI tract of individuals with inflammatory conditions[7]. Thus, it has been suggested that the anti-inflammatory action of NSAIDs depend on the inhibition of COX-2 activity, whereas side effects, such as gastrointestinal damage and renal toxicity, is a consequence of COX-1 inhibition[8,9]. It is believed that COX-2 derived PG play an important role in the healing process of colitis, which is similar to that observed for the mechanisms of gastric ulcers[10,11]. Results of studies on the influence of COX-2 inhibitors in experimental colitis and human IBD thus far have provided conflicting evidence for the exacerbation of colitis, and the attenuation of inflammation by COX-2 inhibitors[12,13].
The mechanism of ulcerogenic activity of NSAIDs on the healing of the intestinal and colonic lesions has not been fully explained. Reuter et al[12] reported that NSAIDs can exacerbate colitis by a mechanism attribu-table to the suppression of COX-2 derived PG synthesis. Tanaka et al[14] showed that the non-selective COX inhibitor indomethacin, suppressed the mucosal prostaglandin E2 (PGE2) level, which in turn caused intestinal damage that was accompanied by the upregulation of COX-2 expression. It should be emphasized that neither a selective COX-1 inhibitor [5-(4-chlorophenyl)-1-(4-methoxyphenyl)-3-(trifluoromethyl)-1H-pyrazole (SC-560)], nor a COX-2 selective inhibitor (rofecoxib) when applied alone caused intestinal damage[8,15,16]. Recent evidence indicates that incorporation of a nitric oxide (NO) generating moiety into the basic structure of NSAIDs, such as aspirin, attenuates the ulcerogenic activity of native NSAID[17]. Under basal conditions, NO derived from the activity of constitutive NO synthase (cNOS) contributes to the maintenance of intestinal integrity and the control of intestinal motility[2,18].
In human IBD and experimental colitis, the inducible form of nitric oxide synthase (iNOS), excessive production of NO derived from iNOS and abundant amounts of toxic peroxynitrite are observed[2]. On the other hand, NO was shown to possesses anti-inflammatory properties and contributes to the resolution of intestinal inflammation[18]. Moreover, iNOS deficient mice with colitis were more susceptible to inflammation as compared to animals with normal iNOS expression[3]. The role of NO released from NO-NSAIDs in the mechanism of gastroprotection has been well documented[19-21], but the efficacy of NO-aspirin (ASA) in the healing of experimental colitis has yet to be determined.
In this study, we focused mainly on the mechanism of healing of colitis by NO-ASA and therefore we compared the effect of vehicle, conventional NSAIDs such as ASA and indomethacin, SC-560 (a selective COX-1 inhibitor), celecoxib (a selective COX-2 inhibitor) with the new derivative of ASA, NO-releasing ASA, on the intensity of inflammation and the accompanying alterations in the colonic blood flow (CBF), myeloperoxidase (MPO) activity and expression of mRNA for COX-2, IL-1β, tumor necrosis factor (TNF)-α and iNOS and their activities in rats with trinitrobenzenesulfonic acid (TNBS)-induced colitis. An attempt was made to determine the involvement of NO in the mechanism of healing of colitis by using NO-ASA and ASA-treated rats with 2-(4-carboxyphenyl)-4, 5-dihydro-4, 4, 5, 5-tetramethyl-1H-imidazolyl-1-oxy-3-oxide, onopotassium salt (carboxy-PTIO), which is a NO scavenger and glyceryl trinitrate (GTN), an NO donor, respectively. Also, the importance of sensory nerve neurotransmitters in the healing of colitis were determined in rats by the functional ablation of sensory nerves by capsaicin, in the absence and the presence of NO-derivative of ASA.
Animal studies were carried out on male Wistar rats weighing 180-220 g. The animals had free access to water and food and were adapted to laboratory conditions and 12 h day/night cycles for 10 d after TNBS administration. The study was approved by the local Ethical Committee at the Jagiellonian University Medical College in Cracow, Poland and run in accordance with the Helsinki declaration.
Colitis in rats was induced by rectal administration of TNBS (Sigma, Slough, United Kingdom) at a dose of 10 mg/kg, dissolved in 50% solution of ethanol as reported in our previous study[22]. Briefly, the animals were anaesthetized with phenobarbital (60 mg/kg ip) and TNBS was administered into the colon in a volume of 0.25 mL per rat at a depth of 8 cm from the rectum with the use of a soft polyethylene catheter. Until the moment of awakening the rats were positioned in the Trendelenburg position so as to avoid loss of the TNBS solution via the rectum. Animals in the control group were given 0.9% saline or in some cases 50% ethanol in the same volume, corresponding to the rats that were administered TNBS.
Animals with TNBS-induced colitis were randomized into 8 experimental groups (A-H), consisting of 6-10 rats per group. Rats received ig the following daily treatments: A: vehicle (saline); B: ASA (80 mg/kg) or indomethacin (5 mg/kg) suspended in 0.25% carboxy methylcellulose (CMC); C: SC-560 (5 mg/kg); D: celecoxib (10 mg/kg); E: NO-ASA (80 mg/kg; NicOx SA, Sophia Antipolis, France); F: PGE2 (5 μg/kg) combined with non-selective COX-1 (ASA) and selective COX-2 (celecoxib) inhibitors; G: GTN (10 mg/kg ig), a NO donor combined with ASA and carboxy-PTIO (5 mg/kg ig) combined with NO-ASA, both NSAID administered in a dose of 80 mg/kg. In series H consisting of 30 rats, capsaicin was administered in a large neurotoxic dose of 125 mg/kg applied in three doses of 25, 50 and 50 mg/kg 14 d prior to TNBS administration to induce the functional ablation of sensory nerves as described previously[23]. On day 15, capsaicin-denervated rats received TNBS and were subsequently given NO-ASA (80 mg/kg ig) with or without calcitonin gene related peptide (CGRP) (10 μg/kg sc), and administration was similar to those treated with vehicle (saline). The doses of NO-ASA and selective COX-1 and COX-2 inhibitors were selected on the basis of our group and others published evidence[14,15,19-21]. In the doses used in this study, the COX-1 and COX-2 selective inhibitors failed to produce gastrointestinal lesions after single or prolonged administration. The dose of 80 mg/kg of ASA was selected based on our preliminary determination of the dose-dependency of this conventional ASA applied in graded doses starting from 10 mg/kg up to 160 mg/kg on healing of TNBS colitis. ASA given in a dose of 10 mg/kg failed to significantly affect the healing of colitis but when this NSAID was administered ig in graded doses of 40 mg/kg, 80 mg/kg and 160 mg/kg, the area of colonic damage was significantly increased by 23%, 42% and 74%, respectively, at day 10 upon TNBS administration (data not shown). We have previously published that 80 mg/kg of ASA is equimolar to 128 mg/kg of NO-ASA and this dose of NO-ASA by itself does not influence the gastrointestinal integrity but markedly increases organ blood flow[21].
After 1, 3, 10 and 14 d from induction of colonic lesions with TNBS, the animals were weighed and anaesthetized to determine CBF using the H2-gas clearance technique[22,23]. The abdominal cavity was opened and after separation of the colon, the CBF in the areas of the mucosa not affected by inflammatory lesions was measured. CBF was expressed as a percentage of the CBF in the vehicle-control rats without TNBS administration.
At the termination of the experiment, the entire colon was removed, isolated from surrounding tissues, opened along the antimesenteric border, rinsed, weighed, and processed for gross and histology determinations. The areas of colonic damage were evaluated planimetrically (Morphomat, Carl Zeiss, Berlin, Germany) by two independent researchers. Subsequently, fragments of the colon (2 mm × 10 mm) with colonic lesions were sampled, fixed with formaldehyde, embedded in paraffin and routinely stained with haematoxilin and eosin for histological assessment.
The presence and intensity of histological changes was evaluated for the following criteria: presence, area and depth of ulceration, presence and intensity of inflammatory infiltrations, ulcerations and fibrosis[24].
Determination of plasma IL-1βand TNF-αlevels, NO concentration the mucosal generation of prostaglandin E2 and gastric mucosal MPO activity
Immediately after CBF measurements, a venous blood sample was drawn from the vena cava and placed into EDTA-containing vials and used for the determination of plasma IL-1β and TNF-α. Blood was collected and placed into sterile, plastic syringes, kept in ice till centrifugation. The blood samples were centrifuged at a speed of 1000 g for 10 min at a temperature of 15 °C temperature and the sera were stored at -80 °C. The serum levels of proinflammatory cytokines IL-1β and TNF-α were evaluated with high sensitive enzyme-linked immunosorbent assay (ELISA) (Quantikine HS, R and D Systems, Minneapolis, Minn., United States) according to manufacturer’s instructions. Intensity of the color reaction was estimated in the spectrophotometer Stat Fax 2100 (Awareness Technology Inc., Pal City, FL, United States) at 490 nm. The intra-and inter-assay coefficients of variation were 8.5% and 10.6%, respectively, for TNF-α, and 10.2% and 10.4%, respectively, for IL-1β. The plasma NO concentration was quantified indirectly as nitrate (NO3-) and nitrite (NO2-) levels using the nitrate/nitrite kit purchased from Cayman Lab, Michigan, United States as described before[25,26]. This method is based on the Griess reaction and the generation of a chromophore absorbing at 595 nm, according to the original procedure reported previously[26]. Since NO in the colonic mucosa is quickly transformed into NO3- and NO3-[25], the total nitrate and nitrite concentration (NOx) is routinely used as an index of NO production. In order to determine NOx, the blood was withdrawn and centrifuged for 10 min at 3000 r/min, the samples were mixed with Griess reagent from the commercially available kit.
