Published online Sep 28, 2011. doi: 10.3748/wjg.v17.i36.4090
Revised: September 5, 2011
Accepted: September 12, 2011
Published online: September 28, 2011
AIM: To explore the role of high-mobility group box 1 (HMGB1) protein during liver fibrogenesis and investigate the functional effects of HMGB1 gene silencing in hepatic stellate cells (HSCs) using siRNA.
METHODS: Hepatic fibrosis in rats was induced throu-gh serial subcutaneous injections of dimethylnitrosamine, and expression of HMGB1 was detected by immunohistochemistry. HMGB1 siRNAs were developed and transiently transfected into HSC-T6 cells using Lipofectamine 2000. HMGB1 expression was evaluated by real-time polymerase chain reaction (PCR) and Western blotting analysis. Expression of α-smooth muscle actin (α-SMA) and collagen types I and III was evaluated by real-time PCR. Cell proliferation and the cell cycle were determined using the methyl thiazolyl tetrazolium method. Finally, collagen content in HSC supernatant was evaluated by an enzyme-linked immunosorbent assay.
RESULTS: The results showed that HMGB1 was upregulated during liver fibrosis and that its expression was closely correlated with the deposition of collagen. siRNA molecules were successfully transfected into HSCs and induced inhibition of HMGB1 expression in a time-dependent manner. Moreover, HMGB1 siRNA treatment inhibited synthesis of α-SMA and collagen types I and III in transfected HSCs.
CONCLUSION: This study suggests a significant fun-ctional role for HMGB1 in the development of liver fibrosis. It also demonstrates that downregulation of HMGB1 expression might be a potential strategy to treat liver fibrosis.
- Citation: Ge WS, Wu JX, Fan JG, Wang YJ, Chen YW. Inhibition of high-mobility group box 1 expression by siRNA in rat hepatic stellate cells. World J Gastroenterol 2011; 17(36): 4090-4098
- URL: https://www.wjgnet.com/1007-9327/full/v17/i36/4090.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i36.4090
Hepatic fibrosis is a major medical problem associated with significant morbidity and mortality. Regardless of the underlying aetiology[1], hepatic fibrosis is characterized by the accumulation of excess extracellular matrix (ECM). The amount of matrix deposition depends on the balance between its synthesis and degradation. When synthesis of ECM exceeds its degradation, the pathological accumulation of ECM leads to liver fibrosis. Therefore, a critical balance must be achieved between maintaining the proper amount of ECM for homeostasis, while at the same time, providing a means of ensuring that excess or improper accumulation does not occur.
High-mobility group box 1 (HMGB1) protein was originally identified as a nuclear nonhistone protein with DNA-binding domains, and it has been implicated as an important endogenous danger signaling molecule. In addition, it can be secreted from cells and exert extracellular functions as a proinflammatory cytokine[2,3]. Increasing evidence now points to multiple functions of HMGB1 in infection, tissue injury, inflammation, apoptosis, and the immune response[4]. HMGB1 can be released both through active secretion from various cells, including activated monocytes/macrophages, neutrophils, and endothelial cells, and through passive release from necrotic cells[3-7]. HMGB1 can directly promote the secretion of proinflammatory cytokines [tumor necrosis factor (TNF), interleukin (IL)-1A/B, IL-6 and IL-8] and chemokines (macrophage inflammatory protein-1A/B) by peripheral blood mononuclear cells (PBMCs)[8,9]. In turn, PBMCs also produce different cytokines that are potentially involved in virus-induced liver damage. HMGB1 acts as a chemoattractant for fibroblasts and endothelial and smooth muscle cells, which are cell types that significantly contribute to wound repair[9,10]. Consequently, HMGB1 can directly stimulate fibroblast proliferation and participate in fibrogenesis[4]. Additionally, inhibitors of HMGB1 significantly reduce tissue damage[5,6]. Moreover, Hamada et al[4] have reported that inhibition of HMGB1 may be beneficial in pulmonary fibrosis. Therefore, we postulated that inhibiting the upregulation of HMGB1 during liver fibrogenesis could be a potential strategy for treating liver fibrosis.
RNA interference is known as a powerful tool for post-transcriptional gene silencing[11] and has opened new avenues in gene therapy. In this study, we induced hepatic fibrosis in rats through serial subcutaneous injections of dimethylnitrosamine (DMN) for 4 wk and evaluated the expression of HMGB1 during the process of hepatic fibrogenesis. Additionally, siRNA molecules targeting the sequences within the rat HMGB1 gene were transfected into hepatic stellate cell (HSC)-T6 cells. The results show that the expression of HMGB1 was correlated with collagen deposition during hepatic fibrosis and that downregulating HMGB1 expression could prohibit collagen production and enhance collagen degradation.
Thirty-two 6-wk-old male Sprague-Dawley rats (230-260 g) were purchased from the Shanghai Laboratory Animal Centre of Chinese Academy of Sciences and fed ad libitum with standard laboratory chow. All rats received humane care according to the Guide for the Care and Use of Laboratory Animals by the Chinese Academy of Sciences. Hepatic fibrosis was induced by intraperitoneal injections of 1% DMN (1 mL/kg body weight) for three consecutive days per week for up to 4 wk[11]. Rats were sacrificed at 1, 2 and 3 wk from the first DMN injection. Liver tissues were either snap-frozen in liquid nitrogen or fixed in 10% formalin for histology and immunostaining.
Liver tissue sections were stained with hematoxylin-eosin (HE) for histopathological examination. Immunohistochemical examination was performed to detect the expression of HMGB1 and collagen types I and III in liver tissues. Briefly, the paraffin sections of left median hepatic lobes were incubated with 3% H2O2 in methanol at 37 °C for 10 min to quench endogenous peroxidase activity. After blocking at room temperature for 20 min, the sections were incubated with antibodies against HMGB1 (R and D Systems, Germany), collagen type I or collagen type III (Boster, Wuhan, China) overnight at 4 °C followed by incubation with horseradish-peroxidase-conjugated secondary antibody (Dako, Kyoto, Japan) at 37 °C for 20 min. Finally, the signals were detected using the Diaminobenzidine Substrate Kit (Vector Laboratories, Burlingame, CA, United States), and a positive outcome was indicated by brown staining in the cytoplasm or nucleus. For the semiquantitative analysis of HMGB1 and collagen expression, the brown-stained tissues in immunohistostaining sections were measured on an image analyzer by a technician blinded to the samples. Five fields were selected randomly from each of two sections, and six rats from each group were examined.