In rats with colitis treated with or without concurrent COX-1 and COX-2 inhibitors, the mucosal samples were taken by biopsy (about 200 mg) from unchanged colon mucosa without mucosal lesions to determine PGE2 generation by radioimmunoassay (RIA) as described previously[19,21]. Briefly, the mucosal samples were placed in preweighed Eppendorf vial with 1 mL of Tris buffer (50 mmol/L, at pH 9.5) added to each vial. The samples were finally minced (15 s) with scissors, washed and centrifuged for 10 s, with the pellet being resuspended again in 1 mL of Tris. Then, each sample was incubated on a vortex mixer for 1 min and centrifuged for 15 s. The pellets were weighed and the supernatant was transferred to a second Eppendorf vial containing indomethacin (10 mmol/L) and kept at -20 °C until the time for RIA. The capability of the mucosa to generate PGE2 was expressed in nanograms of wet tissue weight.
Fragments of colonic tissue weighing about 200 mg were collected and frozen in -70 °C for the determination of MPO activity by ELISA as reported before[22].
Expression of COX-2, IL-1β, TNF-αand iNOS transcripts in the rat colonic mucosa determined by reverse transcriptase-polymerase chain reaction
The mRNA expression for COX-2, IL-1β, TNF-α and iNOS were determined by reverse transcriptase-polymerase chain reaction (RT-PCR) in the unchanged colon mucosa of intact rats or those with TNBS colitis given vehicle, ASA and NO-ASA. Samples of the colon mucosa (about 200 mg) were scrapped off into ice using glass slides and then immediately snapped frozen in liquid nitrogen and stored at -80 °C. The total RNA was isolated from the colon mucosa according to the technique using Trizol Reagent (Invitrogen, Carlsbad, United States) and the manufacturer’s protocol[27]. The first strand of cDNA was synthesized from total cellular RNA (2 μg) using a Reverse Transcription System (Promega, Madison, United States). The PCR was carried out in an automatic DNA thermal cycler, using 1 μg of cDNA and Promega PCR reagents. For amplification of β-actin, COX-2, TNF-α, IL-1β and iNOS cDNA, and gene-specific primers (SIGMA-Aldrich St. Louis, United States) were used. The sequences for primers used in this study are presented in Table 1. Primer annealing was carried out as follows: at 56 °C, 60 °C, 60 °C and 58 °C for COX-2, IL-1β, TNF-α and iNOS, respectively. Amplification of the control rat β-actin was performed on the same samples to verify the RNA integrity. PCR products were separated by electrophoresis in 2% agarose gel containing 0.5 μg/mL ethidium bromide and then visualized under UV light. Location of the predicted PCR product was confirmed by using O’Gene Ruler 50 bp DNA ladder (Fermentas, Life Sciences, San Francisco, United States) as a standard marker. Comparison between different treatment groups was made by determination of COX-2, IL-1β, TNF-α and the iNOS/β-actin ratio of the immunoreactive area by densitometry (Gel-Pro Analyzer, Fotodyne Incorporated, Hartland, WI, United States).
| Gene | Primer | |
| β-actin | Upstream | 5’-TTG TAA CCA ACT GGG ACG ATA TGG-3’ |
| Downstream | 5’-GAT CTT GAT CTT CAT GGT GCT AGG-3’ | |
| IL-1β | Upstream | 5’-GCT ACC TAT GTC TTG CCC GT-3’ |
| Downstream | 5’–GAC CAT TGC TGT TTC CTA GG-3’ | |
| TNF-α | Upstream | 5’-TAC TGA ACT TCG GGG TGA TTG GTC C-3’ |
| Downstream | 5’–CAG CCT TGT CCC TTG AAG AGA ACC-3’ | |
| COX-2 | Upstream | 5’–ACA ACA TTC CCT TCC TTC-3’ |
| Downstream | 5’–CCT TAT TTC CTT TCA CAC C-3’ | |
| iNOS | Upstream | 5’–CCA CAA TAG TAC AAT ACT ACT TGG–3’ |
| Downstream | 5’–ACG AGG TGT TCA GCG TGC TCC ACG–3’ |
Results are expressed as mean ± SE. Statistical analysis was done using Student t test or analysis of variance and two-way ANOVA test with Tukey post hoc test where appropriate. Differences of P < 0.05 were considered significant.
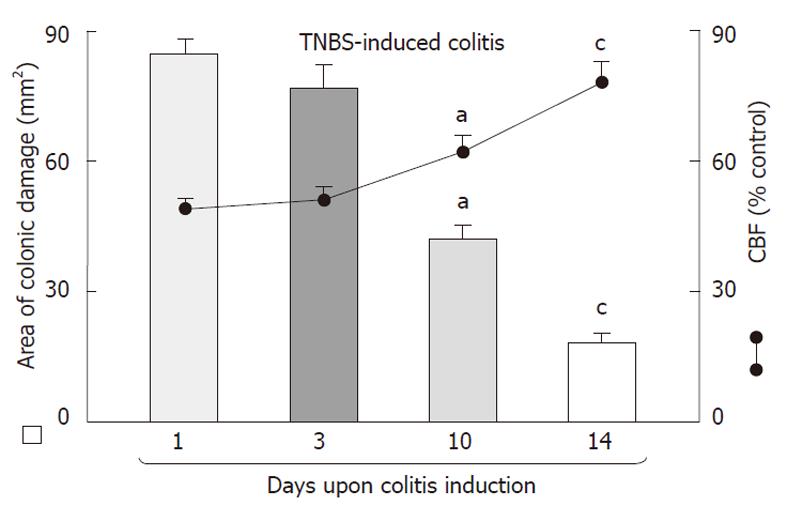
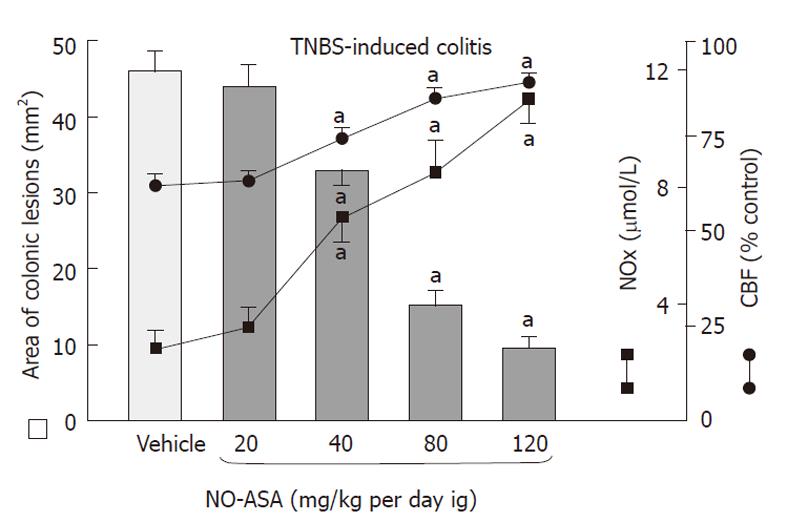
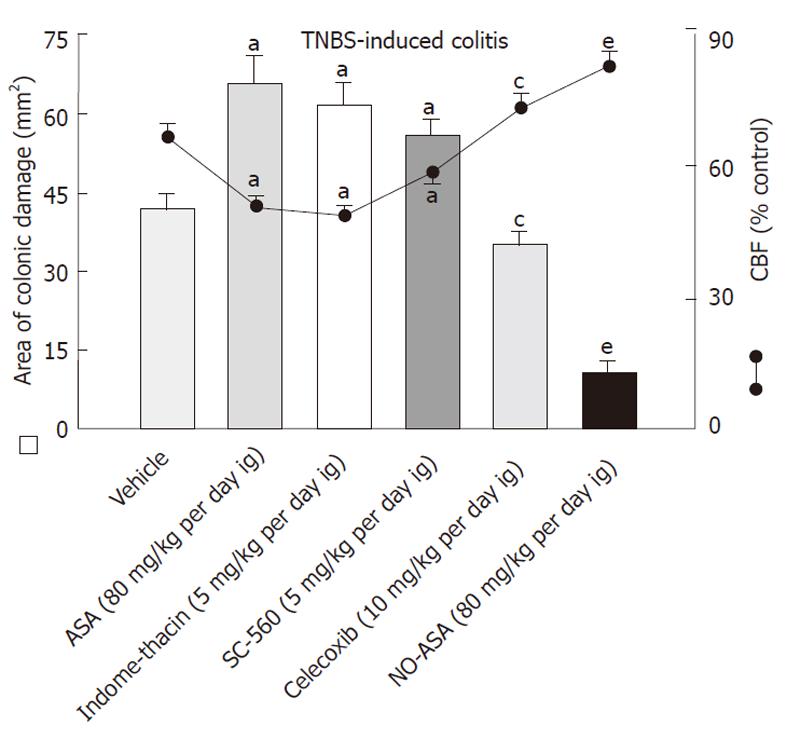
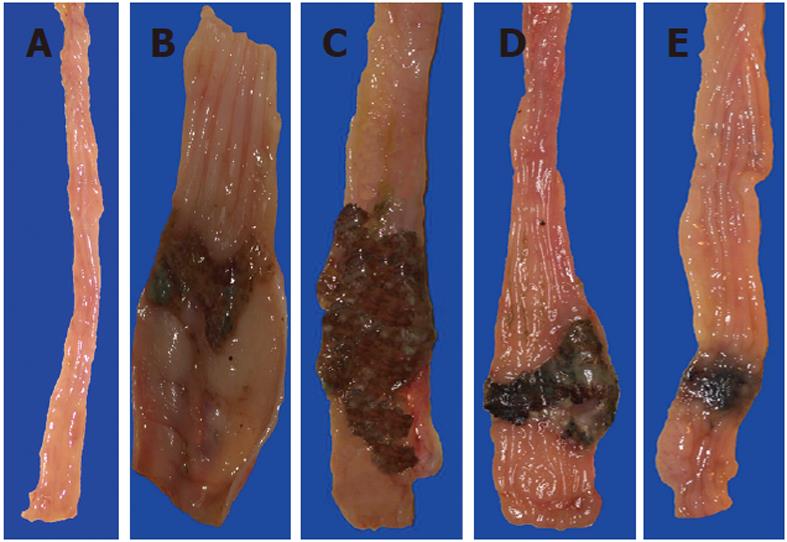
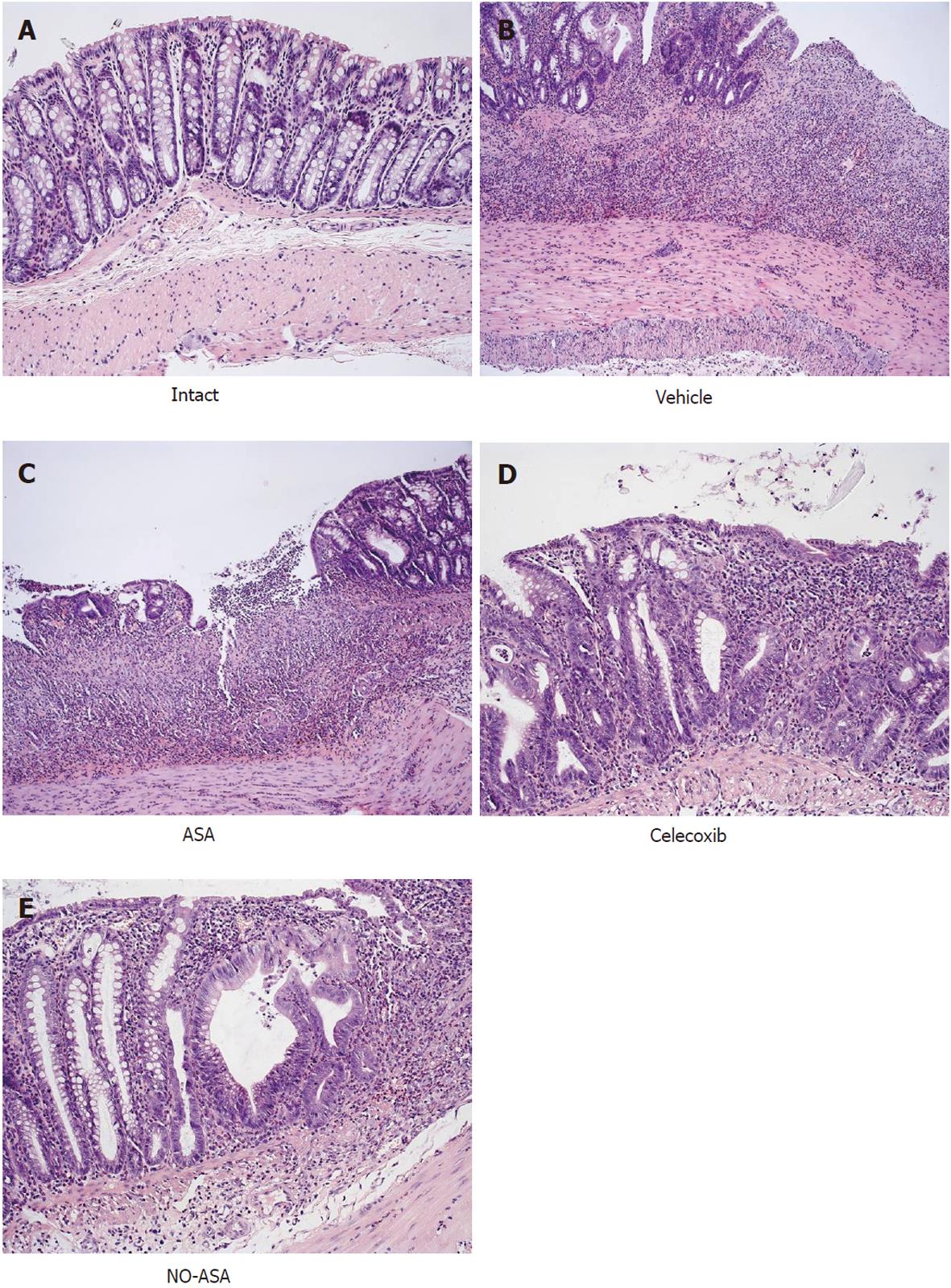
Intrarectal administration of TNBS caused severe damage to the colonic mucosa manifested by inflammatory changes in the colon with extensive ulcerations of the mucosa. The area of these lesions was at a maximum at 24 h, it was not significantly decreased on day 3 but then it significantly declined at day 10 and day 14 (Figure 1). The CBF was decreased by about 46% and 43% on day 1 and 3, respectively, but it was significantly increased on day 10 and 14 (Figure 1). The 10 d administration of NO-ASA applied ig in gradual concentrations ranging from 20 mg/kg up to 120 mg/kg produced a dose-dependent decrease in the area of colonic lesions and this effect was accompanied by a significant rise in plasma NOx concentrations and CBF (Figure 2). In contrast, after 10 d of ASA, indomethacin and SC-560 administration, a significant aggravation of the area of colonic lesions and a significant fall in CBF when compared with vehicle was observed (Figure 3). Similarly as shown in Figure 2, the treatment with NO-ASA applied in a dose of 80 mg/kg ig produced a significant decrease in the area of colonic damage and significantly increased the CBF comparing to vehicle-control (Figure 3). Treatment with celecoxib also significantly decreased the area of colonic damage and produced a significant increase in CBF; however, these changes were significantly less pronounced as compared to those achieved with NO-releasing ASA (Figure 3). Ten days after colitis induced by TNBS treatment all animals had a significant reduction in body weight compared with the control rats without TNBS administration and this loss of body weight was counteracted by treatment with NO-ASA. In contrast, treatment with ASA and indomethacin failed to attenuate the loss of weight induced by TNBS administration (data not shown). As shown in Figure 4A, the intact colonic mucosa showed a normal macroscopic appearance. At day 10 after TNBS administration, colonic damage was still observed in vehicle-control animals (Figure 4B). This gross damage, as reflected by the area of ulceration, was exacerbated by treatment with ASA but significantly reduced by treatment with celecoxib. The area of TNBS-induced damage was significantly smaller in NO-ASA-treated animals as compared to that in rats treated with vehicle or celecoxib (Figure 4E). By histology, intact colonic mucosa showed regular colonic architecture and continuity with no signs of inflammation (Figure 5A). In vehicle-treated TNBS rats on day 10 deep ulcerations and an intense infiltrate with the presence of numerous neutrophils penetrating the muscularis mucosa and submucosa were observed. In addition, features of regeneration, adjacent to the ulcer margin were clearly visible (Figure 5B). In ASA-treated rats with colitis (Figure 5C) there was a deep ulceration with necrosis and an intense inflammation followed by severe neutrophil infiltration and formation of granulation tissue penetrating the muscle layer of the muscularis propria. Less regeneration was observed with ASA (Figure 5C) than in vehicle (control) colonic mucosa (Figure 5B). The area of colonic damage was significantly smaller in celecoxib-treated animals and in those treated with ASA and vehicle (Figure 5B and D). Partially healed epithelium and abnormal crypt architecture possessing a more pronounced regeneration were observed in colonic mucosa of the rat with colitis treated with celecoxib (Figure 5D) when compared to those receiving ASA. By histology, the area of colonic damage was significantly decreased in NO-ASA treated colitis rats as compared to all other COX inhibitors and vehicle-treated rats (Figure 5E). NO-ASA treated rats showed the most advanced healing of colitis, as reflected by the degree of epithelial regeneration and the significantly smaller infiltration of neutrophils. Part of the crypts, which were distant to the scar, displayed a more normal appearance (Figure 5E). In the indomethacin-group, similar to the ASA-group, the massive hemorrhagic lesions and transmural necrosis coexisting with acute inflammatory infiltrate were observed (data not shown).