Double immunostaining of HMGB1 andα-smooth muscle actin
Liver sections were blocked with 5% normal goat serum after fixing and then simultaneously incubated with both monoclonal anti-HMGB1 (R and D Systems, Germany) and polyclonal α-smooth muscle actin (α-SMA) (Fremont, CA, United States) antibodies prepared in phosphate-buffered saline (PBS). The sections were incubated overnight at 4 °C or 1 h at room temperature and then washed with PBS. Sections were then simultaneously incubated with fluorescein-isothiocyanate-conjugated secondary antibody and rhodamine-conjugated secondary antibody for 30 min at 37 °C in the dark. Both primary antibodies were produced in different species. Antibody labeling was examined under a Zeiss LSM-510 laser scanning confocal microscope.
The HSC-T6 cell line, an immortalized rat HSC line, which has a stable phenotype and biochemical characteristics, was kindly provided by Dr. SL Friedman (Division of Liver Diseases, Mount Sinai School of Medicine, New York, NY, United States). All cells were cultured in RPMI1640 medium supplemented with 10% fetal bovine serum and 5% antibiotics and incubated at 37 °C in a humidified atmosphere of 5% CO2. Cells were seeded at 2 × 105 per well in six-well plates 24 h before transfection. The amount of siRNA and transfection reagent was calculated according to the manufacturer’s instructions.
HSC-T6 cells were cultured for 24 h on glass coverslips and fixed in 4% formaldehyde for 30 min at room temperature prior to detergent extraction with 0.1% Triton X-100 for 10 min at 4 °C. Coverslips were saturated with PBS containing 2% bovine serum albumin (BSA) for 1 h at room temperature. Next, cells were incubated with the specific primary antibody for HMGB1 (R and D Systems, Germany) in 1% BSA for 1 h, washed, and incubated with secondary antibody (TRITC AffiniPure Goat Anti-Rabbit IgG, EarthOx, LLC, United States). Finally, cells were stained for 30 min at room temperature with 4,6-diamidino-2-phenylindole. Slides were viewed with a Zeiss LSM-510 laser scanning confocal microscope.
The siRNAs for rat HMGB1 mRNA were designed and synthesised by Invitrogen Life Technologies. We prepared three siRNAs, and the most effective one was selected for construction of the siRNA expression vector. The siRNA sequences used are shown in Table 1. Negative control siRNAs were used to assess non-specific gene silencing effects, and the mock group was the non-transfection group. Cells were transfected with a mixture of plasmid DNA and Lipofectamine 2000 (Invitrogen) in Opti-MEM I medium without serum as recommended by the manufacturer. The medium was then replaced with standard RPMI medium (containing 10% FBS and gentamicin) 24 h post-transfection.
| Plasmid constructs | Target sequence in mRNA(5’-3’) |
| HMGB1-1 (shRNAH1) | GCAAATGACTCAATCTGATT |
| HMGB1-2 (shRNAH2) | AATAGGAAAAGGATATTGCT |
| HMGB1-3 (shRNAH3) | ACCCGGATGCTTCTGTCAAC |
Total RNA was extracted at different time points after siRNA transfection using the Trizol kit (Gibco/Life Technologies) according to the manufacturer’s protocol. The mixture of RNA and primers was loaded into the polymerase chain reaction (PCR) amplifier. The PCR protocol was as follows: predenaturate setting at 95 °C for 5 min, 94 °C for 45 s, annealing at 50 °C for 1 min, and extension at 72 °C for 1 min. The PCR was performed for 40 cycles followed by a final extension at 72 °C for 10 min. We then visualized the PCR product by running it on a 1.5% agarose gel and quantitatively analysed it with Lab Works 4.5 analysis software.
The same quantities of cells were collected from the four groups, and the protein was extracted from the cells at the 24, 48 and 72 h after transfection. The protein content in the supernatant was detected using the bicinchoninic acid method. An equal amount of protein was used for sodium dodecyl sulfate polyacrylamide gel electrophoresis electrophoresis and transferred onto a polyvinylidene fluoride (PVDF) membrane. The PVDF membrane was incubated overnight at 4 °C with monoclonal anti-human HMGB1 (1:300) and was then incubated for 2 h with a secondary antibody (1:5000). Finally, after staining and fixing, the film was analyzed using the Image Analysis System.
Commercial kits (Sigma, St. Louis, MO, United States) were used to quantitate the amount of collagen types I and III in the culture supernatant of HSCs at different time points after siRNA transfection.
The cell suspension was inoculated into 96-well plates at 1000 cells per well with eight ambi-wells and incubated for 1, 2, 3, 4 and 5 d after transfection. Cells were incubated with 20 μL methyl thiazolyl tetrazolium for 4 h. After centrifugation, 150 μL dimethyl sulfoxide was added to the precipitate, and the absorbance of the enzyme was measured at 490 nm. Cell growth rates (average absorbance of each transfected group/non-transfected group) were then calculated.
Continuous data were expressed as the mean ± SD and were analyzed using the Student’s t test. Correlations among the study variables were tested using Pearson’s correlation coefficients. P < 0.05 were considered statistically significant. All calculations were performed using SPSS version 13.0 (SPSS Inc., Chicago, IL, United States).