As shown in Table 2, treatments with ASA, indomethacin and SC-560 were accompanied by a greater increase in the weight of colonic tissue and a significant rise in MPO activity in TNBS-treated animals as compared to vehicle control. In contrast, treatment with celecoxib decreased the weight of colonic tissue and MPO activity below those in the vehicle group; however, these changes were significantly smaller than those caused by NO-ASA (Table 2). In contrast, treatment with NO-ASA resulted in a significant decrease in the area of colonic lesions and colonic tissue weight, accompanied by a significant rise in CBF and a fall in MPO activity compared to the respective values in rats treated with vehicle or those treated with non-selective and selective COX-1 and COX-2 inhibitors (Table 2, Figure 3).
| Treatment | Weight (mg) | MPO (ng/mL) | IL1-β(pg/mL) | TNF-α(pg/mL) |
| Vehicle | 1150 ± 23.1 | 51 ± 8.1 | 42 ± 3.4 | 4.8 ± 0.6 |
| ASA (80 mg/kg) | 1580 ± 41.4a | 78 ± 9.3a | 65 ± 5.1a | 8.5 ± 0.95a |
| Indomethacin (5 mg/kg) | 1450 ± 45.8a | 65 ± 5.2a | 58 ± 4.1a | 7.8 ± 0.62a |
| SC-560 (5 mg/kg) | 1310 ± 32.4a | 52 ± 6.8a | 53 ± 3.9a | 7.5 ± 0.46a |
| Celecoxib (10 mg/kg) | 1082 ± 14.9c | 44 ± 4.3c | 36 ± 2.2c | 3.8 ± 0.33c |
| NO-ASA (80 mg/kg) | 710 ± 12.3e | 35 ± 3.2e | 18 ± 1.5e | 2.4 ± 0.21e |
Plasma levels of proinflammatory cytokines IL-1β and TNF-α, which were negligible in intact rats (data not shown), were markedly elevated in animals administered with ASA, indomethacin and SC-560, when compared to those treated with vehicle (Table 2). Plasma IL-1β and TNF-α levels were significantly attenuated by treatment with the COX-2 inhibitor (celecoxib) when compared to the levels of those cytokines achieved in animals treated with non-selective COX inhibitors (ASA and indomethacin). NO-ASA was administered at a similar dose to that of native ASA, which accelerated the healing of colonic damage, and significantly attenuated plasma IL-1β and TNF-α levels as compared to those achieved with non-selective COX inhibitors (Table 2).
As shown in Table 3, the intestinal level of PGE2 increased in the colonic mucosa of animals with TNBS colitis as compared to that measured in vehicle-controls that did not receive TNBS. Administration of ASA and indomethacin significantly reduced the colonic PGE2 content when compared to the respective value in vehicle-treated control animals. Treatment with ASA and SC-560, the selective COX-1 inhibitor, also significantly reduced PGE2 generation; however this inhibition was less potent than in the case of ASA and indomethacin (Table 3). Administration of the selective COX-2 inhibitor celecoxib, and NO-ASA significantly inhibited the colonic PGE2 generation when compared to the value measured in vehicle-control rats (Table 3).
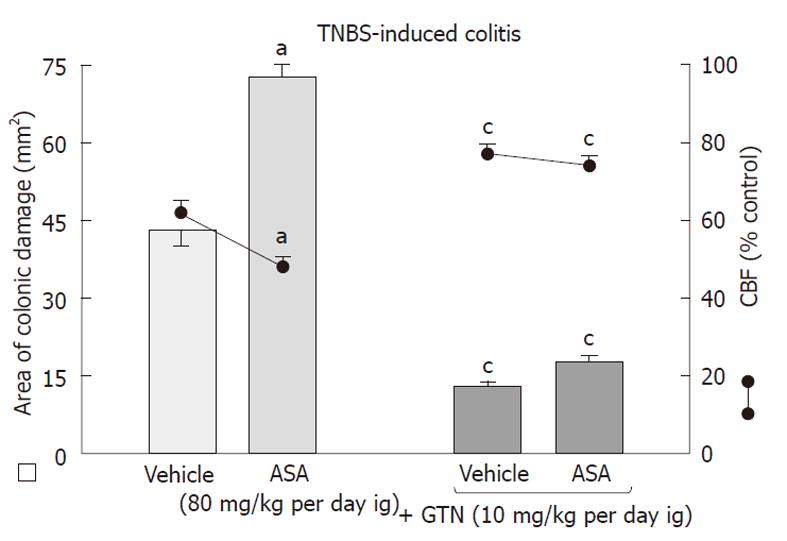
As shown in Figure 6, the area of colonic damage was significantly increased in animals treated for 10 d with ASA and this effect was accompanied by a significant fall in CBF as compared with the respective values observed in vehicle-control animals. Concurrent treatment with GTN, which by itself significantly attenuated the area of colonic damage markedly decreased these lesions and significantly improved the CBF.
Figure 7 shows the effect of 10 d administration of NO-ASA applied ig at a dose of 80 mg/kg on the area of colonic damage and accompanying alterations in CBF. Treatment with NO-ASA caused a similar decrease in area of colonic damage and a significant rise in CBF as presented in Figure 2. The concomitant treatment of carboxy-PTIO, the NO scavenger, which by itself had no influence on the CBF, completely abolished the beneficial effect of NO-ASA on healing of TNBS-induced colonic lesions and reversed an increase in CBF induced by this agent (Figure 7).
Effect of replacement therapy with exogenous PGE2 on TNBS-induced colonic damage and changes in the weight of colonic tissue, CBF, plasma IL-1βand TNF-αlevels in rats treated with COX inhibitors
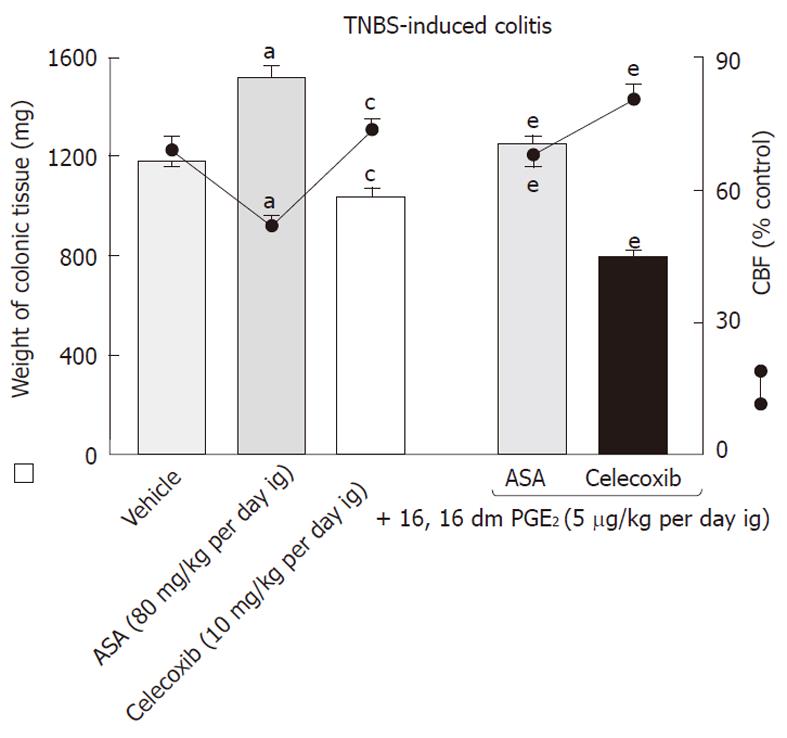
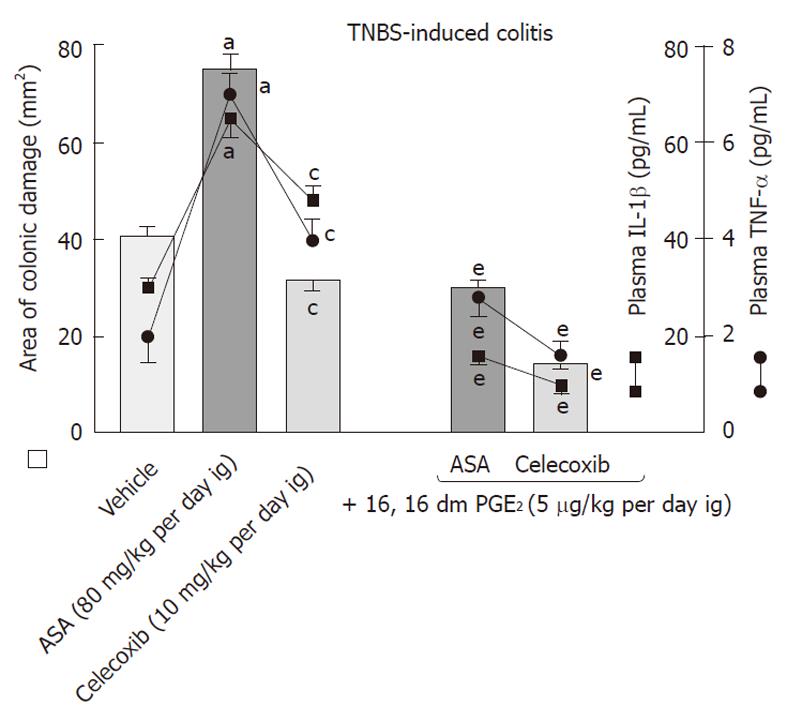
The role of PGE2 in the process of healing of colonic lesions in TNBS-induced colitis animals given ASA and celecoxib was determined using rats exogenously administered with PGE2 in doses of 5 μg/kg added to both COX-1 and COX-2 inhibitors. An increase in both the area of colonic lesions and colonic weight as well as a significant fall in CBF induced by ASA and celecoxib were counteracted by concomitant treatment with exogenous PGE2 (Figures 8 and 9). Moreover, the administration of this synthetic analogue of PGE2 not only reduced the area of colonic damage but also significantly suppressed the rise in plasma IL-1β and TNF-α compared to those in ASA- or celecoxib-treated rats without PGE2 administration (Figure 8).