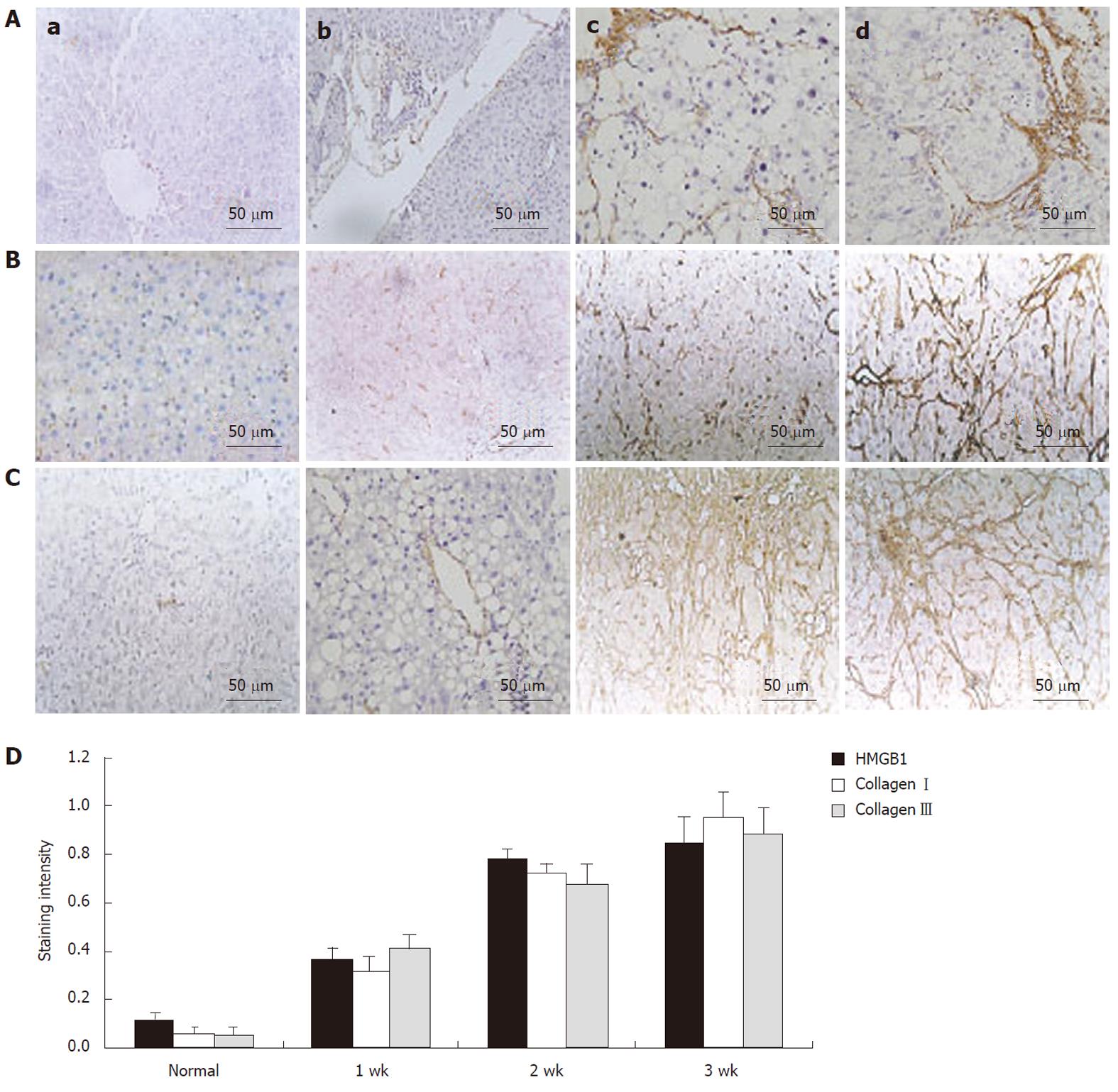
To investigate the expression of HMGB1 during liver fibrosis, liver sections were analysed by HE staining and immunohistochemistry. We localized HMGB1 and collagen types I and III in liver specimens by immunohistochemistry. None of these proteins were observed in control rat livers. In fibrotic rat livers, HMGB1 was markedly increased during liver fibrogenesis and was correlated with the expression of collagen types I and III. Immunohistochemistry indicated that the intensity of HMGB1 immunostaining was stronger in the fibrotic samples (DMN week 1) than in the control group. After DMN injection for 2-3 wk, greater HMGB1 staining was found around the portal tracts and fibrotic septa (Figure 1A). With the development of hepatic fibrosis, there was an enhanced expression of HMGB1, correlating with collagen typesI and III expression, which was mainly located within the mesenchymal (Figure 1B and C). Statistical analysis showed that the expression of HMGB1 was completely correlated with the expression of collagen types I and III during the development of hepatic fibrosis (Figure 1D) (P < 0.05).
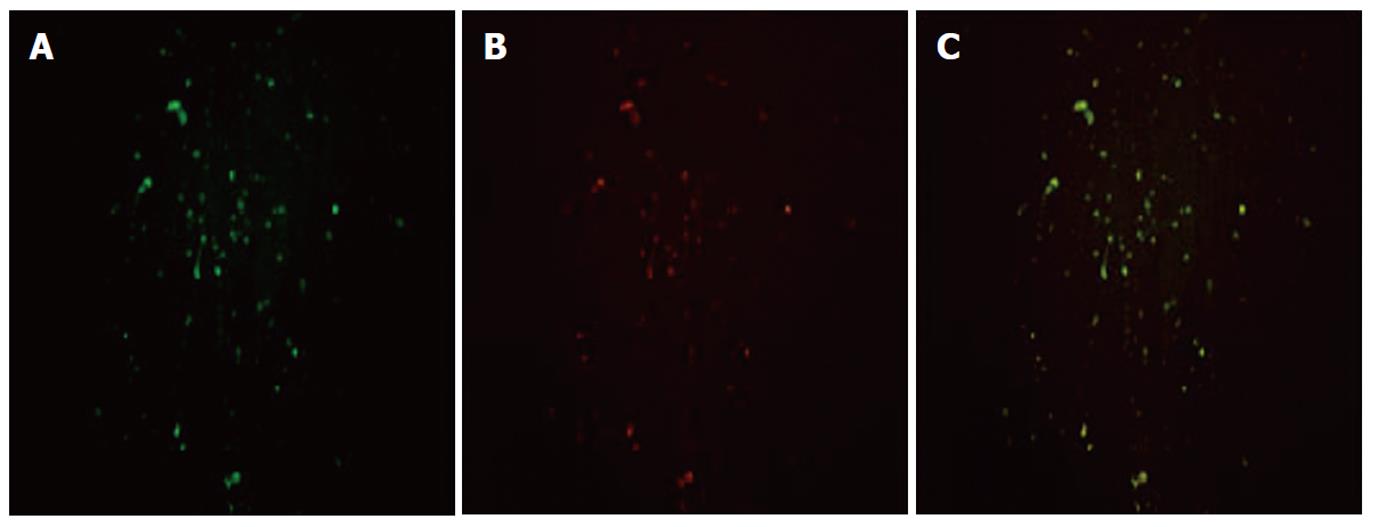
α-SMA, a typical marker of activated HSCs, was selected to determine the cellular localization of HMGB1 in hepatic fibrosis tissue. The localization of HMGB1 and α-SMA was visualized by immunofluorescent double labeling and laser scanning confocal microscopy. The image analysis showed a diffused distribution of HMGB1 throughout the hepatic fibrosis tissue (Figure 2A), and a similar distribution was observed for α-SMA (Figure 2B). When the two images were merged, there was a very high degree of co-localization of HMGB1 with α-SMA throughout the hepatic fibrosis tissue (Figure 2C).