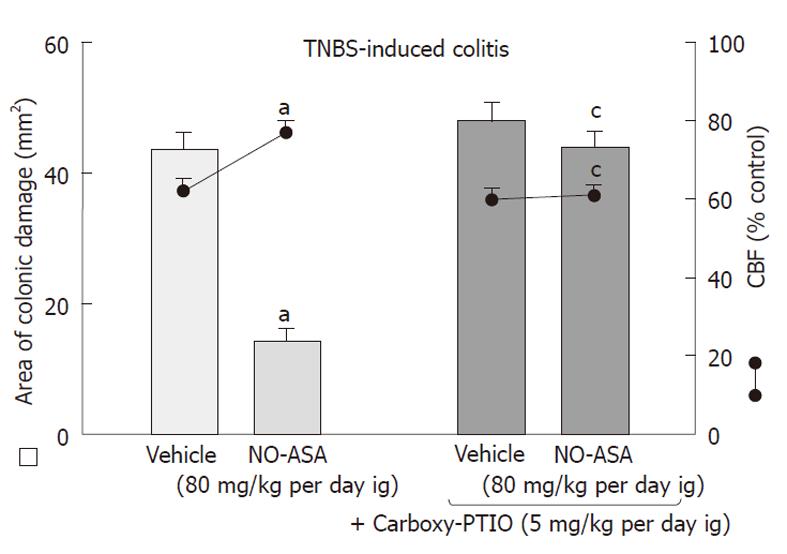
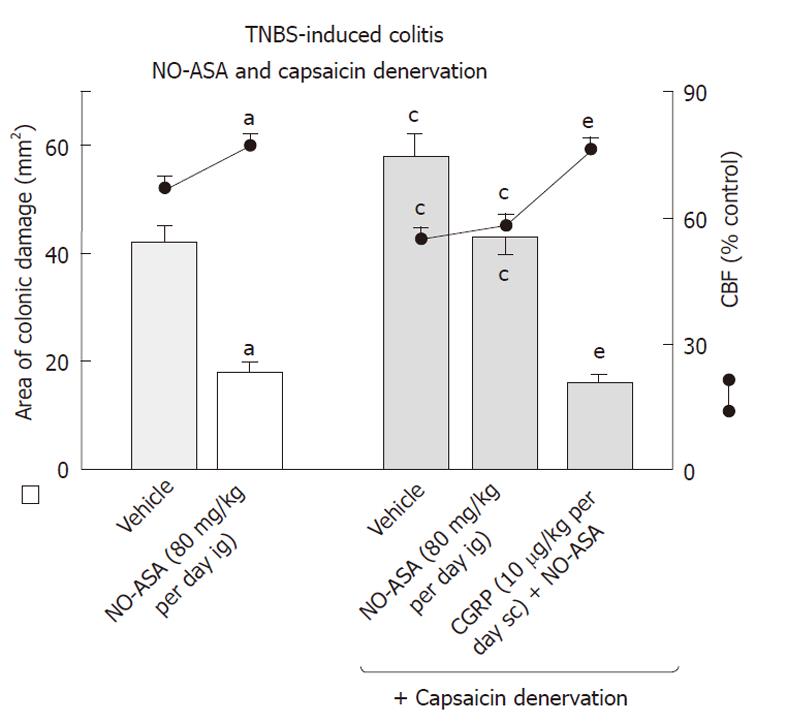
Capsaicin-deactivation of sensory nerves, which by itself increased the area of colonic lesions and produced a significant fall in CBF when compared to those in vehicle-controlled rats, significantly attenuated the NO-ASA induced acceleration of healing of these colonic lesions and the accompanying increase in CBF (Figure 10). Concurrent administration of CGRP (10 μg/kg sc) with NO-ASA restored the healing of colonic damage as reflected by the significant decrease in colonic damage and the increase in CBF induced by this NO-derivative of ASA in rats with capsaicin denervation.
Effect of vehicle, ASA and NO-ASA treatments on the mucosal expression of COX-2, IL-1β, TNF-αand iNOS in rats with colitis
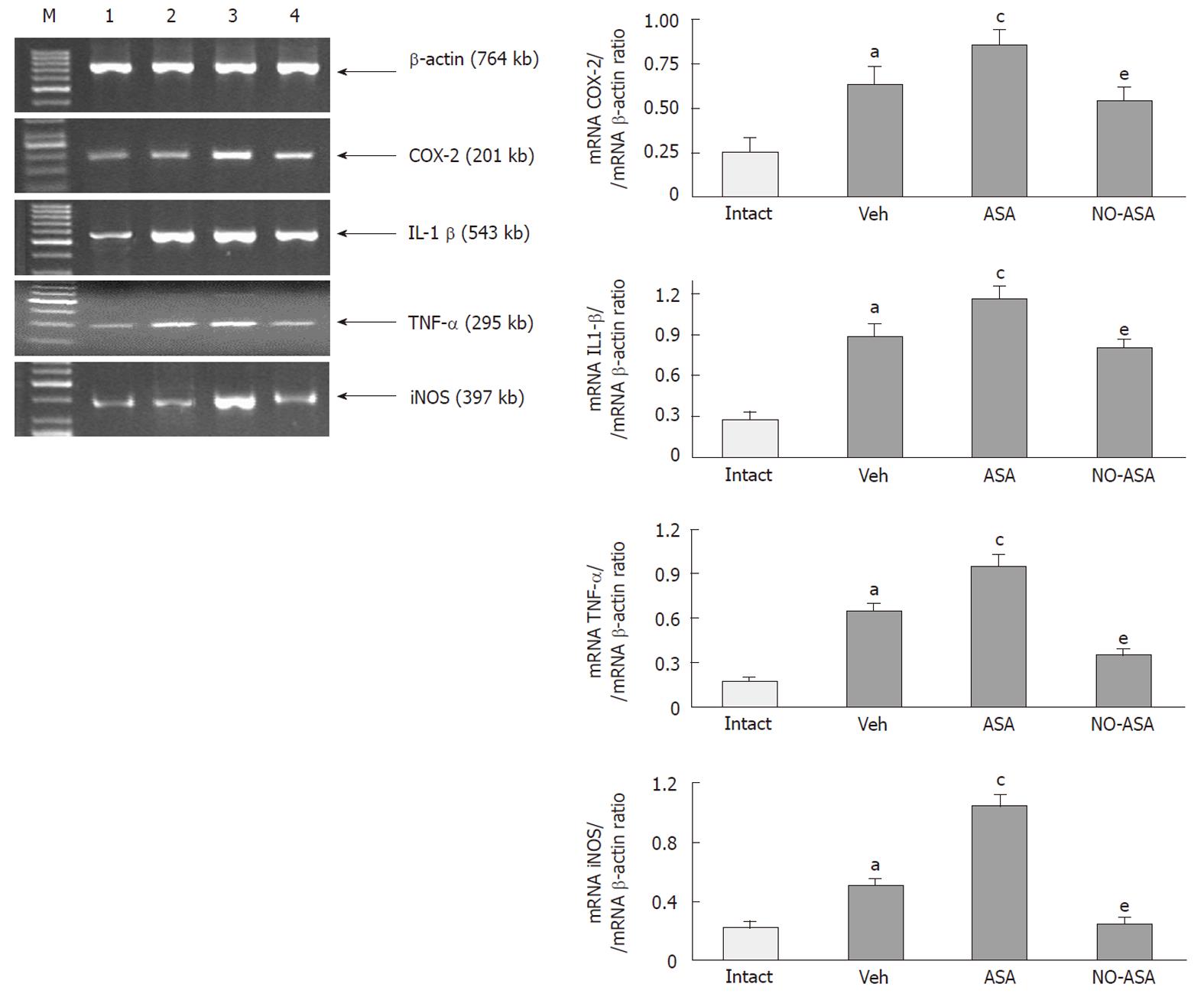
As shown in Figure 11 (left panel), the signal for the expression of COX-2, IL-1β, TNF-α and iNOS was significantly increased in vehicle-treated colonic mucosa (lane 2) in rats with colitis when compared to that in the intact mucosa (lane 1). The ratio of COX-2, IL-1β, TNF-α and iNOS mRNA over β-actin mRNA, confirmed that expression of COX-2, IL-1β, TNF-α and iNOS mRNAs were significantly elevated in TNBS-treated animals (Figure 11, right panel). Treatment with ASA resulted in a strong signal of mRNAs for COX-2, IL-1β, TNF-α and iNOS. The semi-quantitative ratio of COX-2, IL-1β, TNF-α and iNOS (lane 3) confirmed that the expression of these inflammatory markers was significantly increased in the colonic mucosa of rats treated with ASA (Figure 11, right panel). In NO-ASA-treated animals the signal for COX-2, IL-1β, TNF-α and iNOS mRNAs was less pronounced (lane 4) and the determination of the ratio of COX-2, IL-1β, TNF-α and iNOS confirmed that expression of mRNAs for these inflammatory factors was significantly inhibited as compared to those recorded in ASA-treated animals (Figure 11, right panel).
Worldwide, NSAIDs are among the most widely prescribed medications, and are often the drugs of choice for the treatment of various inflammatory conditions. Currently, NSAIDs, including ASA, are recommended as a prophylactic therapy against neurological and cardiologic disorders including strokes and heart infarcts[28]. In humans, UC is a chronic relapsing disorder, characterized by colon mucosa inflammation, ulcerations, diarrhea, bloody stools and abdominal pain[1,3]. The inflamed mucosa of the lower GI tract produces a high amount of PG derived from COX-2 expression and activity in response to stimulation by proinflammatory cytokines and growth factors, also co-expressed at a site of inflammation[14]. Although the NSAIDs effect on the upper GI tract is well documented, the mechanisms by which NSAIDs and their new NO-releasing derivatives affect the course and the healing of colitis in humans and experimental animals has not been fully explored. In the present study, using a rodent model of colitis we determined the effect of the new ASA derivative NO-ASA, and selective and non-selective COX-1 and COX-1 inhibitors on the healing process of this colonic damage, and the effects on weight of colonic tissue, the CBF and MPO activity. Moreover, we assessed the colonic expression of COX-2 which in contrast to COX-1 expression is negligible in normal GI mucosa, but has been shown to be significantly upregulated in most GI-related disorders such as gastritis, gastric mucosal damage, ulcers and ulcerative colitis[3,11,14,29].
Interestingly, the inhibition of COX-2 activity by selective COX-2 inhibitors enhances gastric damage induced by stress and ischemia-reperfusion and delays the healing process of gastric ulcers in the GI tract[29,30]. Moreover, a single application of either COX-1 or COX-2 inhibitors does not cause GI damage, but concurrent treatment with inhibitors of COX-1 and COX-2 activity, resulted in both gastric and intestinal damage. For instance, administration of celecoxib alone or SC-560 alone failed to cause gastric damage, but administration of both selective COX-1 together with COX-2 inhibitors resulted in the formation of gastric mucosal injury. It was concluded that both COX-1 and COX-2 are essential for the maintenance of the integrity of the upper GI-tract[7,31]. Tanaka et al[31] have shown that only TNF-α was influenced by the administration of SC-560 or celecoxib but other cytokines were not affected in mice models of dextran sulphate (DSS)-induced colitis. In their model of colitis produced by adding 3% DSS to drinking water, the inhibition of both COX-1 and COX-2 resulted in exacerbation by these NSAIDs of a widespread intestinal inflammation in mice. This model has, however, different histological appearance characteristics and time course of pathology[31] from that used in our present study[31]. We have utilized a colitis model with TNBS which rather mimics some features of human UC and Crohn’s disease (CD) which do not readily apply to the DSS model.
Expression of TNF-α is considered an important pathogenic feature of human IBD, especially CD[13]. Another cytokine, IL-1β, plays an immunoregulatory role in amplifying the inflammatory response by inducing the cascade activation of immune cells. In high doses, IL-1β is responsible for the formation of epithelial cell necrosis, edema and neutrophil infiltration[24]. In our study TNBS-induced colonic damage was accompanied by a prominent increase in colon tissue weight as well as the rise in the MPO, the gene expression and the plasma levels of both IL-1β and TNF-α. This data is in keeping with previous observations[12] that pathogenesis of colitis is associated with an increase in expression and activity of TNF-α and IL-1β. Furthermore, treatment with the non-selective COX-1 and COX-2 inhibitors such as ASA or indomethacin in our study produced a further rise in plasma IL-1β and TNF-α levels. In contrast, the administration of celecoxib moderately improved the healing of colitis followed by a minor increase in plasma IL-1β and TNF-α levels, while a significant improvement of this healing, accompanied by the suppression of these proinflammatory cytokines, was observed in NO-ASA-treated animals. We have also found significant differences not only in macroscopic and microscopic appearance of the colonic mucosa treated with the selective COX-2 inhibitor and NO-releasing ASA vs the conventional NSAID (aspirin) on healing of colonic lesions but also in functional alterations such as CBF and MPO activity.
We documented that an increase in plasma levels of IL-1β and TNF-α in colitis was reduced to a greater extent by treatment with NO-ASA and to a lesser extent by a COX-2 inhibitor, which was not the case with the non-selective COX inhibitor, ASA. In ASA-treated rats, the expression and activity of these cytokines were both enhanced. The involvement of PG in healing of colitis is supported by our finding that an exogenously administered synthetic analog of PGE2 reversed the delay in this healing, the increase in colonic weight and the rise in plasma levels of both IL-1β and TNF-α induced by ASA and celecoxib. It is concluded that the lack of intestinal PGE2 plays a crucial role in the pathomechanism of exacerbation of inflammatory colonic lesions and the perturbations in CBF in animals administered non-selective COX inhibitors but not to the same extent as in the case of selective COX-2 inhibitors. This is in keeping with the observation that PGE2 is essential to the process of regeneration of epithelial crypts during the time course of DSS-induced colitis[32]. In another report, PGE2 was shown to inhibit production of proinflammatory cytokines, particularly that of TNF-α[33]. We found an apparent increase in MPO activity, which is considered to be an indicator of neutrophil-induced inflammatory infiltration, which was significantly elevated in colitis. The colonic MPO activity was further enhanced by non-selective COX inhibitors and selective COX-1 inhibitors such as ASA, indomethacin and SC-560, respectively, while both celecoxib and especially NO-ASA attenuated MPO activity in colitis rats treated with vehicle. This deleterious effect of non-selective COX-inhibitors, ASA and indomethacin, observed in our study could not be attributed to GI toxicity of these NSAIDs, since e.g. indomethacin was used in our study in a dose which by itself failed to induce gastric mucosal lesions but as shown before, prolonged the healing of acute and chronic gastric ulcers, mostly due to inhibition of protective PG[19,21]. Indomethacin was previously reported to induce experimental colitis at doses up to 10 mg/kg[34] that was higher than that in our present study.
We were particularly interested in exploring the me-chanism of accelerated colonic healing of the new class of so-called “safer NSAID” such as NO-ASA. In our study the TNBS-induced colitis was associated with a fall in CBF, but this impairment in the colonic microcirculation was worsened by native ASA and indomethacin. Celecoxib moderately enhanced colonic healing and slightly increased CBF, suggesting that this COX-2 inhibitor exerts the opposite effect on colonic microcirculation than non-selective COX inhibitors. In clear contrast, NO-ASA greatly improved CBF and reduced both MPO activity and the expression of mRNAs and reduced plasma IL-1β and TNF-α levels thus contributing to the process of healing of inflammatory lesions. We conclude that these healing and anti-inflammatory effects could be attributed to NO being released from NO-ASA, which ultimately was responsible for the improvement of CBF observed in our study. This is supported by the fact that GTN which is an NO donor, when co-administered with ASA, significantly reduced colonic damage and counteracted the fall in CBF induced by this NSAID. Moreover, the mechanism of the acceleration of colonic healing involves the release of NO from NO-ASA. This notion is supported by the observation that NO-ASA dose-dependently accelerated healing of these lesions followed by an increase in plasma NOx levels. Second, carboxy-PTIO, an NO-scavenger, abolished the healing efficacy of this NO-derivative of ASA and the accompanying increase in CBF. In addition, NO released from NO-ASA could contribute to the intestinal elimination of bacteria, protozoa and fungi as reported previously[35]. In other studies, the addition of a NO-releasing moiety to mesalamine exerted an immunomodulatory effect and suppressed intestinal inflammation by inhibiting T-helper cells while stimulating the activity of the antiinflammatory cytokine IL-10, TGFβ and mucosal Treg pathway whereas standard mesalamine was less effective[36].
It is known that the maintenance of GI mucosal integrity depends on different protective mechanisms against injury, which involves the stimulation of two populations of afferent nerves, vagal and spinal. As shown previously, these sensory neurons are involved in GI protection via activation of vasodilatory and anti-inflammatory reflexes[37]. It was reported that the afferent neurons from the dorsal root ganglia play a role in the local regulation of GI circulation, secretion, motility and the process of mucosal repair and healing after injury[37]. CGRP, a neurotransmitter, is released from the peripheral fibers of sensory neurons and plays an important role in GI mucosal defense[37]. Capsaicin, an active ingredient of red pepper has been found to act on the capsaicin sensitive afferent nerves releasing CGRP[38]. When applied in small doses, capsaicin induces a protective response, but a large dose of this neurotoxin renders the gastric mucosa more susceptible to damage induced by indomethacin, ischemia and reperfusion and cold stress[23,26]. This has also been shown to result in a delay in the process of gastric ulcer healing, an event associated with decreased tissue levels of gastric CGRP[37].
In our study, capsaicin-induced functional ablation of sensory nerves with capsaicin, which by itself markedly prolonged the healing process of colonic lesions, attenuated the increase in the healing of these lesions and the accompanying rise in CBF induced by NO-ASA. The impaired healing and evident fall in CBF were restored by concurrent administration of CGRP with NO-ASA in capsaicin-denervated rats. These findings suggest that sensory nerve neuropeptides such as CGRP may contribute to the healing effect and hyperemia induced by NO-ASA. Both CGRP and NO contribute to hyperemia in the GI tract mucosa and facilitate other mechanisms of defense, such as bicarbonate secretion[21,23]. Existing evidence in the lower GI tract revealed an increased inflammatory reaction in experimental colitis of transient receptor potential vanilloid-1 (TRPV-1) knockout mice[37,38]. TRPV-1 is expressed by many afferent nerves and its activation results in the release of vasodilatory peptides such as CGRP[38,39].