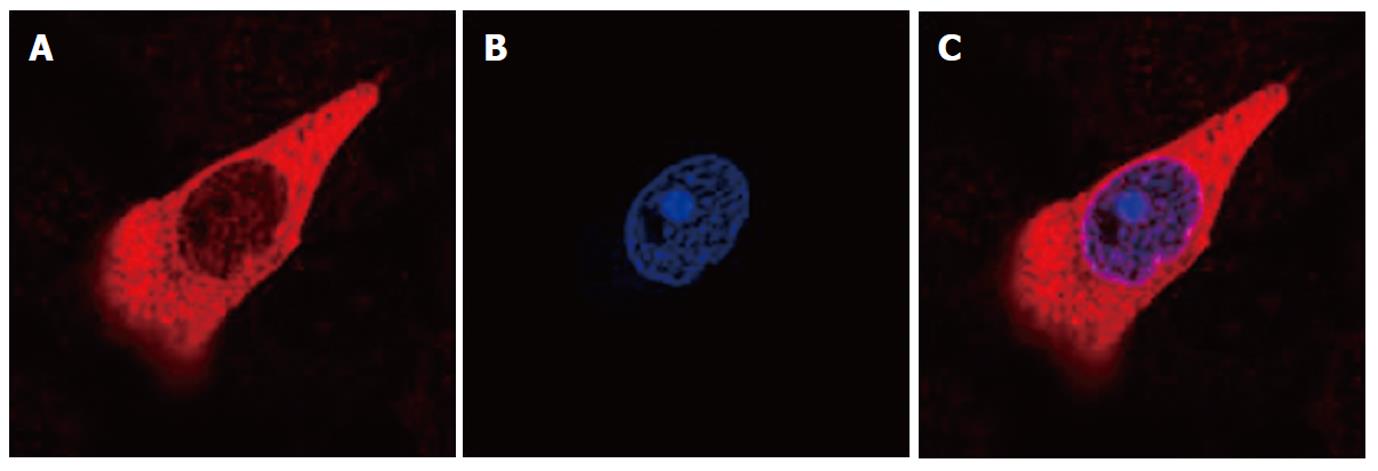
An immunofluorescence study of HSC-T6 cells after 24 h of culture demonstrated the intracellular localization of HMGB1. We evaluated the subcellular localization of HMGB1 by separating bulk nuclei and cytosolic fractions, and HMGB1 was detected primarily within the cytosol of activated HSC-T6 cells (Figure 3).
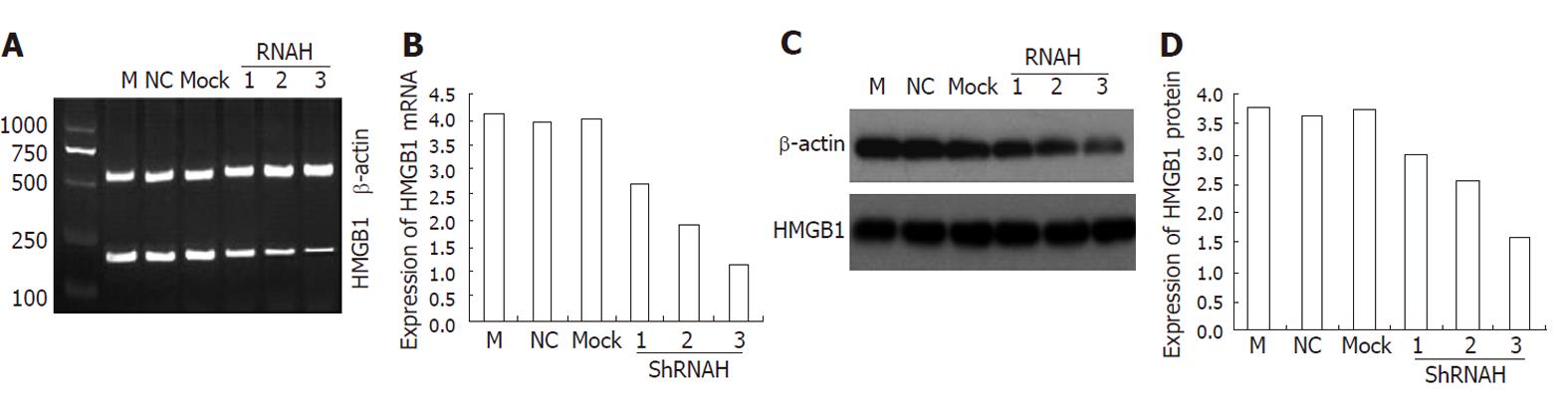
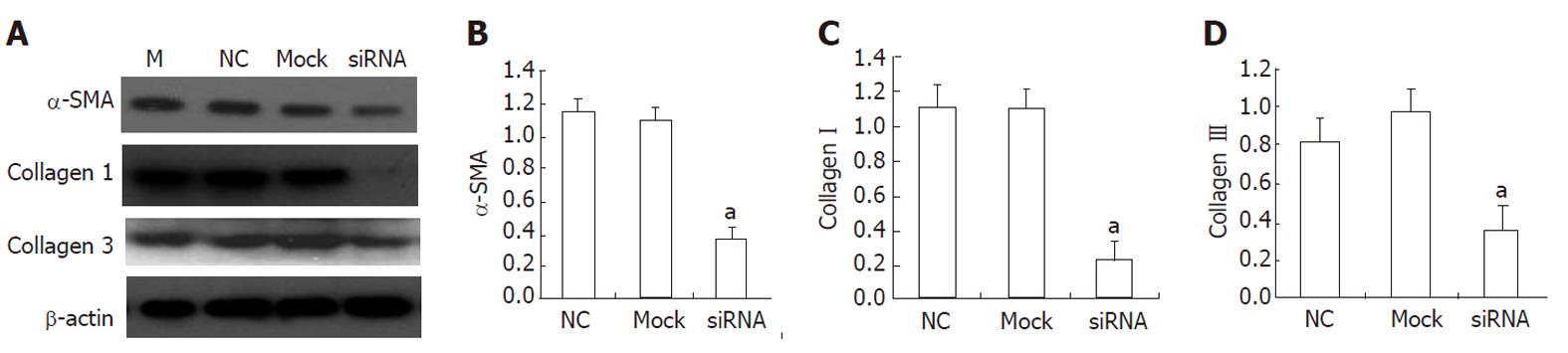
As shown in Table 1, a total of three candidate siRNA sequences were chosen to be complementary to various regions of the rat HMGB1 gene. In a set of preliminary experiments designed to identify the most appropriate sequence for further study, these sequences were transfected into HSC-T6 using Lipofectamine. Forty-eight hours after transfection, HMGB1 transcript and protein levels were reduced in transfected cells. This HMGB1 gene-silencing effect was reproducible and was specific in that it failed to knock down the expression of an unrelated protein, β-actin. All three HMGB1 shRNAs tested in this study were able to reduce the HMGB1 expression in HSC-T6 cells compared with the negative control (NC) siRNA transfectants. Although all three HMGB1 shRNA constructs were effective, shRNAH3 was more efficient in reducing the HMGB1 transcript levels than shRNAH2 and shRNAH1 (Figure 4A). Western blotting analysis (Figure 4C) further confirmed the shRNAH3 silencing of the HMGB1 protein in HSC-T6 cells. Semiquantitative analysis of the real-time (RT)-PCR and Western blot results (Figure 4B and D) also showed that HMGB1 shRNAH3 decreased the expression of HMGB1 in HSC-T6 cells more efficiently than shRNAH2 and shRNAH1. Accordingly, we chose shRNAH3 for the subsequent experiments.
HMGB1 siRNA downregulated mRNA expression ofα-SMA and types I and III collagen in HSC-T6
To investigate the effect of HMGB1 siRNA on HSCs and its potential molecular mechanisms, we detected the mRNA expression of some profibrogenic markers, including α-SMA and collagen types I and III, in transfected HSC-T6. As shown in Figure 5, HMGB1 siRNA reduced the mRNA levels of α-SMA and collagen types I and III.
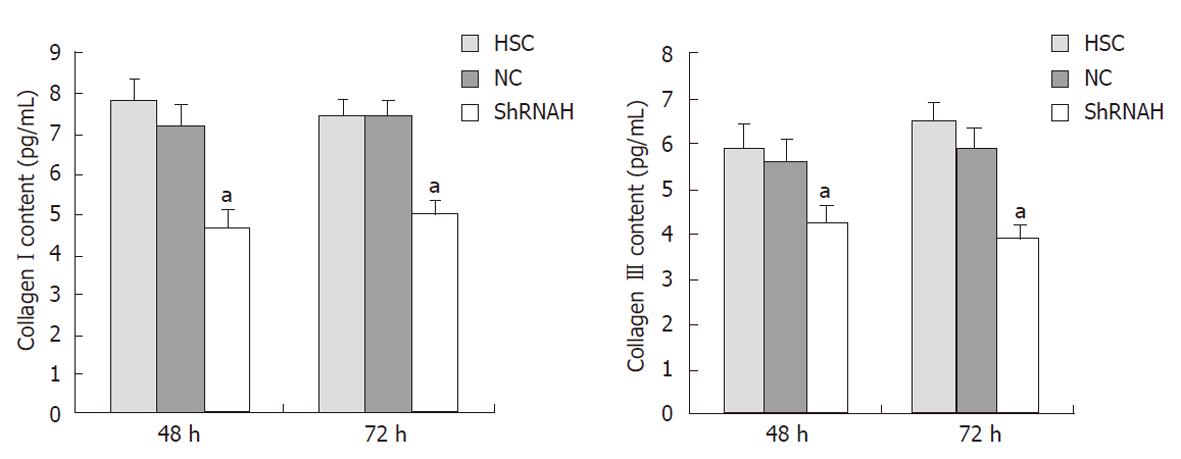
To confirm the effect of HMGB1 siRNA on collagen secretion and degradation, we examined the amount of collagen types I and III in HSCs 48 and 72 h after transfection with shRNAH3 using an ELISA. The results reveal that the content of both collagen types I and III was decreased after transfection with HMGB1 siRNA. Compared with the NC group, the content of collagen types I and III was reduced to 63% and 61%, respectively, 72 h after shRNAH3 transfection (Figure 6).
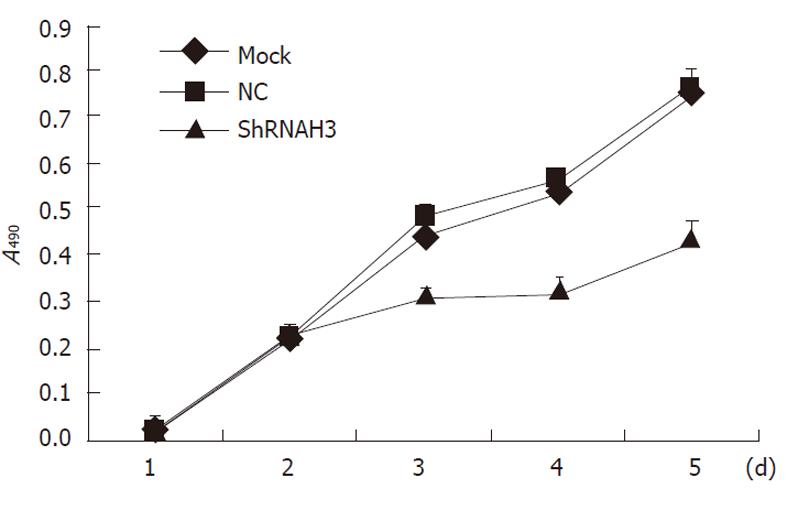
The trypan blue dye test showed that there were no significant differences in the number of cells in the threegroups 2 d after transfection (P > 0.05), but the proliferation in the shRNAH3 group was less than that in the NC group and non-transfection group (Mock group) 3, 4 and 5 d after transfection (P < 0.05, Figure 7). A cell cycle study also indicated that cells were arrested in the G0/G1 phase and that the proportion of cells in the S phase was significantly reduced after downregulation of HMGB1 in HSCs (Table 2).
| Cell cycle phases(%) | ShRNAH3 group | NC group |
| G0/G1 phase | 58.31% ± 0.48%a | 44.25% ± 0.63% |
| S phase | 29.12% ± 1.26%a | 41.32% ± 1.58% |
| G2/M phase | 12.57% ± 1.04% | 14.53% ± 1.28% |
Liver fibrosis is highly associated with chronic hepatocellular injury and the subsequent inflammatory response that produces inflammatory cytokines and recruits inflammatory leukocytes to the injured site. This inflammatory circumstance in the liver drives the activation of HSCs through various fibrogenic mediators[12,13]. Acti-vated HSCs transdifferentiate into myofibroblasts, which then produce excessive amounts of ECM proteins, including collagen types I, III and IV. This leads to irreversible collagen deposition, resulting in liver fibrosis[12,13]. Many studies have suggested that enhancement of matrix degradation may prove particularly valuable in response to injury caused by matrix deposition[14-17]. Some studies have shown that HMGB1 can stimulate proinflammatory cytokine synthesis and directly stimulate fibroblast proliferation and participate in fibrogenesis[8-10].