In summary, conventional NSAIDs, such as ASA and indomethacin or selective COX-1 inhibitors such as SC-560[40], delay the healing of experimental colitis and this effect is accompanied by a fall in CBF and an enhancement in gene expression and release of proinflammatory cytokines IL-1β and TNF-α. These deleterious effects are less pronounced with the use of the selective COX-2 inhibitor, celecoxib, which differs with respect to colonic healing with that of conventional ASA. NO-ASA exerts the opposite effects to those of native ASA and selective COX-1 inhibitors on the delay in healing of TNBS colitis and accompanying fall in colonic microcirculation induced by these agents. The beneficial healing action of NO-ASA, involves the NO mediated suppression of proinflammatory cytokines and the activation of sensory nerves resulting in a local release of sensory vasodilatatory neuropeptides such as CGRP.
This article contains data from the paper presented in preliminary form by Zwolinska-Wcislo M, Kwiecien S, Sliwowski Z, Brzozowski T, Drozdowicz D, Mitis-Musiol M, Konturek SJ, Pawlik WW. (2008) “Classic aspirin, COX-2 selective inhibitor and NO derivative of aspirin in the mechanism of healing of ulcerative colitis” GUT 32: Abstract A124 awarded first prize at Gastroenterology XXV UEGW Meeting, Vienna 2008. Authors would like to acknowledge the careful reading of the manuscript by Mrs. Nily Osman.
Nonsteroidal anti-inflammatory drugs (NSAIDs), including aspirin, are among the most prescribed drugs because of their anti-inflammatory, antipyretic, analgesic effects. Inhibition of prostaglandin synthesis seems to be the major mechanism implicated in both the beneficial and adverse effects of NSAIDs. Aside from the systemic activity of NSAIDs, resulting from the inhibition of mainly cyclooxygenase (COX)-1 activity, the NSAIDs exert a deleterious influence on the upper and lower gastrointestinal (GI) mucosal integrity by affecting the local inflammatory mechanisms, such as neutrophil recruitment, the activation of proinflammatory cytokines, impairment of microcirculation and the release of free oxygen metabolites. A new class of nitric oxide (NO)-releasing NSAIDs was developed to limit the serious adverse effects associated with NSAIDs ingestion, but their effect in lower GI healing with respect to colitis in animal models has not been carefully investigated. Sensory neurons releasing neuropeptides such as calcitonin gene related peptide (CGRP) are involved in the mechanism of GI protection and ulcer healing due to activation of vasodilatatory mediators such as CGRP. Currently, it is unknown whether these sensory neuropeptides could influence the healing of ulcerative colitis (UC) in animals treated with NO-releasing aspirin (ASA).
Both UC and Crohn’s disease belong to the category of inflammatory bowel diseases (IBD). It is believed that three major factors influence the pathogenesis of these two diseases: an individual’s susceptibility, microflora of the gastrointestinal tract and immunological properties of the gastrointestinal mucosa. UC is a disease of the colon which is characterized in humans by chronic inflammation in both the mucosal and submucosal layer with a cellular and humoral immunological response. Patients suffering from UC frequently require anti-inflammatory analgesics such as NSAIDs because of inflammatory conditions such as arthritis, sacroilitis or osteoporosis-related fractures. On the other hand conventional NSAIDs may either induce development of colitis in the healthy colon or exacerbate preexisting colitis. Previous studies revealed that COX exists in the following two isoforms: the inducible COX-2, which is detected at the site of inflammation, and COX-1, which functions in a housekeeping fashion, is present in the majority of human tissues and organs, and is responsible for homeostasis and GI tract integrity. PGs induced by COX-2 are involved in maintaining the intestinal mucosa integrity, in the healing of gastrointestinal ulcers and the modulation of IBD. But the inhibition of both COX-1 and COX-2 isoforms seems to be necessary to induce significant intestinal damage. The new class of NSAIDs containing the NO moiety, such as NO-releasing ASA, could be an alternative strategy in the attenuation of GI side effects of conventional NSAIDs such as ASA. This is due to the fact that NO released from ASA possesses anti-inflammatory properties and contributes to the resolution of intestinal inflammation.
The authors attempted to determine the involvement of NO released from NO-ASA and its role in the mechanism of the healing of colitis. This was accomplished by using NO-ASA and glyceryl trinitrate (GTN), an NO donor, added to classic ASA to mimic the protective action of NO-ASA. Treatment with NO-ASA improved the healing of colitis, with these effects as well as the anti-inflammatory properties of NO-ASA being attributed to the NO released from NO-ASA, which was ultimately responsible for the improvement of colonic blood flow (CBF) observed in this study. Furthermore, the authors found that both NO and the activation of sensory nerve neuropeptides such as CGRP may contribute to the healing effects and the hyperemia induced by NO-ASA. This could be responsible for the attenuation of the expression and the release of pro-inflammatory cytokines such as IL-1β and tumor necrosis factor (TNF)-α. This is supported by the fact that GTN, which is an NO donor, when co-administered with ASA, significantly decreased colonic damage and counteracted the decrease in CBF induced by this NSAID. The study demonstrates that NO-ASA is beneficial when compared to its parent drug ASA in the healing of experimental colitis. Furthermore, celecoxib, the selective COX-2 inhibitor, showed greater efficacy in healing colitis than was observed with non-selective COX-1 and COX-2 inhibitors (conventional ASA, indomethacin). However, the prolonged use of coxibs in clinical practice may be associated with prothrombotic action and increased risk of acute myocardial infarction. This significant finding of the authors with respect to selective COX-2 inhibitors and healing of ulcerative colitis requires further investigation and confirmation in clinical trials.
(1) Inhibition of both COX-1 and COX-2 isoforms by non-selective and selective COX-1 inhibitors exacerbates colonic damage and leads to functional impairment of the colonic mucosa blood flow during the process of healing of colitis; (2) The importance of PG inhibition by NSAIDs in the pathogenesis of colitis is confirmed by the finding that supplementation with exogenous PGs of animals concurrently treated with COX-1 or COX-2 inhibitors attenuated the colonic damage and the decrease of CBF induced by these agents; and (3) The NO released from ASA may be an alternative option to native ASA in patients with lower GI tract disorders such as UC.
Incorporation of NO generating moiety into the basic structure of NSAIDs, such as aspirin (NO-ASA) attenuates the ulcerogenic activity of native NSAID. Under basal conditions, NO derived from the activity of constitutive NO synthase (cNOS) contributes to the maintenance of intestinal integrity and the control of intestinal motility. NO and CGRP exhibit a protective action against NSAIDs induced impairment of colonic healing. GTN, an NO donor, and 2-(4-carboxyphenyl)-4,5-dihydro-4,4,5,5-tetramethyl-1H-imidazolyl-1-oxy-3-oxide, monopotassium salt, an NO scavenger were used for the evaluation of the involvement of NO in the process of colonic healing in rats with colitis. CGRP is a sensory neurotransmitter released from the peripheral endings of afferent sensory neurons implicated in the defense mechanism of the GI mucosa. Capsaicin, an active ingredient of red pepper, has been found to induce a functional ablation of the capsaicin-sensitive afferent nerves releasing CGRP. Previous studies revealed that when applied in small doses capsaicin exerts a protective action, but large doses of this neurotoxin render the GI mucosa more susceptible to damage induced by various ulcerogens and stressors.
This work is good, with proper design of research and interesting results.