Increased expression of HMGB1 has been reported in several liver diseases, including Con A-induced hepatitis[18], hepatic ischemia[2], and orthotopic liver transplantation (OLT)[19]. In the present study, we evaluated HMGB1 expression in the DMN rat model. We found that the level of HMGB1 was upregulated during DMN injection. Moreover, the expression of HMGB1 was closely correlated with the expression of collagen types I and III and was mainly localized to the nonparenchymal cells, especially HSCs. These results suggest that HMGB1 is involved in hepatic fibrogenesis and may play a critical role in the reversal process of liver fibrosis.
HMGB1 was originally identified as a nuclear nonhistone protein with DNA-binding domains and was implicated as an important endogenous danger signaling molecule. Although predominantly located in the nucleus of quiescent cells, HMGB1 can be actively secreted in response to exogenous and endogenous infla-mmatory stimuli such as endotoxin, TNF-α, IL-1, and interferon-γ[20,21]. In addition, extracellular HMGB1 mediates a wide range of inflammatory responses and promotes cell proliferation, migration, and differentiation[10,22]. The cytoplasmic localization of HMGB1 in our study may suggest that HMGB1 plays extra nuclear roles in liver fibrosis and that HSC-T6 cells may even secrete HMGB1 to promote extracellular functions. The subcellular location of HMGB1 in monocytic cells is known to be dependent on the acetylation status of the nuclear localization signal (NLS) of the HMGB1 protein[23]. Inflammatory signals promote acetylation of the NLS, leading to cytoplasmic accumulation of HMGB1 in secretory lysosomes in the monocytic cells[24]. These secretory lysosomes are subsequently exocytosed when the monocytic cells are triggered by a second inflammatory stimulus. Whether the subcellular location of HMGB1 in HSC-T6 cells is regulated in a similar way remains to be investigated.
It has become apparent in recent years that HMGB1 is instrumental in mediating a response to tissue damage and infection. HMGB1 released from necrotic or damaged cells not only triggers inflammation as a non-specific proinflammatory cytokine but also triggers the adaptive immune response[25,26]. Extracellular HMGB1 functions as a damage-associated molecular pattern molecule and activates proinflammatory signaling pathways by activating pattern-recognition receptors including toll-like receptor 4 (TLR4) and the receptor for advanced glycation end-products (RAGE)[27,28]. A previous report showed that RAGE expression in fibrotic livers is restricted to HSCs; its expression is up regulated during cellular activation and transition to myofibroblasts[29], strongly suggesting that HMGB1 is involved in the pathogenesis of liver fibrosis. TLR4 has been suggested to be a receptor for extracellular HMGB1[30,31], and previous studies have indicated that the interaction of HMGB1 with TLR4 plays a critical role in hepatic fibrosis[32]. To date, little has been reported about the pathogenic interactions between HMGB1 and HSCs in terms of profibrogenic propensity. Kao provided evidence that HMGB1 up regulates α-SMA expression and suppresses the activity of the collagen-degrading enzyme matrix metalloproteinase-2[33]. That study also implied that HMGB1, once it is released during rejection of OLT, activates HSCs and exhibits profibrogenic effects either by increasing the HSC population and ECM deposition in liver grafts or by transforming HSCs into myofibroblasts. In contrast, neutralization with an anti-HMGB1 antibody may be a therapeutic modality to prevent fibrogenesis in post-OLT liver grafts[33].
siRNA has become a powerful tool for functional genetic studies and gene therapy in mammals[34,35]. Although gene knockdown by siRNA is highly effective, the off-target effect of siRNA may represent a major obstacle for therapeutic applications. However, the potential off-target effects could be minimized by choosing an siRNA with maximal sequence divergence from the list of genes with partial sequence identity to the intended mRNA target[36]. Software was used to choose a maximal sequence identity of HMGB1 siRNA, and three siRNA sequences were designed. In preliminary experiments, we identified the fact that shRNAH3 had certain interference effects. Our results show that this sequence was more efficient in reducing the HMGB1 transcript levels.
In the present study, we found that after HMGB1 was downregulated in HSCs by siRNA, there was an inhibitory effect on the mRNA levels of α-SMA and collagen types I and III, suggesting that inhibition of HMGB1 could directly result in suppression of HSC activation and collagen production. We also discovered that HMGB1 siRNA prohibited HSC proliferation, and a cell cycle analysis revealed that downregulation of HMGB1 arrested cells at the G0/G1 phase, which confirmed the effect of HMGB1 on cell proliferation; however, the definitive mechanism responsible is still uncertain because HMGB1 is multifunctional and has multiple molecular interactions.
In conclusion, HMGB1 was upregulated during liver fibrogenesis, and downregulating HMGB1 expression in HSCs by siRNA prohibited the activity of HSCs and collagen synthesis and enhanced collagen degradation. The results of our study indicate a significant functional role for HMGB1 in the development of liver fibrosis, and downregulating HMGB1 expression with siRNA could be an effective way to treat liver fibrosis.
Hepatic fibrosis is a response to injury in the liver. It is characterized by both a quantitative and qualitative change in the extracellular matrix (ECM). The activated hepatic stellate cell (HSC) is primarily responsible for excessive ECM deposition during liver fibrosis. It has been shown that high-mobility group box 1 (HMGB1) expression is up regulated during myofibroblast cellular activation and involved in the pathogenesis of hepatic fibrosis. This suggests that HMGB1 is a promising molecular target for hepatic fibrosis gene therapy. Inhibition of abnormal expression of HMGB1 may be an effective strategy for biological therapy of hepatic fibrosis.