| 1. | Shanahan F. Inflammatory bowel disease: immunodiagnostics, immunotherapeutics, and ecotherapeutics. Gastroenterology. 2001;120:622-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 243] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 2. | Porras M, Martín MT, Torres R, Vergara P. Cyclical upregulated iNOS and long-term downregulated nNOS are the bases for relapse and quiescent phases in a rat model of IBD. Am J Physiol Gastrointest Liver Physiol. 2006;290:G423-G430. [PubMed] |
| 3. | Dudhgaonkar SP, Tandan SK, Kumar D, Raviprakash V, Kataria M. Influence of simultaneous inhibition of cyclooxygenase-2 and inducible nitric oxide synthase in experimental colitis in rats. Inflammopharmacology. 2007;15:188-195. [PubMed] |
| 4. | Kefalakes H, Stylianides TJ, Amanakis G, Kolios G. Exacerbation of inflammatory bowel diseases associated with the use of nonsteroidal anti-inflammatory drugs: myth or reality? Eur J Clin Pharmacol. 2009;65:963-970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 89] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 5. | Cipolla G, Crema F, Sacco S, Moro E, de Ponti F, Frigo G. Nonsteroidal anti-inflammatory drugs and inflammatory bowel disease: current perspectives. Pharmacol Res. 2002;46:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 1.9] [Reference Citation Analysis (1)] |
| 6. | Vane JR, Bakhle YS, Botting RM. Cyclooxygenases 1 and 2. Annu Rev Pharmacol Toxicol. 1998;38:97-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2045] [Cited by in RCA: 2054] [Article Influence: 73.4] [Reference Citation Analysis (0)] |
| 7. | Wallace JL. COX-2: a pivotal enzyme in mucosal protection and resolution of inflammation. ScientificWorldJournal. 2006;6:577-588. [PubMed] |
| 8. | Wallace JL, McKnight W, Reuter BK, Vergnolle N. NSAID-induced gastric damage in rats: requirement for inhibition of both cyclooxygenase 1 and 2. Gastroenterology. 2000;119:706-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 488] [Cited by in RCA: 497] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 9. | Bertolini A, Ottani A, Sandrini M. Dual acting anti-inflammatory drugs: a reappraisal. Pharmacol Res. 2001;44:437-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 144] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 10. | Cuzzocrea S, Mazzon E, Serraino I, Dugo L, Centorrino T, Ciccolo A, Sautebin L, Caputi AP. Celecoxib, a selective cyclo-oxygenase-2 inhibitor reduces the severity of experimental colitis induced by dinitrobenzene sulfonic acid in rats. Eur J Pharmacol. 2001;431:91-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Schmassmann A, Peskar BM, Stettler C, Netzer P, Stroff T, Flogerzi B, Halter F. Effects of inhibition of prostaglandin endoperoxide synthase-2 in chronic gastro-intestinal ulcer models in rats. Br J Pharmacol. 1998;123:795-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 217] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 12. | Reuter BK, Asfaha S, Buret A, Sharkey KA, Wallace JL. Exacerbation of inflammation-associated colonic injury in rat through inhibition of cyclooxygenase-2. J Clin Invest. 1996;98:2076-2085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 319] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 13. | Matuk R, Crawford J, Abreu MT, Targan SR, Vasiliauskas EA, Papadakis KA. The spectrum of gastrointestinal toxicity and effect on disease activity of selective cyclooxygenase-2 inhibitors in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2004;10:352-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 73] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 14. | Tanaka A, Hase S, Miyazawa T, Ohno R, Takeuchi K. Role of cyclooxygenase (COX)-1 and COX-2 inhibition in nonsteroidal anti-inflammatory drug-induced intestinal damage in rats: relation to various pathogenic events. J Pharmacol Exp Ther. 2002;303:1248-1254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 104] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 15. | Takeuchi K, Tanaka A, Ohno R, Yokota A. Role of COX inhibition in pathogenesis of NSAID-induced small intestinal damage. J Physiol Pharmacol. 2003;54 Suppl 4:165-182. [PubMed] |
| 16. | Yokota A, Taniguchi M, Takahira Y, Tanaka A, Takeuchi K. Rofecoxib produces intestinal but not gastric damage in the presence of a low dose of indomethacin in rats. J Pharmacol Exp Ther. 2005;314:302-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Wallace JL, Zamuner SR, McKnight W, Dicay M, Mencarelli A, del Soldato P, Fiorucci S. Aspirin, but not NO-releasing aspirin (NCX-4016), interacts with selective COX-2 inhibitors to aggravate gastric damage and inflammation. Am J Physiol Gastrointest Liver Physiol. 2004;286:G76-G81. [PubMed] |
| 18. | McCafferty DM, Mudgett JS, Swain MG, Kubes P. Inducible nitric oxide synthase plays a critical role in resolving intestinal inflammation. Gastroenterology. 1997;112:1022-1027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 176] [Article Influence: 6.1] [Reference Citation Analysis (1)] |
| 19. | Brzozowski T, Konturek PC, Konturek SJ, Sliwowski Z, Pajdo R, Drozdowicz D, Ptak A, Hahn EG. Classic NSAID and selective cyclooxygenase (COX)-1 and COX-2 inhibitors in healing of chronic gastric ulcers. Microsc Res Tech. 2001;53:343-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 89] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 20. | Konturek SJ, Brzozowski T, Pajdo R, Konturek PC, Kwiecień S, Sliwowski Z, Pawlik M, Ptak A, Drozdowicz D, Hahn EG. Gastric preconditioning induced by short ischemia: the role of prostaglandins, nitric oxide and adenosine. Med Sci Monit. 2001;7:610-621. [PubMed] |
| 21. | Konturek PC, Brzozowski T, Ptak A, Kania J, Kwiecień S, Hahn EG, Konturek SJ. Nitric oxide releasing aspirin protects the gastric mucosa against stress and promotes healing of stress-induced gastric mucosal damage: role of heat shock protein 70. Digestion. 2002;66:160-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 22. | Zwolinska-Wcislo M, Brzozowski T, Budak A, Kwiecien S, Sliwowski Z, Drozdowicz D, Trojanowska D, Rudnicka-Sosin L, Mach T, Konturek SJ. Effect of Candida colonization on human ulcerative colitis and the healing of inflammatory changes of the colon in the experimental model of colitis ulcerosa. J Physiol Pharmacol. 2009;60:107-118. [PubMed] |
| 23. | Brzozowski T, Konturek SJ, Sliwowski Z, Pytko-Polończyk J, Szlachcic A, Drozdowicz D. Role of capsaicin-sensitive sensory nerves in gastroprotection against acid-independent and acid-dependent ulcerogens. Digestion. 1996;57:424-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 45] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Vilaseca J, Salas A, Guarner F, Rodriguez R, Malagelada JR. Participation of thromboxane and other eicosanoid synthesis in the course of experimental inflammatory colitis. Gastroenterology. 1990;98:269-277. [PubMed] |
| 25. | Green LC, Tannenbaum SR, Goldman P. Nitrate synthesis in the germfree and conventional rat. Science. 1981;212:56-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 378] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 26. | Pajdo R, Brzozowski T, Konturek PC, Kwiecien S, Konturek SJ, Sliwowski Z, Pawlik M, Ptak A, Drozdowicz D, Hahn EG. Ischemic preconditioning, the most effective gastroprotective intervention: involvement of prostaglandins, nitric oxide, adenosine and sensory nerves. Eur J Pharmacol. 2001;427:263-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 27. | Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156-159. [PubMed] |
| 28. | Laine L. The gastrointestinal effects of nonselective NSAIDs and COX-2-selective inhibitors. Semin Arthritis Rheum. 2002;32:25-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 100] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 29. | Brzozowski T, Konturek PC, Konturek SJ, Sliwowski Z, Drozdowicz D, Stachura J, Pajdo R, Hahn EG. Role of prostaglandins generated by cyclooxygenase-1 and cyclooxygenase-2 in healing of ischemia-reperfusion-induced gastric lesions. Eur J Pharmacol. 1999;385:47-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 74] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 30. | S Kwiecien S, Pawlik MW, Brzozowski T, Konturek PC, Sliwowski Z, Pawlik WW, Konturek SJ. Nitric oxide (NO)-releasing aspirin and (NO) donors in protection of gastric mucosa against stress. J Physiol Pharmacol. 2008;59 Suppl 2:103-115. [PubMed] |
| 31. | Tanaka K, Suemasu S, Ishihara T, Tasaka Y, Arai Y, Mizushima T. Inhibition of both COX-1 and COX-2 and resulting decrease in the level of prostaglandins E2 is responsible for non-steroidal anti-inflammatory drug (NSAID)-dependent exacerbation of colitis. Eur J Pharmacol. 2009;603:120-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 32. | Cohn SM, Schloemann S, Tessner T, Seibert K, Stenson WF. Crypt stem cell survival in the mouse intestinal epithelium is regulated by prostaglandins synthesized through cyclooxygenase-1. J Clin Invest. 1997;99:1367-1379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 168] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 33. | Kabashima K, Saji T, Murata T, Nagamachi M, Matsuoka T, Segi E, Tsuboi K, Sugimoto Y, Kobayashi T, Miyachi Y. The prostaglandin receptor EP4 suppresses colitis, mucosal damage and CD4 cell activation in the gut. J Clin Invest. 2002;109:883-893. [PubMed] |
| 34. | Kankuri E, Vaali K, Korpela R, Paakkari I, Vapaatalo H, Moilanen E. Effects of a COX-2 preferential agent nimesulide on TNBS-induced acute inflammation in the gut. Inflammation. 2001;25:301-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 35. | Vazquez-Torres A, Jones-Carson J, Warner T, Balish E. Nitric oxide enhances resistance of SCID mice to mucosal candidiasis. J Infect Dis. 1995;172:192-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 36. | Santucci L, Wallace J, Mencarelli A, Farneti S, Morelli A, Fiorucci S. Different sensitivity of lamina propria T-cell subsets to nitric oxide-induced apoptosis explains immunomodulatory activity of a nitric oxide-releasing derivative of mesalamine in rodent colitis. Gastroenterology. 2005;128:1243-1257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 37. | Holzer P. Role of visceral afferent neurons in mucosal inflammation and defense. Curr Opin Pharmacol. 2007;7:563-569. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 83] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 38. | Mózsik G, Szolcsányi J, Dömötör A. Capsaicin research as a new tool to approach of the human gastrointestinal physiology, pathology and pharmacology. Inflammopharmacology. 2007;15:232-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 39. | Evangelista S. Role of sensory neurons in restitution and healing of gastric ulcers. Curr Pharm Des. 2006;12:2977-2984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 40. | Lanas A, Scarpignato C. Microbial flora in NSAID-induced intestinal damage: a role for antibiotics? Digestion. 2006;73 Suppl 1:136-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 67] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
Peer reviewers: Dr. Paulino Martínez Hernández Magro, Department of Colon and Rectal Surgery, Hospital San José de Celaya, Eje Vial Norponiente No 200-509, Colonia Villas de la Hacienda, 38010 Celaya, México; Benjamin Perakath, Professor, Dr., Department of Surgery Unit 5, Christian Medical College, Vellore 632004, Tamil Nadu, India
S- Editor Sun H L- Editor O’Neill M E- Editor Zhang DN