HMGB1 is a major component of mammalian chromatin endowed with an architectural function. Increasing evidence now points to multiple functions of HMGB1 in infection, tissue injury, inflammation, apoptosis and the immune response. It has been reported in several liver diseases, including hepatitis, hepatic ischemia, and orthotopic liver transplantation. HMGB1 has been implicated in the pathogenesis of several liver diseases, including Con-A-induced hepatitis, hepatic ischemia, and orthotopic liver transplantation. However, the role of HMGB1 and how to inhibit its expressiong in hepatic fibrosis has yet to be fully elucidated. In this study, the authors demonstrate that the overexpression of HMGB1 could be a potential mechanism for mediating collagen expression and downregulating HMGB1 expression might present as a potential strategy to treat liver fibrosis.
Studies of targeting in vitro and in vivo over expressed genes in hepatic fibrosis by RNA interference, including transforming growth factor-β, connective tissue growth factor and p90RSK, have been reported. However, there has been still no report about targeting HMGB1 by siRNA in hepatic fibrosis. In the present study, the authors used siRNA approach to block HMGB1 expression in HSC-T6 cells, to determine the role of constitutively activated HMGB1 during hepatic fibrosis pathogenesis, and to explore the role and molecular mechanism of targeting HMGB1 in hepatic fibrosis therapy.
By investigating the effect of silencing HMGB1 expression by siRNA on the collagen synthesis and proliferation of HSC-T6 cells, this study may provides a new strategy for biological therapy of liver fibrosis by targeting HMGB1.
HMGB1 was originally identified as a nuclear nonhistone protein with DNA-binding domains and implicated as an important endogenous danger signaling molecule. But it can also be secreted from cells and exert extracellular functions as a proinflammatory cytokine. HSCs are a minor and quiescent cell type in the liver that usually reside in the space of Disse, but which undergo activation after hepatic injury to produce large quantities of fibrillar collagens.
The authors demonstrated the increase of HMGB1 expression in fibrotic livers. Then, they investigated the effect of HMGB1 silencing by siRNA on stellate cell activation and proliferation. The results show that siRNA for HMGB1 significantly inhibits collagen expression and stellate cell proliferation.
| 1. | Moreira RK. Hepatic stellate cells and liver fibrosis. Arch Pathol Lab Med. 2007;131:1728-1734. [PubMed] |
| 2. | Tsung A, Sahai R, Tanaka H, Nakao A, Fink MP, Lotze MT, Yang H, Li J, Tracey KJ, Geller DA. The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J Exp Med. 2005;201:1135-1143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 845] [Cited by in RCA: 952] [Article Influence: 45.3] [Reference Citation Analysis (19)] |
| 3. | Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2606] [Cited by in RCA: 2725] [Article Influence: 100.9] [Reference Citation Analysis (3)] |
| 4. | Hamada N, Maeyama T, Kawaguchi T, Yoshimi M, Fukumoto J, Yamada M, Yamada S, Kuwano K, Nakanishi Y. The role of high mobility group box1 in pulmonary fibrosis. Am J Respir Cell Mol Biol. 2008;39:440-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 170] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 5. | Ilmakunnas M, Tukiainen EM, Rouhiainen A, Rauvala H, Arola J, Nordin A, Mäkisalo H, Höckerstedt K, Isoniemi H. High mobility group box 1 protein as a marker of hepatocellular injury in human liver transplantation. Liver Transpl. 2008;14:1517-1525. [PubMed] |
| 6. | Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3079] [Cited by in RCA: 3335] [Article Influence: 139.0] [Reference Citation Analysis (0)] |
| 7. | Ito I, Fukazawa J, Yoshida M. Post-translational methylation of high mobility group box 1 (HMGB1) causes its cytoplasmic localization in neutrophils. J Biol Chem. 2007;282:16336-16344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 189] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 8. | Andersson U, Wang H, Palmblad K, Aveberger AC, Bloom O, Erlandsson-Harris H, Janson A, Kokkola R, Zhang M, Yang H. High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J Exp Med. 2000;192:565-570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1100] [Cited by in RCA: 1184] [Article Influence: 45.5] [Reference Citation Analysis (14)] |
| 9. | Raucci A, Palumbo R, Bianchi ME. HMGB1: a signal of necrosis. Autoimmunity. 2007;40:285-289. [PubMed] |
| 10. | Mitola S, Belleri M, Urbinati C, Coltrini D, Sparatore B, Pedrazzi M, Melloni E, Presta M. Cutting edge: extracellular high mobility group box-1 protein is a proangiogenic cytokine. J Immunol. 2006;176:12-15. [PubMed] |
| 11. | Chen SW, Chen YX, Zhang XR, Qian H, Chen WZ, Xie WF. Targeted inhibition of platelet-derived growth factor receptor-beta subunit in hepatic stellate cells ameliorates hepatic fibrosis in rats. Gene Ther. 2008;15:1424-1435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209-218. [PubMed] |
| 13. | Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655-1669. [PubMed] |
| 14. | Hu YB, Li DG, Lu HM. Modified synthetic siRNA targeting tissue inhibitor of metalloproteinase-2 inhibits hepatic fibrogenesis in rats. J Gene Med. 2007;9:217-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 15. | Uchinami H, Seki E, Brenner DA, D'Armiento J. Loss of MMP 13 attenuates murine hepatic injury and fibrosis during cholestasis. Hepatology. 2006;44:420-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 166] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 16. | Roderfeld M, Weiskirchen R, Wagner S, Berres ML, Henkel C, Grötzinger J, Gressner AM, Matern S, Roeb E. Inhibition of hepatic fibrogenesis by matrix metalloproteinase-9 mutants in mice. FASEB J. 2006;20:444-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 114] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 17. | González-Cuevas J, Bueno-Topete M, Armendariz-Borunda J. Urokinase plasminogen activator stimulates function of active forms of stromelysin and gelatinases (MMP-2 and MMP-9) in cirrhotic tissue. J Gastroenterol Hepatol. 2006;21:1544-1554. [PubMed] [DOI] [Full Text] |
| 18. | Gong Q, Zhang H, Li JH, Duan LH, Zhong S, Kong XL, Zheng F, Tan Z, Xiong P, Chen G. High-mobility group box 1 exacerbates concanavalin A-induced hepatic injury in mice. J Mol Med (Berl). 2010;88:1289-1298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 19. | Nakano T, Goto S, Lai CY, Hsu LW, Kao YH, Lin YC, Kawamoto S, Chiang KC, Ohmori N, Goto T. Experimental and clinical significance of antinuclear antibodies in liver transplantation. Transplantation. 2007;83:1122-1125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Rendon-Mitchell B, Ochani M, Li J, Han J, Wang H, Yang H, Susarla S, Czura C, Mitchell RA, Chen G. IFN-gamma induces high mobility group box 1 protein release partly through a TNF-dependent mechanism. J Immunol. 2003;170:3890-3897. [PubMed] |
| 21. | Wang H, Vishnubhakat JM, Bloom O, Zhang M, Ombrellino M, Sama A, Tracey KJ. Proinflammatory cytokines (tumor necrosis factor and interleukin 1) stimulate release of high mobility group protein-1 by pituicytes. Surgery. 1999;126:389-392. [PubMed] |
| 22. | Palumbo R, Sampaolesi M, De Marchis F, Tonlorenzi R, Colombetti S, Mondino A, Cossu G, Bianchi ME. Extracellular HMGB1, a signal of tissue damage, induces mesoangioblast migration and proliferation. J Cell Biol. 2004;164:441-449. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 338] [Cited by in RCA: 379] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 23. | Bonaldi T, Talamo F, Scaffidi P, Ferrera D, Porto A, Bachi A, Rubartelli A, Agresti A, Bianchi ME. Monocytic cells hyperacetylate chromatin protein HMGB1 to redirect it towards secretion. EMBO J. 2003;22:5551-5560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 950] [Cited by in RCA: 1037] [Article Influence: 45.1] [Reference Citation Analysis (0)] |
| 24. | Gardella S, Andrei C, Ferrera D, Lotti LV, Torrisi MR, Bianchi ME, Rubartelli A. The nuclear protein HMGB1 is secreted by monocytes via a non-classical, vesicle-mediated secretory pathway. EMBO Rep. 2002;3:995-1001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 682] [Cited by in RCA: 749] [Article Influence: 31.2] [Reference Citation Analysis (12)] |
| 25. | Dumitriu IE, Baruah P, Valentinis B, Voll RE, Herrmann M, Nawroth PP, Arnold B, Bianchi ME, Manfredi AA, Rovere-Querini P. Release of high mobility group box 1 by dendritic cells controls T cell activation via the receptor for advanced glycation end products. J Immunol. 2005;174:7506-7515. [PubMed] |
| 26. | Yang D, Chen Q, Yang H, Tracey KJ, Bustin M, Oppenheim JJ. High mobility group box-1 protein induces the migration and activation of human dendritic cells and acts as an alarmin. J Leukoc Biol. 2007;81:59-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 305] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 27. | Hori O, Brett J, Slattery T, Cao R, Zhang J, Chen JX, Nagashima M, Lundh ER, Vijay S, Nitecki D. The receptor for advanced glycation end products (RAGE) is a cellular binding site for amphoterin. Mediation of neurite outgrowth and co-expression of rage and amphoterin in the developing nervous system. J Biol Chem. 1995;270:25752-25761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 855] [Cited by in RCA: 924] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 28. | Park JS, Svetkauskaite D, He Q, Kim JY, Strassheim D, Ishizaka A, Abraham E. Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J Biol Chem. 2004;279:7370-7377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1174] [Cited by in RCA: 1264] [Article Influence: 57.5] [Reference Citation Analysis (0)] |
| 29. | Fehrenbach H, Weiskirchen R, Kasper M, Gressner AM. Up-regulated expression of the receptor for advanced glycation end products in cultured rat hepatic stellate cells during transdifferentiation to myofibroblasts. Hepatology. 2001;34:943-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 129] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 30. | Yang H, Hreggvidsdottir HS, Palmblad K, Wang H, Ochani M, Li J, Lu B, Chavan S, Rosas-Ballina M, Al-Abed Y. A critical cysteine is required for HMGB1 binding to Toll-like receptor 4 and activation of macrophage cytokine release. Proc Natl Acad Sci USA. 2010;107:11942-11947. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 660] [Cited by in RCA: 661] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 31. | Han J, Zhong J, Wei W, Wang Y, Huang Y, Yang P, Purohit S, Dong Z, Wang MH, She JX. Extracellular high-mobility group box 1 acts as an innate immune mediator to enhance autoimmune progression and diabetes onset in NOD mice. Diabetes. 2008;57:2118-2127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 85] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 32. | Tsung A, Klune JR, Zhang X, Jeyabalan G, Cao Z, Peng X, Stolz DB, Geller DA, Rosengart MR, Billiar TR. HMGB1 release induced by liver ischemia involves Toll-like receptor 4 dependent reactive oxygen species production and calcium-mediated signaling. J Exp Med. 2007;204:2913-2923. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 455] [Cited by in RCA: 523] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 33. | Kao YH, Jawan B, Goto S, Hung CT, Lin YC, Nakano T, Hsu LW, Lai CY, Tai MH, Chen CL. High-mobility group box 1 protein activates hepatic stellate cells in vitro. Transplant Proc. 2008;40:2704-2705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 34. | Jackson AL, Burchard J, Schelter J, Chau BN, Cleary M, Lim L, Linsley PS. Widespread siRNA "off-target" transcript silencing mediated by seed region sequence complementarity. RNA. 2006;12:1179-1187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 694] [Cited by in RCA: 729] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 35. | Tschuch C, Schulz A, Pscherer A, Werft W, Benner A, Hotz-Wagenblatt A, Barrionuevo LS, Lichter P, Mertens D. Off-target effects of siRNA specific for GFP. BMC Mol Biol. 2008;9:60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 36. | De Paula D, Bentley MV, Mahato RI. Hydrophobization and bioconjugation for enhanced siRNA delivery and targeting. RNA. 2007;13:431-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 165] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
Peer reviewers: Ekihiro Seki, MD, PhD, Department of Med-icine, University of California San Diego, Leichag Biomedical Research Building Rm 349H, 9500 Gilman Drive MC#0702, La Jolla, CA 92093-0702, United States; London Lucien Ooi, Professor, Chairman, Division of Surgery, Singapore General Hospital, 1 Hospital Drive, 169608, Singapore
S- Editor Tian L L- Editor Kerr C E- Editor Li JY